Abstract
Aim:
The aim of this study was to explore the effects and mechanism of berbamine on imatinib-resistant BCR-ABL-positive human leukemia K562 (K562-r) cells in vitro and in vivo.
Methods:
Cell viability was measured by MTT assay, and apoptotic morphology changes were detected by fluorescence microscopy. The apoptosis rate was measured by flow cytometric assay. mdr-1 mRNA levels were determined by RT-PCR. Bcl-2 family proteins, cytochrome c(cyt C), poly (ADP-ribose) polymerase (PARP), and P-glycoprotein were detected by Western blot. BALB/c nu/nu mice were injected with K562-r cells subcutaneously. Tumor-bearing mice were treated intravenously with berbamine.
Results:
MTT tests revealed that berbamine significantly inhibited K562-r cell proliferation and increased the chemo-sensitivity of K562-r cells to imatinib. The apoptosis rate was significantly increased following treatment with 21.2 μmol/L berbamine; formation of typical apoptotic blebs was apparent, as observed by fluorescence microscopy. Expression levels of mdr-1 mRNA and P-gp protein were high in untreated K562-r cells and significantly down-regulated by berbamine treatment. Berbamine-treated K562-r cells also exhibited down-regulated expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL, up-regulated expression of the apoptotic proteins Bax and cytoplasmic cyt C, and stimulated proteolytic cleavage of PARP. In addition, berbamine also suppressed the growth of K562-r xenotransplanted tumors in vivo.
Conclusion:
Berbamine inhibited proliferation of K562-r cells both in vitro and in vivo. Berbamine-induced apoptosis in K562-r cells appeared to occur through a mechanism involving Bcl-2 family proteins, as well as mdr-1 mRNA and P-gp protein. Berbamine in combination with imatinib restored the chemo-sensitivity of K562-r cells to imatinib. Our findings suggest that berbamine may be useful in treating imatinib-resistant CML patients.
Keywords: berbamine, chronic myeloid leukemia, imatinib, mdr-1, Bcl-2
Introduction
Chronic myeloid leukemia (CML) is a pluripotent hematopoietic stem cell disorder. Approximately 95% of CML patients carry a characteristic Philadelphia chromosome (Ph)1, a genetic abnormality resulting from a reciprocal translocation between the long arms of chromosomes 9 and 22. The molecular consequence of this translocation is the generation of the fusion gene bcr-abl, which encodes a constitutively active tyrosine kinase2. The BCR-ABL kinase signals to multiple downstream survival pathways, including Ras/Raf/MEK/ERK, PI3K/AKT, JAK-STAT, and nuclear factor κB (NF-κB), which contribute to the pathogenesis of CML3, 4.
Imatinib mesylate (STI571; Gleevec), an inhibitor of the tyrosine-kinase activity of BCR-ABL, has been proven highly effective in patients with chronic-phase CML5. Unfortunately, resistance to imatinib mesylate has become a significant clinical problem, especially in patients with accelerated and blastic-phase disease6. Several approaches have been devised to overcome clinical resistance to imatinib. The proteasome inhibitor, bortezomib (Velcade), farnesyltransferase inhibitors such as SCH66326 and the pyrido-pyrimidine-type kinase inhibitor PD166326 were shown to have growth inhibitory effects on some but not all imatinib-resistant leukemias7, 8. Therefore, it is important to develop alternative treatment strategies.
Berbamine, a natural compound from berberis, is a bis-benzylisoquinoline alkaloid and has been widely used in China for leukopenia treatment over the past decades. Berbamine is a calmodulin (CaM) antagonist. Recent evidence suggests that calmodulin plays an important role in cellular proliferation and that its function may be altered in malignancy9. In our previous studies, we found that berbamine inhibited the growth of leukemia cells such as K562 cells, Jurkat cells and NB4 cells, but had little or no effect on normal bone marrow cells10, 11, 12. Our results suggested that the major effect of berbamine was apoptosis induction, which may be accomplished by down-regulation of bcr-abl oncogene expression and activation of caspase-3 in leukemia K562 cells11, 13. In the present study, we examined the effects of berbamine on imatinib-resistant K562 (K562-r) cells and found that berbamine was effective in inhibiting K562-r cell growth and reversing imatinib resistance.
