Abstract
Background
Modifications of proteins by O-glycosylation determine many of the properties and functions of proteins. We wish to understand the mechanisms of O-glycosylation and develop inhibitors that could affect glycoprotein functions and alter cellular behavior.
Methods
We expressed recombinant soluble human Gal- and GlcNAc-transferases that synthesize the O-glycan cores 1 to 4 and are critical for the overall structures of O-glycans. We determined the properties and substrate specificities of these enzymes using synthetic acceptor substrate analogs. Compounds that were inactive as substrates were tested as inhibitors.
Results
Enzymes significantly differed in their recognition of the sugar moieties and aglycone groups of substrates. Core 1 synthase was active with glycopeptide substrates but GlcNAc-transferases preferred substrates with hydrophobic aglycone groups. Chemical modifications of the acceptors shed light on enzyme–substrate interactions. Core 1 synthase was weakly inhibited by its substrate analog benzyl 2-butanamido-2-deoxy-α-D-galactoside while two of the three GlcNAc-transferases were selectively and potently inhibited by bis-imidazolium salts which are not substrate analogs.
Conclusions
This work delineates the distinct specificities and properties of the enzymes that synthesize the common O-glycan core structures 1 to 4. New inhibitors were found that could selectively inhibit the synthesis of cores 1, 2 and 3 but not core 4.
General significance
These studies help our understanding of the mechanisms of action of enzymes critical for O-glycosylation. The results may be useful for the re-engineering of O-glycosylation to determine the roles of O-glycans and the enzymes critical for O-glycosylation, and for biotechnology with potential therapeutic applications.
Keywords: O-Glycans, Specificity, Inhibitors, C1GalT, C3GnT, C2GnT
1. Introduction
The GalNAcα-Ser/Thr based mucin type O-glycans of glycoproteins and mucins have a number of important functions. They ensure resistance to proteases, and affect physical, chemical and antigenic properties of proteins [1]. Carbohydrate structures linked to O-glycans form blood group and tissue antigens and ligands for protein interactions which control cell adhesion, migration and cell death [2–4]. Alterations of O-glycosylation can therefore dramatically affect the functions of glycoproteins and cellular behavior. Our interest is to understand the detailed mechanisms of O-glycosylation and to develop technologies to change O-glycosylation for further studies of the role of O-glycans.
A large family of polypeptide GalNAc-transferases initiates O-glycan biosynthesis [5], followed by various glycosyltransferases that sequentially or competitively add sugar residues to GalNAcα-Ser/Thr to synthesize different core subtypes [6]. Polypeptide GalNAc-transferases have overlapping specificities towards their peptide or glycopeptide substrates [5,6]. The most common second step of O-glycosylation is the addition of Gal to GalNAc by core 1 β1,3-Gal-transferase (C1GalT, core 1 synthase, T-synthase) that synthesizes the core 1 structure Galβ1–3GalNAcα-Ser/Thr (Fig. 1) [6,7]. Core 1, also known as the T antigen, is often present in the unmodified form in cancer cells but is further modified in most cell types [8]. A deficiency of core 1 production is associated with several human diseases and disorders, and with certain cancer cells that express terminal GalNAc (Tn antigen) or sialylα2–6GalNAc (sialyl-Tn antigen) [9]. This may be due to a deficiency in the chaperone Cosmc that normally ensures the expression of active C1GalT [10–14].
Fig. 1.
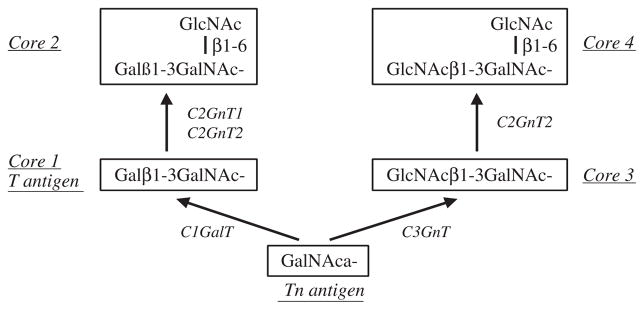
Synthesis of the common O-glycan core structures 1 to 4. C1GalT and C3GnT both act on GalNAc-substrates to synthesize core 1 and core 3, respectively. Core 1 is branched by either C2GnT1 or C2GnT2 to form core 2. Core 3 is branched only by C2GnT2 to form core 4. Core 1–4 structures can be extended and terminated in many different ways.
In specific tissues such as the colon, GalNAc in GalNAcα-Ser/Thr can also be converted to core 3 (Fig. 1), GlcNAcβ1–3GalNAc-, by core 3 β1,3-GlcNAc-transferase (C3GnT) [15,16]. C3GnT activity is low or undetectable in many cancer cells [17,18]. Metastatic colon and prostate cancer cells transfected with the gene encoding C3GnT were less capable of migration and invasion through extracellular matrix components, and suppressed tumor formation and metastasis in mice [19,20]. C3GnT gene knockout mice further showed that core 3 glycans have a protective function in normal gastro-intestinal epithelia [21].
C1GalT and C3GnT utilize GalNAcα-Ser/Thr-peptides as natural acceptor substrates but can be assayed using synthetic GalNAc-derivatives [14–16,22–24]. However, the detailed specificities of human C1GalT and C3GnT have not yet been determined.
Core 1 can be branched to form core 2, GlcNAcβ1–6(Galβ1–3) GalNAc-, by a family of core 2 β1,6-GlcNAc-transferases (C2GnT) (Fig. 1). While C2GnT1 is highly specific and only acts on core 1 [25–27], the related enzyme, core 2/4 β1,6-GlcNAc-transferase (C2GnT2), has a broader specificity and can also synthesize core 4, GlcNAcβ1–6(GlcNAcβ1–3)GalNAc-, from core 3 [15,28,29]. The levels of expression or activities of C2GnT1 have been shown to be abnormal and variable in cancer cells [8,18]. Core 2 O-glycans act as a scaffold structure for sialyl-Lewis x (SLex) which plays a critical role in cell adhesion and cell migration [30,31]. Moreover, MUC1 mucin, which contains core 2 O-glycans, functions as a molecular shield against immune cell attacks, facilitating bladder tumor metastasis [32]. Transfection experiments showed that the expression of C2GnT2 in colon cancer cells HCT116 is associated with reduced cell growth, increased apoptotic cell death and reduced tumor formation in nude mice [33].
Knowledge of the substrate recognition of glycosyltransferases is the basis for the rational design of biologically applicable glycosylation inhibitors that allow studies of the biological and pathological functions of glycans. We previously described a potent UV-activated inhibitor for C2GnT1, core 1 p-nitrophenyl (pnp) [34]. Recently, bis-imidazolium salts were examined in glycosyltransferase assays [35]. These bivalent imidazolium salt compounds contain two positively charged imidazolium groups linked by an aliphatic chain, and also had been reported to be potent and specific inhibitors of Plasmodium replication [36]. The structures of these bis-imidazolium inhibitors are not related to glycosyltransferase substrates and represent a new class of glycosyltransferase inhibitors. We have now studied the inhibition of the enzymes involved in the synthesis of O-glycan core 1 to 4 structures in more detail.
2. Material and methods
2.1. Materials
Materials were purchased from Sigma unless otherwise stated. Gal- and GlcNAc-analogs, core 1 and core 3 disaccharide-containing compounds were synthesized as previously reported [26,27,37–40]. Synthetic glycopeptides [41] and many other sugar derivatives were synthesized and kindly provided by Hans Paulsen (University Hamburg, Germany). The intactness of glycopeptides was confirmed by MALDI-TOF mass spectrometry in the negative or positive ion modes as previously described [27].
