Abstract
Mesenchymal stem cells (MSCs) have been used experimentally for treating inflammatory disorders, partly due to their immunosuppressive properties. Although interleukin-1β (IL-1β) is one of the most important inflammatory mediators, growing evidence indicates that IL-1β signaling elicits the immunosuppressive properties of MSCs. However, it remains unclear how IL-1β signaling accomplishes this activity. Here, we focus on the therapeutic efficacy of IL-1β-primed MSCs in the dextran sulfate sodium (DSS)-induced colitis model, in addition to the underlining mechanisms. We first found that IL-1β-primed MSCs, without any observable phenotype change in vitro, significantly attenuated the development of DSS-induced murine colitis. Moreover, IL-1β-primed MSCs modulated the balance of immune cells in the spleen and the mesenteric lymph nodes (MLNs) through elevating cyclooxygenase-2 (COX-2), IL-6 and IL-8 expression and influencing the polarization of peritoneal macrophages. Importantly, IL-1β-primed MSCs possessed an enhanced ability to migrate to the inflammatory site of the gut via upregulation of chemokine receptor type 4 (CXCR4) expression. In summary, IL-1β-primed MSCs have improved efficacy in treating DSS-induced colitis, which at least partly depends on their increased immunosuppressive capacities and enhanced migration ability.
Keywords: IL-1β, mesenchymal stem cells, ulcerative colitis
Introduction
Mesenchymal stem cells (MSCs) are derived from a variety of tissues and have the capacity for self-renewing and differentiation.1,2 MSC-based therapies have provided convincing evidence for their potential anti-inflammatory and immunomodulatory effects in treating a variety of inflammatory and autoimmune diseases, including ulcerative colitis (UC),3 rheumatoid arthritis4 and sepsis.5 MSCs can migrate to sites of tissue damage,6,7 where they are activated by inflammatory cytokines (such as IFN-γ) and several chemokines, which can recruit or suppress lymphocytes.8,9,10,11
Interleukin-1β (IL-1β) is an important inflammatory factor that is expressed abundantly during the active phase of UC.12,13,14 The UC mice induced by DSS treatment exhibit many symptoms similar to human UC, such as diarrhea, bloody feces, body weight loss, mucosal ulceration and shortening of the large intestine.13 The mechanism behind DSS-induced UC can be attributed to abundant IL-1β secretion from macrophages in lamina propria following DSS stimulation, which initiates an intestinal inflammatory cascade.15 Moreover, administration of anti-IL-1β antibodies improves DSS-induced colitis,14 and mice that are deficient in the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome, a caspase-1-activating complex which regulates IL-1β maturation, are relatively resistant to intestinal inflammation.15
However, growing evidence has indicated that IL-1β signaling supports colonic homeostasis and attenuated colonic inflammation. IL-1β signaling can protect mice from intestinal damage after Citrobacter rodentium infection16 and from DSS-induced colitis.17 Mice that are deficient in IL-1 receptor 1 (IL-1R1) exhibit exacerbated DSS-induced colitis, which is indicated by a higher disease activity index score and an increased mortality compared with wild-type mice. Moreover, blocking IL-1β activity with anakinra, a recombinant form of IL-1R antagonist, enhances the severity of DSS-induced colitis in wild-type mice.18 IL-1β induces the production of cyclooxygenase-2 (COX-2), which promotes the expression of prostaglandin E2 (PGE2).19,20 PGE2, in turn, has been reported to inhibit mucosal inflammation during DSS-induced colitis in mice and rats.21,22 Similarly, pre-treatment with a low dose of IL-1β has also been shown to suppress colonic inflammation in rabbits via the production of PGE2.23
Notably, several studies have indicated that MSCs activated by IL-1β can exhibit immunosuppressive properties. First, IL-1R1, required for IL-1β signal transduction, is abundantly expressed in human MSCs. Second, Groh et al.24 showed that T-cell activation could be inhibited by the cell-free supernatant from MSCs treated with IL-1β for 24 h. Third, IL-1β secreted from CD14 monocytes has been reported to activate MSCs. In addition, IL-6, a hallmark of MSC activity, is vigorously secreted by MSCs following stimulation with IL-1β in a time- and dose-dependent manner, which requires the activation of the p38 MAPK and ERK pathways.25 All of these data suggest that IL-1β could elicit the immunosuppressive properties of MSCs through IL-1R1. However, the mechanism through which IL-1β elicits these immunosuppressive properties is still unclear.
In the present study, our results demonstrated that pre-treatment with IL-1β enhanced the efficacy of MSC transplantation in DSS-induced colitis. Pre-treating MSCs with IL-1β also modulated the balance of immune cells through elevating COX-2 and IL-8 expression. IL-1β also promoted MSC migration to inflammatory sites through upregulating CXCR4 expression.