Materials and methods
Reagents and antibodies
Berbamine powder for in vitro experiments was provided by Fushun Chinese Herb Factory and berbamine liquid preparation for in vivo injection experiments was provided by Zhongfang Phamaceutical Co Ltd. The formula of berbamine is shown elsewhere10, and its molecular weight is 753.80. Imatinib was kindly provided by Professor Jia-yi DING (Cancer Institute, School of Medicine, Zhejiang University). A 1.3 mmol/L berbamine stock solution was prepared by dissolving berbamine powder in isotonic saline and stored at −20 °C. A 1 mmol/L imatinib stock solution was prepared by dissolving imatinib in DMSO (Sigma, St Louis, MO) and stored at −20 °C. Mouse anti-β-actin, anti-Bcl-2, anti-cyt-C, anti-PARP, anti-P-gp, anti-Bax, and anti-Bcl-xL monoclonal antibodies and horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA).
Cell and cell culture
Ph chromosome-positive human CML blast crisis imatinib-sensitive (K562-s) and imatinib-resistant (K562-r) cells were obtained from the Cancer Institute, School of Medicine, Zhejiang University. The imatinib-resistant (K562-r) cells are also resistant to doxorubicin10. Cells were cultured in RPMI-1640 medium (Gibco) containing 10% fetal calf serum (Sijiqing, Hangzhou, China) and 2 mmol/L glutamine.
Staining of cells with Hoechst 33258
Berbamine-treated and control cells were washed with phosphate-buffered saline (PBS) and resuspended in the same buffer. One hundred microliters of cell suspension (1×106 cells/mL) was incubated with 1 μL of Hoechst 33258 (1 g/L in ddH2O) for 10 min. Cell apoptosis was evaluated by fluorescence microscopy.
Apoptosis assessment by annexin-V staining
After drug treatment, cells were washed with PBS and resuspended in 100 μL staining solution containing annexin-V fluorescein and PI in a HEPES buffer (annexin-V-Fluos Staining Kit; Boehringer-Mannheim, Indianapolis, IN). After a 15-minincubation at room temperature, cells were analyzed by flow cytometry. The assay was based on the binding properties of the fluorescent dyes and physiological status of the cells: Annexin-V binds to those cells that express phosphatidylserine on the outer layer of the cell membrane, and PI stains the cellular DNA of cells that have a compromised membrane. This allows discrimination between live cells (unstained with either fluorochrome) and apoptotic (stained only with annexin-V) and necrotic cells (stained with both annexin-V and PI).
MTT (viability) assay
Cells were seeded in 96-well plates (5×104 cells/mL) and drugs were added at required concentrations in a final volume of 200 μL medium. After 24 and 48 h incubation, 20 μL of tetrazolium dye 3-4,5-dimethylthiazol-2,5-diphenyltetrazolium (MTT reagent, Amrecso: stock 5 g/L) was added and the cells were incubated for an additional 4 h. The optical density (570 nm) of each sample was determined with a plate reader (Bio-TEK, USA). The viability (%) was calculated according to the following formula and the IC50 value was determined by a straight line regression fit: Viability (%)=(Mean Absorbance of Sample/Mean Absorbance of Control)×100.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of mdr-1 mRNA and kinase domain sequence
Total cellular RNA was isolated from cells using Trizol reagent (Invitrogen, Carlsbad, CA) and treated with RNase-free DNase I (Promega, Madison, WI). Reverse transcription was performed with 2 μg RNA. cDNA was used as template for PCR. A long PCR method was used to amplify the ABL kinase domain of the BCR-ABL allele with forward primer BCRF (5′-TGACCAACTCGTGTGTGAAACTC-3′) and reverse primer ABLKinaseR (5′-TCCACTTCGTCTGAGATACTGGATT-3′). A second-stage PCR was performed using forward primer ABLKinaseF (5′-CGCAACAAGCCCACTGTCT-3′) and reverse primer ABLkinaseR. The entire kinase domain was sequenced along both DNA strands; the kinase domain spanned 863 bases (GenBank accession number M14752)14. mdr-1 forward primer (5′-CCCAGCATTGCAATAGCAGG-3′), reverse primer (5′-GTTCAAACTTCTGCTCCTCA-3′) and β-actin forward primer (5′-CTTCAACACCCCAGCCATGTA-3′), and reverse primer (5′-TAGAAGCATTTGCGGTGGACG-3′) were used in the reactions. All primers were synthesized by Shanghai ShenGong. β-Actin was co-amplified as an endogenous control to standardize the amount of the sample RNA added to the reaction. Amplified products were separated by electrophoresis on 1.6% agarose gels and the results were analyzed using Kodak Digital 3.0 software.