2.2. Enzymes
Active, soluble human recombinant core 1 β1,3-Gal-transferase (C1GalT) was prepared in insect Hi-5 cells by co-expression with human Cosmc as previously described [11] and the crude cell extracts were used as the enzyme source. His-tagged soluble human recombinant core 2 β1,6-GlcNAc-transferase (C2GnT1) was produced in insect cells as described and used as the crude cell extract [42]. Soluble human recombinant core 3 β3GlcNAc-transferase (C3GnT) and core 2/4 β6GlcNAc-transferase (C2GnT2) containing His-tags were also produced in Sf9 insect cells [43; http://glycoenzymes.ccrc.uga.edu/]. C3GnT and C2GnT2 activities were barely detectable before purification. Therefore, both enzyme proteins were purified by Ni2+-nitrilotriacetic acid (Ni2+-NTA) affinity chromatography. Briefly, the insect cell supernatants were dialyzed against dialysis buffer (50 mM NaH2PO4, 500 mM NaCl; pH 8.0) for 18 h at 4 °C with a buffer change after the first 6 h. Ni2+-NTA resin (Thermo Scientific) was first equilibrated with dialysis buffer. The dialyzed insect cell supernatant was then incubated with the equilibrated resin at room temperature for 3 h with gentle agitation. The mixture was transferred into an empty column and the resin was allowed to settle. The resin was washed with 10 column volumes of dialysis buffer containing 20 mM imidazole, which was gradually increased to 50 mM. Enzyme was eluted with 5 column volumes of dialysis buffer containing 250 to 500 mM imidazole. The eluted fractions were concentrated with polyethylene glycol at 4 °C, and then dialyzed against HEPES buffer (20 mM HEPES, 1 mM MgCl2, 20 mM NaCl, 1 mM DTT) and 1 mL protease inhibitor (Sigma Protease inhibitor cocktail for general use) for 3 h at 4 °C. Aliquots of purified enzyme solutions were adjusted to 20% glycerol and stored at −80 °C. The protein concentrations of the enzyme stock solutions were determined by the Bio-Rad (Bradford) protein assay method using bovine serum albumin as the standard.
Western blot analysis was performed with mouse monoclonal anti-His antibody against the His-tag as the primary antibody (Cell Biolabs, Inc.) and horseradish peroxidase-conjugated goat anti-mouse IgG as the secondary antibody (Santa Cruz Biotechnology). Labeling was visualized with Western blot detection system (iNtRON Biotechnology).
2.3. Glycosyltransferase assays
All glycosyltransferase assays were carried out in at least duplicate determinations with less than 10% difference between assays [14,27,44]. The standard assay mixtures for human recombinant C1GalT contained in a total volume of 40 μL: 5 μL of insect cell supernatant containing C1GalT (0.036 mg protein), 0.125 M MES, pH 7.0, 12.5 mM MnCl2, 10 mM AMP, 0.4 mM UDP-[3H]Gal (2000–3000 cpm nmol−1) and 0.5 mM GalNAcα-Bn. Control assays contained no acceptor substrate or no inhibitor.
Affinity purified human recombinant C3GnT was assayed in mixtures containing 10 μL C3GnT solution (0.003 mg protein), 0.125 M MES buffer, pH 7.0, 10 mM AMP, 0.125 M GlcNAc, 12.5 mM MnCl2 1.05 mM UDP-[3H]GlcNAc (5800 cpm/nmol) and acceptors as indicated in the Tables, or water in negative controls.
Purified human recombinant C2GnT2 was assayed similarly as C3GnT but without MnCl2. 15 μL purified C2GnT2 (0.002 mg protein) was used in each assay and Galβ1–3GalNAcα-pnp (Core 1-pnp) or GlcNAcβ1–3GalNAcα-pnp (Core 3-pnp) were employed as standard acceptor substrates. C2GnT1 produced in insect cells was assayed as described [27].
For inhibition studies, inhibitors were present at the same concentration as the acceptor in the assays, or as indicated in figure legends and tables. Assay mixtures were incubated for 1 h at 37 °C and reactions were quenched with 600 μL of cold water. Reaction products were isolated using 0.4 mL AG1 × 8 ion exchange resin (100–200 mesh). Radioactivity of the eluate was determined by scintillation counting. The Origin Pro 8.0 program was used to determine kinetic parameters KM, Vmax and IC50.
3. Results
3.1. Characterization of Core1 β1,3-Gal-transferase (C1GalT)
Human recombinant C1GalT was highly active with GalNAcα-Bn as acceptor substrate. The reaction was linear with respect to protein concentration up to 0.8 mg/mL and incubation time up to 1.5 h. The optimal reaction pH was 7.0. The conserved presence of the DxD motif in the C1GalT sequence suggested the involvement of divalent metal ions as cofactors in Gal-transferase catalysis. Of several divalent metal ions studied, Mn2+ at 12.5 mM concentration was shown to be an efficient cofactor (taken as 100% activity), while 15% activity was observed in the presence of 12.5 mM Co2+. None of the other metal ions tested, including Mg2+, Ca2+, Pb2+, Ni2+, Zn2+ and Cu2+, was effective in stimulating C1GalT activity (Supplementary Fig. 1), and no activity was observed in the presence of 12.5 mM ethylenediamine tetraacetic acid (EDTA). Half-maximal activity was reached at 0.25 mM MnCl2 concentration (Supplementary Fig. 2A). C1GalT was specific for UDP-Gal as the donor substrate (Fig. 2). The apparent KM for UDP-Gal was 1.0 mM, and Vmax was 0.16 μmol/h/mg with 5 mM GalNAcα-Bn as acceptor substrate. The apparent KM for GalNAcα-Bn was 0.8 mM and Vmax was 0.14 μmol/h/mg (Table 1, Supplementary Fig. 3).
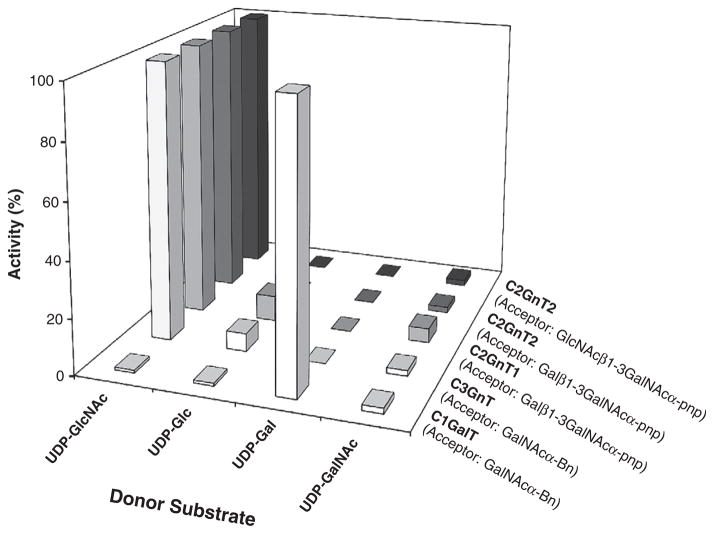
Fig. 2.
Donor specificities of C2GnT2, C2GnT1, C3GnT, and C1GalT. Glycosyl transfer to acceptor substrate was measured as a function of nucleotide sugar donor. Enzymes were assayed as described in the Methods section, except in the presence of different nucleotide sugars replacing the standard donor substrates; 0.40 mM UDP-Gal, 2266 cpm/nmol; 0.345 mM UDP-Glc, 5549 cpm/nmol; 0.5 mM UDP-GlcNAc, 5876 cpm/nmol; or 0.6 mM UDP-GalNAc, 1708 cpm/nmol. The activity with the nucleotide sugar donor specific for each enzyme was set to 100%. C1GalT and C3GnT activities were assayed using GalNAcα-Bn as acceptor substrate; C2GnT1 activity was assayed using Galβ1–3GalNAcα-pnp as acceptor substrate; C2GnT2 activity was assayed using Galβ1–3GalNAcα-pnp and GlcNAcβ1–3GalNAcα-pnp as acceptor substrates.
Table 1.
Kinetic parameters of glycosyltransferases for acceptor and donor substrates. Assays were carried out as described in the Material and methods section, using the indicated acceptor substrates to determine the KM and Vmax values (Origin Pro 8.0). Bn, benzyl; pnp, p-nitrophenyl.