Materials and methods
Isolation and culture of umbilical cord-derived MSCs
MSCs were isolated using previously described methods.26 Briefly, human umbilical cords were obtained from full-term deliveries with informed consent of the mother after caesarian section. The cords were aseptically stored at 4 °C in sterile phosphate-buffered saline (PBS) supplemented with 200 U/ml penicillin-streptomycin (Sigma Aldrich, St. Louis MO, USA). Tissue collection for research was approved by the Ethics Committee of the Drum Tower Hospital of Nanjing University Medical School, China. Fresh human umbilical cords were collected and rinsed in PBS at 4 °C, and umbilical arteries and veins were removed and cut into 1- to 2-mm pieces in DMEM/F-12 medium (Gibco, Grand Island, NY, USA). The pieces of cords were digested with mixed enzymes, including type II collagenase, neutral protease and hyaluronidase at 37 °C for 3 h. After three washes with PBS, umbilical cord-derived MSCs were plated into a culture flask. The medium was replaced every 3 days. Colonies of fibroblast-like cells formed approximately 7 days later, and the cultures were trypsinized and passaged into a new flask for further expansion.
MSC treatment
MSCs were pre-treated with 10 ng/ml murine IL-1β (Peprotech, Rocky Hill, NJ, USA) for 48 h to activate IL-1β signaling. IL-1β signaling in MSCs was inhibited by downregulating IL-1R1 expression with small RNA interference (siRNA). Specific sequence for human IL-1R1 siRNA was designed and synthesized by Ribobio (GuangZhou, China). Two suitable sequences were selected (IL-1R1–siRNA#1, GAACACAAAGGCACUAUAAdTdT, and IL-1R1–siRNA#2, GCAGCAUA UAUCCAGUUAA dTdT) and mixed as siRNA for the following experiments. MSCs were cultured to 40% confluency and transfected with the siRNA duplex and negative control siRNA using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommended protocol. Then, 10 nM siRNA was used for 10-cm plates. After 24 h of transfection, the cells were stimulated with 10 ng/ml murine IL-1β for another 48 h and harvested for reverse transcription-PCR and western blotting. In vitro models were divided into five groups (Table 1).
Table 1. Grouping of MSCs used in vitro experiment.
| Group (MSCs) | Treatment |
|---|---|
| Con | No treatment |
| siRNA Con | Negative control siRNA transfection |
| IL-1β | 10 ng/ml IL-1β |
| IL-1R1 siRNA | si-IL-1R1 transfection |
| IL-1R1 siRNA+IL-1β | After IL-1R1 siRNA transfection, 10 ng/ml IL-1β was added |
Abbreviations: IL-1β, interleukin-1β IL-1R1, IL-1 receptor 1; MSC, mesenchymal stem cell; siRNA, small RNA interference.
DSS-induced mice colitis
Acute colitis was induced in C57BL/6 male mice (8-week-old) by administering 3% DSS (molecular weight 40 000 Da; Sigma) from day 0 to day 7 in the drinking water ad libitum and changing to regular water from day 8 onward, as previously described.20,30
C57BL/6 mice were randomly divided into the following five groups (n=10 mice/group): (i) control group; (ii) DSS treatment; (iii) DSS with MSC treatment; (iv) DSS with IL-1β-primed MSC treatment; (v) DSS with IL-1R1 siRNA-transfected MSC treatment (Table 2). At day 1, mouse tails were intravenously injected with 2×106 MSCs (MSCs, IL-1β-primed or IL-1R1 siRNA-transfected MSCs) diluted in 200 µl PBS or a vehicle control (PBS alone). Mice were killed at day 14, except for the mice in the DSS treatment group, which were killed at day 11.
Table 2. Grouping of mice used in vivo experiment.
| Group (mice) | DSS treatment | MSC treatment |
|---|---|---|
| Control | – | – |
| DSS | + | – |
| DSS+MSC | + | MSCs |
| DSS+IL-1β MSC | + | IL-1β-primed MSCs |
| DSS+IL-1R1 siRNA MSC | + | IL-1R1 siRNA-transfected MSCs |
Abbreviations: DSS, dextran sulfate sodium; IL-1β, interleukin-1β IL-1R1, IL-1 receptor 1; MSC, mesenchymal stem cell; siRNA, small RNA interference.
Colitis severity was assessed daily by scoring the clinical disease activity (0–4), including evaluating stool consistency, presence of fecal blood and weight loss. The entire colon was removed from the cecum to the anus, and colon length and weight were measured as indirect inflammation markers. For histopathological analysis, colon segments were fixed in 10% buffered formalin phosphate, and paraffin-embedded sections were prepared for hematoxylin and eosin (H&E) staining. Histological scores were blindly determined. For tissue inflammation, increased numbers of inflammatory cells in the lamina propria were scored as 1, confluence of inflammatory cells extending into the submucosa was scored as 2 and transmural extension of the infiltrate was scored as 3. For tissue damage, discrete lymphoepithelial lesions were scored as 1, mucosal erosions were scored as 2 and extension through deeper structures of the bowel wall was scored as 3. The combined histological colitis severity score ranged from 0 to 6.
