Abstract
Background
Management of hepatocellular carcinoma (HCC) often involves many subspecialist providers, as well as a broad range of treatment options. This study sought to evaluate referral and treatment patterns among patients with HCC at a large academic medical center.
Methods
Data from our cancer registry between 2003–2011 were abstracted on 394 patients who were primarily diagnosed/treated for HCC at Johns Hopkins Hospital (JHH); data on patients who were diagnosed/treated with HCC elsewhere and who received secondary treatment at JHH (n=391) were also abstracted for comparison purposes.
Results
Among the main cohort, the most common specialties to be consulted were surgery (n=225, 57.1 %), gastroenterology (n=225, 57.1 %), and interventional radiologist (n=206, 52.3 %), while only 96 (24.4 %) were referred to medical oncology. Factors associated with surgical consultation included younger age (odds ratio (OR) 3.35, 95 % CI 1.62–6.92), tumor size <5 cm (OR 1.82, 1.09–3.02), and unilobar disease (OR 2.94, 1.31–6.59) (all P<0.05). Patients initially diagnosed/treated elsewhere had larger tumors (4 vs. 6 cm), bilateral disease (19.2 vs. 26.8 %), and were more likely to be seen by interventional radiology (all P<0.05)
Conclusions
Most patients were seen by surgeons, gastroenterologists, or interventional radiologists, with only a minority being seen by medical oncologists. Referral patterns depended on patient-level factors, as well as extent of disease.
Keywords: Hepatocellular, Carcinoma, Referral patterns, Consultation
Introduction
Worldwide, hepatocellular carcinoma (HCC) represents 5 % of all cancers, with annual cases exceeding 500,000.1 In the US, the incidence of HCC has been rising over the past three decades and currently represents the fastest growing cause of cancer-related deaths among men.2,3 Patients with HCC have a broad range of management options including resection, ablation, transplantation, chemoembolization, systemic chemotherapy, as well as the possible need for antiviral therapies for patients with hepatitis. As such, many patients with HCC may benefit from seeing a wide scope of physician specialists. In fact, HCC frequently requires multidisciplinary care involving expertise from specialists including surgical oncologists, transplant surgeons, interventional radiologists, gastroenterologists, hepatologists, radiation oncologists, and medical oncologists.4
To date, there has been a lack of comprehensive information on factors affecting referral to specialists and subsequent receipt of treatment in patients with HCC. A recent survey-based study of surgeons treating HCC revealed that choice of therapy for patients with early HCC was influenced by both provider level factors, including practice type, patient volume, and provider subspecialty.5 Other studies have suggested that nonclinical factors can also significantly impact choice of therapy for HCC and account for some of the variation in therapy choice across providers that are not accounted for by differences in tumor-specific factors.6 Variation in choice of therapy and its relation to specialist referral is particularly relevant to understanding patterns of care for patients with HCC. Unlike the management of many other malignancies, in which there is general consensus on treatment recommendations, the National Comprehensive Cancer Network’s clinical practice guidelines in oncology recommends an array of potential treatment options for both resectable (e.g., ablation, resection, transplantation, etc.) and nonresectable (intraarterial therapy (IAT), sorafenib, etc.) HCC.7 In fact, large geographic and institutional variations in the treatment of HCC have been noted.8 The underlying relation of provider referral patterns and treatment recommendations and choice of therapy for HCC, however, remains poorly understood.
Understanding processes of care, as well as referral patterns, has been suggested as both a means to standardize and optimize cancer care.9 Accordingly, studies aimed at defining the pattern of care and the overall trajectory of medical and surgical care for patients with HCC from the time of diagnosis through treatment may help provide insight into understanding practice pattern variation. In turn, such studies may better elucidate some of the underlying causes of heterogeneity in patient care around HCC. With this in mind, the current study sought to evaluate the referral and treatment patterns among patients with HCC utilizing an institution-based tumor registry.
Methods
Study Design
Data was abstracted from the Johns Hopkins Hospital (JHH) cancer registry for patients diagnosed with liver cancer between the years 2003 and 2011. This registry contains data on all cancer patients who were diagnosed at, treated at, or underwent some combination of diagnostic workup or treatment at JHH. The registry is continually updated by trained personnel according to the Commission on Cancer’s 2012 Facility Oncology Registry Standards.10 Data from the registry are accessible by request from investigators at Johns Hopkins Medical Institutions. The study protocol was approved by the Johns Hopkins medicine institutional review board.
Data were abstracted on the 394 patients who were primarily diagnosed and treated at the JHH; data on patients who were diagnosed and/or treated elsewhere and received secondary consultation or treatment at JHH (n=391) were also abstracted for comparison purposes. Patient identifiers provided within the registry data were used to access institutional medical records for each patient in order to obtain variables that were not recorded in the registry, including details of HCC staging and treatment, dates of specialist visits, and dates of procedures.
Technical Information
Treatment referred to the cancer-directed therapy for HCC management during the course of disease. Limited disease was defined by a previous validated algorithm11 and included patients without any of the following: metastatic disease, tumors larger than 5 cm, nodal metastasis, extrahepatic extension, and major vascular invasion. To summarize burden of comorbid diseases, a modified Elixhauser classification system was used to calculate a single numeric score for patient comorbidities.12 Those with a modified Elixhauser score at the 75th percentile or greater were defined as having a high comorbidity burden.
