Abstract
Interplay between macrophages and dendritic cells in the processing and presentation of bacterial antigens for T-cell immune responses remains poorly understood. Using a Listeria monocytogenes (Lm) infection model, we demonstrate that dendritic cells (DCs) require the support of macrophages to elicit protective immunity against Lm infection. DCs themselves were inefficient at taking up Lm but capable of taking up microparticles (MPs) released by Lm-infected macrophages. These MPs transferred Lm antigens to DCs, allowing DCs to present Lm antigen to effector T cells. MP-mediated Lm antigen transfer required MHC class I participation, since MHC class I deficiency in macrophages resulted in a significant reduction of T-cell activation. Moreover, the vaccination of mice with MPs from Lm-infected macrophages produced strong protective immunity against Lm infection. We here identify an intrinsic antigen transfer program between macrophages and DCs during Lm infection, and emphasize that macrophages also play an essential role in DC-elicited Lm-specific T-cell responses.
Keywords: dendritic cell, Listeria monocytogenes, microparticles, macrophage, protective immunity
Introduction
The mutual interaction between innate and adaptive immune responses plays a crucial role in the optimal clearance of invading pathogens.1,2,3 Innate immune cells respond first to infection but are frequently insufficient to overcome the virulence mechanisms of pathogens; thus, the adaptive immune responses are activated. Macrophages and dendritic cells are two key innate immune cell types involved in phagocytosis and presentation of antigen, respectively, upon bacterial infection.4,5 It is accepted that both macrophages and dendritic cells contribute to the activation of T cells;6,7 however, the interplay between these cells in the processing and presentation of bacterial antigens for the goal of activating T cells remains poorly understood.
Listeria monocytogenes (Lm) is an intracellular parasitic bacterial pathogen in both humans and animals that is widely used in infectious disease models to study adaptive immune responses.2 Upon infection, macrophages actively phagocytose Lm; however, Lm is capable of escaping the phagosome allowing it to spread from cell to cell. As a result, adaptive immune responses have to be elicited to eliminate bacteria. A critical role for DCs in mounting adaptive immunity against Lm was demonstrated years ago. Jung et al.8 showed that dendritic cells (DCs) are required to elicit anti-Lm CTL responses, whereas macrophages fail to initiate anti-Listeria CTL responses in the absence of DC. However, Kolb-Mäurer et al.9 showed that human monocyte-derived immature DCs poorly phagocytose Lm in vitro in the absence of plasma antibodies against listerial p60; such antibodies are thought to act as an opsonin for Lm phagocytosis by DCs. In addition, it was reported that upon injection of fluorescent heat-killed bacteria into mice, only a low frequency of CD11chigh DCs take up particles and a sizeable fraction of F4/80high CD11clow macrophages show the intense fluorescence.10 Such discrepancies suggest that a mutual interaction might exist between macrophages and dendritic cells in the processing and presentation of antigens to T cells in the induction of a protective immune response. However, to date, a cross-talk between these two cell types has not been definitively described.
It has been demonstrated that eukaryotic cells may shed components of the plasma membranes encapsulating cytoplasmic elements into the extracellular space when activated or during apoptosis.11,12,13 These vesicles vary from 100 to 1000 nm in size and are known as microparticles (MPs).14 In the present study, we show that both macrophages and DCs are essential for the induction of Lm-specific T-cell responses but with different responsibilities. Macrophages phagocytose and release Lm antigens-containing MPs, which are subsequently captured by DCs leading to priming T-cell responses. Here we provide evidence demonstrating that DCs require the help of macrophages to elicit the adaptive immunity against Lm infection.
Materials and methods
Mice
BALB/c and C57BL/6 mice (8 weeks) were purchased from the Center of Medical Experimental Animals of Hubei Province (Wuhan, China) and the Center of Experimental Animals of Chinese Academy of Medical Science (Beijing, China) for studies approved by the Animal Care and Use Committee of Tongji Medical College. MHC-class I−/−, MHC-class II−/− and MyD88−/− mice were maintained in the barrier facility at the Mount Sinai School of Medicine.
Preparation of Lm bacteria
Lm 104035, a virulent strain,15 was grown in Brain Heart Infusion Broth (BD Biosciences, San Jose, CA, USA) at 37°C for 16 h, washed repeatedly, suspended in phosphate-buffered saline (PBS) and stored at −80°C until use.
Generation of bone marrow-derived DCs
Bone marrow cells were harvested from femurs of mice and cultured in RPMI 1640 supplemented with 10% fetal bovine serum(FBS), 2 mM L-glutamine, 1 mM sodium pyruvate, 1 mM HEPES, 50 µM 2-ME, 100 U/ml penicillin and 100 µg/ml streptomycin. The cells were cultured in six-well plates with 20 ng/ml GM-CSF (PeproTech, Rocky Hill, NJ, USA), 10 ng/ml IL-4 (PeproTech) and cytokines were replenished on day 3. On day 6, the nonadherent cells were harvested and the CD11c+ DCs were purified for the experiments.
In vivo depletion of macrophages
To deplete macrophages, mice were intraperitoneally (i.p.) injected with 30 µg anti-mouse depleting anti-F4/80 Ab (A3-1, SeroT) or PBS, or with Clodrolip or PBS-containing liposomes (provided by Dr Reto A Schwendener, University of Zurich). When indicated, the anti-F4/80 depleting monoclonal antibody (mAb) or liposomes were injected at days −2 and 0 after Lm injection. The final clodronate liposome suspension contained 5 mg of clodrolip/ml.
Assay for cytokines
The amounts of interferon (IFN)-γ and IL-22 in the supernatants were determined by ELISA kits (R&D Systems, Minneapolis, MN, USA).
Isolation of MPs
Supernatants of cultured macrophages were used to isolate MPs as described before.16 Briefly, supernatants were centrifuged at 300g×5 min, 500g×5 min, 1500g×5 min and 5000g×5 min for removal of cells and debris. The supernatant was passed through a 1.2 µm filter in order to remove bacteria, and then further centrifuged for 60 min at 14 000g to pellet MPs.
Labelling of MPs
Bacteria were stained with carboxyfluorescein succinimidyl ester (CFSE; Sigma, St Louis, MO, USA) and used to infect macrophages. The released MPs were isolated as described. In some cases, MPs isolated from macrophages were labeled with a red-fluorescent cell linker (PKH26; Sigma) according to the manufacturer's protocol. Such fluorescent MPs were observed under two-photon fluorescent microscopy (LSM 710 and ConfoCor 3 Microscope Systems; Carl Zeiss, Jena, Germany) or analyzed by flow cytometry, as described previously.17
Count of MPs
Isolated MPs were suspended in 250 µl PBS with 1 µl Polystyrene Latex Beads (LB-30; Sigma). After mixing, the number of MPs was counted by a flow cytometer in accordance with their respective bead sizes.