In vivo bioluminescence and CM-DiI fluorescent imaging
For bioluminescence imaging and fluorescent imaging, luciferase-expressing MSCs were labeled with CM-DiI dye (Molecular Probes, Eugene, Oregon, USA) according to the manufacturer's protocol. C57BL/6 mice were randomly divided into the following groups (n=5 each group): (i) DSS with MSC treatment; and (ii) DSS with IL-1β-primed MSC treatment. At day 1, C57BL6 mice were treated with 3% DSS. At day 2, mice were injected 2.0×106 MSCs via tail vein. At day 4, mice were given an intraperitoneal injection of 0.2 mg D-Luciferin Potassium Salt (Caliper, Hopkinton, USA) and killed 10 min later. Spleen, mesenteric lymph nodes (MLNs), and colon were obtained and imaged with an IVIS Lumina XR System (Caliper) and then frozen for cryosectioning. For visualizing the localization of labeled MSCs based on fluorescence of CM-DiI dye (red), cryosectioned tissue was counter-stained with 4′-6-diamidino-2-phenylindole, and fluorescent images were collected with a confocal scanning laser system (Olympus FluoView 1000; Olympus Corp., Lake Success, NY, USA) attached to an inverted microscope (IX81; Olympus Corp., Tokyo, Japan).
Flow cytometric analysis
Monoclonal antibodies against human CD11b, CD106, CD14, CD19, CD31, CD34, CD45, HLA-DR, CD29, CD44, CD73, CD90 and isotype-matched controls, as well as monoclonal antibodies against mouse CD206, CD11c, F4/80, CD3, CD4, IL-17A, IL-4, IFN-γ, Foxp3 and isotype-matched controls, were obtained from eBiosciences, San Diego, CA, USA. For the detection of intracellular cytokines, cells were incubated with phorbol myristate acetate (50 ng/ml) and ionomycin (750 ng/ml) for 4 h. Labeled cells were analyzed on FACS Calibur flow cytometer BD Biosciences, San Diego, USA and data were analyzed using CellQuest software (BD Biosciences).
Quantitative PCR (Q-PCR) analysis
Q-PCR for GAPDH, COX-2, IL-6, IL-8 and CXCR4 was performed on an Applied Biosystems 7300 Sequence Detection System (Applied Biosystems, Foster city, CA, USA) using SYBR green dye (Bio-red). For quantification, the relative mRNA level of specific gene expression was obtained using the 2−ΔCt method.
Transwell migration assay
The migratory ability of MSCs was evaluated using Transwell plates (Corning Costar, Tewksbury, USA), which were 6.5 mm in diameter with 8-µm pore filters. In brief, MSCs were suspended in serum-free medium and seeded into the upper well, and 10 ng/ml murine IL-1β containing medium was placed in the lower well of a Transwell plate. Following incubation for 12 h at 37 °C, cells that had not migrated from the upper side of the filter were scraped off with a cotton swab, and filters were stained with crystal violet. The number of cells that had migrated to the lower side of the filter was counted under a light microscope at ×4 magnification in five randomly selected fields.
Statistical analysis
Results were presented as mean±s.e.m. Student's t-test was used to compare between two groups. One-way ANOVA analysis of variance was used to compare among three or more groups. Differences were considered to be significant with P values of <0.05. Statistical calculations and survival curves were performed with GraphPad Prism software (San Diego, CA, USA).
Results
The effect of IL-1β signaling on the morphology, phenotype and viability of MSCs
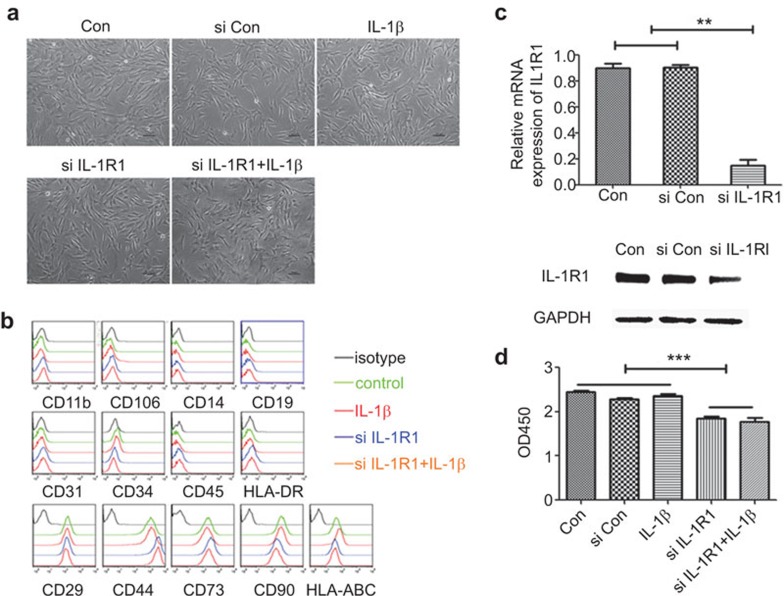
MSCs were generated from human umbilical cord tissue, and their colonies visually appeared as a homogenous population of cells after four passages (Figure 1a). The MSC cultures were then assayed routinely for the presence of MSC-related cell surface antigens by flow cytometry analysis. The results showed that the phenotype of MSCs was positive for CD29, CD44, CD73, CD90 and HLA-ABC (MHC-I) and was negative for CD11b, CD106, CD14, CD19, CD31, CD34, CD45 and HLA-DR (MHC-II) (Figure 1b).