| Enzyme | Substrate | KM (mM) | Vmax (μmol/h/mg) |
|---|---|---|---|
| C1GalT | GalNAcα-Bn | 0.8 | 0.14 |
| UDP-Gal (using GalNAcα-Bn) | 1.0 | 0.16 | |
| C3GnT | GalNAcα-Bn | 3.0 | 0.95 |
| UDP-GlcNAc (using GalNAcα-Bn) | 2.8 | 1.30 | |
| GalNAcα-perillyl | 0.3 | 0.40 | |
| Ac-A-(GalNAcα)TG-NH2 | 0.7 | 1.10 | |
| C2GnT1 | Galβ1–3GalNAcα-pnp | 1.1 | 0.08 |
| UDP-GlcNAc (using Galβ1–3GalNAcα-pnp) | 3.2 | 0.10 | |
| C2GnT2 | Galβ1–3GalNAcα-pnp | 2.1 | 1.50 |
| UDP-GlcNAc (using Galβ1–3GalNAcα-pnp) | 2.2 | 1.70 | |
| GlcNAcβ1–3GalNAcα-pnp | 5.8 | 1.00 | |
| UDP-GlcNAc (using GlcNAcβ1–3GalNAcα-pnp) | 2.1 | 0.80 |
A series of synthetic compounds was employed to study the acceptor substrate specificity of C1GalT (Table 2). The activity of C1GalT with 0.5 mM of GalNAcα-Bn as substrate was 0.042 μmol/h/mg (taken as 100% activity). GalNAc derivatives were variably active as substrates when the aglycone groups were benzyl (Bn), p-nitrophenyl (pnp), perillyl or peptide. The diphosphate aglycone group prevented activity. GalNAcα-peptide substrates exhibited up to 5.64-fold the activity observed with GalNAcα-Bn. The most active substrate was the acetyl and amide-protected glycopeptide Ac-GHA-(GalNAcα) TSLPVTG-NH2, derived from the tandem repeat sequence of MUC4 mucin. Free glycopeptides that had a sequence of 10 amino acids derived from mucins MUC2 and MUC3 were also very active (Table 2) and the activity levels varied according to the position of GalNAc in the glycopeptide. However, a MUC1 mucin-derived glycopeptide having 21 amino acids with GalNAc attached to Ser-6 was only 39% active compared to GalNAcα-Bn. No activity was detected with the short glycopeptide A-(GalNAcα)T. Free GalNAc showed only 6% activity, indicating that the presence of a hydrophobic or peptide aglycone group was beneficial. In contrast to the GalNAcα-anomer, the β-anomer, GalNAcβ-pnp, was not a substrate for C1GalT. In addition, removal or substitution of the hydroxyl group at the 3- or 4-position of GalNAc yielded analogs that were inactive as substrates. However, the hydroxyl group at the 6-position of GalNAc was not required in an active substrate. Thus, deletion or modifications of the 6-hydroxyl, or substitutions with a variety of substituents including fluoro groups or saccharide moieties yielded substrates with activities of 49 to 191%, compared to GalNAcα-Bn. However, no activity was detected with the glycopeptide TE-(GlcNAcβ1–6GalNAcα-)TTSHSTPG, although the corresponding glycopeptide containing a single GalNAcα-residue was very active with 3.7 times the activity observed with GalNAcα-Bn (Table 2). These results show that the activity is determined not only by the sugar moiety but also by its position within the peptide, its substitution, by the amino acid composition and sequence as well as the length of the peptide.
Table 2.
Acceptor substrate specificity of human C1GalT and C3GnT. C1GalT and C3GnT were assayed as described in Material and methods with 0.5 mM GalNAcα-Bn as the standard acceptor substrate (set to 100% activity), or with other GalNAc derivatives as substrates. Bn, benzyl; GalN, D-galactosamine; nd, not done; pnp, p-nitrophenyl; onp, o-nitrophenyl; bold T or S in glycopeptides indicate the attachment sites for an O-glycan.
| Compound name (0.5 mM in assays) | C1GalT activity (%) | C3GnT activity (%) |
|---|---|---|
| GalNAcα-Bn | 100a | 100b |
| Section I: modifications of the aglycone | ||
| GalNAcα-phenyl | 181 | 37 |
| GalNAcα-pnp | 204 | 88 |
| GalNAcα-perillyl | 102 | 153 |
| GalNAcβ-pnp | <1 | <1 |
| GalNAcα-O-PO3-PO3-(CH2)11-O-Ph | <1 | <1 |
| GalNAc | 6 | <1 |
| GalNAcβ1–4GlcNAcβ-Bn | <1 | <1 |
| Section II: modifications of the ring substituents | ||
| 2-N-Propionyl-GalNα-Bn | 60 | 62 |
| 2-N-Butyryl-GalNα-Bn | <1 | <1 |
| 2-Deoxy-Galα-Bn | <1 | <1 |
| 3-Deoxy-GalNAcα-Bn | <1 | <1 |
| 3-O-Ethyl-GalNAcα-Bn | <1 | <1 |
| 3-O-Propyl-GalNAcα-Bn | <1 | <1 |
| 4-Deoxy-GalNAcα-Bn | <1 | <1 |
| 6-Deoxy-GalNAcα-Bn | 68 | <1 |
| 6-O-(4,5-Anhydro)pentyl-GalNAcα-Bn | 191 | <1 |
| GlcNAcβ1–6-GalNAcα-Bn (Core 6-Bn) | 74 | <1 |
| Galβ1–4GlcNAcβ1–6GalNAcα-onp | 38 | <1 |
| Galβ1–3(6-deoxy)GalNAcα-Bn | <1 | <1 |
| Galβ1–6GalNAcα-Bn | 30 | <1 |
| Galβ1–4Glcβ1–6GalNAcα-Bn | 63 | <1 |
| 4-F-4-Deoxy-GlcNAcβ1–6-GalNAcα-Bn | 88 | <1 |
| Galβ1–4GlcNAcβ1–6Galα-(2-naphthyl) | <1 | <1 |
| GlcNAcβ1–6-Galβ-OCD3 | <1 | <1 |
| Section III: glycopeptides | ||
| A-(GalNAcα)T | <1 | 15 |
| Ac-V-(GalNAcα)TP-NH2 | 71 | 36 |
| Ac-A-(GalNAcα)TG-NH2 | 143 | 170 |
| Ac-GHA-(GalNAcα)TSLPVTG-NH2 | 564 | 22 |
| TTTVTP-(GalNAcα)TPTG | 259 | 5 |
| TTTV-(GalNAcα)TPTPTG | 270 | 10 |
| TT-(GalNAcα)TVTPTPTG | 99 | 8 |
| T-(GalNAcα)TTVTPTPTG | 231 | nd |
| TET-(GalNAcα)TSHSTPG | 210 | nd |
| TE-(GalNAcα)TTSHSTPG | 373 | nd |
| TE-(GlcNAcβ1–6GalNAcα)TTSHSTPG | <1 | nd |
| AHGVT-(GalNAcα)SAPDTRPAPGSTAPPA | 39 | 15 |
100% C1GalT activity corresponds to 0.042 μmol/h/mg.
100% C3GnT activity corresponds to 0.190 μmol/h/mg.
No C1GalT activity was detected when GalNAc was replaced by derivatives of Gal, galactosamine (GalN) or GlcNAc which suggested a requirement for the N-acyl group as well as the galacto configuration in the substrate. 2-N-Propionyl-GalNα-Bn showed 60% activity compared to GalNAcα-Bn, while the bulky 2-N-butyryl substituent prevented enzyme activity.
3.2. Characterization of core 3 β1,3-GlcNAc-transferase (C3GnT)
Purified human recombinant C3GnT showed a major protein band at 56 kDa in the elution fractions seen by SDS-PAGE (Supplementary Fig. 4A). Western blotting revealed a single protein band at 56 kDa for both the crude enzyme preparation and the purified enzyme (Supplementary Fig. 4B) which had an activity of 0.19 μmol/h/mg with 0.5 mM GalNAcα-Bn as acceptor substrate. The conserved DxD motif in the C3GnT sequence suggested the requirement for divalent metal ion as a cofactor for GlcNAc transfer. Mn2+ was the most effective cofactor, while Co2+ gave 18% activity compared to Mn2+. None of the other metal ions tested including Mg2+, Ca2+, Pb2+, Ni2+, Zn2+ and Cu2+ was effective in stimulating C3GnT activity (Supplementary Fig. 1) and there was no activity in the presence of EDTA. This pattern was similar to that for C1GalT. However, the effect of Mn2+ on C3GnT differed from its effect on C1GalT, since it required a higher concentration of MnCl2 to saturate the reaction (Supplementary Fig. 2B).
C3GnT was most active with UDP-GlcNAc as the donor substrate (Fig. 2), but also showed 0.8% activity with UDP-Glc. This may be an in vitro phenomenon since the Glcβ1–3GalNAc- structure has never been reported in human mucin O-glycans. The apparent KM for UDP-GlcNAc was 2.8 mM, and Vmax was 1.30 μmol/h/mg (Supplementary Fig. 5A, Table 1).
The acceptor substrate specificity of C3GnT was investigated with a series of synthetic compounds and compared to that of C1GalT (Table 2). The aglycone groups of GalNAc derivatives had a large effect on activity. The apparent KM value for GalNAcα-Bn was 3.0 mM with a Vmax of 0.95 μmol/h/mg while the apparent KM for GalNAcα-perillyl was 0.3 mM with a Vmax of 0.4 μmol/h/mg (Table 1, Supplementary Fig. 5B). Similar to C1GalT, the β-anomer GalNAcβ-pnp was not recognized as a substrate for C3GnT. In addition, removal or substitution of the hydroxyl group at the 3- or 4-position of GalNAc yielded inactive substrates. Modifications at position 2 of GalNAc led to similar results for both C1GalT and C3GnT. However, in contrast to C1GalT, the 6-hydroxyl of GalNAc was found to be absolutely essential for C3GnT activity. The reactivity towards glycopeptide substrates containing GalNAcα-Thr was generally lower for C3GnT, compared to C1GalT (Table 2). In addition, these two enzymes had very different reactivity ratios using GalNAcα-glycopeptide substrates. Some of the glycopeptides that had very high activity with C1GalT were poorly active with C3GnT. The glycopeptide Ac-A-(GA)TG-NH2 was the best substrate with an apparent KM of 0.7 mM and a Vmax of 1.1 μmol/h/mg. The MUC4-derived glycopeptide Ac-GHA-(GalNAcα)TSLPVTG-NH2, was only 22% active compared to GalNAcα-Bn, and the MUC2-derived glycopeptides were poorly active while no activity was observed with MUC3-derived glycopeptides. It is interesting that the glycopeptide A-(GalNAcα)T that was inactive with C1GalT showed 15% activity with C3GnT. Thus, the recognition of these GalNAc-glycopeptides was not only a function of the glycopeptide structure and composition but was specific for each enzyme.