Western blot analysis
Cells were collected and washed with cold PBS. Total cellular proteins were extracted using M-PER mammalian protein extraction reagent (Pierce Chemical Co, Rockford, IL) according to the manufacturer's protocol. For analyses of cyt C, cells were fractionated as previously reported15, 16. Protein concentrations were determined by BCA Protein Assay (Pierce Chemical Co). Equal amounts of protein were subjected to SDS-PAGE (10% polyacrylamide gels) and then transferred to a PVDF membrane (BioRad Laboratories, Hercules, CA). After the protein transfer, the membrane was blocked with TBST containing 5% fat-free milk for 2 h and then reacted with primary antibodies overnight at 4 °C. After being washed with TBST three times, the membranes were probed with a horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. Immunoreactive proteins were visualized with ECL plus enhanced chemiluminescence reagents (Pierce Chemical Co).
Animal studies
All animal procedures were approved by the institution's Ethics Committee. BABL/C nu/nu mice (5–7 weeks old) were obtained from the Laboratory Animal Center of Shanghai Institute for Biology Science. All mice used in this study were bred and maintained in a specific pathogen-free environment. For this study, 5×107 K562-r cells in 0.2 mL of medium were injected subcutaneously in the midline dorsal region of nude mice on d 0. At twenty-four hours post injection, mice were randomly assigned to two groups (treated group and control group, 6 mice per group). Berbamine was administered at a dose of 60 mg/kg (in 0.3 mL) twice a day, at 8 am and 4 pm, for 3 weeks. The mice in the control group were given equal volumes of isotonic saline. The experiment was repeated two times. Tumor volumes were measured with calipers every five days. Mice were euthanized on d 30 and peripheral blood, tumors, livers, and spleens were harvested from each mouse.
Histopathology
Murine tissues were fixed for at least 72 h in 10% neutral buffered formalin (Sigma), dehydrated in alcohol, cleared in xylene, and infiltrated with paraffin. Four-micrometer-thick tissue sections from paraffin-embedded tissue blocks were placed on charged slides, deparaffinized in xylene, rehydrated through graded alcohol solutions, and stained with hematoxylin/eosin.
Statistical analysis
Unless otherwise indicated, statistical analysis of raw data was performed using Student's t test implemented in software package SPSS 12.0 for Windows (Chicago, IL). P values less than 0.05 were considered statistically significant. Each experiment was repeated at least three times. All data are expressed as the mean±SD.
Results
Berbamine inhibits K562 cell growth and restores the sensitivity of K562-r cells to imatinib
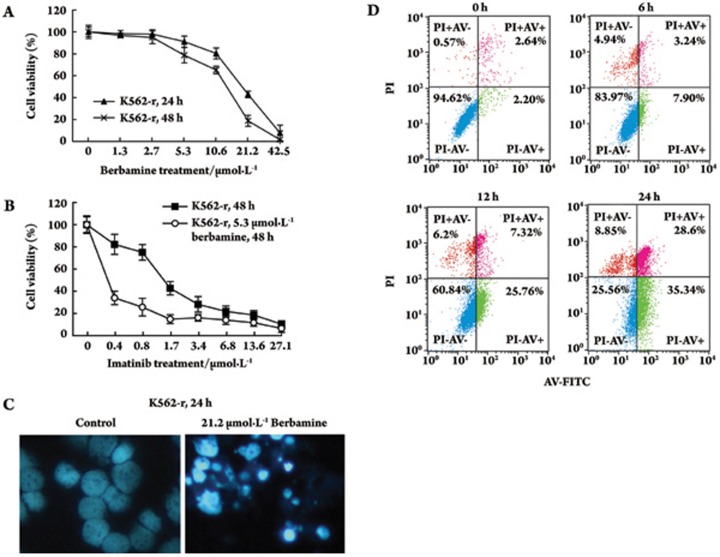
We investigated the effects of berbamine on CML cells. Leukemia cells (K562) were exposed to berbamine at different concentrations for 24 or 48 h. At each concentration, the percentage of viable K562-r cells at 48 h was lower than at 24 h (Figure 1A). The IC50 values of berbamine in K562-r cells were 17.1 μmol/L and 11.1 μmol/L for 24 h and 48 h, respectively. These data indicated that berbamine inhibited K562-r cell growth in a dose- and time-dependent manner. The IC50 of imatinib was 0.29 μmol/L against K562-s cells and 2.23 μmol/L against K562-r cells for 48 h (Figure 1B). The imatinib resistance level of K562-r cells was 7.7 times higher than that of K562-s cells. To investigate whether berbamine could restore the sensitivity of K562-r cells to imatinib, we treated K562-r cells with a combination of different concentrations of imatinib and 5.3 μmol/L berbamine (IC10 berbamine to K562-r at 48 h) for 48 h. The IC50 of imatinib was 0.35 μmol/L (Figure 1B), revealing a 6.3-fold increase in chemo-sensitivity to imatinib in berbamine-treated K562-r cells.