Flow cytometric analysis
For DC analysis, cells were incubated with 1 µg/106 cells of Fc receptor-blocking Ab (clone 24G2; American Type Culture Collection, Manassas, VA, USA), and then stained with FITC-conjugated anti-mouse CD80, CD86 and MHC class II, respectively. For T-cell analysis, after 24G2 blockade, cells were stained with allophycocyanin (APC)-conjugated anti-mouse CD3 and phycoerythrin (PE)-conjugated anti-mouse CD4 for surface staining, and then fixed and permeabilized with Fix/Perm solution for intracellular staining with FITC-conjugated anti-mouse IFN-γ antibody. All fluorophore-conjugated antibodies and the corresponding isotypes were purchased from eBioscience (San Diego, CA). In addition, MPs were suspended in PBS and mixed in microbeads of 3 µm in diameter (Sigma-Aldrich, Saint Louis, MO). Optimal instrument settings and MP gate were selected based on the microbeads. Total event counts of MPs were determined within the MP gate.
Western blot
Cell lysates and prestained molecular weight markers were separated by SDS-PAGE followed by transfer onto nitrocellulose membranes. The membranes were blocked in TBST (Tris-buffered saline with 0.5% of Triton X-100) containing 5% non-fat milk, and probed with antibodies. After incubation with the secondary antibody conjugated with horseradish peroxidase, membranes were extensively washed, and the immunoreactivity was visualized by enhanced chemiluminescence according to the manufacturer's protocol (ECL kit; Santa Cruz, Santa Cruz, CA, USA). All antibodies were purchased from Santa Cruz.
In vivo Lm protection assay
In total 5×106 macrophages in 2 ml culture media were treated with PBS or 5×107 viable Lm (100 µg/ml gentamycin added 30 min later) for 48 h. Additionally, 5×107 viable Lm were incubated in 2 ml culture media alone with gentamycin. Each 2 ml supernatant was used for MP isolation and this quantity was used for one mouse injection. Mice were immunized subcutaneously with MPs mixed with rehydragel adjuvants for 7 days and challenged by intravenous (i.v.) injection of 1×105 viable Lm. Survival was monitored for 10 days. Six mice were used per group.
Cytotoxicity assay
The cytotoxicity was done as described before.18 Briefly, H22 hepatocarcinoma tumor cells were infected by incubation with Lm (1:100 ratio) for 2 h, and then cultured with 100 µg/ml of gentamycin for 24 h. Tumor cells were labeled with CFSE and used as target cells. After incubation with MPs isolated from the supernatants of Lm-infected macrophages, bone marrow-derived DCs were cultured with T cells for 10 days. The activated T cells were harvested and used as effector cells.
Statistic analysis
Data were expressed as mean value±s.d. and interpreted by ANOVA test. Differences were considered to be statistically significant when P<0.05.
Results
Macrophages are required for DC-elicited anti-Lm T-cell response in vivo
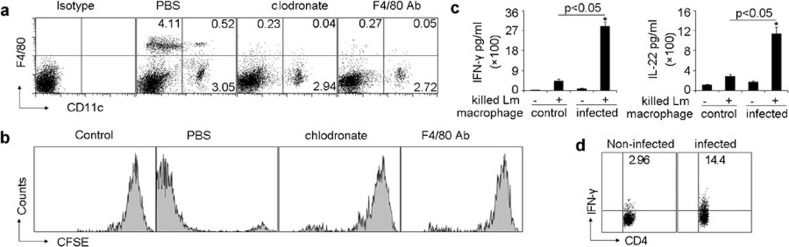
Upon Lm infection, macrophages phagocytose bacteria and secrete a variety of cytokines to mediate innate immune responses, while DCs present Lm antigen to induce adaptive immune responses. Whether and how macrophages participate in the induction by DCs of anti-Lm T-cell response remains unclear. Previously, Jung et al.8 clearly showed that the depletion of DCs resulted in the failure to elicit anti-Lm CTL responses in mice. In addition, they also found that DC depletion affected neither bacterial uptake nor killing by macrophages,8 suggesting that macrophages do not directly present Lm peptides to T cells. These results prompted us to investigate whether depletion of macrophages would affect the DC-induced Lm-specific T-cell responses. Splenic macrophages, but not DCs, were depleted in mice by i.p. injecting liposomal clodronate or anti-F4/80 antibody (Figure 1a). Under such conditions, BALB/c nude mice were adoptively transferred with T cells isolated from the spleens of Lm-infected mice. We found that the depletion of macrophages abrogated the in vivo proliferative response of adoptively transferred T cells 60 h after the i.v. injection of 1.0×103 viable Lm (Figure 1b). To confirm these results, we transferred the Lm-infected macrophages into naive C57BL/6 mice for 7 days and the spleen cells were cultured with killed bacteria for measurement of cytokine production by ELISA assay. As expected, the inoculation of Lm-infected macrophages into C57BL/6 mice strongly induced the production of IFN-γ and IL-22 (Figure 1c), two potent mediators of cellular inflammatory responses against bacterial pathogens.19,20,21 In addition, IFN-γ-producing CD4+ T cells were analyzed by FACS and showed to be consistently present (Figure 1d). This result was not due to the contamination of live Lm escaping from the infected macrophages, since the mice were treated with gentamicin before and after adoptive transfer. These findings suggested that the initial infection of macrophages by Lm is required for the generation of protective immune responses. Thus, although macrophages do not directly present Lm peptides, they seem to participate in the induction of Lm specific T-cell responses.
Figure 1.
Macrophages are required in DC-elicited anti-Lm T-cell responses. (a) BALB/c mice were i.p. injected with clodrolip or anti-F4/80 depleting mAb for macrophage depletion. Forty-eight hours later, splenic cells were stained with FITC-conjugated CD11c and PE-conjugated F4/80 mAbs or their isotypes and analyzed by flow cytometry. This result was the representative from four mice in each group. (b) BALB/c nude mice (n=6) with or without macrophage depletion were adoptively transferred with CFSE-labeled T cells isolated from the spleens of Lm-infected mice or naive mice (control), and 1.0×103 viable Lm were injected into these mice after 6 h. Sixty hours later, the proliferation of adoptively transferred T cells in the spleen was determined by flow cytometry. (c, d) Thioglycolate-elicited peritoneal macrophages from C57BL/6 mice (n=6) were infected with Lm for 2 h in culture dish and the cells (5×106) were then adoptively transferred to gentamycin-treated C57BL/6 mice. Seven days later, mice were killed and splenic cells were restimulated with heat-killed Lm for 24 h. The supernatants were harvested and assayed for IFN-γ and IL-22 by ELISA (c). The cells were stained and IFN-γ expression in CD4+ T cells was analyzed by flow cytometry (d). DC, dendritic cell; IFN, interferon; i.p., intraperitoneally; Lm, Listeria monocytogenes; mAb, monoclonal antibody; PE, phycoerythrin.