Figure 1.
The effects of IL-1β signaling on MSC phenotype, morphology and vitality. (a) The morphology of MSCs was observed by microscopy. (b) The effect of IL-1β signaling on the phenotype of MSC. Expression of phenotypic surface antigens on MSCs was detected using FACS. There was no significant difference between the different treatment groups of MSCs. (c) Knockdown of IL-1R1 with siRNA. MSCs were either mock transfected or transfected with the mixture of two different siRNAs against IL-1R1. The cells were then harvested at day 2 and analyzed at the mRNA and protein level. (d) IL-1β signaling affects the viability of MSCs. The viability of MSCs was measured using the Cell Counting Kit-8 method. IL-1β, interleukin-1β IL-1R1, IL-1 receptor 1; MSC, mesenchymal stem cell; siRNA, small RNA interference.
IL-1β, one of the most important soluble inflammatory mediators, has been found to be enhanced in UC. IL-1R1, the major receptor for IL-1β, is expressed in MSCs. To study the impact of IL-1β signaling on MSCs, we first downregulated the expression of IL-1R1 on MSCs using siRNA. The two siRNAs mixtures targeted against different regions of IL-1R1 mRNA were cotransfected into MSCs, and 48 h later the cells were harvested for Q-PCR and western blot analysis. The results showed that IL-1R1 was significantly downregulated at both the mRNA and protein levels (Figure 1c).
Importantly, after being cultured for 2 days with or without IL-1β (10 ng/ml), the five experimental conditions for MSCs (control, si control, IL-1β, IL-1R1 siRNA, IL-1R1 siRNA+IL-1β) retained their homogeneous fibroblastoid cell morphology, with a smooth border and unaffected expression of surface markers (Figure 1a and b). Moreover, IL-1β did not affect the viability of MSCs, but IL-1R1 siRNA significantly reduced viability of MSCs either in the absence or presence of IL-1β (Figure 1d). These data indicate that IL-1β signaling does not affect the morphology and phenotype of MSCs, but IL-1β signaling is important to maintain the viability of MSCs.
IL-1β-primed MSCs attenuate the development of DSS-Induced colitis
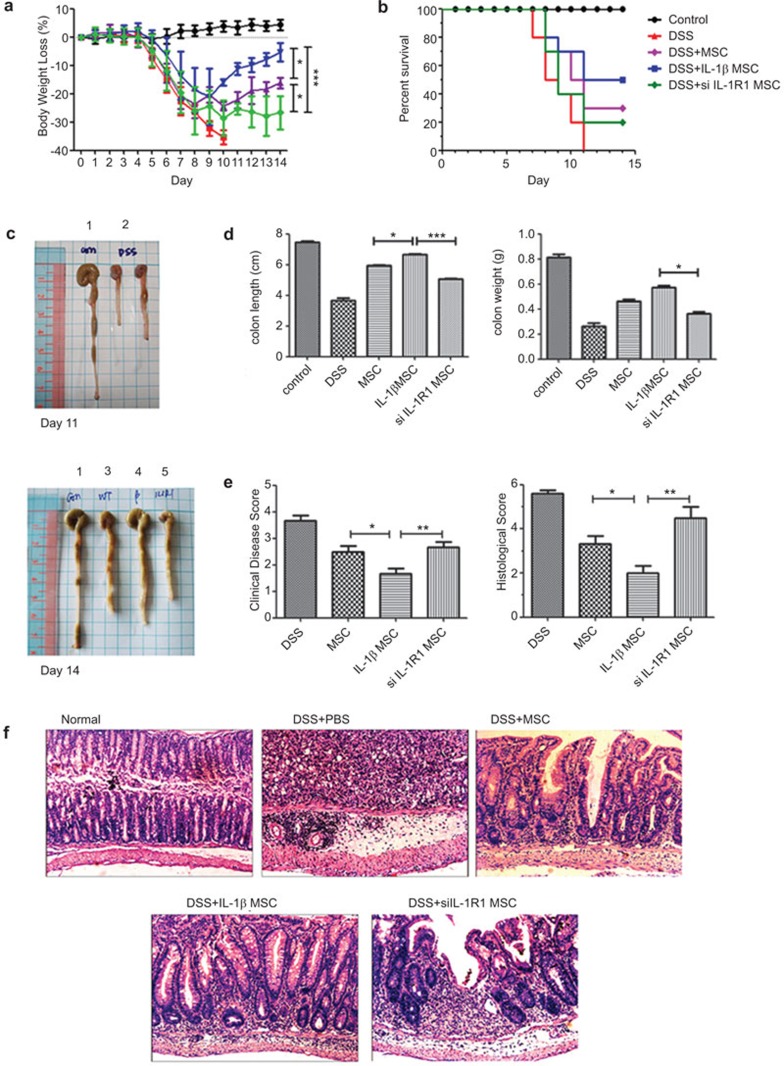
Similar to previous reports,27,28 we successfully induced colitis in C57BL/6 mice by oral administration of 3% DSS. The mouse colitis was characterized by sustained bloody diarrhea, weight loss, 100% mortality at day 11 (Figure 2a, b and d), shorter and lighter colons (Figure 2c), histological changes with severe colonic transmural inflammation, increased wall thickness, localized inflammatory cell infiltration, epithelial ulceration with degeneration of crypt architecture and loss of goblet cells (Figure 2e and f). The next day, following oral administration with 3% DSS, the mice were intravenously injected through the tail vein with PBS, MSCs, IL-1β-primed MSCs, or IL-1R1 siRNA transiently transfected MSCs. At day 14, the mice showed ameliorated colitis and improved survival rates to different degrees compared with those in the PBS group (Figure 2a–f). Strikingly, compared to the mice treated with MSCs or IL-1R1 siRNA transiently transfected MSCs, IL-1β-primed MSC-treated mice had a significantly improved survival rate, lower weight loss and clinical disease scores in addition to longer length and heavier colons (Figure 2a–e). Histologically, IL-1β-primed MSCs exhibited significantly ameliorated colonic transmural inflammation and decreased wall thickness, restored goblet cells and suppressed mucosal ulceration and focal loss of crypts, all of which was associated with a decrease in disease score and histological score (Figure 2e and f).