Patients were considered to have consulted a specialist if medical record documentation of a provider visit was present on or subsequent to the date of diagnosis. Surgeon consultation included general surgeons, surgical oncologists, and transplant surgeons. Visits to a gastroenterologist were recorded only if relevant to liver pathology. Other specialties recorded were interventional radiology, medical oncology, and radiation oncology. The order of specialists seen, from first to fifth, was recorded; however, we did not distinguish the order of multiple consultations on a single day. Time to specialist visit and time to treatment were measured in days from the recorded date of diagnosis. Type of IAT and associated IAT–chemotherapy regimens were recorded from procedure notes.
Statistical Analysis
Data were organized via summary statistics using medians and percentages as appropriate with corresponding interquartile range (IQR) or as frequency distributions for continuous and categorical variables, respectively. Rates of consultation and treatments were determined for the whole cohort of abstracted patients, as well as for subgroups determined by whether initial HCC diagnosis was made at JHH versus an outside hospital. Differences in patient, demographic, and disease-related characteristics were assessed with the chi-square test and the Wilcoxon rank sum test, as appropriate. Logistic regression was used to evaluate the association of multiple variables on referral and receipt of treatment. Association of patient survival and other variables were analyzed using Cox regression analysis and reported as hazard ratios (HR) and corresponding 95 % confidence intervals (95 % CI). Statistical analysis was performed using SAS version 9.3 software (SAS Institute, Cary, NC). A P value of less than 0.05 was considered to be statistically significant, and all P values were two-sided.
Results
Patient Characteristics
Characteristics and demographics of the study cohort are shown in Table 1. Among the 394 patients who were primarily diagnosed and treated at the JHH, the majority was male (n=306, 77.7 %), and median age at diagnosis was 57 years (IQR 52–65 years). The most common race in the cohort was white (n=235, 59.6 %). Local patients (living in Delaware, Maryland, Pennsylvania, Virginia, or District of Columbia) comprised the majority of cases (n=367, 93.2 %). Married patients (n=227, 57.6 %) were more common than single, divorced, or widowed patients. The cohort exhibited a high prevalence of history of tobacco use (n=254, 64.5 %) and history of alcohol use (n=228, 57.9 %). The annual number of patients who were primarily diagnosed and treated at the Johns Hopkins Hospital was 44; there was a trend toward an increasing number of new HCC cases per year over the study period (2003–2005, 31/year vs. 2005–2006, 44/year vs. 2007–2008, 44/year vs. 2009–2011, 52/year; P<0.05 for trend).
Table 1.
Comparison of patient characteristics based on diagnosis at JHH versus elsewhere
| Diagnosis at JHH (n=394) | Diagnosis elsewhere (n=391) | P value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age at diagnosis, years, median (IQR) | 57 (52–65) | 62 (55–71) | <0.001 |
| Male gender | 306 | 298 | 0.63 |
| Race | |||
| White | 235 | 266 | <0.001 |
| Black | 128 | 74 | |
| Asian | 27 | 43 | |
| Other/unknown | 4 | 8 | |
| Marital status | |||
| Married | 227 | 264 | 0.06 |
| Single, never married | 86 | 68 | |
| Divorced or separated | 45 | 30 | |
| Widowed | 23 | 21 | |
| Unknown | 13 | 8 | |
| Clinical characteristics | |||
| Any history of tobacco use | 254 | 241 | 0.02 |
| Any history of alcohol use | 228 | 236 | 0.37 |
| Comorbidities | |||
| Hepatitis | |||
| HCV infection | 138 | 104 | 0.04 |
| HBV infection | 38 | 38 | |
| Other or unspecified hepatitis | 22 | 12 | |
| Cirrhosis | |||
| Alcoholic cirrhosis | 43 | 38 | 0.85 |
| Nonalcoholic cirrhosis | 101 | 102 | |
| Ascites | 10 | 13 | 0.51 |
| Cancer characteristics | |||
| Staging | |||
| Nodal disease | 44 | 62 | 0.05 |
| Extrahepatic disease | 44 | 58 | 0.13 |
| Metastatic disease | 37 | 53 | 0.07 |
| Multiple tumor foci | 111 | 158 | <0.001 |
| Bilobar disease | 76 | 102 | 0.02 |
| Size ≥ 5 cm | 146 | 238 | <0.001 |
| Limited/potentially curable disease | 210 | 124 | <0.001 |
Most patients had one or more comorbidities documented (n=258, 65.5 %) and a high comorbidity burden (n=181, 45.9 %) as represented by a modified Elixhauser comorbidity score of 11 or greater. Liver-related comorbidities were common, with viral hepatitis present in 186 (47.2 %) and cirrhosis in 144 (36.5 %) patients. Roughly one half of patients had early stage HCC disease (n=210, 53.2 %). Patients with advanced disease had metastatic disease (n=37, 20.1 %), tumors larger than 5 cm (n=147, 80.8 %), nodal metastasis (n=44, 23.9 %), and extrahepatic extension (n=44, 23.9 %).
Compared with patients who were initially diagnosed and managed at JHH (n=394), patients who were initially diagnosed and/or treated at an outside hospital and only subsequently referred to JHH (n=391) had a number of different clinical and tumor-specific characteristics (Table 1). Specifically, patients diagnosed elsewhere were older (median age, 57 vs. 62 years) and were more likely to be White (59.6 vs. 68.0 %) (both P<0.05). Patients initially diagnosed and/or treated elsewhere were also more likely to have advanced disease (46.7 vs. 68.3 %), larger tumors (4 vs. 6 cm), bilateral disease (19.2 vs. 26.8 %), and multifocal disease (28.2 vs. 40.4 %) (all P<0.05).
