Abstract
One of the most important metabolic hallmarks of cancer cells is enhanced lipogenesis. Depending on the tumor type, tumor cells synthesize up to 95% of saturated and mono-unsaturated fatty acids (FA) de novo in spite of sufficient dietary lipid supply. This lipogenic conversion starts early when cells become cancerous and further expands as the tumor cells become more malignant. It is suggested that activation of FA synthesis is required for carcinogenesis and for tumor cell survival. These observations suggest that the enzymes involved in FA synthesis would be rational therapeutic targets for cancer treatment. However, several recent reports have shown that the anti-tumor effects, following inhibition of endogenous FA synthesis in cancer cell lines may be obviated by adding exogenous FAs. Additionally, high intake of dietary fat is reported to be a potential risk factor for development and poor prognosis for certain cancers. Recently it was reported that breast and liposarcoma tumors are equipped for both de novo fatty acid synthesis pathway as well as LPL-mediated extracellular lipolysis. These observations indicate that lipolytically acquired FAs may provide an additional source of FAs for cancer. This review focuses on our current understanding of lipogenic and lipolytic pathways in cancer cell progression.
Keywords: Cancer, Fatty acid synthase, Fatty acid synthesis, Lipolysis, Lipogenesis, Lipoprotein Lipase
Introduction
Cancer cells are characterized by their ability to divide more frequently than normal cells. Rapidly proliferating cancer cells exhibit increased demands for energy and macromolecules. To cope with these elevated requirements cancer cells undergo major metabolic modifications. Since the 1920s, it has been known that, in contrast to most normal tissues, cancer cells show avid glucose uptake and tend to convert glucose to lactate through the glycolytic pathway regardless of whether oxygen is present (aerobic glycolysis; Warburg Effect) [1]. Glucose metabolism via the glycolytic pathway provides not only energy, but also a carbon source for anabolic synthesis of critical biochemical precursors. It is now widely recognized that tumors frequently exhibit an increased ability to synthesize lipids [2, 3], and that this lipogenesis is tightly coupled to glucose metabolism.
Several lines of evidence suggest that activation of the de novo fatty acid (FA) synthesis pathway is required for carcinogenesis [4–6]. The FA synthesis pathway is extensively studied in the context of cancer biology and is currently thought to be the major pathway exploited by the cancer cells for the acquisition of FAs [5]. However, recent findings suggest that certain cancer cells/tissues can utilize both lipogenic and lipolytic pathways to acquire fatty acids that, in turn, promote cancer cell proliferation and survival [7, 8].
This review focuses on our current understanding of the roles of both the lipogenic and lipolytic pathways in mediating tumor growth and the therapeutic benefits that could possibly be achieved by targeting these pathways.
Fatty acids support various aspects of tumorigenesis
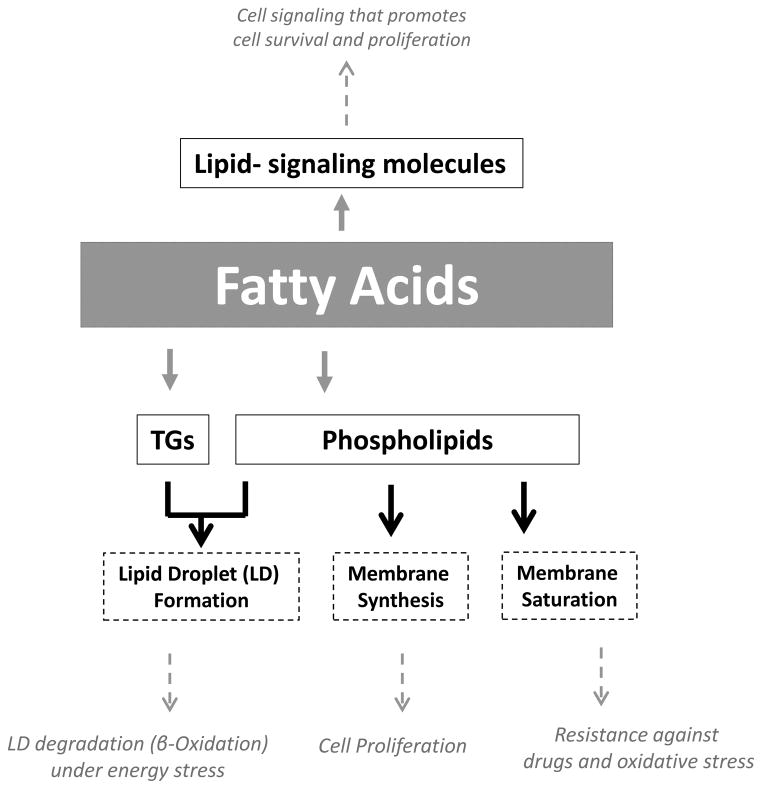
Fatty acids may contribute to cancer progression by multiple mechanisms (Figure 1). The most widely discussed aspect of FA-biochemistry with respect to tumor biology is their role as building blocks for newly-synthesized membrane phospholipids. Large amounts of FAs are required to accommodate high rates of proliferation in cancer cells [5]. Cancer cells can acquire FAs through lipogenesis and/or lipolysis to support their growth and proliferation.