Figure 2.
IL-1β-primed MSCs attenuate the development of DSS-induced colitis. (a) The body weight changes were calculated as a percentage of initial body weight on day 0. (b) Survival rates were compared among normal mice, DSS-treated mice, DSS with MSC-treated mice, DSS with IL-1β-primed MSC-treated mice and DSS with IL-1R1 siRNA transiently transfected MSC mice. (c) Macroscopic images of representative mouse colons harvested on days 11 and 14. The numbers on the top of the picture indicated as follows: 1, normal mouse; 2, DSS-treated mouse; 3, DSS with MSC-treated mouse; 4, DSS with IL-1β-primed MSC-treated mouse; 5, DSS with IL-1R1 siRNA transiently transfected MSC mice. (d) Assessment of colonic weight and length upon killing. (e) Colitis scores and Histology scores indicated that IL-1β primed MSCs prevented DSS-induced pathology. Colitis scores were based on the presence of loose stool, bleeding, and macroscopic inflammation. Histology scores were derived from microscopic analyses of longitudinal colon sections from each mouse. (f) Photomicrographs (magnification ×10) of an H&E-stained paraffin section of a representative mouse colon from each treatment group. DSS, dextran sulfate sodium; H&E, hematoxylin and eosin; IL-1β, interleukin-1β IL-1R1, IL-1 receptor 1; MSC, mesenchymal stem cell; siRNA, small RNA interference.
These data consistently demonstrated that IL-1β-primed MSCs were more effective in the prevention of DSS-induced colitis than unprimed MSCs or the IL-1R1 siRNA transiently transfected MSCs. This finding suggests that IL-1β signaling is important for the therapeutic efficacy of MSCs in treating DSS-induced colitis.
IL-1β-primed MSCs alters the balance of immune cells
MSCs have been previously shown to have immunomodulatory effects. They can both suppress the proliferation and activation of innate and adaptive immune cells and activate regulatory T cells (Tregs). Because of these properties, we next investigated the effects of IL-1β-primed MSCs on the profile of immune cells in DSS-induced colitis mice.
Flow cytometry revealed that CD11c+ M1 macrophages were significantly increased in the peritoneal cavity of DSS-induced colitis mice and were significantly decreased in IL-1β-primed MSC-treated mice, but there was no notable difference among the mice treated with MSCs, IL-1β-primed MSCs, or IL-1R1 siRNA transiently transfected MSCs (Figure 3a).
Figure 3.
IL-1β-primed MSCs decrease systemic and local inflammatory responses in acute colitis. (a) M1 and M2 macrophage levels in mice peritoneal cavity. (c–e) Th1, Th2, Th17 and Treg cell levels in spleens and MLNs of mice. IL-1β, interleukin-1β MSC, mesenchymal stem cell; Treg, regulatory T cell; MLN, mesenteric lymph node.
However, CD206+ M2 macrophages were decreased in DSS-treated mice but significantly increased to the baseline level in IL-1β-primed MSC-treated mice. Moreover, M2 macrophages were significantly more prevalent in the IL-1β-primed MSC-treated mice than in the IL-1R1 siRNA transiently transfected MSC-treated mice (Figure 3a).
Moreover, IL-1β-primed MSC-treated colitis mice displayed significantly increased numbers of Treg cells and Th2 cells, in addition to decreased numbers of Th1 and Th17 cells in their spleens and MLNs compared with those in DSS-treated mice (Figure 3b–e). Notably, there were significantly elevated Treg cell numbers in IL-1β-primed MSC-treated mice compared to MSC-treated mice.
MSCs can inhibit T-cell activation and proliferation in vitro through soluble factors. Recent studies suggest that these soluble factors, including PGE2, IL-6 and IL-8, are critical for the immunomodulatory function of MSCs. COX-2 (a key enzyme in the synthesis of PGE2),31 IL-832 and IL-633 are known to be downstream transcripts of the IL-1β signaling pathway, but it is unclear whether this phenomenon is also the case for MSCs. We found that IL-1β could significantly elevate COX-2, IL-6 and IL-8 mRNA expression and COX-2 protein expression, while IL-1R1 siRNA-transfected MSCs showed abrogated IL-1β signaling effects (Figure 4).