Referral Patterns
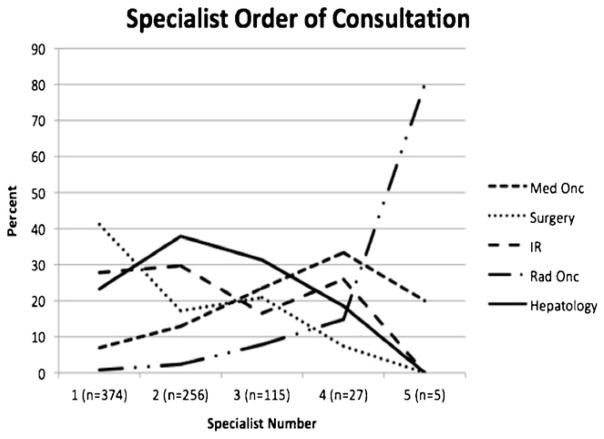
Patient consultations with provider specialists are shown in Table 2. Among the 394 patients who were primarily diagnosed and treated at JHH, the most common specialties to be consulted following diagnosis were surgery (n=225, 57.1 %) and gastroenterology (n=225, 57.1 %). While over half of patients saw an interventional radiologist (n=206, 52.3 %), only 96 (24.4 %) were referred to medical oncology. Eighty-three patients (21.1 %) saw one specialist, 151 (38.3 %) saw two specialists, 93 (23.6 %) saw three specialists, 22 (5.6 %) saw four specialists, and 5 (1.3 %) saw all five specialists. Among patients who saw multiple specialists, the most common combination was surgery and interventional radiology (13.7 %). For patients who saw multiple specialists, the average time between the first and second consultation with any specialist was 98 days (range, 0 to 1,167 days; median, 64 days). The order in which patients were seen by the different specialists is shown in Fig. 1. Of note, surgeons, interventional radiologists, and gastroenterologists were more likely to be the first and second specialists consulted, whereas medical oncologists and radiation oncologists were more likely to be consulted later in the patient’s clinical course (e.g., fourth and fifth specialists). Compared with patients who were initially diagnosed and managed at JHH, patients initially diagnosed and/or treated elsewhere had different patterns of care (Table 2). Specifically, patients initially diagnosed and/or treated at an outside hospital were more likely to have a consult with an interventional radiologist (52.3 vs. 79.8 %) and were less likely to have a consult with a gastroenterologist (57.1 vs. 43.4 %), when seen at JHH (both P<0.05).
Table 2.
Comparison of consultations and treatment based on diagnosis at JHH versus elsewhere
| Diagnosis at JHH (n=394) | Diagnosis elsewhere (n=391) | P value | |
|---|---|---|---|
| Types of specialists consulted following diagnosis | |||
| Seen by surgeon | 225 | 214 | 0.50 |
| Seen by interventional radiologist | 206 | 312 | <0.001 |
| Seen by medical oncologist | 96 | 105 | 0.42 |
| Seen by radiation oncologist | 26 | 36 | 0.18 |
| Seen by gastroenterologist | 225 | 171 | <0.001 |
| Treatment | |||
| Surgery | |||
| Transplant | 93 | 34 | <0.001 |
| Lobectomy | 20 | 23 | |
| Wedge or segmental resection | 41 | 37 | |
| Other treatment | |||
| nterventional radiology (IAT, TACE, TAE) | 148 | 261 | <0.001 |
| Percutaneous ablation | 7 | 8 | |
| Radiation | 3 | 0 | |
| Supportive care only | 75 | 20 | |
Fig. 1.
Percentage of specialist consultations representing the first through fifth referral for each patient, by specialist type
Several factors were associated with consultation patterns (Table 3). Among the 210 patients categorized with early-stage disease, a lower proportion was seen by an interventional radiologist (47.2 %), while a higher proportion saw a surgeon (73.3 %). Proportionally, patients who were younger and who had smaller tumor diameter were more likely to see a surgeon compared with a medical oncologist. In contrast, more patients with advanced disease were referred to interventional radiology (58.2 %) or best supportive care (21.7 %). On further analysis, several factors were noted to be associated with the odds of referral to a specific specialist (Table 4). Surgical consultation was more common among younger patients (age 50–55 year old, odds ratio (OR)=3.35, 95 % CI 1/62–6.92 vs. >66 years) (P=0.01). While younger age was also associated with a higher likelihood of referral to interventional radiology, older patients seemed to be more commonly referred to medical oncology or best supportive care. One of the factors most strongly associated with referral pattern was extent of disease. Patients with tumors <5 cm (OR=1.82, 95 % CI 1.09–3.02) and unilateral disease (OR=2.94, 95 % CI 1.31–6.59) were more likely to be seen by a surgeon than patients with more advanced disease (all P<0.05). Among those patients with early stage disease, 56 (26.7 %) were never referred to a surgeon; most of these patients had a significant comorbidity burden (n=40, 71.4 %). In contrast, patients with multifocal disease (OR=2.37, 95 % CI 1.17–4.78) and those with bilobar disease (OR 2.60, 95 % CI 1.19–5.67) were more likely to be seen by interventional radiology (both P<0.05). The proportion of patients with a high comorbidity burden who were referred to surgery was higher than interventional radiology (56.9 vs. 49.7 %; P=0.01).