3.3. Characterization of core 2 β1,6-GlcNAc-transferase (C2GnT1) and core 2/4 β1,6-GlcNAc-transferase (C2GnT2)
We previously examined the specificities of recombinant human C2GnT1 [27] and the enzyme from human leukemia cells [26] that synthesizes the core 2 structure. In the present work we expanded on the specificity of the enzyme towards additional substrates having hydrophobic aglycone groups, as well as glycopeptides carrying the core 1 structure (Table 3). We compared this with the specificity of human recombinant C2GnT2 that synthesizes both the core 2 and core 4 structures. After purification, C2GnT2 showed a major protein band at 53 kDa by SDS-PAGE and Western blot analysis (Supplementary Fig. 6A, B) with a specific activity of 0.22 μmol/h/mg.
Table 3.
Acceptor substrate specificity of human C2GnT1 and C2GnT2. C2GnT1 and C2GnT2 were assayed as described in Material and methods with 0.5 mM Galβ1–3GalNAcα-pnp as the standard acceptor substrate (set to 100% activity). Bn, benzyl; GalN, D-galactosamine; pnp, p-nitrophenyl; onp, o-nitrophenyl. Bold S and T in glycopeptides indicate the attachment of O-glycans.
| Compound name (0.5 mM in assays) | C2GnT1 activity (%) | C2GnT2 activity (%) |
|---|---|---|
| Galβ1–3GalNAcα-pnp (Core1-pnp) | 100a | 100b |
| Section I: modifications of the aglycone | ||
| Galβ1–3GalNAcα-Bn (Core1-Bn) | 64 | 35 |
| Galβ1–3GalNAcα-perillyl | 135 | 86 |
| Galβ1–3GalNAcα-onp | 79 | 100 |
| Section II: modifications of the sugar moiety | ||
| GalNAcα-Bn | <1 | <1 |
| 2-N-Butyryl-GalNα-Bn | <1 | <1 |
| GlcNAcβ1–3-Galβ-methyl | <1 | <1 |
| GlcNAcβ1–3GalNAcα-pnp (Core 3-pnp) | <1 | 32 |
| GlcNAcβ1–3GalNAcα-allyl | <1 | 27 |
| Galβ1–3GlcNAcα-Bn | <1 | <1 |
| Fucα1–2Galβ1–3GalNAcα-methyl | <1 | <1 |
| 3-Deoxy-GalNAcα-Bn | <1 | <1 |
| Galβ1–3(6-deoxy)GalNAcα-Bn | <1 | <1 |
| GlcNAcβ1–6GalNAcα-Bn (Core 6-Bn) | <1 | <1 |
| Galβ1–3(4-deoxy)GalNAcα-Bn | <1 | <1 |
| Galβ1–3(6-O-methyl)GalNAcα-Bn | <1 | <1 |
| 3-Deoxy-Galβ1–3GalNAcα-Bn | 90 | 52 |
| 3-O-Methyl-Galβ1–3GalNAcα-Bn | 104 | 41 |
| Galβ1–3GlcNAcβ1–3Galβ1–3GalNAcα-Bn | <1 | <1 |
| 4-Deoxy-Galβ1–3GalNAcα-Bn | <1 | <1 |
| 4-F-4-Deoxy-Galβ1–3GalNAcα-Bn | <1 | <1 |
| 6-Deoxy-Galβ1–3GalNAcαBn | <1 | <1 |
| GlcNAcβ1–6(GlcNAcβ1–3)GalNAcα-Bn (Core 4-Bn) | <1 | <1 |
| Section III: glycopeptides | ||
| (Galβ1–3GalNAcα)TAGV | 92 | <1 |
| T-(GlcNAcβ1–3GalNAcα)TTVTPTPTG | <1 | <1 |
| TETTSHS-(GlcNAcβ1–3GalNAcα)TPG | <1 | <1 |
| TET-(GlcNAcβ1–3GalNAcα)TSHSTPG | <1 | <1 |
| TE-(GlcNAcβ1–3GalNAcα)TTSHSTPG | <1 | <1 |
| TE-(GlcNAcβ1–6GalNAcα)TTSHSTPG | <1 | <1 |
| TT-(Galβ1–3GalNAcα)TVTPTPTG | 13 | <1 |
| T-(Galβ1–3GalNAcα)TTVTPTPTG | 11 | <1 |
| TT-(Galβ1–3GalNAcα)TVTP-(Galβ1–3GalNAcα)TPTG | 12 | <1 |
| TETTSHS-(Galβ1–3GalNAcα)TPG | 70 | 7 |
| TET-(Galβ1–3GalNAcα)TSHSTPG | 27 | <1 |
| Ac-PTT-(Galβ1–3GalNAcα)TGIST-NH2 | 36 | <1 |
| Ac-PT-(Galβ1–3GalNAcα)TTGIST-NH2 | 49 | 6 |
| Ac-P-(Galβ1–3GalNAcα)TTTGIST-NH2 | 7 | <1 |
| Ac-GTT-(Galβ1–3GalNAcα)TPIST-NH2 | 14 | <1 |
| Ac-GT-(Galβ1–3GalNAcα)TTPIST-NH2 | 13 | 5 |
| Ac-G-(Galβ1–3GalNAcα)TTTPIST-NH2 | 12 | 8 |
| Ac-PT-(Galβ1–3GalNAcβ)TTPIST-NH2 | 43 | 15 |
| Ac-P-(Galβ1–3GalNAcβ)TTTPIST-NH2 | 72 | 32 |
| AHGVT-(GalNAcα)SAPDTRPAPGSTAPPA | <1 | <1 |
| AHGVT-(Galβ1–3GalNAcα)SAPDTRPAPGSTAPNA | 9 | <1 |
| AHGVT-(Galβ1–3GalNAcα)SAPESRPAPGSTAPNA | 9 | <1 |
| AHGVT-(Galβ1–3GalNAcα)SAPDTRPAPGSTAPTA | 9 | <1 |
| AHGVT-(Galβ1–3GalNAcα)SAPETRPAPGSTAPTA | 11 | <1 |
| AHGVT-(Galβ1–3GalNAcα)SAPDTRPAPGSTAP- (Galβ1–3GalNAcα)TA | 18 | <1 |
| AHGVT-(Galβ1–3GalNAcα)SAPDTRPAPGS- (Galβ1–3GalNAcα)SAPPA | 9 | <1 |
100% C2GnT1 activity corresponds to 0.034 μmol/h/mg.
100% C2GnT2 activity corresponds to 0.220 μmol/h/mg.
Although both C2GnT1 and 2 have a SPDE sequence resembling a DxD motif which was shown for C2GnT1 to contain the catalytic base Glu320 [45,46], a metal ion cofactor was not required for catalysis. Interestingly, the presence of 12.5 mM Mn2+ in the assay led to an 83–85% reduction of C2GnT2 activity using core 1 or core 3 substrates. Similarly, C2GnT1 activity was reduced by 61% in the presence of 12.5 mM Mn2+ (Supplementary Fig. 7A, B).
Both, C2GnT1 and C2GnT2 were found to be specific for UDP-GlcNAc as the sugar donor substrate (Fig. 2). For C2GnT1, the apparent KM for UDP-GlcNAc with 5 mM Galβ1–3GalNAcα-pnp as acceptor was 3.2 and Vmax was 0.1 μmol/h/mg (Table 1, Supplementary Fig. 8A). For C2GnT2, the apparent KM for UDP-GlcNAc with 5 mM Galβ1–3GalNAcα-pnp as acceptor was 2.2 mM and Vmax was 1.7 μmol/h/mg. With 5 mM GlcNAcβ1–3GalNAcα-pnp as the acceptor substrate, the apparent KM for UDP-GlcNAc was 2.1 mM and Vmax was 0.8 μmol/h/mg (Table 1, Supplementary Fig. 9A).