Figure 4.
The effect of IL-1β signaling on the immunomodulation of MSCs. (a) After treatment with 10 ng/ml IL-1β for 24 and 48 h, the COX-2 expression of MSCs was evaluated by Q-PCR and western blot. (b, c) IL-1R1 siRNA-transfected MSCs were treated with or without IL-1β for 24 h, after which IL-8 and IL-6 expression was analyzed. COX-2, cyclooxygenase-2; IL-1β, interleukin-1β IL-1R1, IL-1 receptor 1; MSC, mesenchymal stem cell; Q-PCR, quantitative PCR; siRNA, small RNA interference.
Our results indicated that the marked attenuation of IL-1β-primed MSCs on the development of DSS-induced colitis may be related to modulating the immune cell profile though elevated COX2, IL-6 and IL-8 expression.
IL-1β signaling promotes the migration of MSCs
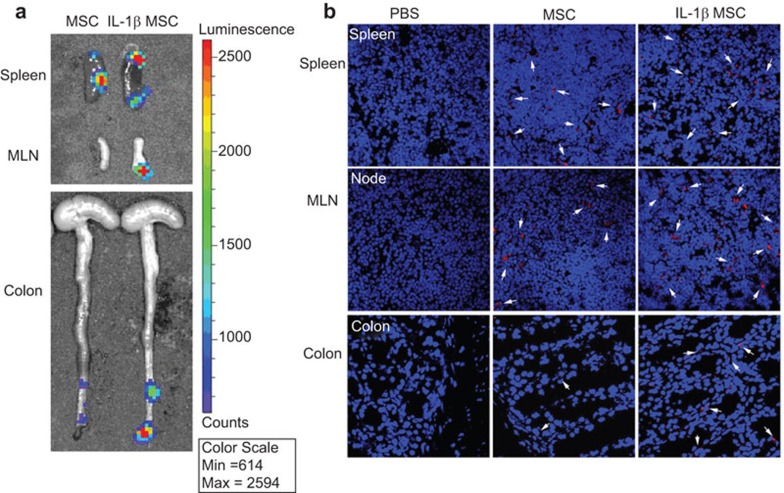
Given the migratory ability of MSCs to travel to inflammatory or injured sites, we assessed whether the IL-1β-primed MSCs had improved migration in vivo. The day after being treated with DSS, the mice were intravenously injected with luciferase-expressing MSCs, which were dual-labeled with CM-DIL and IL-1β-primed. On the fifth day, Luciferase substrate was injected into the peritoneal cavity. The mice were killed after 10 min, and the organs were immediately collected to image bioluminescence. IL-1β-primed MSCs showed stronger bioluminescence signal in the spleen, MLN and colon than unprimed MSCs (Figure 5a).
Figure 5.
In vivo bioluminescence imaging of fluc-Mscs delivery. (a) Representative bioluminescence images. IL-1β-primed MSC-injected mice showed stronger signal in spleens, MLNs and colons than unprimed MSCs. (b) Ex vivo imaging of CM-DIL dye-labeled MSCs in spleens, MLNs and colons. Arrows indicate MSCs in organs of MSC-injected mice. IL-1β, interleukin-1β MLN, mesenteric lymph node; MSC, mesenchymal stem cell.
To confirm the localization of luminescent MSCs in each organ, frozen sections of the organs were imaged. Representative fluorescent microscopic images showed that IL-1β-primed MSCs labeled with CM-DIL (red) were readily detected in the spleen, MLN and colon, whereas less bioluminescence signal was detected in MSCs labeled with CM-DIL and injected into mice, and no signal was detected in PBS injected mice (Figure 5b). Our results indicated that pretreating MSCs with IL-1β could improve the in vivo localization of MSCs to inflammatory sites during DSS induced colitis.
The enhanced migratory ability of IL-1β-primed MSCs is related to upregulation of CXCR4 expression
Given the greater migratory ability of IL-1β-primed MSCs to the inflammatory or injured sites and that overexpression of CXCR4 receptor could promote the migration of MSCs to the injured tissues,34 we investigated whether IL-1β could elevate MSC migration in vitro and enhance CXCR4 expression. We used a transwell migration assay to answer this question. As expected, our results showed that the migratory response of MSCs to IL-1β was more pronounced than those in the culture medium without IL-1β, while the IL-1R1 siRNA-transfected MSCs showed unchanged migration ability either in the absence or presence of IL-1β (Figure 6a). Moreover, Q-PCR and western blot analysis showed that both CXCR4 mRNA and protein expressions were significantly elevated in MSCs cultured with IL-1β (10 ng/ml) for 48 h (Figure 6b and c), and the IL-1R1 siRNA-transfected MSCs could not promote CXCR4 expression even after IL-1β stimulation. Together, these results suggested that the influence of IL-1β on the migratory ability of MSCs may be attributed to inducing enhanced CXCR4 expression.