Table 3.
Comparison of patient and disease characteristics by specialists seen and therapy received among patients primarily diagnosed and treated at JHH.
| Demographic characteristics | Seen by surgeon (n=225) | Surgery (n=166) | Seen by interventional radiologist (n=206) | Intraarterial therapy (n=201) | Seen by medical oncologist (n=96) |
|---|---|---|---|---|---|
| Age at diagnosis, years | |||||
| ≤50 | 50 (22.2) | 35 (21.1) | 34 (16.5) | 35 (17.4) | 16 (16.7) |
| 51–55 | 50 (22.2) | 41 (24.7) | 39 (18.9) | 36 (17.9) | 18 (18.8) |
| 56–60 | 45 (20.0) | 31 (18.7) | 41 (19.9) | 42 (20.9) | 21 (21.9) |
| 61–65 | 41 (18.2) | 30 (18.1) | 39 (18.9) | 37 (18.4) | 18 (18.8) |
| ≥66 | 39 (17.3) | 29 (17.5) | 53 (25.7) | 51 (26.4) | 23 (23.9) |
| Male gender | 161 (71.6) | 112 (67.5) | 164 (79.6) | 162 (80.6) | 70 (72.9) |
| Race | |||||
| White | 153 (68.0) | 116 (69.9) | 121 (58.7) | 117 (58.2) | 58 (60.4) |
| Black | 51 (22.7) | 33 (19.9) | 68 (33.0) | 68 (33.8) | 30 (31.3) |
| Asian | 19 (8.4) | 17 (10.2) | 15 (7.3) | 13 (6.5) | 7 (7.3) |
| Married | 154 (68.4) | 122 (73.5) | 121 (58.7) | 120 (59.7) | 62 (64.6) |
| Instate | 205 (91.1) | 150 (90.4) | 193 (93.7) | 188 (93.5) | 85 (88.5) |
| Clinical characteristics | |||||
| History of tobacco use | 124 (55.1) | 93 (56.0) | 133 (64.6) | 131 (65.2) | 61 (63.5) |
| History of alcohol use | 129 (57.3) | 90 (54.2) | 110 (53.4) | 109 (54.2) | 46 (47.9) |
| Viral hepatitis | 92 (40.9) | 68 (41.0) | 89 (43.2) | 86 (42.8) | 38 (39.6) |
| Cirrhosis | |||||
| Alcoholic cirrhosis | 23 (10.2) | 18 (10.8) | 17 (8.3) | 18 (9.0) | 5 (5.2) |
| Nonalcoholic cirrhosis | 60 (20.5) | 46 (27.7) | 57 (27.7) | 54 (26.9) | 21 (21.9) |
| Ascites | 4 (1.8) | 3 (1.8) | 4 (1.9) | 4 (2.0) | 1 (1.0) |
| Cancer characteristics | |||||
| Staging | |||||
| Nodal disease | 11 (5.0) | 4 (2.4) | 22 (10.7) | 23 (11.4) | 15 (15.6) |
| Extrahepatic disease | 11 (5.0) | 4 (2.4) | 22 (10.7) | 24 (11.9) | 15 (15.6) |
| Metastatic disease | 3 (1.3) | 0 (0.0) | 16 (7.8) | 16 (8.0) | 15 (15.6) |
| Multiple tumor foci | 40 (17.8) | 18 (10.8) | 67 (32.5) | 70 (34.8) | 28 (29.2) |
| Bilobar disease | 20 (8.9) | 9 (5.4) | 39 (18.9) | 42 (20.9) | 22 (22.9) |
| Size ≥ 5 cm | 60 (26.7) | 34 (20.5) | 88 (42.9) | 90 (45.0) | 48 (50.1) |
| Limited disease | 154 (68.4) | 129 (77.7) | 99 (48.0) | 93 (46.3) | 35 (36.4) |
Figures in bold were significant at P<0.05
Table 4.
