Abstract
Context
Abuse of synthetic stimulant compounds resulting in significant toxicity is being increasingly reported by poison centers. Toxicologic assessment is complicated by inconsistent manufacturing processes and limited laboratory testing. We describe a case of self-reported exposure to 25-I (25I-NBOMe), a novel phenethylamine derivative, with subsequent quantification in serum.
Case details
An 18-year-old male presented to the emergency department (ED) with severe agitation and hallucinations after jumping out of a moving car. He was tachycardiac (150–160 bpm) and hypertensive (150–170 mm Hg systolic and 110 mg Hg diastolic), and required physical restraints and treatment with intravenous lorazepam administration. His symptoms gradually improved and vital signs returned to normal over 48 h, though he continued to have episodes of aggressiveness. An assay was developed by our analytical toxicology laboratory for 25-I, and serum obtained during ED evaluation and treatment was found to contain 0.76 ng/ml of 25-I.
Case discussion
For 25I-NBOMe, 25-I is a common abbreviation for 25I-NBOMe, which is a (n-benzyl) phenethylamine in the 2C “family.” Initially synthesized for research, cases of self-reported use of 25-I have recently appeared in the literature, some of which contain qualitative urine conf rmation. There are no commercially available quantitative assays, and no previous reports have published serum concentrations. 25-I is a potent new synthetic drug with apparent significant behavioral toxicity that can be detected and quantified in serum.
Keywords: CNS/Psychological, Organ/tissue specific, Complications of poisoning, Heart, Organ/tissue specific, Complications of poisoning
Introduction
In recent years, a large number of illicit congeners of amphetamine and cathinone have been sold as bath salts, plant food or fertilizer, insect repellent, pond cleaner, or vacuum freshener. Many of these new synthetic drugs belong to the class of beta-keto derivatives of amphetamine: methcathinone, mephedrone, ethcathinone, and pentedrone, as well as the methylenedioxy ring derivatives of amphetamine similar to methylenedioxymethamphetamine (MDMA, “Ecstasy”): methylone, ethylone, butylone, and pentylone.1 These drugs are available in small packets containing milligrams to gram quantities. They have become available via the internet or at convenience stores, gas stations, truck stops, tattoo parlors, and discount tobacco outlets, and are commonly sold as and referred to as bath salts with the disclaimer, “Not For Human Consumption.”1–3
Over the last 2–3 years, poison centers and emergency departments have reported numerous cases of severe poisonings in young adults who have ingested or smoked substituted amphetamine or cathinone designer drugs. 4,5 In most cases, diagnosis is presumptive since many patients are unable or refuse to provide a specific history. Additionally, timely laboratory testing to identify compounds in bath salts is not presently available. In response to this rising epidemic of drug abuse, the DEA issued in October 2011 a temporary schedule I status for the most commonly encountered drugs: mephedrone, methylone, and 3,4-methylenedioxypyrovalerone (MDPV). 5 Following adoption of new regulations, maufacturers of bath salts and other “disguised” commercial products change the composition of the products to new compounds, including non-regulated congeners of regulated drugs such as the “2C” family of drugs. 6,7
We report a case of self-reported use of a new designer drug, 25-I (25I-NBOMe, 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine) with laboratory confirmation in serum by high-pressure liquid chromatography with tandem mass spectrometry (LC/MS/MS). The structure of this designer compound is presented in Fig. 1. 25-I is one of the class of N-benzyl phenethylamine 2C derivatives that are potent serotonin 2A (5-HT2A) receptor agonists. 8,9 The 5-HT2A receptor has been closely linked to complex behaviors including working memory, cognitive processes, and affective disorders such as schizophrenia. These receptors are believed to mediate the primary effects of hallucinogenic drugs. 10 The synthesis of this group of 5-HT2A receptor agonists, which includes 25-I, dates back to at least the early 1990s, yet reports of human use have appeared in the medical literature only in recent years.11–13 Human or whole animal pharmacokinetic or pharmacological data are not presently available, and abuse of these 5-HT2A agonists has not been documented by toxicology testing until recently, 11–12 though not quantitated in serum until this case.
Fig. 1.