A series of Galβ1–3GalNAc- and GlcNAcβ1–3GalNAc derivatives was used to study the substrate specificity of C2GnT1 and C2GnT2 (Table 3). Both enzymes preferred the core 1 over the core 3 substrate, and the hydrophobic aglycone groups had significant but different effects on the activities. C2GnT1 had an apparent KM value of 1.1 mM for the core 1 substrate Galβ1–3GalNAcα-pnp and Vmax was 0.08 μmol/h/mg (Table 1, Supplementary Fig. 8B). For C2GnT2, the apparent KM value for Galβ1–3GalNAcα-pnp was 2.1 mM with a Vmax of 1.5 μmol/h/mg. The apparent KM for the corresponding core 3 substrate GlcNAcβ1–3GalNAcα-pnp was much higher at 5.8 mM with a Vmax was 1.0 μmol/h/mg (Tables 1, Supplementary Fig. 9B).
As was the case for C2GnT1 [26], the 4- and 6-hydroxyl groups of both Gal and GalNAc residues were absolutely essential for C2GnT2 activity. Galβ1–3GlcNAcα-Bn was not active indicating that the axial position of the 4-hydroxyl was critical for C2GnT2. Substitution of the 2-hydroxyl of Gal with Fuc also prevented activity. The 3-hydroxyl of Gal was not essential for C2GnT1 or C2GnT2. The 3-O-methyl substitution of Gal also had little effect on C2GnT1 and resulted in 41% C2GnT2 activity. However, the extension of the Gal moiety by a GlcNAcβ1–3 residue resulted in inactive substrate, probably by steric hindrance (Table 3).
Both, C2GnT1 and C2GnT2 activities were dramatically reduced when the aglycone group was a peptide derived from MUC1 MUC2, MUC 3or MUC4. Generally, C2GnT2 showed a much lower activity towards glycopeptide substrates containing the core 1 structure, and no detectable activity towards glycopeptides containing the core 3 or core 6 (GlcNAcβ1–6GalNAc-) structure (Table 3). As reported previously, one of the core 1-containing glycopeptides tested, (Galβ1–3GalNAcα-)TAGV, had high activity with C2GnT1. However, C2GnT2 obviously recognized this glycopeptide differently since no C2GnT2 activity was detected (Table 3). MUC1 glycopeptides having 21 amino acids were generally poor substrates for C2GnT1 and showed no activity of C2GnT2. In contrast, acetyl- and amide-protected shorter glycopeptides were more active with C2GnT1 (7 to 49% of the activity with Gal-1–3GalNAc-pnp) and with C2GnT2 (<1 to 8% activity).
Both, C2GnT1 and C2GnT2 were surprisingly active with glycopeptides having the β-linked core 1 structure (Galβ1–3GalNAcβ-Thr) which showed 43 to 72% activity for C2GnT1 and 15 to 32% activity for C2GnT2 (Table 3).
3.4. Inhibition of Gal- and GlcNAc-transferases
Compounds that were inactive as substrates were screened as inhibitors for Gal- and GlcNAc-transferase activities. Substrate analogs, as well as a series of neutral imidazoles and monovalent imidazolium salts [36], and a series of bis-imidazolium salts containing an aliphatic linker-chain were tested. Although some of the bis-imidazolium salts inhibited β3GalT5 and β4GalT1 [35], none of these compounds produced an inhibitory effect on C1GalT activity (Table 4). Naphthyl derivatives of GlcNAc were previously shown to be potent inhibitors of β4GalT1 [37,38] but did not inhibit C1GalT or any of the other three enzymes. In this large series of compounds only benzyl 2-butanamido-2-deoxy-α-D-galactoside (2-N-butyryl-GalNα-Bn) weakly inhibited C1GalT activity with an IC50 of 2.31 mM (Supplementary Fig. 10) but did not inhibit the other enzymes tested (Table 4).
Table 4.
Inhibition of human C1GalT, C3GnT, C2GnT1 and C2GnT2. Human recombinant enzymes were assayed as described in Material and methods with 0.5 mM GalNAcα-Bn (for C1GalT and C3GnT) or Galβ1–3GalNAcα-pnp (for C2GnT1 and C2GnT2) as the acceptor substrate. All inhibitor compounds were inactive as substrates. The inhibitor concentration in the assay was at a 1:1 ratio with the acceptor substrate (0.5 mM). Other bis-imidazolium compounds with similar structure but having aliphatic linker-chains containing 4, 6, 8, 10, 11, 12, 13 or 14 carbons in length were inactive as substrates and as inhibitors.
| Inhibitor (0.5 mM in assays) | C1GalT
|
C3GnT
|
C2GnT1
|
C2GnT2
|
|||
|---|---|---|---|---|---|---|---|
| Inhibition (%) | IC50 (mM) | Inhibition (%) | IC50 (mM) | Inhibition (%) | IC50 (mM) | Inhibition (%) | |
| 1,15-Bis-(3-methyl-1H-imidazolium-1-yl)pentadecane dichloride | <1 | <1 | <1 | <1 | |||
| 1,16-Bis-(3-methyl-1H-imidazolium-1-yl)hexadecane dichloride | <1 | <1 | 81 | 0.23 | <1 | ||
| 1,18-Bis-(3-methyl-1H-imidazolium-1-yl)octadecane dichloride | <1 | <1 | 100 | 0.02 | <1 | ||
| 1,20-Bis-(3-methyl-1H-imidazolium-1-yl)eicosane dichloride | <1 | 95 | 0.14 | 100 | 0.06 | <1 | |
| 1,20-Bis-(3-methyl-1H-imidazolium-1-yl)eicosane dimesylate | <1 | 80 | 0.13 | 100 | <1 | ||
| 1,22-Bis-(3-methyl-1H-imidazolium-1-yl)docosane dimesylate | <1 | 78 | 0.26 | 100 | 0.07 | <1 | |
| 1-Thio-N-butrylGlcNβ-(2-naphthyl) | <1 | <1 | <1 | <1 | |||
| 2-N-Butyryl-GalNα-Bn | 16 | 2.31 | <1 | <1 | <1 | ||
None of the inactive GalNAc derivatives had any inhibitory effect on C3GnT. However, bis-imidazolium salts containing an aliphatic linker-chain of 20 or 22 carbons significantly inhibited C3GnT activity (Table 4). The IC50 of an inhibitor with a 20-carbon chain, 1,20-bis-(3-methyl-1H-imidazolium-1-yl)eicosane dichloride, was 0.14 mM, while the IC50 of the respective dimesylate salt was 0.13 mM. The IC50 of the inhibitor with a 22-carbon chain, 1,22-bis-(3-methyl-1H-imidazolium-1-yl)docosane dimesylate, was 0.26 mM (Table 4, Supplementary Fig. 11). Imidazolium salts with linker-chains of 18 carbons or less did not inhibit C3GnT.
Although none of the sugar derivatives inhibited C2GnT1, the bis-imidazolium salts with aliphatic linker-chains of 16, 18, 20 or 22 carbons in length were potent inhibitors (Table 4, Supplementary Fig. 12). The lowest IC50 value (0.062 mM) was seen with 1,20-bis-(3-methyl-1H-imidazolium-1-yl)eicosane dichloride. Surprisingly, none of these compounds inhibited the related enzyme C2GnT2.
4. Discussion
All four human enzymes studied here are inverting glycosyltransferases that transfer a sugar residue from an α-linkage in UDP-α-Gal or UDP-α-GlcNAc to form β-linkages in the products, which are the major mucin type O-glycan core structures 1 to 4 (Fig. 1, Table 5). These core structures are found in the highly O-glycosylated tandem repeat regions of colonic or lung mucins [6,47]. These enzymes therefore efficiently accept the peptide and glycopeptide moieties of their mucin substrates. However, in vitro, only core 1 synthase has a pronounced preference for mucin-derived glycopeptides and may thus bind preferably to GalNAcα-Thr. The enzymes that assemble core 2, 3 and 4 generally were poorly active using mucin-derived glycopeptides. Although the sugar moieties have a stabilizing effect on peptide conformations, large mucin molecules having rigid ‘bottle brush’ conformations appear to be better substrates for these enzymes, and are efficiently glycosylated in vivo. Mucin-derived glycopeptides have been used in vitro to synthesize the Tn and T antigens [27,48]. However, the relatively short size and flexible nature of glycopeptides used in this study did not allow efficient synthesis of core 3 and 4 O-glycans. The in vitro synthesis of core 3-glycopeptides by recombinant C3GnT has been achieved using MUC1-derived glycopeptide derivatives linked to beads [48]. These solid phase-linked glycopeptides are expected to be more stable in their conformations and may thus be better substrates.