Odds ratios from multivariate logistic regression analysis among patients primarily diagnosed and treated at JHH (n=394)
| Surgery | Seen by Surgeon | Surgery and seen by surgeon | Intraarterial therapy | Seen by IR | Intraarterial therapy and seen by IR | Seen by medical oncologist | Seen by GI | |
|---|---|---|---|---|---|---|---|---|
| Age, years | ||||||||
| ≤50 | 2.18 (1.05–4.52) | 3.35 (1.62–6.92) | 1.46 (0.55–3.85) | 0.67 (0.35–1.28) | 0.56 (0.29–1.08) | 1.09 (0.16–7.49) | 0.92 (0.43–1.97) | 2.57 (1.30–5.05) |
| 51–55 | 1.64 (0.78–3.45) | 1.75 (0.89–3.45) | 2.18 (0.73–6.49) | 0.52 (0.28–0.97) | 0.53 (0.29–1.00) | 0.29 (0.06–1.37) | 0.92 (0.44–1.93) | 2.55 (1.33–4.87) |
| 56–60 | 1.08 (0.50–2.33) | 1.95 (0.96–3.97) | 1.25 (0.43–3.63) | 0.97 (0.51–1.89) | 0.81 (0.42–1.55) | 3.20 (0.30–34.1) | 1.35 (0.64–2.82) | 3.16 (1.59–6.28) |
| 61–65 | 1.39 (0.64–3.01) | 2.15 (1.04–4.46) | 1.31 (0.46–3.70) | 0.88 (0.46–1.71) | 0.88 (0.46–1.71) | 0.64 (0.12–3.33) | 1.03 (0.49–2.20) | 3.92 (1.93–7.98) |
| ≥66 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Male sex | 0.42 (0.24–0.73) | 0.53 (0.30–0.93) | 0.44 (0.20–0.96) | 1.37 (0.83–2.25) | 1.26 (0.77–2.08) | 1.40 (0.38–5.24) | 0.69 (0.39–1.21) | 1.27 (0.75–2.14) |
| White Race | 2.20 (1.35–3.59) | 2.37 (1.49–3.80) | 2.05 (1.03–4.10) | 0.93 (0.61–1.43) | 0.93 (0.61–1.43) | 1.86 (0.60–5.78) | 1.16 (0.70–1.91) | 1.36 (0.87–2.13) |
| Cirrhosis | 1.20 (0.72–2.00) | 1.03 (0.64–1.68) | 1.13 (0.57–2.24) | 1.08 (0.69–1.67) | 1.09 (0.71–1.69) | 1.20 (0.38–3.78) | 0.58 (0.34–0.99) | 1.53 (0.96–2.44) |
| Single tumor | 2.72 (1.26–5.87) | 1.26 (0.62–2.55) | 3.45 (1.20–9.93) | 0.38 (0.19–0.77) | 0.42 (0.21–0.85) | 0.11 (0.01–1.28) | 1.72 (0.79–3.76) | 1.00 (0.51–1.98) |
| Unilobar disease | 2.26 (0.86–5.95) | 2.94 (1.31–6.59) | 1.76 (0.42–7.38) | 2.23 (1.02–4.88) | 2.55 (1.17–5.56) | 3.55 (0.31–40.1) | 0.63 (0.27–1.47) | 1.63 (0.76–3.50) |
| Node negative disease | 6.18 (2.02–18.9) | 3.41 (1.54–7.54) | 3.06 (0.73–12.7) | 1.14 (0.58–2.23) | 1.24 (0.63–2.41) | 1.59 (0.30–8.39) | 0.66 (0.32–1.35) | 1.34 (0.67–2.68) |
| Size <5 cm | 2.19 (1.27–3.76) | 1.82 (1.09–3.02) | 2.14 (1.03–4.46) | 0.60 (0.37–0.96) | 0.66 (0.41–1.06) | 0.72 (0.21–2.49) | 0.46 (0.27–0.79) | 2.47 (1.52–4.02) |
Figures in bold were significant at P<0.05
Patterns of Treatment and Long-term Outcome
Among the 394 patients who were primarily diagnosed and treated at the JHH, the most common type of HCC treatment was surgery (n=166, 42.1 %) (index procedure, n=154 vs. secondary procedure, n=12). Among the 154 patients undergoing surgery as the index procedure, 93 underwent transplantation, while 61 underwent resection. IAT was performed in 148 (37.6 %) patients as the index treatment modality, with the number of IAT procedures ranging from 1 to 9. Among patients who underwent IAT, 59 (39.9 %) had multifocal disease. Of those receiving IAT, the median number of treatments was 2. Median time to first IAT was 36 days. Of all IAT procedures performed in the cohort (n=440), most utilized a combination of cisplatin, doxorubicin, and mitomycinc (n=262, 59.5 %) or doxorubicin-eluding beads (n=160, 36.4 %). Seventy-five (19 %) patients received supportive care only. Of note, in contrast with patients who were initially diagnosed and managed at JHH, patients who were initially diagnosed and/or treated at an outside hospital and only subsequently referred to JHH were more likely to undergo IAT (6.2 vs. 37.6 %) and less likely to undergo surgery (24.1 vs. 39.1 %).
Consultation patterns of patients seeing certain specialists partly explained treatment choices among patients (Table 3). Patients who had a single tumor were over 3-fold more likely to undergo surgery following consultation with a surgeon as compared to those with multifocal disease (OR 3.45, 95 % CI 1.20–9.93). Race also impacted receipt of surgical therapy, as white patients had higher rates of surgery compared with patients of all other races on multivariate analysis (P=0.04). Similarly, female patients were also more likely to receive surgery after consultation with a surgeon (OR 2.26, 95 % CI 1.04–4.94; P=0.04). Tumor diameter ≥5 cm and multifocal disease had the biggest effect on likelihood of receipt of IAT (both P<0.05).
Overall survival was 14.2 months (95 % CI 11.2–19.2 months). Survival among patients undergoing resection or transplantation was 47.0 months (95 % CI 35.0–89.2 months). Tumor-specific factors were the strongest predictors of survival. On univariate analyses, tumor size, number, and the presence of vascular invasion all impacted long-term outcome. Specifically, among patients undergoing resection or transplantation, patients with multiple tumors (HR 1.76, 95 % CI 1.02–3.00) had an increased risk of death (all P<0.05). Following IAT, overall survival was 11.0 months (95 % CI 8.8–13.2 months), and similar factors were associated with outcome. In particular, patients with large tumors (HR 2.19, 95 % CI 1.48–3.25) and those with multifocal disease (HR 1.77, 95 % CI 1.20–2.63) had worse long-term outcomes (both P<0.05). Other covariates such as sex, race, and cirrhosis did not influence survival (all P>0.05) (Table 5).
Table 5.