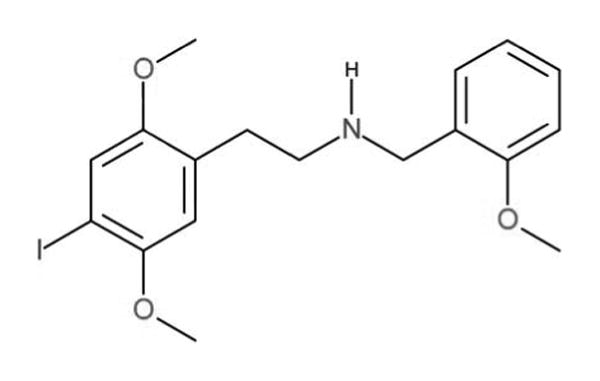
Chemical Structure of 25-I, a new potent 5-HT2A receptor agonist designer drug (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
Case report
An 18-year-old male was brought to an emergency department (ED) in handcuffs by police after reportedly jumping out of a moving car. On arrival, he was being restrained due to severe agitation and hallucinations (not further described). His vital signs were remarkable for tachycardia with the heart rate of 150s bpm and hypertension with the blood pressure of 150–170 mm Hg systolic and 110 mm Hg diastolic. Blood oxygen saturation was 100% on room air, and he was afebrile (initial temperature not recorded). Computerized tomography of the head was normal. An electrocardiogram revealed sinus tachycardia at 138 bpm; PR interval, 138 msec; QRS, 106 msec; QT, 284 msec; and QTc, 432 msec. During a lucid interval in the ED, the patient admitted to using 25-I.
Laboratory studies obtained in the ED included serum Na of 134mEq/L (135–145 mEq/L); K, 2.8 mEq/L (3.6–5.2 mEq/L); Cl, 103 mEq/L (98–108); CO2, 18 mmol/L (21–32 mmol/L); BUN, 14 mg/dL (7–18 vmmol/L); creatinine, 1.4 mg/dL; glucose, 192 mg/dL (136–145 mg/dL); Ca, 8.1 mg/dL (9.2–10.7 mg/dL); aspartate aminotransferase, 21 U/L (up to 40 U/L); alanine aminotransferase, 33 U/L (up to 60 U/L); white blood cell count (18,200 U/L); and INR 1.1. Blood ethanol was 25 mg/dL, serum salicylate was 3.4 mg/dL, and serum acetaminophen was reported as < 2 mg/L. A urine drug screen performed using enzyme immunoassay was positive for cannabinoids and negative for benzoylecgonine, amphetamines, barbiturates, benzodiazepines, opiates, and phencyclidine. Urine analysis was unremarkable.
Initial management consisted of one liter of intravenous normal saline and two doses of 2 mg lorazepam, after which the patient calmed down, and became alert and cooperative with the ED staff. He was admitted to the intensive care unit (ICU) for close observation, and lorazepam was continued with a 1.5 mg/h infusion. Approximately 12 h following arrival, the patient ' s vital signs had stabilized (heart rate, 70 bpm; blood pressure, 116/60 mmHg; respiratory rate, 16 bpm; and temperature, 97.7°F), though he remained restless and agitated when awake. At 24 h after arrival, the patient's pupils were noted to be mydriatric (6–7 mm) and he remained agitated when awake, requiring restraints in addition to the lorazepam infusion (increased to 2 mg/h) and dexmedetomidine 0.7 mcg/kg/h. Over the next 24 h, the sedative infusions were stopped, he continued to have episodes of aggressiveness, and he was started on oral ziprasidone treatment following psychiatric evaluation. It is unclear whether ziprasidone was continued after hospital discharge.
Laboratory analysis
Preparation of calibrators and quality control specimens
In-house drug-free serum provided the matrix for all prepared calibrators and quality control (QC) specimens. Pooled drug-free serum was obtained from updated blood bank units. The pooled serum were found not to contain common drugs of abuse, over-the-counter drugs, and basic extractable drugs such as tricyclic antidepressants, selective serotonin reuptake inhibitors, benzodiazepines, and antihistamines using immunoassay and analyzed using gas chromatography–mass spectrometry. Appropriate volumes of the working solution of 25-I (Cayman Chemical, Ann Arbor, MI) were added to pooled serum to obtain a five-point calibration curve with a range of 0.1–10 ng/ml of 25-I. The calibration curve was prepared in duplicate. The following QC serum specimens for 25-I were prepared and analyzed with the test specimens: limit of quantifcation quality control (LOQC), target concentration of 0.1 ng/ml; low quality control (LQC), target concentration of 0.25 ng/ml; medium control (MQC), target concentration of 2.0 ng/ml; and high quality control (HQC), target concentration of 7.5 ng/mL. A drug-free (negative) control that did not contain 25-I but did contain the internal standard (ISTD), and a double-negative control that did not contain 25-I or ISTD were also run with the patient sample. All QC samples were stored at − 20° C until analysis.
Specimen extraction
To a 1.0-ml aliquot of serum, 100 uL of SKF525A, the ISTD consisting of 1.0 ug/ml (100 ng total) in methanol, was added with mixing. Then, 2.0 ml of 2 N sodium hydroxide was added followed by 2 ml of hexane/ethyl acetate (1:1). The samples were mixed for 5 min and then centrifuged for 10 min at 3000 rpm. The upper organic layer was transferred to a clean test tube and evaporated to dryness under a gentle stream of nitrogen in a 40°C dry bath. The samples were reconstituted with 100 uL of mobile phase and placed in auto-sample (HPLC/MS/MS) vials for analysis.