Figure 1.
Apoptotic effects of berbamine on K562-r cells. (A) Effects of berbamine on K562-r cell growth. Cells were incubated with different concentrations of berbamine, and the total number of viable cells was determined by MTT assay at 24 h and 48 h after treatment. (B) Effects of imatinib with or without 5.3 μmol/L berbamine on K562-r cell growth for 48 h. (C) Fluorescence photomicrographs of cells stained with Hoechst 33258 (400×). (Left panel) control; (right panel) K562-r cells treated with 21.2 μmol/L berbamine for 24 h. (D) Fluorescence-activated sorting analysis of annexin-V-FITC/PI for quantification of berbamine-induced apoptosis in K562-r cells treated with 21.2 μmol/L berbamine for 0, 6, 12, or 24 h. Error bars represent SD.
Berbamine-mediated growth inhibition is due to induction of apoptosis
To determine whether berbamine-mediated growth inhibition is associated with apoptosis, cells were treated with berbamine at 21.2 μmol/L for 24 h. Hoechst 33258 staining was used to identify changes in the cells' nuclei. Control cells showed homogeneous nuclear staining. In contrast, cells treated with berbamine exhibited typical apoptotic bleb phenomena such as irregular staining of nuclei as a result of chromatin condensation and nuclear fragmentation (Figure 1C). FCM was performed to investigate the change of apoptosis rate. The proportion of apoptotic cells increased from 2.2% to 7.9%, 25.76%, and 35.34% at 6, 12, and 24 h, respectively, following berbamine treatment (Figure 1D).
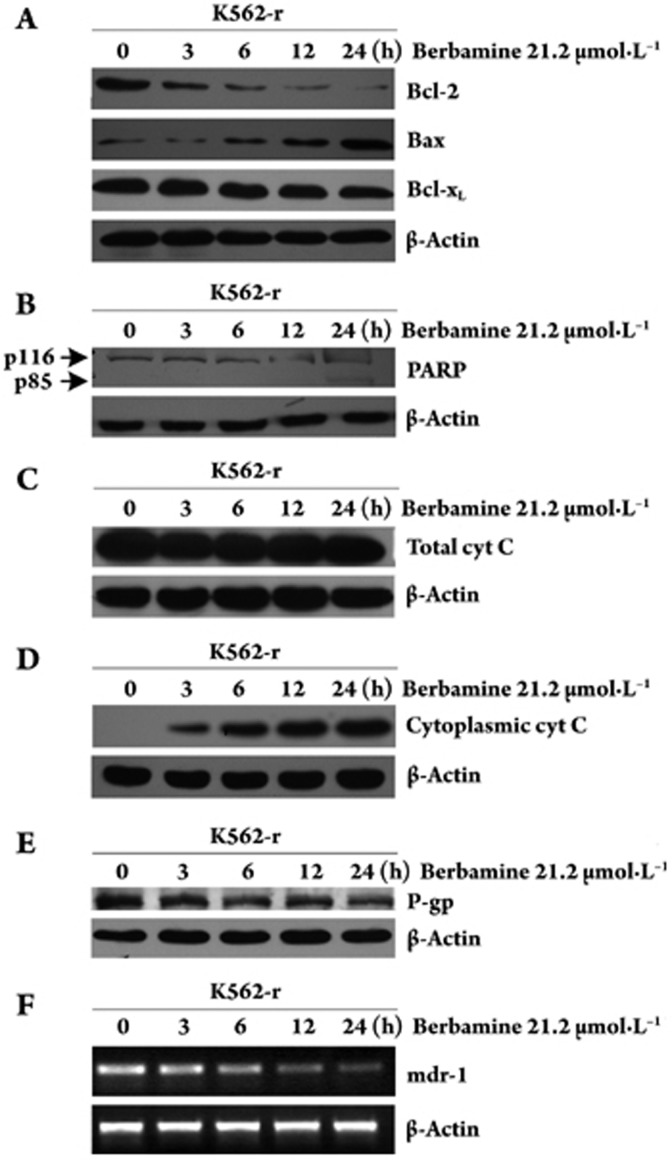
To assess whether berbamine interferes with the expression levels of proteins involved in the control of differentiation, apoptosis and drug resistance, K562-r cells were cultured for different periods in the presence of 21.2 μmol/L berbamine. Sixty micrograms of total cellular proteins was assayed for levels of Bcl-2 family proteins, cyt C, and poly ADP-ribose polymerase (PARP), which are involved in apoptosis of CML. Berbamine treatment decreased the levels of Bcl-2 and Bcl-xL and increased the levels of cytoplasmic cyt C and Bax (Figure 2A), accompanied by cleavage of PARP at 24 h (Figure 2B, 2C, 2D).