Figure 6.
Activation of IL-1β signaling promotes the migration of MSCs. (a) Here, 10 ng/ml IL-1β was added into the lower chamber of the Transwell plates, and magnification photographs of representative stained filters were taken at 12 h; ×4 darker areas are due to higher cell density. The average number of migrating cells per field was assessed by counting at least four high-power random fields per filter. (b, c) The gene and protein levels of CXCR4 in MSCs were checked after IL-1β treatment by Q-PCR and western blot, respectively. Results are mean±s.d. from three experiments each performed in duplicate. IL-1β, interleukin-1β MSC, mesenchymal stem cell; Q-PCR, quantitative PCR.
Discussion
Increasingly more studies have revealed the beneficial effects of MSC therapy in treating many diseases, including UC. MSCs can be derived from different human tissues and organs and, in the last several years, the human umbilical cord has gained increasing attention as a possible clinical alternative source for MSCs other than bone marrow.2 IL-1β is not only the key inflammatory factor in UC, but also mediates the development of DSS-induced UC. IL-1β can elicit the immunosuppressive properties of MSCs, but it is unclear whether the therapeutic efficacy of MSCs during UC is related to IL-1β. Here, we determined that IL-1β-primed MSCs had better efficacy in treating DSS-induced mice colitis, which might be due to the altered properties of MSCs following stimulation of IL-1β or one of several other proteins.
MSCs have the inherent function of repairing injured tissue. Our results showed that all of the surviving mice treated with MSCs (MSCs, IL-1β-primed MSCs, or IL-1R1 siRNA transiently transfected MSCs) exhibited therapeutic effects following treatment. The IL-1β-primed MSCs displayed the best efficacy, while the IL-1R1 siRNA transiently transfected MSCs displayed the worst efficacy. These results suggest that activation of the IL-1β signaling pathway in MSCs is beneficial for their therapeutic efficacy during murine colitis. We hypothesize that the MSCs that were primed by IL-1β were already activated in vitro before tail intravenous injection, but unprimed or IL-1R1 siRNA transiently transfected MSCs are activated by in vivo IL-1β following tail vein injection, as IL-1β is present in UC lesions.
IL-1β can drive the enhanced invasiveness of MSCs (derived from ovarian endometrioma),29 and MSCs are preferentially recruited by lymphoid organs and the inflamed colon in the UC mice.2 Our results showed that IL-1β-primed MSCs were recruited to the inflamed site (spleen, MLN and colon) in a greater numbers than other MSCs. This finding indicated that IL-1β could facilitate the recruitment of MSCs to the inflamed sites. Furthermore, IL-1β could also promote the migration of MSCs in vitro. The SDF-1/CXCR4 axis has been implicated in the migration of MSCs to the impaired brain after hypoglossal nerve injury in a rat model, resulting in tissue regeneration,30 and over expression of CXCR4 improves MSC migration to infracted myocardium and ameliorates cardiac performance.31 Our results showed that IL-1β-primed MSCs significantly increased expression of CXCR4 at both the mRNA and protein levels in vitro. Altogether, these results suggest that IL-1β could enhance the ability of MSCs to target and localize to inflammatory sites and elevate their migration ability through increasing CXCR4 expression, all of which could render MSCs more efficacious in cell-based repair therapy.
MSCs have extensive immunomodulatory functions on activated immunocytes. Our results showed that MSCs could both shape the host response to effectively polarize macrophage from M1 to M2 and switch T cells from a Th1/Th17 immune profile towards a Th2/Treg immune profile. All of these results are consistent with previous reports. However, the ability of MSCs to modulate the activation of immunocytes is not entirely innate. For example, the immunosuppressive ability of MSCs is induced by inflammatory cytokines such as IFN-γ, TNF-α and IL-1β.32 We also found that IL-1β could significantly elevate COX-2, IL-8 and IL-6 expression in MSCs, which was abrogated following knockdown of IL-1R1. COX-2 is a key enzyme in the synthesis of PGE2, and MSC-derived PGE2 is involved in skewing the inflammatory environment towards an anti-inflammatory profile, altering the cytokine secretion profile of T-cell subsets (Th1, Th2, or Tregs).27,33 IL-8 is known as a specific neutrophil-attracting chemokine, and activated MSCs can mediate neutrophil recruitment through IL-8.34 IL-6 could promote MSC secretion of PGE235 as well as maintain the proliferative and undifferentiated state of bone marrow-derived MSCs.36 Altogether, these data suggest that IL-1β signaling enhances the ability of MSCs to produce COX-2, IL-8 and IL-6 and may facilitate MSCs survival, neutrophil recruitment and immunosuppressive capacity.
Although most of the immunocytes detected above did not show significant difference between IL-1β-primed MSC mice and those treated with other MSCs, IL-1β induced elevated expression of COX-2, IL-8 and IL-6 in MSCs. This result may potentially be attributed to the timing of immunocyte collection. We collected immunocytes when all of the surviving mice were recovered from colitis, a time when the three types of MSCs may have already carried their immunomodulatory function, so that the immune profile could be the same among the three types of MSC-treated mice.