HPLC/MS/MS analysis
25-I was identifed and quantified using an Applied Bio systems 3200 Q trap with a turbo V source for TurbolonSpray (Ontario, Canada) attached to a Shimadzu SCL HPLC system (Kyoto, Japan) controlled by analyst 1.4.2 software and run in multiple reaction monitoring (MRM). The chromatographic separation was performed using a Zorbaz eclipse XDB-C18 column, 4.6×75 mm, 3.5 micron (Agilent Technologies, USA). The mobile phase was 20:80 (v/v) water with 1 g/L ammonium acetate and 0.1% formic acid/methanol with a flow rate of 0.3 ml/min. Total run time was 10.00 min. The injection volume was 10 μL, and the autosampler temperature was set at 5°C. Under these conditions, the retention time for 25-I was 2.9 min and 4.5 min for the ISTD. The following transition ions for 25-I and SKF525A, respectively, were monitored: 428 > 121; 428 > 91 and 354 > 167; 354 > 209 m/z. The patient ' s serum, obtained in the ED, was determined to contain 0.76 ng/mL of 25-I.
Assay validation
A minimal assay validation was performed prior to testing due to the necessity of insuring the reliability of the analytical results particularly in a single case such as this. Each calibrator concentration of the duplicate curves was determined to be within ± 15% of the expected value. The linear regression correlation coefficients (r2) for the calibration curves yielded a mean r2 of 0.996. The lower limit of quantification (LOQ) and the lower limit of detection (LOD) for 25-I were administratively set at a concentration of 0.1 ng/ml. The LOD of 25-I had a response at least five times the signal-to-noise ratio of the response to drug-free pooled serum. The accuracy/bias of the assay for the 25-I at the LOQC (0.1 ng/ml) ranged from 87% to 117%, at a LQC (0.25 ng/ml) from 88% to 108%, at a MQC (2.0 ng/ml) from 83% to 111%, and at a HQC (7.5 ng/ml) from 95% to 106%. The criteria for acceptable assay accuracy/bias were quantified for 25-I results within ± 20% of the target value of the prepared QC samples. The intra-day precision for 25-I at the four different QC concentrations ranged between 9.6 and 16%. The QC testing yielded the following results: LOD/LOQC target, 0.1 ng/ml (mean, 0.11 ±0.2 ng/ml, CV = 16%, n = 4); LQC target, 0.25 ng/ml (mean, 0.24 ±0.2 ng/ml, CV=10%, n = 3); MQC target, 2.0 ng/ml (mean, 2.0 ± 2.8 ng/ml, CV=14%, n = 3); and HQCtarget, 7.5 ng/ml (mean, 7.7 ±0.37 ng/ml, CV = 4.8%, n = 3). Sample carryover was evaluated using two different procedures. For the carryover study, the high calibrator containing 10 ng/ml of 25-I was prepared in drug-free pooled serum. First, immediately following the injection of an extracted high calibrator, an extract of a drug-free serum was injected. The rejection criterion for carryover was set at the detection of 25-I at a concentration less than 20% of the LOD/LOQC. Following injection, 25-I was not detected in the injected drug-free serum sample. An additional procedure to evaluate possible 25-I carryover during analysis involved injecting an extracted HQC (7.5 ng/ml) immediately followed by an LQC (0.25 ng/ml). This analysis was repeated consecutively four times. The rejection criterion for carryover was set at a 25-I concentration with a bias of less than 20% of the target value of the LOD/LOQC. Lack of carryover was confirmed as 25-I did not demonstrate a significant quantified bias.
Discussion
The patient displayed initial signs and symptoms consistent with sympathomimetic toxidrome (tachycardia, hypertension, mydriasis, agitation, and hypokalemia) plus hallucinations and bizarre behavior likely associated with serotonergic toxicity. Clinical experience with intoxication due to 2C compounds is limited. Diminished mental status, agitation, hypothermia, emesis, urinary incontinence, severe hypertension, vasoconstriction, extensor posturing and intraventricular hemorrhage were reported in a 39-year-old woman who took MDA (methylenedioxyamphet-amine) and 2C-I, which were confirmed in urine by assay validation.11 The contribution of the drugs to the cerebral hemorrhage is confounded by the f nding of Moyamoya on cerebral angiography. Two case series of 25-I exposures of 4 and 10 patients, respectively, were presented at the 2012 North American Congress of Clinical Toxicology.12,13 Though reported only in abstract format, the most common effects in these patients were tachycardia (13 of 14), agitation (10 of 14), and hypertension (8 of 14). Five of 14 patients experienced tonic/clonic seizures and one suffered a cerebral hemorrhage. There appears to be a significant overlap in clinical effects observed in patients exposed to 2C compounds compared to patients exposed to amphetamine and cathinone derivatives.3–5 The most notable effects in this patient following 25-I ingestion were tachycardia and the persistence of agitation requiring continuous lorazepam infusion for approximately three days.