Hazard ratios from survival analysis among patients primarily diagnosed and treated at JHH (n=394)
| HR univariate | HR adjusted | HR adjusted for Tx | |
|---|---|---|---|
| ≤50 | 0.65 (0.44–0.96) | 0.64 (0.43–0.95) | 0.64 (0.42–0.98) |
| 51–55 | 0.85 (0.59–1.21) | 0.83 (0.57–1.19) | 0.75 (0.51–1.12) |
| 56–60 | 1.01 (0.70–1.45) | 0.99 (0.68–1.43) | 0.94 (0.64–1.39) |
| 61–65 | 0.81 (0.55–1.21) | 0.88 (0.59–1.31) | 0.89 (0.59–1.35) |
| ≥66 | Reference | Reference | Reference |
| Male sex | 1.68 (1.22–2.31) | 1.38 (0.99–1.93) | 1.11 (0.79–1.56) |
| White race | 0.79 (0.61–1.01) | 0.80 (0.62–1.03) | 0.97 (0.75–1.27) |
| Cirrhosis | 1.26 (0.98–1.62) | 1.26 (0.97–1.64) | 1.23 (0.94–1.61) |
| Multiple tumors | 2.19 (1.68–2.84) | 1.31 (0.89–1.92) | 1.25 (0.87–1.80) |
| Bilobar disease | 2.63 (1.98–3.50) | 1.41 (0.93–2.14) | 0.97 (0.65–1.44) |
| Node positive disease | 2.74 (1.93–3.90) | 2.00 (1.39–2.90) | 1.26 (0.84–1.88) |
| Size ≥5 cm | 1.98 (1.54–2.54) | 1.63 (1.23–2.16) | 1.66 (1.23–2.23) |
Discussion
Understanding referral patterns of patients to cancer specialists may have important implications for utilization of health care resources, optimization of clinical outcomes, and reduction of unwarranted variation. Unfortunately, knowledge of referral patterns for specialty care is uncommon. In one study, Haymart et al. examined the referral patterns for patients with high risk thyroid cancer, while in a separate study, Mandl reported on who should be referred for total hip and knee replacements.13–17 Surprisingly, little data have been reported on the referral patterns among patients with cancer. Patients with cancer frequently benefit from a multi-disciplinary approach with physician input from a range of providers including surgery, medical oncology, and radiation oncology, among others. The case for multidisciplinary care is even more pronounced for patients with HCC.18 Treatment of HCC often includes possible surgical, intraarterial, and systemic options. As such, patients often benefit from being seen by various providers. Despite this, some studies have suggested that up to 20–50 % of patients—even with early stage disease—receive no therapy for HCC.11,19 The current study is important because we define the referral and treatment patterns among patients with HCC utilizing an institution-based tumor registry at a major cancer center. We found that over one half of patients had consultation with surgery and/or interventional radiology, while only about 25 % had a referral to medical oncology. Of note, although patients with early stage disease were more likely to be referred to surgery, about one quarter never was referred to a surgeon. Understanding variations in patient care, referral patterns, and utilization of physician services is important and can help ensure optimal delivery of care. In the current study, we noted that up to one quarter of patients with early HCC did not get referred to a surgeon. Previous work from our group using population-based SEER data had similarly suggested that there is a significant missed opportunity to improve survival of patients with early HCC through the use of surgical therapy.11 The data in the current study are important not only to surgeons, but also other health care providers/referring doctors who care for patients with HCC. The data elucidate current referral patterns, while highlighting which factors are associated with referral and receipt of therapy for HCC.
A particular strength of the current study was the use of our institution-based tumor registry. The use of registries has been highlighted as an important tool for monitoring and improving health care delivery through several means, including studies on the patterns of care.20 Tumor registries allow for more accurate data collection and management, as the registry is continually updated by trained personnel according to the Commission on Cancer’s 2012 Facility Oncology Registry Standards.21 Unlike some traditional department-based databases that are procedure based (e.g., surgery, IAT, etc.), the cancer registry allowed us to survey all HCC cases across the institution, independent of which provider saw the patient. In addition to the cancer registry data, we also augmented the data with re-review of the medical records. Previous studies have demonstrated the importance and usefulness of registry studies to examine provider practices.22 As such, we were able to analyze a comprehensive and truly representative population of HCC patients seen at our institution. In turn, we were able to explore patterns of care and elucidate provider-specific consultation patterns across the continuum of care in a “real-world” cancer center setting.