Table 5.
Glycosyltransferases used in this study. All of the enzymes used are human, inverting transferases having a predicted GT-A fold [52]. The GT family assignment is from the CAZy data bank. The DxD motif may contain one or more catalytically active acidic amino acids. Although N-glycosylation sites are present, their occupation by N-glycans and their function in enzyme stability has only been shown for C2GnT1 [42]. Multiple Cys residues function in forming protein dimers (C1GalT) [14] and/or disulfide bonds (C1GnT1) [45]. -T, -transferase.
| Enzyme names | Accession # | EC# | GT family | Fold | DxD motif | N-Glycan sites | Cys |
|---|---|---|---|---|---|---|---|
| C1GalT, core 1 β1,3-Gal-T, Core 1 synthase, T-synthase, Core 1-T | NP_064541 | 2.4.1.122 | GT31 | GT-A | DAD | 0 | 7 |
| C3GnT, core 3 β1,3-GlcNAc-T, Core 3 synthase, β3GnT6 | Q6ZMB0 | 2.4.1.147 | GT31 | GT-A | DDD | 3 | 3 |
| C2GnT1, core 2 β1,6-GlcNAc-T, Core 2-T | Q02742 | 2.4.1.102 | GT14 | GT-A | DE, DVDVD | 2 | 2 |
| C2GnT2, core 2/4 β1,6-GlcNAc-T, Core 4-T | AAD10824 | 2.4.1.148 | GT14 | GT-A | DE, DSD, DID | 2 | 2 |
Polypeptide GalNAc-transferases act as the first enzymes in the O-glycan core synthesis pathways to synthesize the Tn antigen and bind the peptide moieties in the substrate binding site [49–51]. The crystal structures of human T2 and T10 [49,50] as well as mouse T1 [51] reveal a lectin binding domain, which suggests that the enzymes can bind a glycopeptide as the acceptor substrate. Of the four enzymes that extend the Tn antigen, only the crystal structure of mouse C2GnT1 is known, with and without the acceptor or UDP [45,46]. It appears that the peptide moiety of glycopeptides is not bound to the enzyme but extrudes into the solution. Thus, most glycopeptides are poor acceptor substrates for C2GnT1, independent of the α- or β-configurations of core 1, while hydrophobic groups may assist substrate binding. It is remarkable that core 1-TAGV is an excellent substrate and may uniquely bind to hydrophobic amino acids near the acceptor binding site of C2GnT1 but not to the similar enzyme C2GnT2.
Human C1GalT has a distinct substrate specificity recognizing the GalNAc-ring in α-configuration as well as the aglycone group, with a preference for peptide. GlcNAcβ1–6GalNAcα-Bn is a good substrate for C1GalT but the MUC3 mucin-derived glycopeptide with this disaccharide structure is not. This suggests that glycopeptides can have unfavorable conformations or cause poor accessibility of the acceptor to the catalytic site. GalNAc in β-configuration also does not bind to the enzyme and future protein structure of C1GalT may reveal a steric hindrance of β-linked GalNAc.
C1GalT does not require the presence of the 6-hydroxyl of GalNAc for substrate binding but it does require the 2-N-acyl group. While 2-N-propyl GalNα-Bn shows reduced activity, 2-N-butyryl-GalNα-Bn is inactive but binds and inhibits the enzyme, probably by steric hindrance of catalysis. This is the first report of a substrate analog inhibitor of C1GalT.
C3GnT also requires GalNAc substrate in the α-configuration as well as all of the substituents of the GalNAc sugar ring. While the N-propyl group allows significant activity, the N-butyryl group prevents binding of the compound to the enzyme. The aglycone groups of the GalNAc-substrates are also important. Glycopeptides showed variable activity and it appears that the length and amino acid composition of peptides determine the efficiency of GlcNAc transfer.
Human core 1 and core 3 synthases are related to Gal-transferase families. Both of them are within the GT31 family of glycosyltransferases with a GT-A fold. They have DxD motifs (Table 4) which may provide the catalytic base to activate the acceptor sugar by de-protonation. This motif may also coordinate the Mn2+ ion and thus interact with the phosphate of UDP-Gal/UDP-GlcNAc [52]. The MnCl2 requirement of human C1GalT (Supplementary Fig. 2A) resembles that of the rat liver enzyme which has an optimum at 10 mM MnCl2 [14]. This in vitro concentration is much higher than the Mn2+ concentration in the Golgi which is less than 1 mM. Thus Mn2+ regulates glycoprotein glycosylation reactions but also glycoprotein trafficking and turnover [53]. C2GnT1 and C2GnT2 do not require divalent metal ions for activity. The inhibition of these enzymes by the unphysiologically high concentration of 12.5 mM Mn2+ in vitro may be a result of altered protein structure.
Human C2GnT1 and C2GnT2 are members of the β1,6-GlcNAc-transferase family and have 57% sequence identity and 73% similarity. Both enzymes are in the GT14 family (Table 5). The structure of mouse C2GnT1 suggests that Arg378 and Lys401 residues are in proximity to the β-phosphate of UDP-GlcNAc, thus replacing the function of the Mn2+ ion in catalysis [45]. The interactions may serve to stabilize the UDP leaving group after GlcNAc transfer. The human counterpart of C2GnT1 also has Arg378 and Lys401 residues close to the DxD motif near the C-terminus that may replace the function of a divalent metal ion.
A conserved SPDE motif resembling the DxD motif is present in mouse and human C2GnT1 as well as in human C2GnT2. The Glu320 of this sequence in mouse C2GnT1 is near the O-4 and O-6 of GalNAc of bound substrate while Arg254 forms a hydrogen-bond with O-4 of Gal [45,46]. Glu243 also binds to O-4 as well as the O-6 of the Gal residue. This explains the absolute requirement of C2GnT1 for the Galβ1–3GalNAc sequence where the 4- and 6-hydroxyls of both sugar residues have to be unmodified. The acceptor binding site of C2GnT2, in addition to core 1, also accommodates the GlcNAc residue of core 3. This could be due to the replacement of Glu253 with Thr in C2GnT2 that may allow the binding of the equatorial O-4 of GlcNAc. Tyr358 is in the vicinity of O-2 of Gal in mouse C2GnT1, but this residue appears to be replaced by the smaller Gly368 residue in C2GnT2, explaining why the bulkier GlcNAc is accepted instead of Gal at that site. However, the core 3 substrate GlcNAcβ1–3GalNAcα-pnp showed an almost 3-fold higher KM value compared to the core 1 substrate, suggesting that core 3 does not bind as well as core 1 to C2GnT2.
A third enzyme of the C2GnT family, C2GnT3, which is primarily expressed in the thymus was shown to be specific for UDP-GlcNAc as the sugar donor substrate and also has the SPDE motif [54]. The enzyme has the acceptor specificity of C2GnT1 and acts on core 1 but not on core 3, although a very low distal I-antigen GlcNAc-transferase (IGnT) activity was detected with the i-antigenic GlcNAcβ1–3Galβ-methyl substrate. Interestingly, Hashimoto et al. [55] reported that mouse C2GnT2 acted on core 1, core 3 and GlcNAcβ1–3Gal-substrates and thus has the core 2-, core 4- and IGnT activities, similar to that of human C2GnT2 [29] and the crude M-enzyme (C2GnT2) in rat colon [15,26]. However, in our current study we did not observe the IGnT activity of human C2GnT2, and our observations correspond to the specificity of human C2GnT2 cloned by Schwientek et al. [28]. Thus, depending on the assay conditions, the enzyme might have a very low activity towards the GlcNAcβ1–3Gal-substrate.
It is interesting that imidazolium salts potently inhibit C3GnT and C2GnT1 but not C1GalT or C2GnT2. These inhibitors are not substrate analogs and also inhibit β1,3- and β1,4-Gal-transferases [35] in a mixed non-competitive/uncompetitive fashion. This selective mode of inhibition does not appear to involve binding of the positively charged salts to UDP-sugars, since only selected transferases are inhibited. It will have to be shown if there are hydrophobic and negatively charged amino acid residues on the surface of enzyme proteins in an appropriate configuration that allows the binding of imidazolium salts with spacers of specific lengths (20 to 22 carbons for C3GnT and 16 to 22 carbons for C2GnT1). This binding then may cause an unfavorable protein conformation or block access to the substrate binding site. The glycosyltransferases studied here are among those affected by cancer and other diseases [8], and inhibitors have therapeutic potential. Future knowledge of the structures of these glycosyltransferases will help our understanding of substrate recognition and aid in further development of inhibitors.