Figure 2.
K562-r cells were exposed to berbamine at 21.2 μmol/L for different time periods and Bcl-2, Bax, Bcl-xL, PARP, total cyt C, cytoplasmic cyt C, and P-glycoprotein levels (A, B, C, D, E,) were determined by Western blot. mdr-1 mRNA levels (F) were determined by RT-PCR. β-actin was used as a loading control.
Berbamine down-regulates the expression of mdr-1 mRNA and P-glycoprotein
Various mechanisms of resistance to imatinib exist, including BCR-ABL kinase domain mutations and increased imatinib efflux mediated by the multidrug resistance P-glycoprotein (P-gp). We used RT-PCR to amplify the ABL kinase domain of BCR-ABL and then sequenced the domain to identify mutations involved in K562-r cell imatinib resistance. The results demonstrated that K562-r cells had no mutations in the ATP-binding region of BCR-ABL (data not shown). We also tested the levels of mdr-1 mRNA and P-gp protein expressed in K562-r cells. Both mdr-1 mRNA and P-gp protein were highly expressed in K562-r cells and berbamine treatment significantly decreased the levels of mdr-1 mRNA and P-gp protein (Figure 2E, 2F).
Activity and toxicity of berbamine in vivo
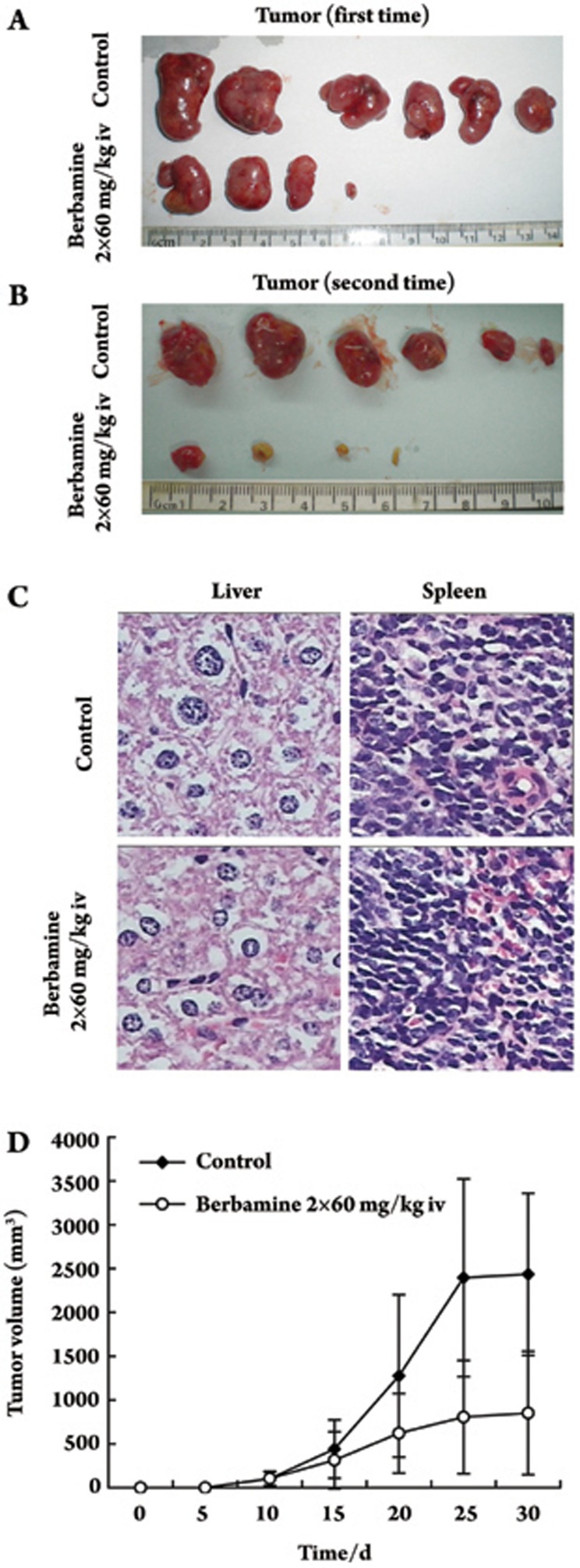
To assess the possible therapeutic activity of berbamine against K562-r cells in vivo, BALB/c nu/nu mice were treated with berbamine 24 h after subcutaneous implantation of K562-r cells. A preliminary set of tests established the dose of berbamine needed to achieve maximal therapeutic effect with tolerated side effects (data not shown). Based on these findings, we chose a dose of 60 mg/kg berbamine for experiments with mice. Due to berbamine pharmacokinetics in vivo, berbamine was excised after 6 h17. For this reason, 60 mg/kg berbamine was administered twice daily in 0.3 mL, at 8 am and 4 pm, for 3 weeks. Mice in the control group were given equal volumes of isotonic saline. The dose and schedule were tolerated. The tumor incidence within 10 days was 100% in the control group and 66% (8/12) in the treated group. In addition, tumor growth was significantly inhibited in the treated group. The mean volume of the tumors was smaller in the treated group than in the control group (P<0.05). The mean tumor weight was lower in the treated group (0.969±0.705 g) compared with the controls (2.47±1.03 g). The tumor growth inhibitory rate of the treated group was 60.43% relative to the control group (Figure 3A, 3B, 3D).
Figure 3.
Effect of berbemine on the growth of K562-r cells in vivo. Berbamine was administered iv at 60 mg/kg body weight in two daily injections (8:00 and 16:00) 24 h after the subcutaneous injection of 5×107 K562-r cells. Control mice were treated with equivalent volumes of saline instead of drug. Each experimental group contained six mice. Tumor volume at d 30, both (A) and (B); (C) liver and spleen HE staining. Results are presented as average tumor volume (D); error bars represent 95% confidence intervals and are displayed only when they exceed 5% of the respective mean.