Figure 1. Fatty acids promote various aspects of tumor cell development, progression and survival.
Fatty acids provide the cancer cells with membrane building blocks, signaling molecules and energy source that supports their rapid proliferation and survival. (See text for more details)
The source of FAs may determine the phospholipid composition of membranes. In this context, it is important to consider that mammalian cells have a limited ability to synthesize polyunsaturated fatty acids de novo, as they lack the Δ 12 desaturase. Therefore, enhanced de novo lipogenesis enriches the cancer cell membranes with saturated and/or mono-unsaturated fatty acids [9]. As these FAs are less prone to lipid peroxidation than polyunsaturated acyl chains, de novo FA synthesis was proposed to make cancer cells more resistant to oxidative stress-induced cell death [9]. Moreover, as saturated lipids pack more densely, increased lipogenesis also alters lateral and transverse membrane dynamics that may limit the uptake of drugs, making the cancer cells more resistant to therapy [9].
Fatty acids may also be used to supply energy. Most tumors show a high rate of glucose uptake that supports their energetic as well as biosynthetic requirements [10]. However, certain types of tumors, including prostate tumors, display increased dependence on β-oxidation of fatty acids as their main source of energy. Prostate tumors exhibit low rates of glucose consumption [11, 12], increased fatty acid uptake [13] and overexpression of certain enzymes involved in β-oxidation [14]. Likewise, human leukemia cells have been shown to require β-oxidation for their proliferation and survival [15].
Fatty acids can also be used for the biosynthesis of an array of protumorigenic lipid-signaling molecules. A lipid messenger considered to be particularly important in contributing to cancer is phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P3], a molecule that is formed by the action of phosphatidylinositol-3-kinase and activates protein kinase B/Akt to stimulate cell proliferation and survival [16, 17]. Other prominent examples of lipid messengers are lysophosphatidic acid (LPA) that signals through a family of G protein-coupled receptors to promote cancer aggressiveness [18], and prostaglandins, a class of lipid messengers that are formed by cyclooxygenases and support migration and tumor-host interactions [19, 20].
Lipogenesis versus lipolysis
Various tumor types display increased endogenous FA biosynthesis irrespective of extracellular lipid availability [21], whereas most normal cells, even those with comparatively high proliferation rates, preferentially use dietary/exogenous lipids for synthesis of new structural lipids [5, 21]. A few normal tissues such as adipocytes, hepatocytes, hormone-sensitive cells [22], the cycling endometrium, and fetal lung tissue [23] may have a very active FA-synthesis pathway. However, de novo FA synthesis is suppressed in most normal cells. The upregulated FA synthesis in tumor cells is reflected by a significant increase in expression and activity of various enzymes involved in the lipogenic pathway [21]. For example, elevated levels of fatty acid synthase (FASN), the major enzyme responsible for fatty acid biosynthesis, are correlated with poor prognosis in breast cancer patients [4, 5]. Increases in both FASN expression and activity are observed early in oncogenesis and correlate with cancer progression [5], with FASN-overexpressing tumors exhibiting more aggressive phenotypes [5]. The upregulated FA-synthesis fuels membrane biogenesis in rapidly proliferating cancer cells and renders membrane fatty acids more saturated (Figure 2) [9]. This affects fundamental cellular processes including signal transduction, gene expression, ciliogenesis [24], and therapeutic responsiveness [9].