Macrophages participate in DC presenting Lm antigens in vitro
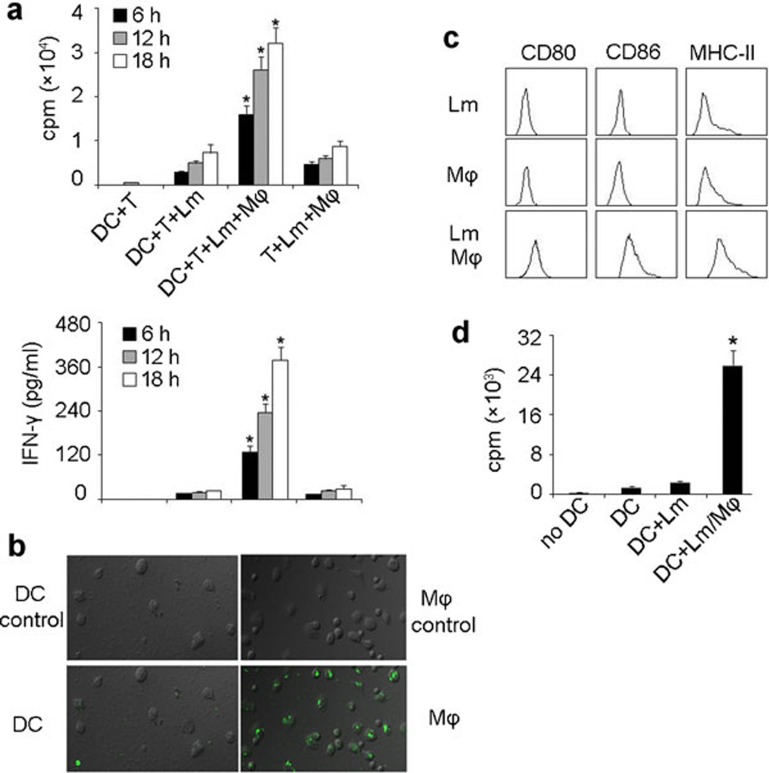
The culture of DC precursors from bone marrow in either GM-CSF/IL-4 or Flt3L has widely been used; however, the former is more related to infection and the latter is more related to the steady-state.22 Therefore, in this study, we used GM-CSF/IL-4-induced DCs to further confirm the involvement of macrophages in DCs presenting Lm antigen to T cells. Bone marrow-derived DCs were incubated with viable Lm (gentamicin was added 30 min later) in the presence or absence of peritoneal macrophages for various time intervals (6, 12 and 18 h). DCs were then isolated and incubated with T cells for Lm antigen presentation. The result showed that DCs cultured without macrophages induced weak T-cell proliferation; however, DCs cultured with macrophages induced strong T cell proliferation as well as IFN-γ production (Figure 2a). In addition, Lm-infected macrophages were not capable of effectively inducing T cell proliferation in the absence of DCs (Figure 2a). In line with these data, we found that DCs were not effective at directly capturing Lm, since CFSE-labeled Lm were mainly present in macrophages and few bacteria were able to enter DCs (Figure 2b). These findings suggested that macrophages participate in DC presentation of Lm antigens, and implied that Lm antigens may be transferred from macrophages to DCs for Lm-specific T-cell activation.
Figure 2.
Macrophages are involved in DCs presenting Lm antigen in vitro. (a) Macrophages were required for DC-elicited Lm-specific T-cell proliferation. Bone marrow-derived DCs (BALB/c background) were incubated with viable Lm (100 µg/ml gentamycin added 30 min later) in the presence or absence of peritoneal macrophages for various time intervals (6, 12 and 18 h). 1×105 splenic T cells, isolated from the spleens of Lm-infected BALB/c mice, were cocultured with those DCs (1×104) or Lm-infected macrophages. T-cell proliferation was measured 72 h later by incorporation of [3H]-thymidine (top) and the supernatants were used for IFN-γ detection by ELISA kit (bottom). *P<0.05, compared with the other corresponding groups. (b) Lm was poorly taken up by DCs. DCs (left) or peritoneal macrophages (right) were incubated with CFSE-labeled Lm for 1 h and the uptake of bacteria was detected by fluorescent microscopy. The top was shown as the background fluorescence. (c, d) DC maturation and presenting of Lm antigens were conferred by the supernatants from Lm-infected macrophage cell cultures. DCs were incubated with the supernatants of Lm-infected macrophages, uninfected macrophages or Lm alone for 24 h. After incubation with Fc receptor-blocking Ab, cells were stained with CD80, CD86 and MHC class II mAbs, respectively, and analyzed by flow cytometry (c). Meanwhile, T cells, isolated from the spleens of Lm-infected BALB/c mice, were incubated with DCs that had been treated with the supernatants of Lm-infected macrophages, Lm-infected DCs or untreated DCs. T-cell proliferation was measured 72 h later by incorporation of [3H]-thymidine (d). Experiments were repeated at least three times. *P<0.05, compared with macrophage-absent groups. DC, dendritic cell; IFN, interferon; Lm, Listeria monocytogenes; Ab, antibody.
Supernatants from Lm-infected macrophage cell cultures confer DC maturation and presentation of Lm antigens
Next, we investigated how macrophages conferred DC presentation of Lm antigens to T cells. In this regard, thioglycollate-elicited macrophages were infected with viable Lm (gentamycin was added 30 min later) for 24 h and supernatants were harvested by centrifugation at 3000 r.p.m. for 15 min and 5000 r.p.m. for 5 min in order to remove bacteria and cellular debris. The supernatants were passed through a 1.2 µm filter to further remove bacteria and cellular debris, and then incubated with DCs for 24 h. As shown in Figure 2c, the expression of CD80, CD86 and MHC class II on DCs was upregulated by the supernatants from Lm-infected macrophages compared to cells incubated with supernatants from uninfected macrophages. Such DC activation was not ascribed to the bacterial contamination, since the supernatant from the above Lm alone did not affect DC maturation (Figure 2c). Moreover, we found that DCs treated with supernatants of Lm-infected macrophages effectively induced T cell proliferation (Figure 2d). These findings suggested that factors released from Lm-infected macrophages are capable of eliciting the maturation and conferring the immunogenicity of DCs against Lm infection.