The weight of the animals in the treated groups was similar to that of the controls. White blood cell and platelet counts were not statistically different between the two groups. Animals were also subjected to histopathologic analysis. No abnormal findings were noted in liver and spleen at necropsy (Figure 3C). The only abnormalities observed in all treated animals were phlebitis, tachypnea, and cardiopalmus, which all disappeared after 30 min.
Discussion
In the present study, we found that berbamine was able to inhibit the growth of K562-r cells and increase the chemo-sensitivity of K562-r cells to imatinib. We also found that the berbamine-mediated K562-r growth inhibition was associated with apoptosis.
Recent studies have shown that overexpression of anti-apoptosis proteins may induce cancer cell resistance to chemotherapeutic drugs18. The most studied apoptosis-related proteins are Bcl-2 family proteins, which fall into two groups that generally either repress apoptosis (Bcl-2 and Bcl-xL) or promote apoptosis (Bax, Bak, and Bad). These proteins play key roles in controlling activation of caspases, in part by regulating the release of cyt C from mitochondria19, 20, 21. Campose et al analyzed 82 samples of newly diagnosed acute myeloid leukemia and found that high levels of Bcl-2 protein expression in acute myeloid leukemia cells were associated with poor response to chemotherapy and poor survival22. Increased expression of Bcl-2, Bcl-xL, and P-gp and decreased expression of the bax gene have been reported for various drug-resistant cancer cells23, 24, 25. Ibrado et al reported that high Bcl-2 and Bcl-xL levels in HL-60/Bcl-2 and HL-60/Bcl-xL cells, which were created by transfection of the cDNA of Bcl-2 or Bcl-xL, antagonized drug-induced apoptosis26. In addition, inappropriate expression of the mdr-1 gene encoding the P-gp has been frequently implicated in the resistance to different chemotherapeutic drugs. A P-gp-dependent decline of intracellular imatinib levels was observed in a model of K562 cells with gradually increasing P-gp expression. Furthermore, decreased imatinib levels were associated with retention of the phosphorylation pattern of the BCR-ABL target Crkl and a loss of effectiveness of imatinib on cellular proliferation and apoptosis27. Rumpold et al found that P-gp-mediated resistance to imatinib and anthracyclines could be durably reversed by nonviral, transposon-based knockdown of P-gp in malignant cells to restore imatinib sensitivity in resistant CML cell lines28.