The chemical composition of disguised commercial products such as bath salts appears to frequently change. They may contain a single drug or a mixture of several amphetamine-related compounds.4,6,7 Thus, these products may differ greatly in composition and potency, leading to unpredictable clinical effects and toxicity. For this reason, an intelligible discussion of bath salt intoxication in man should begin with, as in this case, absolute identification of the particular intoxicant.
The emergence of 25-I on the illicit drug market demonstrates how quickly the manufacturers and suppliers of these designer drugs respond to changes in federal or state laws. Initially available through internet sources, these compounds may now be obtained from “dealers” or manufactured in local laboratories. The ten cases presented in reference 13 all occurred within a 2- to 3-month period (first quarter of 2012) in one metropolitan area. In September 2012, following MDPV and methylone, 25-I was the third most encountered drug exhibit handled by the Commonwealth of Virginia Department of Forensic Sciences.14 25-I used by this patient is believed to have been produced locally, thus demonstrating the regional nature of drugs of abuse. Several states including Virginia and Louisiana have enacted laws to classify many designer drugs, including 25-I, into Schedule I. In July 2012, nine 2C hallucinogens, mephedrone, MDPV, and most synthetic cannabinoids were added to federal Schedule I under the Food & Drug Administration Safety and Innovation Act.15
Conclusion
This case report illustrates clinical toxicity associated with a documented serum concentration of 25-I, a potent new synthetic phenethylamine compound with potentially significant behavioral toxicity.
Footnotes
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Gibbons S. “Legal Highs” – novel and emerging psychoactive drugs: a chemical overview for the toxicologist. Clin Toxicol. 2012;50:15–24. doi: 10.3109/15563650.2011.645952. [DOI] [PubMed] [Google Scholar]
- 2.Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): legal status and patterns of abuse. Ann Pharmacother. 2012;46:436–441. doi: 10.1345/aph.1Q628. [DOI] [PubMed] [Google Scholar]
- 4.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 5.Murphy CM, Dulaney AR, Beuhler MC, Kacinko S. “Bath Salts” and “Plant Food” products: the experience of one regional US poison center. J Med Toxicol. 2012 doi: 10.1007/s13181-012-0243-1. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuba D, Sekula K. Identification and characterization of 2,5-dimethoxy-3,4-dimethyl-B-phenethylamine (2C-G) – a new designer drug. Drug Test Anal. 2012 doi: 10.1002/dta.1396. www.drugtestinganalysis.com. [DOI] [PubMed]
- 7.Zuba D, Sekula K, Buczek A. 25C-NBOMe – new potent hallucinogenic substance identified on the drug market. Forensic Sci Int. 2012 doi: 10.1016/j.forsciint.2012.08.027. in press. http://dx.doi.org/10.1016/j.forsciint.2012.08.027. [DOI] [PubMed]
- 8.Nichols DE, Frescasa SP, Chemela BR, Rehderb KS, Zhongb D, Lewin AH. High specific activity tritium-labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): A high-affinity 5-HT2A receptor-selective agonist radioligand. Bioorg Med Chem. 2008;16:6116–6123. doi: 10.1016/j.bmc.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(651) and Phe340(652) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- 10.Aghaajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropyschopharmacol. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 11.Drees J, Stone JA, Wu AHB. Morbidity involving the hallucinogenic designer amines MDA and 2C-I. J Forensic Sci. 2009;54:1485–1487. doi: 10.1111/j.1556-4029.2009.01199.x. [DOI] [PubMed] [Google Scholar]
- 12.Kelly A, Eisenga B, Riley B, Judge B. Case series of 25I-NBOMe exposures with laboratory confirmation. Clin Toxicol. 2012;50:702. abstract. [Google Scholar]
- 13.Rose SR, Cumpston KL, Stromberg PE, Wills BK. Severe poisoning following self-reported use of 25-I, a novel substituted amphetamine. Clin Toxicol. 2012;50:707–708. abstract. [Google Scholar]
- 14.Jackson L. Commonwealth of Virginia Department of Forensic Science. Scientific Advisory Committee Open Meeting; October 9, 2012. [Google Scholar]
- 15.Hogue C. US criminalizes designer drugs. Chem Eng News. 2012;90:28–29. [Google Scholar]