Among patients who were primarily diagnosed and treated at the JHH, the overall distribution of referrals among the various specialists was predominantly surgery (57.1 %), gastroenterology (57.1 %), and interventional radiologist (52.3 %). Perhaps not surprisingly, one of the factors that impacted referral to a surgeon the most was stage of disease (Table 3). In fact, patients with early stage of disease were more often seen by a surgeon, as surgical consultation was roughly 2-fold more likely among patients with a small, unilateral HCC. Interestingly, however, up to one quarter of patients with early stage disease never saw a surgeon. While the reason for this is undoubtedly multifactorial, it was probably due in part to the fact that this subset of patients with early HCC had a significant comorbidity burden and may have been deemed not appropriate for surgical consideration based on medical factors. When examining patients with advanced disease, these individuals were more likely to be seen by interventional radiology. Both the presence of multi-focal tumors and bilobar disease were associated with over a 2-fold higher referral rate to interventional radiology compared with another subspecialty. In fact, 58.2 % of patients with advanced disease were seen by an interventional radiologist at some point in their care compared with only 33.2 % for medical oncology. In looking at the overall pattern of referral, of particular interest was the high number of patients who saw multiple providers (78.9 %), with many patients seeing two (38.3 %) or three (23.6 %) specialists. Within the last few years, the Johns Hopkins Liver Tumor Center established a single-day multidisciplinary clinic in response to this need of patients to be seen by a range of specialists.18 The multidisciplinary liver clinic has had a significant impact on management of patients with liver tumors, resulting in alternations to imaging and pathology interpretation, diagnosis, and the management plan in a subset of patients.18
Differences in referral patterns were noted for both age and race. In contrast to using claims data such as Medicare, we were able to include patients of all ages with HCC. Interestingly, the most common age group of HCC diagnosis in the present cohort was 51–55 years, and 74 % of patients were <65 at the time of diagnosis. We noted that patients ≤ 50 years old were more likely to either see a surgeon or have surgery than those older than 65 years. Some of these differences could be explained by the fact that few patients ≤ 50 years old (34.5 %) had significant medical comorbidities compared with patients older than 65 years of age (56.2 %). Prior research has noted that older patient age has a negative impact on likelihood that patients will receive the standard of care in cancer.23 Moreover, in a separate study of HCC among older patients, other authors noted an underutilization of curative intent therapy.24 Significant differences in referral patterns were also noted based on race. Black patients were less likely to be seen by a surgeon. In addition, among those seen by a surgeon, race also impacted receipt of surgical therapy, as white patients had higher rates of surgery compared with patients of all other races on multivariate analysis (P=0.04). Racial disparities have previously been well-documented in the use of various cancer screening programs and therapies.25–28 In fact, in their study examining referral patterns and therapy choices among patients with esophageal cancer, Steyerberg et al. similarly noted that both age and race were significant factors that impacted disparities in treatment and outcome.15 The reasons underlying racial disparities among cancer patients remain poorly defined, but are undoubtedly multifactorial and related to differences in medical access, stage at presentation, as well as complex social and economic factors.
The current study had several limitations. We were unable to collect and analyze data on provider-level factors. Previous data have suggested that provider specific characteristics, such as years in practice and sex, can influence care patterns.13,14 Data on the types, dosing, and timing of system chemotherapy were also difficult to ascertain. Given the level of heterogeneity around systemic chemotherapy, we were not able to comment on chemotherapy details. The current study also did not take into account patient preferences regarding referral with a specialist and subsequent treatment. Patient preferences are difficult, if not impossible, to determine in a retrospective analysis and therefore were not included. Finally, the current study focused on referral patterns of patients who were able to gain access to our health care system. As noted, compared with patients who were initially diagnosed and managed at JHH, patients who were initially diagnosed and/or treated at an outside hospital and only subsequently referred to JHH had different clinical and tumor-specific characteristics, as well as referral patterns. Data from the current study cannot be extrapolated to the population at large, as many of these patients may not have access to a high volume quaternary hepatopancreato-biliary cancer center.
In conclusion, specific patient and disease characteristics dictated patterns of care in HCC cancer management. Most patients were seen by surgeons, gastroenterologists, or interventional radiologists, with only a minority being seen by medical oncologists. Referral patterns for patients with HCC depended not only on the extent of disease but also on demographic factors including age and race. Given that specialist consultation and subsequent receipt of treatment has been correlated in other cancer types,29,30 data on referral patterns from the current study may help explain some variations in treatment among patients with HCC.
Contributor Information
Sylvie Stacy, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Omar Hyder, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
David Cosgrove, Department of Medical Oncology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Joseph M. Herman, Department of Radiation Oncology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Ihab Kamel, Department of Radiology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Jean-Francois H. Geschwind, Department of Interventional Radiology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Ahmet Gurakar, Department of Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Robert Anders, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Andrew Cameron, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Timothy M. Pawlik, Email: tpawlik1@jhmi.edu, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD, USA. Division of Surgical Oncology, Department of Surgery, Johns Hopkins Hospital, 600 N. Wolfe Street, Blalock 688, Baltimore, MD 21287, USA