Supplementary Material
Acknowledgments
The synthesis of many sugar derivatives and glycopeptides used in these studies was accomplished in the laboratory of Professor Hans Paulsen, Hamburg, Germany, over a period of more than 20 years. Many of his students and postdoctoral fellows were involved in this work. This paper is therefore dedicated to Hans Paulsen on the occasion of his 90th birthday in appreciation of his essential contributions to the field of glycosyltransferases. This work was supported by the CIHR (to I.B. and W.A.S.), an NSERC discovery grant (to I.B) and grants from the Prostate Cancer Fight Foundation ‘Motorcycle Ride for Dad’ (to I.B). The glycorepository project was supported by NIH P41GM103390 and P41RR005351.
Abbreviations
- Ac-
acetyl
- C1GalT
core 1 β1,3-Gal-transferase
- C3GnT
core 3 β1,3-GlcNAc-transferase
- C2GnT1
core 2 β1,6-GlcNAc-transferase
- C2GnT2
core 2/4 β1,6-GlcNAc-transferase
- Gn
GlcNAc
- onp
o-nitrophenyl
- pnp
p-nitrophenyl
Footnotes
Dedicated to Professor Hans Paulsen on the occasion of his 90th birthday.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbagen.2013.04.001.
References
- 1.Hollingsworth MA, Swansson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 2.Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20:169–177. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Schultz MJ, Swindall AF, Bellis SL. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012;31:501–518. doi: 10.1007/s10555-012-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patsos G, Hebbe-Viton V, Robbe-Masselot C, Masselot D, San Martin R, Greenwood R, Paraskeva C, Klein A, Graessmann M, Michalski JC, Gallagher T, Corfield A. O-glycan inhibitors generate aryl-glycans, induce apoptosis and lead to growth inhibition in colorectal cancer cell lines. Glycobiology. 2009;19:382–398. doi: 10.1093/glycob/cwn149. [DOI] [PubMed] [Google Scholar]
- 5.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockhausen I. Biosynthesis of complex mucin-type O-glycans, comprehensive natural products II chemistry and biology. In: Mander L, Lui H-W, Wang PG, editors. Carbohydrates, nucleosides and nucleic acids. Vol. 6. Elsevier; Oxford: 2010. pp. 315–350. Chapter 11. [Google Scholar]
- 7.Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl. 2011;50:1770–1791. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brockhausen I. Glycosyltransferases involved in the synthesis of mucin type O-glycans in human cancer cells. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai PR. Immunoreactive T and Tn antigens in malignancy: role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus Med Rev. 2000;14:312–325. doi: 10.1053/tmrv.2000.16229. [DOI] [PubMed] [Google Scholar]
- 10.Mi R, Song L, Wang Y, Ding X, Zeng J, Lehoux S, Aryal RP, Wang J, Crew VK, van Die I, Chapman AB, Cummings RD, Ju T. Epigenetic silencing of the chaperone Cosmc in human leukocytes expressing tn antigen. J Biol Chem. 2012;287:41523–41533. doi: 10.1074/jbc.M112.371989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, He M, Cummings RD. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci U S A. 2010;107:9228–9233. doi: 10.1073/pnas.0914004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J Cell Biol. 2004;164:451–459. doi: 10.1083/jcb.200311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju T, Cummings RD, Canfield WM. Purification, characterization, and subunit structure of rat core 1 beta1,3-galactosyltransferase. J Biol Chem. 2002;277:169–177. doi: 10.1074/jbc.M109056200. [DOI] [PubMed] [Google Scholar]
- 15.Brockhausen I, Matta KL, Orr J, Schachter H. Mucin synthesis. UDP-GlcNAc: GalNAc-R beta 3-N-acetylglucosaminyltransferase and UDP-GlcNAc:GlcNAc beta 1–3GalNAc-R (GlcNAc to GalNAc) beta 6-N-acetylglucosaminyltransferase from pig and rat colon mucosa. Biochemistry. 1985;24:1866–1874. doi: 10.1021/bi00329a010. [DOI] [PubMed] [Google Scholar]
- 16.Iwai T, Inaba N, Naundorf A, Zhang Y, Gotoh M, Iwasaki H, Kudo T, Togayachi A, Ishizuka Y, Nakanishi H, Narimatsu H. Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide beta1,3-N-acetylglucosaminyltransferase (beta 3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. J Biol Chem. 2002;277:12802–12809. doi: 10.1074/jbc.M112457200. [DOI] [PubMed] [Google Scholar]
- 17.Iwai T. Core 3 synthase is down-regulated in colon carcinoma and suppresses the cancer metastasis. Seikagaku. 2006;78:429–433. [PubMed] [Google Scholar]
- 18.Vavasseur F, Yang JM, Dole K, Paulsen H, Brockhausen I. Synthesis of O-glycan core 3: characterization of UDP-GlcNAc: GalNAc-R beta 3-N-acetyl-glucosaminyltransferase activity from colonic mucosal tissues and lack of the activity in human cancer cell lines. Glycobiology. 1995;5:351–357. doi: 10.1093/glycob/5.3.351. [DOI] [PubMed] [Google Scholar]
- 19.Iwai T, Kudo T, Kawamoto R, Kubota T, Togayachi A, Hiruma T, Okada T, Kawamoto T, Morozumi K, Narimatsu H. Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc Natl Acad Sci U S A. 2005;102:4572–4577. doi: 10.1073/pnas.0407983102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SH, Hatakeyama S, Yu SY, Bao X, Ohyama C, Khoo KH, Fukuda MN, Fukuda M. Core3 O-glycan synthase suppresses tumor formation and metastasis of prostate carcinoma PC3 and LNCaP cells through down-regulation of alpha2beta1 integrin complex. J Biol Chem. 2009;284:17157–17169. doi: 10.1074/jbc.M109.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brockhausen I, Möller G, Pollex-Krüger A, Rutz V, Paulsen H, Matta KL. Control of O-glycan synthesis: specificity and inhibition of O-glycan core 1 UDP-galactose: N-acetylgalactosamine-alpha-R beta 3-galactosyltransferase from rat Liver. Biochem Cell Biol. 1992;70:99–108. doi: 10.1139/o92-015. [DOI] [PubMed] [Google Scholar]
- 23.Brockhausen I, Möller G, Merz G, Adermann K, Paulsen H. Control of mucin synthesis: the peptide portion of synthetic O-glycopeptide substrates influences the activity of O-glycan core 1 UDP-galactose: N-acetyl-alpha-galactosaminyl-R β3-galactosyl-transferase. Biochemistry. 1990;29:10206–10212. doi: 10.1021/bi00496a008. [DOI] [PubMed] [Google Scholar]
- 24.Granovsky M, Bielfeldt T, Peters S, Paulsen H, Meldal M, Brockhausen J, Brockhausen I. UDP-galactose: glycoprotein-N-acetyl-D-galactosamine 3-β-D-galactosyltransferase activity synthesizing O-glycan core 1 is controlled by the amino acid sequence and glycosylation of glycopeptide substrates. Eur J Biochem. 1994;221:1039–1046. doi: 10.1111/j.1432-1033.1994.tb18822.x. [DOI] [PubMed] [Google Scholar]
- 25.Bierhuizen MF, Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc: Gal beta 1–3-GalNAc-R (GlcNAc to GalNAc) beta 1–6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci U S A. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhns W, Rutz V, Paulsen H, Matta KL, Baker MA, Barner M, Granovsky M, Brockhausen I. Processing O-glycan core 1, Gal beta 1–3GalNAc alpha-R. Specificities of core 2, UDP-GlcNAc: Gal beta 1–3 GalNAc-R(GlcNAc to GalNAc) beta 6-N-acetylglucosaminyltransferase and CMP-sialic acid: Gal beta 1–3GalNAc-R alpha 3-sialyltransferase. Glycoconj J. 1993;10:381–394. doi: 10.1007/BF00731043. [DOI] [PubMed] [Google Scholar]
- 27.Brockhausen I, Dowler T, Paulsen H. Site directed processing: role of amino acid sequences and glycosylation of acceptor glycopeptides in the assembly of extended mucin type O-glycan core 2. Biochim Biophys Acta. 2009;1790:1244–1257. doi: 10.1016/j.bbagen.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Schwientek T, Nomoto M, Levery SB, Merkx G, van Kessel AG, Bennett EP, Hollingsworth MA, Clausen H. Control of O-glycan branch formation. Molecular cloning of human cDNA encoding a novel beta1,6-N-acetylglucosaminyltransferase forming core 2 and core 4. J Biol Chem. 1999;274:4504–4512. doi: 10.1074/jbc.274.8.4504. [DOI] [PubMed] [Google Scholar]