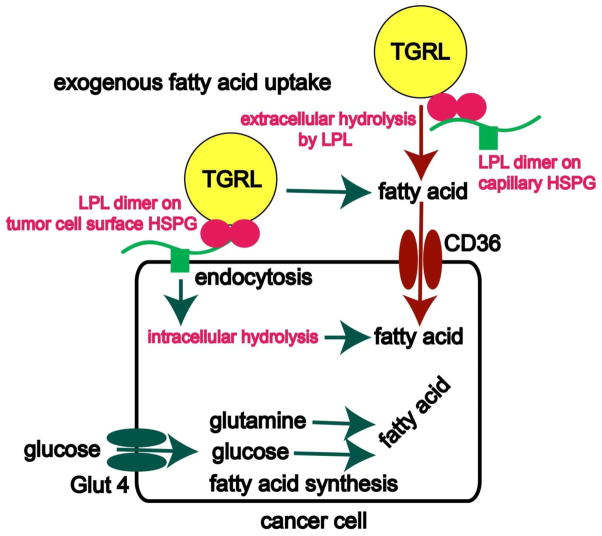
Figure 2. Fatty acid acquisition by tumors.
Glucose and glutamine supply carbon that enzymes of lipogenesis, including fatty acid synthase (FASN), use to synthesize FA. Alternatively, exogenous FA may be acquired by extracellular lipolysis TG from the TG-rich lipoproteins (TGRL) using the secreted enzyme lipoprotein lipase (LPL) bound to a heparin-like heparan sulfate proteoglycan motif on the luminal surface of vascular epithelium. Resultant free FAs enter the cancer cell via CD36, the FA uptake channel. This is the mechanism used by normal adipose and striated muscle cells. The presence of a heparin-releasable pool of LPL and the HSPG motif on the surface of tumor cells raises the novel possibility that malignant cells may decorate their surface with the enzyme, rather than simply secreting it. In this speculative arrangement LPL tumor cell surface-associated LPL could mediate extracellular hydrolysis. Alternatively, LPL could facilitate endocytosis of lipoproteins by serving as a non-enzymatic bridge between the cell surface heparan sulfate binding site and the lipoprotein.
Chemical or RNAi-mediated inhibition of key enzymes involved in FA synthesis, including FASN [5, 21, 25], acetyl-CoA-carboxylase (ACACA) [26] and ATP-citrate lyase (ACLY) [27–30], has been shown to attenuate cancer cell growth and to induce cell death. However, cytotoxic effects of de novo FA synthesis inhibition can be averted by FA supplementation [25, 26, 31, 32]. The ability of exogenous FAs to functionally substitute for endogenously derived FAs in promoting cell viability suggests that cancer cells can incorporate and utilize exogenous lipids as an alternative source of FAs.
Generally, lipogenesis has been considered as the major means of FA acquisition in cancer cells. However, a recent study clearly showed that, in addition to lipogenesis, cancer cells can also use exogenous fatty acids to fuel their growth (Figure 2) [7]. It was reported that the aggressive “triple-negative” breast cancer cell lines express lipoprotein lipase (LPL), the key enzyme for extracellular lipolysis, and the transmembrane channel for exogenous free FA uptake (CD36), together with the classical lipogenic markers such as FASN [7]. In selected cancer cell lines lipolysis is, therefore, an additional pathway for FA acquisition.
In contrast to the cultured breast cancer cells, where LPL is significantly expressed only in triple-negative cell lines, clinical breast tumor tissues universally display LPL, irrespective of their biomarker status [7]. This expression is also seen in liposarcomas and prostate tumor tissues. The recent report by Kuemmerle et al. thus highlights the stark discrepancy in the LPL expression of cancer cell lines in vitro and clinical tumor samples in vivo [7]. Several plausible interpretations were given to explain this inconsistency. First and foremost is the fact that cell lines are passaged over time in culture systems lacking vascular endothelium, a physiologic site of LPL action [7]. In the absence of an exogenous source of FAs, de novo synthesis would be the preferred mechanism for FA-acquisition over lipolysis or receptor-mediated endocytosis.
From the aforementioned work, it is clear that LPL is of functional significance to certain cancer cells. This has been reaffirmed by studies which have identified LPL as a biomarker for poor prognosis in chronic lymphocytic leukemia (CLL) [33–36]. Attempts have been made to assess the functional significance of LPL in CLL cells in vitro. However, these studies employed the drug Orlistat as an inhibitor of LPL enzyme activity. Because Orlistat inhibits both LPL and FASN, these results are difficult to interpret [37].