MPs shed by Lm-infected macrophages are the source for DC immunogenicity
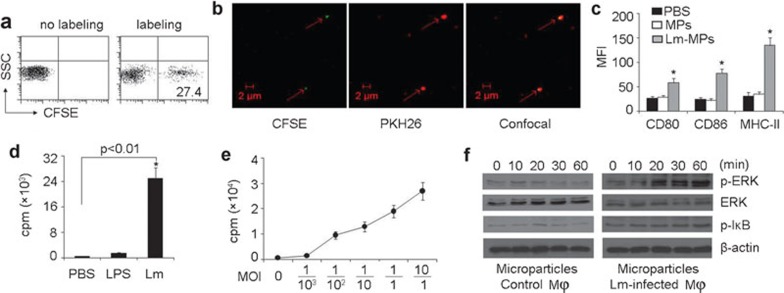
The above data might be interpreted as after taking up Lm, macrophages released certain components that could be captured by DCs, leading to antigen presentation to T cells. We speculated that MPs, which were released from Lm-infected macrophages, might contain Lm components. It is known that following stimulation or during apoptosis, cells change their cytoskeleton structures, leading to plasma membranes encapsulating cytosolic elements that are shed into the extracellular space.11,12,13 We hypothesized that after phagocytosis by macrophages, Lm components were encapsulated into MPs, which were subsequently released into the extracellular space. To test this, macrophages were infected with CFSE-labeled Lm and the released MPs were isolated from the supernatants by centrifugation using a widely accepted method for MP isolation.16 The fluorescence was observed in MPs via both flow cytometry (27.4% CFSE-positive MPs) and under the microscope (Figure 3a and b), indicating the presence of Lm-derived bacterial components in MPs. Consistently, Lm component-containing MPs stimulated DC maturation and resulted in T-cell proliferation (Figure 3c and d). However, neither the MP-absent supernatants nor control MPs isolated from the supernatant of lipopolysaccharide (LPS)- or PBS-stimulated macrophages had an effect on DCs eliciting T-cell proliferation (Figure 3d). To exclude the possibility of live Lm in MPs that might contaminate the result, the above isolated MPs were added into the bacterial culture media and shaken at 37°C, and no Lm grew in the media. Meanwhile, we found that both CFSE-labeled and unlabeled Lm grew in the media very well, suggesting the CFSE labeling do not affect the viability of Lm. Here, we also used Lm with different multiplicities of infection to treat macrophages and assayed the effect of isolated MPs on T cell proliferation. We found that even low numbers of Lm could result in T-cell proliferation via DC antigen presentation and that increased numbers of Lm further promoted T cell proliferation (Figure 3e). To further analyze the effect of MPs on DCs, we examined the activation of mitogen-activated protein kinases (MAPKs) and nuclear factor kappaB (NF-κB), two critical signaling pathways involved in DC activation.23,24 DCs were stimulated with MPs from Lm-infected or control macrophages for various time intervals (10, 30 and 60 min). The activation of MAPK and NF-κB by MPs from Lm-infected macrophages was confirmed by the induction of extracellular signal-regulated proteinkinase (ERK) and inhibitor of kappaB (IκB) phosphorylation (Figure 3f). Consistently, MPs from Lm-infected rather than uninfected macrophages could induce bone marrow-derived DCs to upregulate the expression of IL-12 (Supplementary Figure 1). These data suggested that macrophage-released MPs are involved in DC presentation of Lm antigens.
Figure 3.
Microparticles released by Lm-infected macrophages stimulated DC maturation and resultant T cell proliferation. (a, b) Lm components were present in MPs. Macrophages were infected with CFSE-labeled Lm and the released MPs were isolated from the supernatants by centrifugation. The isolated MPs were analyzed by flow cytometry (a). Or, macrophages were labeled with PKH26 and infected with CFSE-labeled Lm. The green Lm components and red MPs were observed under two-photon laser scanning fluorescence microscopy (b). (c) Lm component-containing MPs stimulated DC maturation. DCs were incubated with PBS, MPs from Lm-infected or control macrophages for 24 h and were stained with CD80, CD86 or MHC class II mAb, the mean fluorescence intensity (MFI) was measured. *P<0.05, compared with PBS group. (d) DCs elicited T-cell activation after incubating with Lm component-containing MPs. Macrophages (5×106) were treated with Lm or LPS and the released MPs were isolated from the supernatants. DCs were incubated with MPs and cocultured with T cells isolated from the spleens of Lm-infected BALB/c mice. The T-cell proliferation was measured. (e) Macrophages were treated with different MOIs, and the isolated MPs were used to treat DCs for T-cell proliferation assay as above. (f) The activation of MAPK and NF-κB of DCs by MPs. Bone marrow-derived DCs were stimulated with MPs from Lm-infected or control macrophages for various time intervals (0–60 min). Western blot was performed for analysis of MAPK ERK and IκB phosphorylation. Results are representative of at least three independent experiments. CFSE, carboxyfluorescein succinimidyl ester; DC, dendritic cell; ERK, extracellular signal-regulated protein kinase; IκB, inhibitor of kappaB; Lm, Listeria monocytogenes; LPS, lipopolysaccharide; mAb, monoclonal antibody; MAPK, mitogen-activated protein kinase; MOI, multiple of infection; MP, microparticle; NF-κB, nuclear factor kappaB; PBS, phosphate-buffered saline.