- 29.Yeh JC, Ong E, Fukuda M. Molecular cloning and expression of a novel beta-1, 6-N-acetylglucosaminyltransferase that forms core 2, core 4, and I branches. J Biol Chem. 1999;274:3215–3221. doi: 10.1074/jbc.274.5.3215. [DOI] [PubMed] [Google Scholar]
- 30.St Hill CA, Baharo-Hassan D, Farooqui M. C2-O-sLeX glycoproteins are E-selectin ligands that regulate invasion of human colon and hepatic carcinoma cells. PLoS One. 2011;6:e16281. doi: 10.1371/journal.pone.0016281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St Hill CA, Farooqui M, Mitcheltree G, Gulbahce HE, Jessurun J, Cao Q, Walcheck B. The high affinity selectin glycan ligand C2-O-sLex and mRNA transcripts of the core 2 beta-1,6-N-acetylglucosaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas. BMC Cancer. 2009;9:79. doi: 10.1186/1471-2407-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki Y, Sutoh M, Hatakeyama S, Mori K, Yamamoto H, Koie T, Saitoh H, Yamaya K, Funyu T, Habuchi T, Arai Y, Fukuda M, Ohyama C, Tsuboi S. MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int J Oncol. 2012;40:1831–1838. doi: 10.3892/ijo.2012.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang MC, Chen HY, Huang HC, Huang J, Liang JT, Shen TL, Lin NY, Ho CC, Cho IM, Hsu SM. C2GnT-M is downregulated in colorectal cancer and its re-expression causes growth inhibition of colon cancer cells. Oncogene. 2006;25:3267–3276. doi: 10.1038/sj.onc.1209350. [DOI] [PubMed] [Google Scholar]
- 34.Toki D, Granovsky MA, Reck F, Kuhns W, Baker MA, Matta KL, Brockhausen I. Inhibition of UDP-GlcNAc:Gal beta 1–3GalNAc-R (GlcNAc to GalNAc) beta 6-N-acetylglucosaminyltransferase from acute myeloid leukaemia cells by photo-reactive nitrophenyl substrate derivatives. Biochem Biophys Res Commun. 1994;198:417–423. doi: 10.1006/bbrc.1994.1061. [DOI] [PubMed] [Google Scholar]
- 35.Gao Y, Vlahakis JZ, Szarek WA, Brockhausen I. Selective inhibition of glycosyltransferases by bivalent imidazolium salts. Bioorg Med Chem. 2013;21:1305–1311. doi: 10.1016/j.bmc.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 36.Vlahakis JZ, Lazar C, Crandall IE, Szarek WA. Anti-Plasmodium activity of imidazolium and triazolium salts. Bioorg Med Chem. 2010;18:6184–6196. doi: 10.1016/j.bmc.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y, Lazar C, Szarek WA, Brockhausen I. Specificity of β1,4-galactosyl-transferase inhibition by 2-naphthyl 2-butanamido-2-deoxy-1-thio-β-D-glucopyranoside. Glycoconj J. 2010;27:673–684. doi: 10.1007/s10719-010-9312-3. [DOI] [PubMed] [Google Scholar]
- 38.Brockhausen I, Benn M, Bhat S, Marone S, Riley JG, Montoya-Peleaz P, Vlahakis JZ, Paulsen H, Schutzbach JS, Szarek WA. UDP-Gal: GlcNAc-R β1,4-galactosyltransferase — a target enzyme for drug design. Acceptor specificity and inhibition of the enzyme. Glycoconj J. 2006;23:523–539. doi: 10.1007/s10719-006-7153-x. [DOI] [PubMed] [Google Scholar]
- 39.Xia J, Xue J, Locke RD, Chandrasekaran EV, Srikrishnan T, Matta KL. Synthesis of fluorinated mucin core 2 branched oligosaccharides with the potential of novel substrates and enzyme inhibitors for glycosyltransferases and sulfotransferases. J Org Chem. 2006;71:3696–3706. doi: 10.1021/jo052626j. [DOI] [PubMed] [Google Scholar]
- 40.Chandrasekaran EV, Xue J, Piskorz C, Locke RD, Tóth K, Slocum HK, Matta KL. Potential tumor markers for human gastric cancer: an elevation of glycan: sulfotransferases and a concomitant loss of alpha1,2-fucosyltransferase activities. J Cancer Res Clin Oncol. 2007;133:599–611. doi: 10.1007/s00432-007-0206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathieux N, Paulsen H, Meldal M, Bock K. Synthesis of glycopeptide sequences of repeating units of the mucins MUC 2 and MUC 3 containing oligosaccharide side-chains with core 1, core 2, core 3, core 4 and core 6 structure. J Chem Soc Perkin Trans. 1997;1:2359–2368. [Google Scholar]
- 42.Toki D, Sarkar M, Yip B, Reck F, Joziasse D, Fukuda M, Schachter H, Brockhausen I. Expression of stable human O-glycan core 2 β1,6-N-acetylglucosaminyltransferase in Sf9 insect cells. Biochem J. 1997;325:63–69. doi: 10.1042/bj3250063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarvis DL. Baculovirus-insect cell expression systems. Methods Enzymol. 2009;463:191–222. doi: 10.1016/S0076-6879(09)63014-7. [DOI] [PubMed] [Google Scholar]
- 44.Gao Y, Chachadi VB, Cheng PW, Brockhausen I. Glycosylation potential of human prostate cancer cell lines. Glycoconj J. 2012;29:525–537. doi: 10.1007/s10719-012-9428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pak JE, Arnoux P, Zhou S, Sivarajah P, Satkunarajah M, Xing X, Rini JM. X-ray crystal structure of leukocyte type core 2 beta1,6-N-acetylglucosaminyltransferase. Evidence for a convergence of metal ion-independent glycosyltransferase mechanism. J Biol Chem. 2006;281:26693–26701. doi: 10.1074/jbc.M603534200. [DOI] [PubMed] [Google Scholar]
- 46.Pak JE, Satkunarajah M, Seetharaman J, Rini JM. Structural and mechanistic characterization of leukocyte-type core 2 β1,6-N-acetylglucosaminyltransferase: a metal-ion-independent GT-A glycosyltransferase. J Mol Biol. 2011;414:798–811. doi: 10.1016/j.jmb.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 47.Robbe C, Capon C, Coddeville B, Michalski JC. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem J. 2004;384:307–316. doi: 10.1042/BJ20040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krakun SK, Clo E, Clausen H, Levery SB, Jensen KJ, Blixt O. Random glycopeptide bead libraries for seromic biomarker discovery. J Proteome Res. 2010;9:6705–67614. doi: 10.1021/pr1008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kubota T, Shiba T, Sugioka S, Furukawa S, Sawaki H, Kato R, Wakatsuki S, Narimatsu H. Structural basis of carbohydrate transfer activity by human UDP-GalNAc: polypeptide alpha-N-acetylgalactosaminyltransferase (pp-GalNAc-T10) J Mol Biol. 2006;359:708–727. doi: 10.1016/j.jmb.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 50.Fritz TA, Raman J, Tabak LA. Dynamic association between the catalytic and lectin domains of human UDP-GalNAc:polypeptide alpha-N-acetylgalactosaminyltransferase-2. J Biol Chem. 2006;281:8613–8619. doi: 10.1074/jbc.M513590200. [DOI] [PubMed] [Google Scholar]
- 51.Fritz TA, Hurley JH, Trinh LB, Shiloach J, Tabak TA. The beginnings of mucin biosynthesis: the crystal structure of UDP-GalNAc:polypeptide alpha-N-acetylgalactosaminyltransferase-T1. Proc Natl Acad Sci U S A. 2004;101:15307–15312. doi: 10.1073/pnas.0405657101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breton C, Fournel-Gigleux S, Palcic MM. Recent structures, evolution and mechanisms of glycosyltransferases. Curr Opin Struct Biol. 2012;22:540–549. doi: 10.1016/j.sbi.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Mukhopadhyay S, Bachert C, Smith DR, Linstedt AD. Manganese-induced trafficking and turnover of the cis-Golgi glycoprotein GPP130. Mol Biol Cell. 2010;21:1282–1292. doi: 10.1091/mbc.E09-11-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwientek T, Yeh JC, Levery SB, Keck B, Merkx G, van Kessel AG, Fukuda M, Clausen H. Control of O-glycan branch formation. Molecular cloning and characterization of a novel thymus-associated core 2 beta1, 6-n-acetylglucosaminyltransferase. J Biol Chem. 2000;275:11106–11113. doi: 10.1074/jbc.275.15.11106. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto M, Tan S, Mori N, Cheng H, Cheng PW. Mucin biosynthesis: Molecular cloning and expression of mouse mucus-type core 2 beta1,6 N-acetylglucosaminyltransferase. Glycobiology. 2007;17:994–1006. doi: 10.1093/glycob/cwm068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.