LPL is a secreted enzyme that hydrolyzes the TGs in chylomicrons or very low-density lipoproteins (VLDL) (reviewed in [38]). In normal tissues, secreted LPL is targeted to local capillary endothelial cells by an escort protein (GPIHBP1), where it is bound to the luminal surface by noncovalent linkage to a specific heparan sulfate proteoglycan (HSPG) motif [39], (reviewed in [40]). The FAs released by hydrolysis of circulating TGs can be taken up by the cells via CD36. At this location on endothelial cells, LPL has also been found to have an additional non-catalytic “bridging” function, allowing for the accumulation and cellular uptake of lipoproteins via receptor-mediated endocytosis [41].
It is important to emphasize that the lipolytic mechanism described above has been elucidated in normal tissues. The deployment of LPL by cancer cells is not fully understood, and may differ from that employed by adipose and striated muscle cells. Kuemmerle and coworkers found a heparin-releasable pool of LPL in both HeLa cells and breast tumor tissue, suggesting that LPL may be bound to the surface of these cells. Moreover, Smits and colleagues have demonstrated the presence of the heparin-like HSPG motif on the surface of ovarian cancer cells [42]. Altered expression of genes coding enzymes involved in HSPG synthesis as well as down-regulation of heparanase expression, have recently been shown in breast tumors [43]. These findings raise the novel possibility that malignant cells may bind the enzyme to their surface, as opposed to simply secreting it.
Another hypothesis that further stipulates the need of paired extra- and intracellular lipolytic pathways for cancer cells is that endogenously synthesized fatty acids are rapidly incorporated into cellular neutral lipid stores. Nomura et al. [8] proposed that in order to release fatty acyl moieties from these reservoirs complementary intracellular lipolytic pathways are required. They demonstrated the role of an intracellular lipase, monoglyceride lipase MGLL (monoacyl glycerol lipase, MGLL) in promoting tumorigenesis. Intracellular lipolysis catalyzes the breakdown of TGs stored in intracellular lipid droplets [44]. MGLL provides, by de-esterification, a stream of intracellular free FA that fuels proliferation, growth, and migration of cancer cells. In a more recent report, it was shown that MGLL also exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer [45]. The disposition of intracellular triglyceride stores in stressed cells has been termed “lipophagy,” and is viewed as a component of the autophagic response that allows cells to adapt to stress and avoid cell death (reviewed in [46]).
A factor that should be considered with respect to increased lipogenesis in cancer cells is that the end-product of this pathway, palmitate (and other palmitate-like saturated FA), is toxic to the cells. Accumulation of FAs and neutral lipids in non-adipose tissue is known to rapidly stimulate apoptosis [47]. Moreover, palmitate excess could feed-back to inhibit endogenous FA synthesis. A balance between lipogenesis, lipid uptake and intracellular lipolysis would, therefore, be required to maintain lipid homeostasis [47].
Increased expression and activity of lipolytic enzymes in tumor cells or the tumor microenvironment is a recently revealed phenomenon. However, increased lipolysis in adipose tissue of patients suffering from cancer cachexia is well-recognized. Many research groups have shown that brisk lipolysis is a key factor behind adipose cachexia in weight-losing cancer patients [48–51]. It has been suggested that tumor load promotes intracellular lipolysis in adipose tissues of cachectic patients, though the underlying mechanisms are just beginning to be elucidated [48]. In the case of malignant melanoma, the tumor secretes an inhibitor of adipose LPL termed melanoma cachexia factor, causing inhibition of extracellular lipolysis and depletion of adipose stores [52]. A recent report also demonstrated homing of ovarian cancer metastases to omental adipose tissue, where the cancer cells elicited release of FA from the adipocytes [53].
Metabolic changes such as elevated levels of circulating free fatty-acids, monoacylglycerides and diacylglycerides have been observed in cachectic cancer patients [54]. These increased levels of fatty-acids can promote tumor growth by fueling the metabolism of cancer cells. Again, to benefit from these metabolites cancer cells must upregulate their FA-uptake pathways.