References
- 1.Bruix J, Sherman M American association for the study of liver diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(Suppl):2S88–94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the US from 1975 to 2005. J Clin Oncol. 2009;27(9):1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23(13):2892–9. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- 5.Nathan H, Bridges JF, Schulick RD, Cameron AM, Hirose K, Edil BH, Wolfgang CL, Segev DL, Choti MA, Pawlik TM. Understanding surgical decision making in early hepatocellular carcinoma. J Clin Oncol. 2011;29(6):619–25. doi: 10.1200/JCO.2010.30.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan H, Segev DL, Bridges JF, Massie AB, Cameron AM, Hirose K, Schulick RD, Choti MA, Pawlik TM. Influence of nonclinical factors on choice of therapy for early hepatocellular carcinoma. Ann Surg Oncol. 2013;20(2):448–56. doi: 10.1245/s10434-012-2619-5. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology - Hepatobiliary Cancers. 2012. Version 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag HB, Siegel AB, Davila JA, Shaib YH, Cayton-Woody M, McBride R, McGlynn KA. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. Journal of Hepatology. 2006;44(1):158–166. doi: 10.1016/j.jhep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35(3):519–24. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-SEER 17 Regs Public Use, Nov. 2010 Sub (2000–2008)-Linked to County Attributes-Total US, 1969–2008 Counties. Bethesda, MD: National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch; 2011. [Google Scholar]
- 11.Nathan H, Hyder O, Mayo SC, Hirose K, Wolfgang CL, Choti MA, Pawlik TM. Surgical Therapy for Early Hepatocellular Carcinoma in the Modern Era: A 10-Year SEER-Medicare Analysis. Ann Surg. 2013 doi: 10.1097/SLA.0b013e31827da749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Medical Care. 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds GA, Chitnis JG, Roland MO. General practitioner outpatient referrals: do good doctors refer more patients to hospital? BMJ. 1991;302(6787):1250–2. doi: 10.1136/bmj.302.6787.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franks P, Clancy CM. Referrals of adult patients from primary care: demographic disparities and their relationship to HMO insurance. J Fam Pract. 1997;45(1):47–53. [PubMed] [Google Scholar]
- 15.Steyerberg EW, Neville B, Weeks JC, Earle CC. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol. 2007;25(17):2389–96. doi: 10.1200/JCO.2006.09.7931. [DOI] [PubMed] [Google Scholar]
- 16.Mandl LA. Determining who should be referred for total hip and knee replacements. Nat Rev Rheumatol. 2013 doi: 10.1038/nrrheum.2013.27. [DOI] [PubMed] [Google Scholar]
- 17.Haymart MR, Banerjee M, Yang D, Stewart AK, Griggs JJ, Sisson JC, Koenig RJ. Referral Patterns for Patients with High-Risk Thyroid Cancer. Endocrine Practice. 2013:1–19. doi: 10.4158/EP12288.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Mavros MN, Cosgrove D, Hirose K, Herman JM, Smallwood-Massey S, Karnel I, Gurakar A, Anders R, Cameron A. Impact of a single-day multidisciplinary clinic on the management of patients with liver tumors. Current Oncology. 2013;20(2):e123. doi: 10.3747/co.20.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cance WG, Stewart AK, Menck HR. The National Cancer Data Base Report on treatment patterns for hepatocellular carcinomas: improved survival of surgically resected patients, 1985–1996. Cancer. 2000;88(4):912–20. doi: 10.1002/(sici)1097-0142(20000215)88:4<912::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Bufalino VJ, Masoudi FA, Stranne SK, Horton K, Albert NM, Beam C, Bonow RO, Vern Davenport RL Girgus M, Ronarow GC, Krumholz HM, Legnini MW, Lewis WR, Nichol G, Peterson ED, Rumsfeld JS, Schwamm LH, Shahian DM, Spertus JA, Woodard PK, Yancy CW. The American Heart Association’s recommendations for expanding the applications of existing and future clinical registries: a policy statement from the American Heart Association. Circulation. 2011;123(19):2167–79. doi: 10.1161/CIR.0b013e3182181529. [DOI] [PubMed] [Google Scholar]
- 21.Facility Oncology Registry Data Standards. Chicago, IL: Commission on Cancer; 2012. [Google Scholar]
- 22.Gliklich R, Dreyer N. Registries for Evaluating Patient Outcomes. Agency for Healthcare Research and Quality; Rockville, MD: 2010. [PubMed] [Google Scholar]
- 23.Kurtz JE, Heitz D, Enderlin P, Imbert F, Nehme H, Bergerat JP, Dufour P. Geriatric oncology, general practitioners and specialists: current opinions and unmet needs. Crit Rev Oncol Hematol. 2010;75(1):47–57. doi: 10.1016/j.critrevonc.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Shah SA, Smith JK, Li Y, Ng SC, Carroll JE, Tseng JF. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer. 2011;117(5):1019–26. doi: 10.1002/cncr.25683. [DOI] [PubMed] [Google Scholar]
- 25.Nathan H, Frederick W, Choti MA, Schulick RD, Pawlik TM. Racial disparity in surgical mortality after major hepatectomy. J Am Coll Surgeons. 2008;207(3):312–319. doi: 10.1016/j.jamcollsurg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andaya AA, Enewold L, Zahm SH, Shriver CD, Stojadinovic A, McGlynn KA, Zhu K. Race and Colon Cancer Survival in an Equal-Access Healthcare System. Cancer Epidem Biomar. 2013 doi: 10.1158/1055-9965.EPI-13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer BA, Insel BJ, Hershman DL, Benson MC, Neugut AI. Racial disparities in the use of palliative therapy for ureteral obstruction among elderly patients with advanced prostate cancer. Support Care Cancer. 2013:1–9. doi: 10.1007/s00520-012-1666-6. [DOI] [PubMed] [Google Scholar]
- 28.Del Carmen MG, Avila-Wallace M. Effect of Health Care Disparities on Screening. Clin Obstet Gynecol. 2013;56(1):65–75. doi: 10.1097/GRF.0b013e31827af75a. [DOI] [PubMed] [Google Scholar]
- 29.Luo R, Giordano SH, Zhang DD, Freeman J, Goodwin JS. The role of the surgeon in whether patients with lymph node-positive colon cancer see a medical oncologist. Cancer. 2007;109(5):975–82. doi: 10.1002/cncr.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkpatrick HM, Aitelli CL, Qin H, Becerra C, Lichliter WE, McCollum AD. Referral patterns and adjuvant chemotherapy use in patients with stage II colon cancer. Clin Colorectal Cancer. 2010;9(3):150–6. doi: 10.3816/CCC.2010.n.020. [DOI] [PubMed] [Google Scholar]