Because elevated levels of Bcl-2, Bcl-xL, and mdr-1 mRNA and its protein product P-gp were observed in K562-r cells, we were interested to learn whether the berbamine-induced apoptosis and changes in imatinib resistance of K562-r cells were also related to altered expression of Bcl-2 family proteins and the mdr-1 gene. Our results from the present study suggest that the molecular mechanisms of berbamine action in leukemia cells are associated with down-regulation of anti-apoptotic protein Bcl-2 and Bcl-xL, up-regulation of Bax, promotion of cytosolic accumulation of cyt C, and induction of proteolytic cleavage of PARP. Our previous results suggested that the mechanism of berbamine-induced apoptosis in K562-s cells was associated with activation of caspase-311, 13. In the present study we found that cleavage of endogenous PARP was apparent 24 h after berbamine treatment in K562-r cells. Because PARP is an innate substrate of caspase-3, our new findings are consistent with our previous conclusion that activation of caspase-3 is one of the key molecular events leading to berbamine-mediated K562 cell growth inhibition/apoptosis. We also found that both mdr-1 mRNA and its protein product P-gp were down-regulated in berbamine-treated K562-r cells. Taken together, it appears that multiple factors, including Bcl-2 family proteins and the mdr-1 gene, mediate the berbamine-inhibited proliferation and reversed resistance to apoptosis of K562-r cells.
In the in vivo study, the tumor incidence in the berbamine-treated group was lower than that of the control group. Furthermore, tumor growth was significantly inhibited when mice were treated with berbamine. The inhibitory rate of the treated group was 60.43% compared with the control group. Mice in the treated group did not exhibit weight loss and their white blood cell and platelet counts were not different from that of the control group. No abnormal histopathology was noted in the liver or spleen at necropsy. These findings indicate that berbamine has significant antileukemic activity with little toxicity in an animal model.
In conclusion, the present study provided evidence to show that berbamine is effective in inhibiting K562-r cell growth through induction of apoptosis. One of the underlying mechanisms of berbamine action is modulation of Bcl-2 family proteins and the mdr-1 gene. The ability of berbamine to restore the chemo-sensitivity to imatinib without obvious side effects makes this natural compound a suitable candidate to be exploited as an antileukemia agent in CML patients with drug resistance.
Author contribution
Xiao-ying ZHAO designed research; Yan-lin WEI and Lei XU performed research; Yun LIANG and Xiao-hua XU analyzed data; Yan-lin WEI and Lei XU wrote the paper
Acknowledgments
This project was supported by the Zhejiang Provincial Natural Science Foundation (No 491020-N20529).