Taken together, these findings indicate that lipogenesis and/or lipolysis could be utilized to varying extents by the cancer cells to fulfill their fatty acid requirements. Such functional lipolytic-lipogenic coupling emphasizes the fundamental importance of characterizing these pathways and their interactions. Another important question to be addressed involves the specific metabolic fates of FAs derived from endogenous and exogenous sources. Currently it is unknown whether FAs derived from the lipolysis of exogenous lipids enter the same pool of free FAs generated through lipogenesis and used for membrane biogenesis or signaling pathways.
The uptake of exogenous FA by cancer cells prompts the idea that LPL may be an important component of the interface between dietary fat and cancer biology. Obesity, a state of raised levels of circulating FA, has been associated with increased incidence of certain cancers [55] and a poor prognosis for established tumors [56]. In a variety of rodent breast cancer models, high-fat feeding promotes cancer incidence and growth [57]. Interventional studies in human breast cancer patients, however, have not revealed a survival advantage of a low fat diet [58].
In the recent past molecular pathological epidemiology of cancer has been an area of interest for many cancer biologists [59]. Molecular pathological epidemiology (MPE) is a transdisciplinary field that examines a relationship between exposures and molecular signatures in tumor, as well as interactive influences of the exposures and molecular features on cancer progression [60, 61]. Utilizing the MPE approach Ogino et al. analyzed independent and combined effect of body mass index (BMI) and FASN expression on clinical outcome in colon cancer patients [62]. They reported that in non-obese colon cancer patients, tumoral FASN overexpression is associated with improved survival, whereas among moderately overweight or obese patients FASN overexpression may predict a worse outcome [62]. Recent work by Kuchiba et al. suggests that obesity is associated with increased risk of FASN-negative colorectal cancers [63]. These findings indicate that cellular FASN status may determine a cell’s dependence on energy balance status for malignant transformation. Further studies are required to investigate the interactive effects of dietary lipids and tumoral molecular features on tumor behavior (prognosis or clinical outcome). The data obtained from these prospective works may help in attributing the effects of dietary variables to a specific molecular subtype of cancer.
Therapeutic targeting of lipid metabolism for cancer treatment and prevention
Preceding attempts to exploit cancer FA requirements for therapeutic benefit mainly focused on de novo FA synthesis. Several research groups have shown that therapeutic targeting of various enzymes of this pathway such as ACLY, FASN and ACACA may result in tumor regression both in vitro and in vivo [5, 21, 25–28]. Recent findings suggest that cancer cells can generate FAs via both lipogenic and lipolytic mechanisms that, in turn, support their proliferation and survival [7, 8]. Hence, lipolysis-derived FAs may be able to attenuate any therapeutic advantage achieved by targeted inhibition of de novo FA synthesis. This recent appreciation of a “lipolytic phenotype” raises the possibility of novel therapeutic targets. Research groups have begun to investigate the therapeutic potential of lipolysis, and Kuemmerle and coworkers found that simultaneous inhibition of both lipogenesis and lipolysis yielded an enhanced anticancer effect in tissue culture.
Zaidi et al. recently reported that cancer cells exhibit increased reliance on de novo FA synthesis for cancer cell growth and survival under lipid-restricted growth conditions [29]. We observed that in several cancer cell lines cytotoxic effects achieved by silencing of lipogenic pathway were enhanced when the cells were cultured in lipid-reduced growth conditions [29]. In addition, we have observed increased fatty acid synthase (FASN) expression following exogenous lipid withdrawal in a variety of cancer types. These findings suggest that the availability of exogenous fatty acids may affect the balance between lipolysis and lipogenesis, and emphasize the potential impact of dietary fat on cancer biology. In order to attain maximum therapeutic benefit from the treatments targeting FA requirements of cancer cells, a better understanding of these two pathways is required. Most importantly the interactions between the two pathways should be studied in detail.