Macrophages/MPs/DCs form an axis to transfer Lm antigenicity
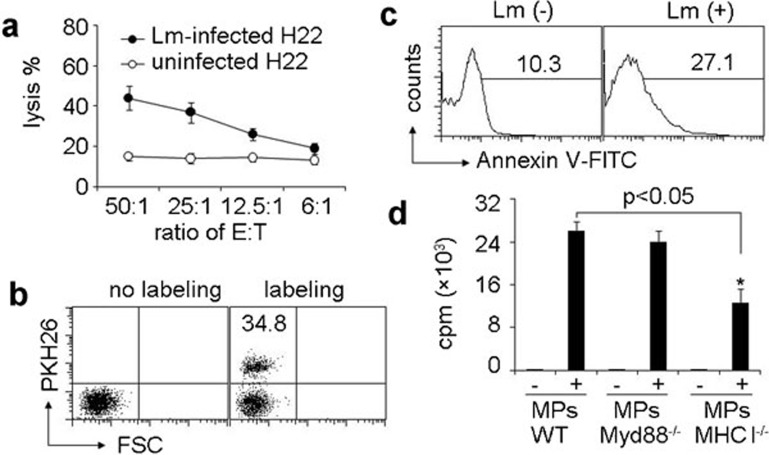
Next, we wondered how MPs by Lm-infected macrophages transferred Lm antigenicity to DCs. To elucidate this, we performed cytotoxicity assays to clarify MPs containing Lm antigenic components. T cells stimulated by MPs-treated DCs were used as effector cells and Lm-infected or uninfected H22 liver tumor cells (BALB/c background) were used as target cells.18 We found that the effector T cells lysed Lm-infected H22 cells but not uninfected H22 cells (Figure 4a). Therefore, MPs from Lm-infected macrophages contained Lm components, which might induce T cells to kill Lm-infected cells. Consistently, we found that DCs effectively took up MPs. By using PKH26 membrane dye to stain MPs, we found that 35% DCs presented red fluorescence (Figure 4b). DCs are known to have the capacity to take up apoptotic cells. Here, we also found that phosphatidylserine was translocated to the outer layer of the membrane of MPs, the marker of apoptosis (Figure 4c). Given DCs acquiring antigens via multiple ways,25,26,27,28 two possibilities were speculated here to further dissect how MPs transferred Lm antigenicity to DCs: (i) DCs use membrane fusion strategy to directly acquire Lm antigen peptide–MHC complexes from MPs; or (ii) DCs internalize MPs, process Lm antigens and present them to the surface. To clarify the pathway, MHC class I-deficient macrophages were infected with Lm to generate MHC class I-deficient MPs. The latter were incubated with wild type DCs for 4 h, followed by the addition of CD8+ T cells. Interestingly, we found that compared to the control, MHC class I-deficient MPs significantly reduced the presentation of Lm antigen by DCs to CD8+ T cells (Figure 4d), suggesting that DCs directly present MHC class I-peptide complexes derived from macrophages and carried by MPs to T cells. Toll-like receptors, which recognize pathogens, are also thought to be important for DC presentation of microbial antigens.29,30 Considering that MyD88 is the main adaptor molecule of the Toll-like receptor (TLR) signaling pathway, we also determined if abrogating TLR-function by disrupting MyD88 would have an effect on T-cell activation. However, we found that MyD88 deficiency in macrophages did not affect the activation of T cells (Figure 4d), suggesting that the TLR signaling pathway in macrophages is not required for the DC acquisition of Lm antigens-containing MPs in this system. In addition, we incubated MHC class II-deficient DCs with wild type MPs for 4 h, and then co-cultured the DCs with CD4+ T cells. We found that MHC class II deficiency in DCs significantly reduced the presentation of MP-derived Lm antigen by DCs to CD4+ T cells, evaluated by both T-cell proliferation and IFN-γ production (Supplementary Figure 2). These data were consistent with a previous report demonstrating that receptors on MPs may be transferred to recipient cell surface;31 however, these data also suggested that DCs may capture MPs through different pathways, including the membrane fusion between DCs and MPs and DC uptake and processing. Nevertheless, the detailed mechanism is worthy of further investigation.
Figure 4.
DCs take up the MPs derived from macrophages and present Lm antigenicity to T cells. (a) Lm components within MPs possessed antigenicity. MPs were isolated from the supernatants of macrophages with or without Lm infection. T cells isolated from Lm-infected mouse spleen were cocultured with MP-treated DCs for 10 days and acted as effector cells. CFSE-labeled H22 cells with or without Lm infection were used as target cells. The cytolysis assay was performed with various ratios of effector to target cells. The lysis was analyzed with flow cytometry. (b) DCs took up MPs. Macrophages were infected with Lm. The isolated MPs were stained with or without PKH26 and incubated with DCs for 6 h. The red fluorescence was determined by flow cytometry. (c) The apoptosis marker phosphatidylserine was present on the surface of MPs derived from Lm-infected macrophages. Macrophages were infected with Lm. The isolated MPs were stained with FITC-Annexin V and analyzed by flow cytometry. (d) Bone marrow-derived DCs were incubated with MPs from Lm-infected macrophages of WT, MHC-I−/− or MyD88−/− mice, respectively. Four hours later, followed by the addition of CD8+ T cells, Lm-infected mouse spleen and [3H]-thymidine incorporation was determined after 48 h of culture. *P<0.05, compared with macrophage-absent groups. CFSE, carboxyfluorescein succinimidyl ester; DC, dendritic cell; Lm, Listeria monocytogenes; LPS, lipopolysaccharide; MP, microparticle; WT, wild-type.
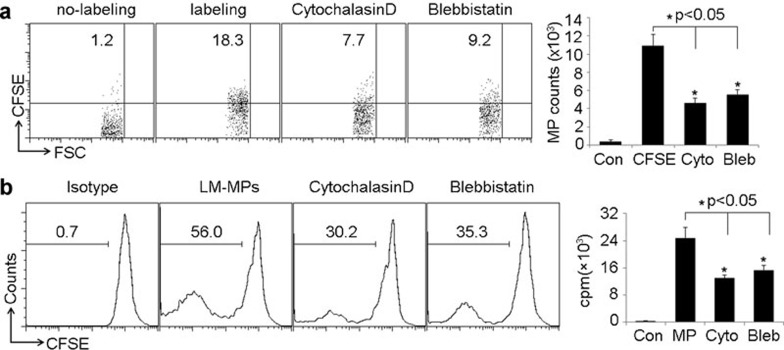
Actin filament is required for the generation of MPs containing Lm components by macrophages
Next, we tried to explore the intracellular event underlying the production of Lm component-containing MPs by macrophages. It is known that the arrangement and deformation of cytoskeleton plays important role in cellular endocytosis and exocytosis. In this regard, we wondered whether cytoskeleton was required for the production of Lm-induced MPs by macrophages. Thus, we treated macrophages with cytochalasin D, an inhibitor of F-actin polymerization, and found that the impairment of actin filament formation resulted in the decreased of MP release by Lm-treated macrophages (Figure 5a). We then further treated macrophages with blebbistatin, an inhibitor of myosin II ATPase activity. Similarly, the inhibition of actin filament motility also led to the decreased MP release (Figure 5a). This phenomenon was also observed in the isolated peritoneal macrophages (data not shown). Consistently, it was found that after the cytochalasin D or blebbistatin treatment, MPs attenuated the effect of Lm components on DCs eliciting T cell proliferation (Figure 5b). Therefore, these findings suggest that myosin II-triggered, actin filament-generated tension might mediate the production of Lm component-containing MPs by macrophages.