In summary, in the present study, we showed that systemically infused IL-1β-primed MSCs could effectively attenuate DSS-induced murine colitis. IL-1β-primed MSCs could mediate their immunosuppressive effect via two modes of action. First, IL-1β enhanced the ability of MSCs to migrate to the inflamed spleen, MLN and colon of DSS-induced colitis mice by elevating CXCR4 expression, where MSCs could repair the injured intestinal mucosa and perform their immunosuppressive functions. Second, IL-1β promoted MSC secretion of IL-8, which is necessary for MSCs to recruit neutrophils and macrophages. IL-1β-pimed MSCs can then more efficiently suppress the excessive inflammatory and immune reactions by direct cell–cell contact or by secretion of PGE2.
References
- Tsagias N, Koliakos I, Karagiannis V, Eleftheriadou M, Koliakos GG. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus Med. 2011;21:253–261. doi: 10.1111/j.1365-3148.2011.01076.x. [DOI] [PubMed] [Google Scholar]
- Liang L, Dong C, Chen X, Fang Z, Xu J, Liu M, et al. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transplant. 2011;20:1395–1408. doi: 10.3727/096368910X557245. [DOI] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- Wojakowski W, Tendera M. Mobilization of bone marrow-derived progenitor cells in acute coronary syndromes. Folia Histochem Cytobiol. 2005;43:229–232. [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Mesenchymal stem cells: their phenotype, differentiation capacity, immunological features and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–171. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, et al. MCP-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KH, Murakami A, Hayashi R, Ohigashi H. Interleukin-1β targets interleukin-6 in progressing dextran sulfate sodium-induced experimental colitis. Biochem Biophys Res Commun. 2005;337 2:647–654. doi: 10.1016/j.bbrc.2005.09.107. [DOI] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Arai Y, Takanashi H, Kitagawa H, Okayasu I. Involvement of interleuking-1 in the development of ulceratie colitis inducted by dextran sulfate sodiium in mice. Cytokine. 1998;10:890–896. doi: 10.1006/cyto.1998.0355. [DOI] [PubMed] [Google Scholar]
- Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- Lebeis SL, Powell KR, Merlin D, Sherman MA, Kalman D. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. . Infect Immun. 2009;77:604–614. doi: 10.1128/IAI.00907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojouharoff G, Hans W, Obermeier F, Männel DN, Andus T, Schölmerich J, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353–358. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Navajas JM, Law J, Nguyen KP, Bhargava M, Corr MP, Varki N, et al. Interleukin 1 receptor signaling regulates DUBA expression and facilitates Toll-like receptor 9-driven antiinflammatory cytokine production. J Exp Med. 2010;207:2799–2807. doi: 10.1084/jem.20101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Neumann D, Hoff T, Resch K, DeWitt DL, Goppelt-Struebe M. Interleukin-1-induced cyclooxygenase 2 expression is suppressed by cyclosporin A in rat mesangial cells. Kidney Int. 1994;45:150–158. doi: 10.1038/ki.1994.18. [DOI] [PubMed] [Google Scholar]
- Mizel SB, Dayer JM, Krane SM, Mergenhagen SE. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1) Proc Natl Acad Sci U S A. 1981;78:2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta M, Hirata I, Toshina K, Murano M, Maemura K, Hamamoto N, et al. Expression of the EP4 prostaglandin E2 receptor subtype with rat dextran sodium sulphate colitis: colitis suppression by a selective agonist, ONO-AE1-329. Scand J Immunol. 2002;56:66–75. doi: 10.1046/j.1365-3083.2002.01096.x. [DOI] [PubMed] [Google Scholar]
- Cominelli F, Nast CC, Llerena R, Dinarello CA, Zipser RD. Interleukin 1 suppresses inflammation in rabbit colitis. Mediation by endogenous prostaglandins. J Clin Invest. 1990;85:582–586. doi: 10.1172/JCI114476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh ME, Maitra B, Szekely E, Koç ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine. 2005;32:270–279. doi: 10.1016/j.cyto.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Lasigliè D, Traggiai E, Federici S, Alessio M, Buoncompagni A, Accogli A, et al. Role of IL-1 beta in the development of human TH17 cells: lesson from NLPR3 mutated patients. PLoS One. 2011;6:e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- Kao AP, Wang KH, Long CY, Chai CY, Tsai CF, Hsieh TH, et al. Interleukin-1β induces cyclooxygenase-2 expression and promotes the invasive ability of human mesenchymal stem cells derived from ovarian endometrioma. Fertil Steril. 2011;96:678–684. doi: 10.1016/j.fertnstert.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Najar M, Raicevic G, Boufker HI, Fayyad Kazan H, de Bruyn C, Meuleman N, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton's Jelly and bone marrow sources. Cell Immunol. 2010;264:171–179. doi: 10.1016/j.cellimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Romieu-Mourez R, François M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol. 2009;182:7963–7973. doi: 10.4049/jimmunol.0803864. [DOI] [PubMed] [Google Scholar]
- Bouffi C, Bony C, Courties G, Jorgensen C, Noël D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. Plos One. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]