Acknowledgments
Work from the author’s laboratories was supported by
Higher Education Commission, Pakistan. (N. Zaidi)
FWO Grant G.0691.12 (J.V. Swinnen)
GOA Grant 11/009 (J.V. Swinnen)
IAP7-32 Grant (J.V. Swinnen)
NIH Grant RO1CA126618 (W.B. Kinlaw)
Norris Cotton Cancer Center Prouty grants (W.B. Kinlaw),
NIH Training Grant DK07508 (N.B. Kuemmerle),
The Program in Experimental and Molecular Medicine at the Geisel School of Medicine at Dartmouth (L. E. Lupien)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Aisenberg AC. The Glycolysis and Respiration of Tumors. Academic Press; New York: 1961. pp. 12–13. [Google Scholar]
- 3.Baron A, et al. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91(1):47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 4.Kuhajda FP, et al. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A. 2000;97(7):3450–4. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67(7):2964–71. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 7.Kuemmerle NB, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10(3):427–36. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nomura DK, et al. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140(1):49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rysman E, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70(20):8117–26. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 10.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price DT, et al. Comparison of [18 F]fluorocholine and [18 F]fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J Urol. 2002;168(1):273–80. [PubMed] [Google Scholar]
- 12.Effert PJ, et al. Metabolic imaging of untreated prostate cancer by positron emission tomography with 18fluorine-labeled deoxyglucose. J Urol. 1996;155(3):994–8. [PubMed] [Google Scholar]
- 13.Liu Y, Zuckier LS, Ghesani NV. Dominant uptake of fatty acid over glucose by prostate cells: a potential new diagnostic and therapeutic approach. Anticancer Res. 2010;30(2):369–74. [PubMed] [Google Scholar]
- 14.Zha S, et al. Peroxisomal branched chain fatty acid beta-oxidation pathway is upregulated in prostate cancer. Prostate. 2005;63(4):316–23. doi: 10.1002/pros.20177. [DOI] [PubMed] [Google Scholar]
- 15.Samudio I, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. 2010;120(1):142–56. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zunder ER, et al. Discovery of drug-resistant and drug-sensitizing mutations in the oncogenic PI3K isoform p110 alpha. Cancer Cell. 2008;14(2):180–92. doi: 10.1016/j.ccr.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren J, et al. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006;66(6):3006–14. doi: 10.1158/0008-5472.CAN-05-1292. [DOI] [PubMed] [Google Scholar]
- 19.Gupta GP, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446(7137):765–70. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 20.Marnett LJ. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992;52(20):5575–89. [PubMed] [Google Scholar]
- 21.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100(9):1369–72. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusakabe T, et al. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem. 2000;48(5):613–22. doi: 10.1177/002215540004800505. [DOI] [PubMed] [Google Scholar]
- 23.Wagle S, et al. Hormonal regulation and cellular localization of fatty acid synthase in human fetal lung. Am J Physiol. 1999;277(2 Pt 1):L381–90. doi: 10.1152/ajplung.1999.277.2.L381. [DOI] [PubMed] [Google Scholar]
- 24.Willemarck N, et al. Aberrant activation of fatty acid synthesis suppresses primary cilium formation and distorts tissue development. Cancer Res. 2010;70(22):9453–62. doi: 10.1158/0008-5472.CAN-10-2324. [DOI] [PubMed] [Google Scholar]
- 25.Kridel SJ, et al. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64(6):2070–5. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 26.Chajes V, et al. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006;66(10):5287–94. doi: 10.1158/0008-5472.CAN-05-1489. [DOI] [PubMed] [Google Scholar]
- 27.Hatzivassiliou G, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8(4):311–21. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Hanai J, et al. Inhibition of lung cancer growth: ATP citrate lyase knockdown and statin treatment leads to dual blockade of mitogen-activated protein Kinase (MAPK) and Phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol. 2011;227(4):1709–20. doi: 10.1002/jcp.22895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaidi N, et al. ATP Citrate Lyase Knockdown Induces Growth Arrest and Apoptosis through Different Cell- and Environment-Dependent Mechanisms. Mol Cancer Ther. 2012;11(9):1925–35. doi: 10.1158/1535-7163.MCT-12-0095. [DOI] [PubMed] [Google Scholar]
- 30.Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72(15):3709–14. doi: 10.1158/0008-5472.CAN-11-4112. [DOI] [PubMed] [Google Scholar]
- 31.Kuhajda FP, et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci U S A. 1994;91(14):6379–83. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen AM, et al. Fatty acid synthesis is a therapeutic target in human liposarcoma. Int J Oncol. 2010;36(5):1309–14. doi: 10.3892/ijo_00000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van’t Veer MB, et al. The predictive value of lipoprotein lipase for survival in chronic lymphocytic leukemia. Haematologica. 2006;91(1):56–63. [PubMed] [Google Scholar]