Figure 5.
Actin cytoskeletons are involved in Lm-induced MP generation by macrophages. (a) Actin cytoskeletons were involved in MP generation by macrophages. Peritoneal macrophages were incubated with CFSE-labeled Lm for 2 h, and then treated with cytochalasin D or blebbistatin, respectively. Twenty-four hours later, MPs in the supernatants were isolated and analyzed by a flow cytometer. The left panel shown here was a representative from three independent experiments. (b) Macrophages (5×106) were treated with Lm or Lm/cytochalasin D or Lm/blebbistatin or control PBS for 24 h, and the released MPs were isolated from the supernatants. DCs were incubated with MPs and cocultured with CFSE-labeled T cells derived from Lm-infected mouse spleen for 72 h. T-cell proliferation was measured by a flow cytometer. The left panel shown here was a representative from three independent experiments. CFSE, carboxyfluorescein succinimidyl ester; DC, dendritic cell; Lm, Listeria monocytogenes; MP, microparticle; PBS, phosphate-buffered saline.
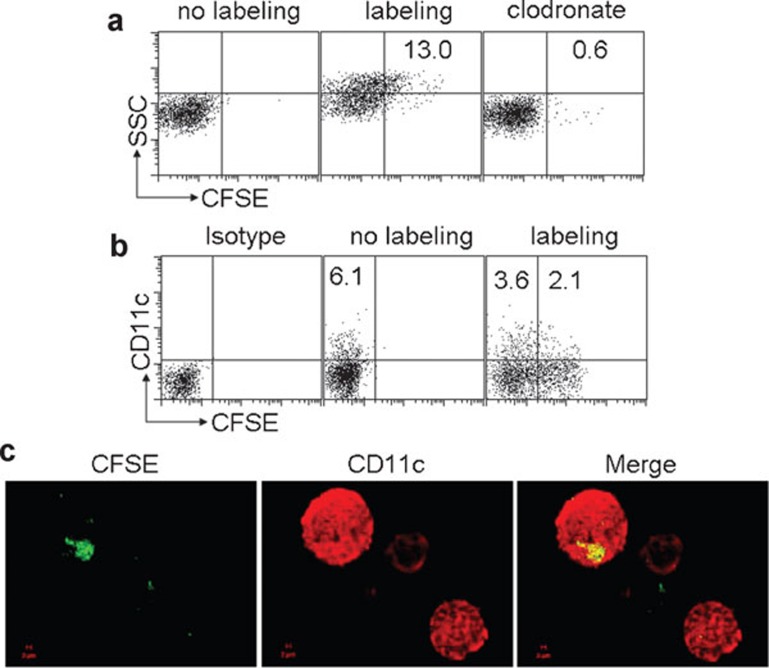
Generation of MPs containing Lm components by macrophages and uptake by DCs in vivo
Next, we validated the in vivo generation of MPs by macrophages under the condition of Lm infection. Using a peritoneal infection model, we i.p. injected 1×107 colony forming unit (CFU) CFSE-labeled Lm to mice. 12 h later, the peritoneal lavage was applied to isolate the MPs. As expected, we found that 13% MPs contained Lm components by flow cytometry (Figure 6a). However, if we previously depleted peritoneal macrophages, we found that Lm infection only resulted in 0.6% CFSE positive MPs (Figure 6a), suggesting that MPs containing Lm components are mainly generated by macrophages after peritoneal Lm infection. To clarify DCs taking up these MPs, MPs were isolated from peritoneal lavage 12 h after CFSE-labeled Lm peritoneal infection, and then i.p. injected into naive mice. Six hours later, we harvested peritoneal cells and found that 3%–6% of peritoneal cells were CD11c+ DCs and ∼40% of these cells were CFSE-positive (Figure 6b). Consistently, the result of confocal microscopy also showed that DCs took up Lm components (Figure 6c). In addition, as shown in Figure 6b, a population of CD11c− cells was also CFSE-positive, which might be macrophages. Therefore, these findings suggested that during Lm infection in vivo, macrophages phagocytose Lm and release Lm component-packaging MPs, leading to the subsequent uptake of the released MPs by DCs.
Figure 6.
Generation of MPs containing Lm components by macrophages and uptake by DC in vivo. (a) MPs were mainly generated by macrophages after peritoneal infection of Lm. BALB/c mice (n=6) with or without 48 h macrophage depletion by clodrolip were i.p. injected with 1×107 CFU CFSE-labeled Lm. Twelve hours later, MPs were isolated from the peritoneal lavage fluids and analyzed by flow cytometry. (b, c) MPs were taken up by DCs in vivo. The isolated CFSE-MPs after 1×107 CFU CFSE-labeled Lm peritoneal infection were i.p. injected into naive BALB/c mice. Six hours later, the peritoneal cells were harvested and stained with CD11c mAb and analyzed by flow cytometry (b) or observed under two-photon laser scanning fluorescent microscope (c). CFSE, carboxyfluorescein succinimidyl ester; CFU, colony forming unit; DC, dendritic cell; i.p., intraperitoneally; Lm, Listeria monocytogenes; mAb, monoclonal antibody; MP, microparticle.