- 34.Van Bockstaele F, et al. Lipoprotein lipase mRNA expression in whole blood is a prognostic marker in B cell chronic lymphocytic leukemia. Clin Chem. 2007;53(2):204–12. doi: 10.1373/clinchem.2006.076331. [DOI] [PubMed] [Google Scholar]
- 35.Oppezzo P, et al. The LPL/ADAM29 expression ratio is a novel prognosis indicator in chronic lymphocytic leukemia. Blood. 2005;106(2):650–7. doi: 10.1182/blood-2004-08-3344. [DOI] [PubMed] [Google Scholar]
- 36.Kaderi MA, et al. LPL is the strongest prognostic factor in a comparative analysis of RNA-based markers in early chronic lymphocytic leukemia. Haematologica. 2011;96(8):1153–60. doi: 10.3324/haematol.2010.039396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heintel D, et al. High expression of lipoprotein lipase in poor risk B-cell chronic lymphocytic leukemia. Leukemia. 2005;19(7):1216–23. doi: 10.1038/sj.leu.2403748. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2009;50(Suppl):S86–90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies B, et al. GPIHP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metabolism. 2010;12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg RD, et al. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest. 1997;99(9):2062–70. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med (Berl) 2002;80(12):753–69. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 42.Smits NC, et al. The heparan sulfate motif (GlcNS6S-IdoA2S)3, common in heparin, has a strict topography and is involved in cell behavior and disease. J Biol Chem. 2010;285(52):41143–51. doi: 10.1074/jbc.M110.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez-Vega I, et al. Specific genes involved in synthesis and editing of heparan sulfate proteoglycans show altered expression patterns in breast cancer. BMC Cancer. 2013;13:24. doi: 10.1186/1471-2407-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zechner R, et al. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15(3):279–91. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura DK, et al. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011;18(7):846–56. doi: 10.1016/j.chembiol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu K, Czaja MJ. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013;20(1):3–11. doi: 10.1038/cdd.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menendez JA. Fine-tuning the lipogenic/lipolytic balance to optimize the metabolic requirements of cancer cell growth: molecular mechanisms and therapeutic perspectives. Biochim Biophys Acta. 2010;1801(3):381–91. doi: 10.1016/j.bbalip.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279(15):2610–23. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 49.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89(2):381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 50.Agustsson T, et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007;67(11):5531–7. doi: 10.1158/0008-5472.CAN-06-4585. [DOI] [PubMed] [Google Scholar]
- 51.Das SK, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333(6039):233–8. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- 52.Mori M, et al. Cancer cachexia syndrome developed in nude mice bearing melanoma cells producing leukemia-inhibitory factor. Cancer Res. 1991;51(24):6656–9. [PubMed] [Google Scholar]
- 53.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gercel-Taylor C, et al. Aberrations in normal systemic lipid metabolism in ovarian cancer patients. Gynecol Oncol. 1996;60:35–41. doi: 10.1006/gyno.1996.0008. [DOI] [PubMed] [Google Scholar]
- 55.Eheman C, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–66. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chlebowski RT. Obesity and breast cancer outcome: adding to the evidence. J Clin Oncol. 2012;30(2):126–8. doi: 10.1200/JCO.2011.39.7877. [DOI] [PubMed] [Google Scholar]
- 57.Freedman LS, Clifford C, Messina M. Analysis of dietary fat, calories, body weight, and the development of mammary tumors in rats and mice: a review. Cancer Res. 1990;50(18):5710–9. [PubMed] [Google Scholar]
- 58.Pierce JP, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogino S, et al. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60(3):397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogino S, Giovannucci E. Commentary: Lifestyle factors and colorectal cancer microsatellite instability--molecular pathological epidemiology science, based on unique tumour principle. Int J Epidemiol. 2012;41(4):1072–4. doi: 10.1093/ije/dys076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102(6):365–7. doi: 10.1093/jnci/djq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogino S, et al. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. 2008;26(35):5713–20. doi: 10.1200/JCO.2008.18.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuchiba A, et al. Body mass index and risk of colorectal cancer according to fatty acid synthase expression in the nurses’ health study. J Natl Cancer Inst. 2012;104(5):415–20. doi: 10.1093/jnci/djr542. [DOI] [PMC free article] [PubMed] [Google Scholar]