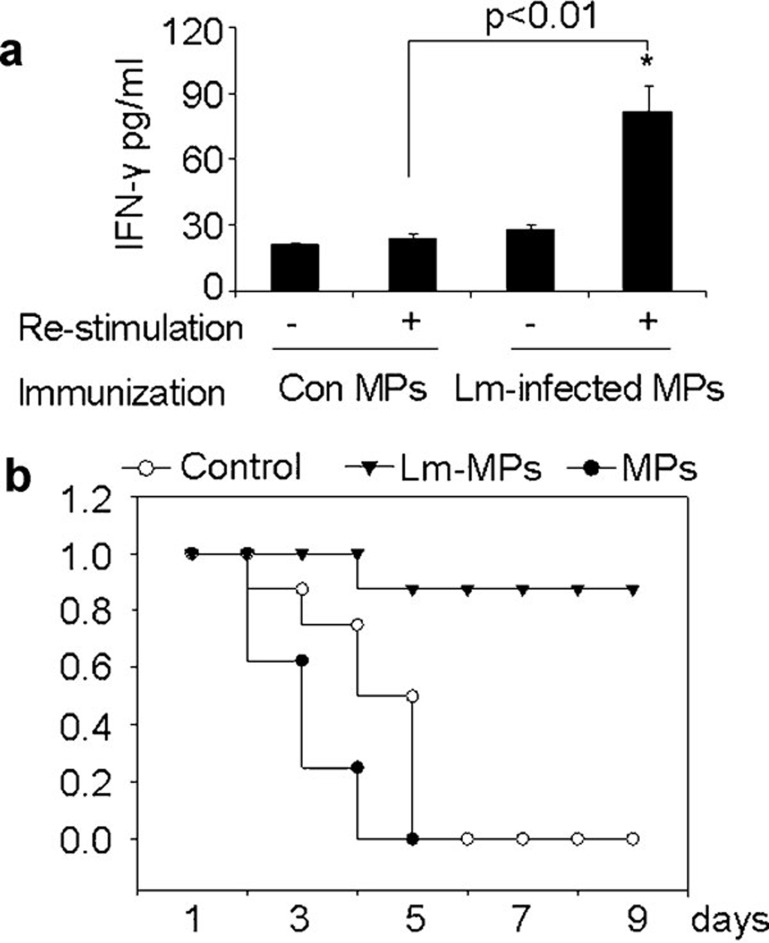
MPs from Lm-infected macrophages elicit protective immunity
Finally, we wondered whether the Lm antigenicity of MPs is able to elicit protective immune response in vivo. To verify this, mice were immunized i.p. with MPs from Lm-infected macrophages mixed with rehydragel adjuvant for 7 days. The mice were killed and splenic cells were activated with MPs for 48 h. The production of IFN-γ was clearly induced in splenocytes from mice immunized with MPs from Lm-infected macrophages compared to those mice immunized with MPs derived from uninfected macrophages (Figure 7a). In a separate experiment, mice were immunized with Lm-MPs and control MPs, respectively, for 7 days followed by challenge by i.v. injection of 1×105 CFU viable Lm. The results showed that most mice immunized with MPs from Lm-infected macrophages survived, as opposed to the mice immunized with control MPs (Figure 7b). However, such protective immunity could not be ascribed to the contamination of Lm in MPs, since after the filtration of the supernatants of single Lm incubation, the centrifugated pellets had no protective effect against Lm challenge (Figure 7b). Furthermore, in these in vivo experiments, we also used gentamicin to treat mice concomitant with MP injection. Together, these data suggested that MPs containing Lm components elicit protective immune responses.
Figure 7.
Lm antigenicity of MPs elicits protective immune response in vivo. (a) IFN-γ production by splenocytes in MPs-immunized mice. BALB/c mice were i.p. immunized with MPs mixed with rehydragel adjuvant for 7 days. Splenic cells were isolated and stimulated by MPs from Lm-infected or uninfected macrophages. Forty-eight hours later, the supernatants were harvested and assayed for IFN-γ by ELISA. (b) MPs by Lm-infected macrophages elicited protective immunity against Lm infection. MPs were isolated from the supernatants of the cultured macrophages in the presence or absence of Lm, and passed through 1.2 µm filter. The supernatants of sole Lm incubation were also experienced such processes and used as control. BALB/c mice (n=6) were immunized with MPs mixed with rehydragel adjuvants for 7 days and challenged with the i.v. injection of 1×105 viable Lm. The survival of mice was observed (P<0.001; Kaplan–Meier analysis). The data shown here were representative of three independent experiments. IFN, interferon; i.p., intraperitoneally; i.v., intravenous; Lm, Listeria monocytogenes; MP, microparticle.
Discussion
Despite the well established roles of dendritic cells and macrophages in the development of adaptive immune responses, it remains unclear if and how these two cell types interact with each other during natural infection. In the present study, we demonstrated that DCs require the participation of macrophages to generate protective immune responses against Lm infection and macrophage-released MPs act as a vector to bridge DCs and macrophages.
DCs are believed to be specialized in capturing and processing antigens in vivo,32,33 converting protein antigens to peptides that are presented on MHC molecules and recognized by T cells. Through interactions with DCs, helper T cells differentiate into either Th1 cells, which produce IFN-γ to resist infection by facultative and obligate intracellular microbes such as Lm, Th2 cells that produce IL-4 and IL-13 to mobilize immune cells to resist helminths, or Th17 cells producing IL-17 and IL-22 to mobilize phagocytic cells to eliminate extracellular bacteria.34,35 One important question that is not answered yet is whether DCs fulfill all of these functions by themselves or if they need other cell types for support during bacterial infection. In the present study, we have obtained evidence in support of the idea that DCs require interactions with macrophages to elicit T cell-mediated immune responses during Lm infection, including (i) depletion of macrophages with liposomal clodronate or F4/80 antibody which led to impaired T cell activation; (ii) inoculation of Lm-infected macrophages into C57BL/6 mice strongly induced T cell-mediated immune responses as measured by the production of IFN-γ and IL-22; and (iii) infection of DCs in the presence of macrophages with viable Lm elicited strong T-cell proliferation and IFN-γ production. However, infecting either one of the cell types only induced poor T-cell activation. These findings clearly indicate that DCs and macrophages need to collaborate with each other for the optimal induction of T cell-mediated immune response during Lm infection. Notably, although DCs mediate T cell responses against Lm infection, Edelson et al.36 recently reported that DCs are required for Lm transport, and DC ablation drastically reduces Lm replication. Such different role of DCs probably strengthens the idea that DCs cooperate with macrophages against Lm infection.
Macrophages are professional phagocytic cells that play a critical role in host defense against Lm infection. After being engulfed by macrophages, Lm survives by disrupting the phagosomal membrane by secreting virulence factors including listerilysin O, and then escaping into the cytoplasm. In response to Lm, macrophages produce a wide variety of pro-inflammatory cytokines, including TNF-α, IL-1, IL-6, IL-12, IL-23, etc.,2,37,38 which are involved in host defense against this pathogen; however, whether macrophages adapt other pathways involved in the protective immunity remains elusive. The expression of the above cytokines by macrophages is due to recognition of bacterial components or products through pathogen-associated molecule pattern receptors, whose signalings commonly result in the activation of NF-κB and MAPK. Interestingly, the present study found that macrophages upon infection with Lm, released MPs, leading to the activation of MAPK and NF-κB in DCs. Moreover, these released MPs contain Lm antigen components and can be taken up by DCs for antigen presentation. Nevertheless, the process of MP-contained antigens by DCs might be very complicated. We found that Lm-infected MPs from MHC class I-deficient macrophages significantly reduced the presentation of Lm antigen by DCs to CD8+ T cells compared to control MPs, suggesting that DCs directly present MHC class I-peptide complexes derived from macrophages and carried by MPs to T cells. Although TLR signaling is shown to be important for DC presentation of microbial antigens,29,30 in our case, MyD88 deficiency in macrophages did not show effect on DCs acquisition of Lm antigen from MPs. Besides, MHC class II deficiency impairs the presentation of the MP-derived Lm antigen by DCs to CD4+ T cells. On the basis of these data, we propose that DCs take up microparticles by (i) internalization of MPs and disintegration of their membrane structure to small fragments; (ii) release of class II antigens from disintegrated MPs that enter into class II antigen presentation processes; and (iii) fusion of MP membrane fragments with MHC complexes followed by antigen presentation to the DC membranes. Therefore, MPs may serve as messengers for the mutual interaction between macrophages and DCs so as to facilitate optimal anti-Lm immune responses.
Killed bacteria have long been thought to be substantial in the generation of protective immune responses against Lm infection, although the exact reasons are not well understood. In the present study, we found that MP-contained Lm components have strong antigenicity, as Lm-infected H22 cells were lysed when effector T cells were induced by DCs treated with MPs. In line with this, we demonstrated that mice immunized with MPs from Lm-infected macrophages mixed with rehydralgel adjuvant induced strong protective immune responses. The results indicate that for the generation of protective immunity, antigens need to be coupled with components from host cells in the forms such as Lm-infected MPs, suggesting that MPs could be used as platforms for future vaccine preparation.
Our present study raises an important issue, that is, whether the MP pathway is generally adapted in animals and humans in response to intracellular bacterial infection. Like Lm, other intracellular bacteria such as mycobacteria also infect macrophages. Inevitably, mycobacterium infection will cause the activation of apoptosis of macrophages. Given the fact that activated or apoptotic cells generate and release MPs, it is without doubt that macrophages would produce MPs, which might encapsulate mycobaterial components. In this regard, the MP pathway is probably a general defense mechanism against intracellular bacterial infections.
In summary, we demonstrated that macrophages transfer Lm antigen to dendritic cells by releasing MPs, and identified an intrinsic antigen transferring program between macrophages and DCs that leads to the induction of protective adaptive immune responses. Notably, the quantity of MPs released by Lm-infected macrophages correlated to the infection level. Only when MPs reach a threshold, does transfer of Lm immunogenicity to DCs occur. This suggests that a threshold signal is required for the induction of an adaptive immune response. In addition, our study may, in part, address a basic immunological issue: how does the immune system sense the magnitude of infection and subsequently elicits adaptive immunity. Therefore, dissecting the intrinsic antigen transfer program between macrophages and dendritic cells will have far reaching implications in the link between innate and adaptive immunity to bacterial infection.
Supplementary Information accompanies the paper on Cellar & Molecular Immunology's website (http://www.nature.com/cmi).
Acknowledgments
This work was supported by the National Basic Research Program of China (2012CB932500), Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (30911120482), the Program for New Century Excellent Talents in University (NCET-08-0219) and the Fundamental Research Funds for the Central Universities (HUST-2010JC024, HUST-2011TS027).
Supplementary Information
References
- Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- Pamer EG. Immune responses to Listeria monocytogenes. . Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for TH1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Jutras I, Desjardins M. Phagocytosis: at the crossroads of innate and adaptive immunity. Annu Rev Cell Dev Biol. 2005;21:511–527. doi: 10.1146/annurev.cellbio.20.010403.102755. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, de los Santos K, Sparwasser T, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb-Mäurer A, Pilgrim S, Kämpgen E, McLellan AD, Bröcker EB, Goebel W, et al. Antibodies against listerial protein 60 act as an opsonin for phagocytosis of Listeria monocytogenes by human dendritic cells. Infect Immun. 2001;69:3100–3109. doi: 10.1128/IAI.69.5.3100-3109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Bode AP, Sandberg H, Dombrose FA, Lentz BR. Association of factor V activity with membranous vesicles released from human platelets: requirement for platelet stimulation. Thromb Res. 1985;39:49–61. doi: 10.1016/0049-3848(85)90121-5. [DOI] [PubMed] [Google Scholar]
- Jurk K, Kehrel BE. Platelets: physiology and biochemistry. Semin Thromb Hemost. 2005;31:381–392. doi: 10.1055/s-2005-916671. [DOI] [PubMed] [Google Scholar]
- VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277–287. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Jiang J, Shen H, Chen YH. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J Immunol. 2004;73:5652–5658. doi: 10.4049/jimmunol.173.9.5652. [DOI] [PubMed] [Google Scholar]
- Tang K, Liu J, Yang Z, Zhang B, Zhang H, Huang C, et al. Microparticles mediate enzyme transfer from platelets to mast cells: a new pathway for lipoxin A4 biosynthesis. Biochem Biophys Res Commun. 2010;400:432–436. doi: 10.1016/j.bbrc.2010.08.095. [DOI] [PubMed] [Google Scholar]
- György B, Módos K, Pállinger E, Pálóczi K, Pásztói M, Misják P, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117:39–48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- Huang B, Zhao J, Shen S, Li H, He KL, Shen GX, et al. Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res. 2007;67:4346–4352. doi: 10.1158/0008-5472.CAN-06-4067. [DOI] [PubMed] [Google Scholar]
- Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577–7584. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
- Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174:8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 2007;28:227–233. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells crossdressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–6024. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- Luketic L, Delanghe J, Sobol PT, Yang P, Frotten E, Mossman KL, et al. Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J Immunol. 2007;179:5024–5032. doi: 10.4049/jimmunol.179.8.5024. [DOI] [PubMed] [Google Scholar]
- Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C, West MA, Zaru R. TLR signalling regulated antigen presentation in dendritic cells. Curr Opin Immunol. 2010;22:124–130. doi: 10.1016/j.coi.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Köppler B, Cohen C, Schlöndorff D, Mack M. Differential mechanisms of microparticle transfer to B cells and monocytes: anti-inflammatory properties of microparticles. Eur J Immunol. 2006;36:648–660. doi: 10.1002/eji.200535435. [DOI] [PubMed] [Google Scholar]
- López-Bravo M, Ardavín C. In vivo induction of immune responses to pathogens by conventional dendritic cells. Immunity. 2008;29:343–351. doi: 10.1016/j.immuni.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. . Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- Zielinski CE, Corti D, Mele F, Pinto D, Lanzavecchia A. Dissecting the human immunologic memory for pathogens. Immunol Rev. 2011;240:40–51. doi: 10.1111/j.1600-065X.2010.01000.x. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Bradstreet TR, Hildner K, Carrero JA, Frederick KE, Kc W, et al. CD8alpha+ dendritic cells are an obligate cellular entry point for productive infection by Listeria monocytogenes. . Immunity. 2011;35:236–248. doi: 10.1016/j.immuni.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of Th1 CD41 T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Meeks D, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. . J Immunol. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.