References
- Nowell P, Hungerford D. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132:1497. doi: 10.1126/science.144.3623.1229. [DOI] [PubMed] [Google Scholar]
- Konopka JB, Watanabe SM, Witte ON. An alteration of the human c-ABL protein in K562 leukemia-cells unmasks associated tyrosine kinase-activity. Cell. 1984;37:1035–42. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Kirchner D, Duyster J, Ottmann O, Schmid RM, Bergmann L, Munzert G. Mechanisms of bcr-Abl-mediated NF-kappa B/Re1 activation. Exp Hematol. 2003;31:504–11. doi: 10.1016/s0301-472x(03)00069-9. [DOI] [PubMed] [Google Scholar]
- Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. New Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Dai Y, Rahmani M, Pei XY, Dent P, Grant S. Bortezomib and flavopiridol interact synergistically to induce apoptosis in chronic myeloid leukemia cells resistant to imatinib mesylate through both Bcr/Abl-dependent and -independent mechanisms. Blood. 2004;104:509–18. doi: 10.1182/blood-2003-12-4121. [DOI] [PubMed] [Google Scholar]
- von Bubnoff N, Veach DR, Miller WT, Li WQ, Peschel C, Bornmann WG, et al. Inhibition of wild-type and mutant Bcr-Abl by pyrido-pyrimidine-type small molecule kinase inhibitors. Cancer Res. 2003;63:6395–404. [PubMed] [Google Scholar]
- Hait WN, Lazo JS. Calmodulin — a potential target for cancer chemotherapeutic-agents. J Clin Oncol. 1986;4:994–1012. doi: 10.1200/JCO.1986.4.6.994. [DOI] [PubMed] [Google Scholar]
- Xu RZ, Dong QH, Yu YZ, Zhao XY, Gan XX, Wu D, et al. Berbamine: A novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity. Leukemia Res. 2006;30:17–23. doi: 10.1016/j.leukres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Wu D, Lin MF, Cai Z, Zhao XY. Effects of berbamine on Jurkat cells and its mechanisms in vitro and in vivo. Chin J Hematol. 2004;25:754–6. [Google Scholar]
- He ZW, Zhao XY, Xu RZ, Wu D. Effects of berbamine on growth of leukemia cell line NB4 and its mechanism. J Zhejiang Univ (Medical Sci) 2006;35:209–14. doi: 10.3785/j.issn.1008-9292.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhao XY, Wu D. Berbamine, a calmodulin antagonist, induces apoptosis in human leukemia K562 cells. Chin J Hematol. 2003;24:261–2. [Google Scholar]
- Branford S, Rudzki Z, Walsh S, Grigg A, Arthur C, Taylor K, et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood. 2002;99:3472–5. doi: 10.1182/blood.v99.9.3472. [DOI] [PubMed] [Google Scholar]
- Chen Q, Takeyama N, Brady G, Watson AJM, Dive C. Blood cells with reduced mitochondrial membrane potential and cytosolic cytochrome c can survive and maintain clonogenicity given appropriate signals to suppress apoptosis. Blood. 1998;92:4545–53. [PubMed] [Google Scholar]
- Amarante-Mendes GP, Kim CN, Liu L, Huang Y, Perkins CL, Green DR, et al. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome c and activation of caspase-3. Blood. 1998;91:1700–5. [PubMed] [Google Scholar]
- Li BX, Gao YR, Li WH. Determination of berbamine in rat plasma by reversed phase high performance liquid chromatography. J Harbin Med Univ. 1995;29:201–3. [Google Scholar]
- Minn AJ, Rudin CM, Boise LH, Thompson CB. Expression of Bcl-x(L) can confer a multidrug-resistance phenotype. Blood. 1995;86:1903–10. [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–45. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EHY, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High expression of Bcl-2 protein in acute myeloid-leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–6. [PubMed] [Google Scholar]
- Kim CN, Wang XD, Huang Y, Ibrado AM, Liu L, Fang GF, et al. Overexpression of Bcl-x (L), inhibits Ara-C-induced mitochondrial loss of cytochrome c and other perturbations that activate the molecular cascade of apoptosis. Cancer Res. 1997;57:3115–20. [PubMed] [Google Scholar]
- Baran Y, Ural AU, Gunduz U. Mechanisms of cellular resistance to imatinib in human chronic myeloid leukemia cells. Hematology. 2007;12:497–503. doi: 10.1080/10245330701384179. [DOI] [PubMed] [Google Scholar]
- Mancini M, Brusa G, Benvenuti M, Mazzacurati L, Campanini F, Barbieri E, et al. The (BCR)-B-p210-ABL tyrosine kinase of chronic myeloid leukemia causes resistance to radio-induced apoptotic death by inhibiting the proapoptotic BAX gene. Leukemia. 2004;18:370–2. doi: 10.1038/sj.leu.2403207. [DOI] [PubMed] [Google Scholar]
- Ibrado AM, Huang Y, Fang GF, Liu L, Bhalla K. Overexpression of Bcl-2 or Bcl-x(L) inhibits Ara-C-induced CPP32/Yama protease activity and apoptosis of human acute myelogenous leukemia HL-60 cells. Cancer Res. 1996;56:4743–8. [PubMed] [Google Scholar]
- Illmer T, Schaich M, Platzbecker U, Freiberg-Richter J, Oelschlagel U, von Bonin M, et al. P-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia. 2004;18:401–8. doi: 10.1038/sj.leu.2403257. [DOI] [PubMed] [Google Scholar]
- Rumpold H, Wolf AM, Gruenewald K, Gastl G, Gunsilius E, Wolf D. RNAi-mediated knockdown of P-glycoprotein using a transposon-based vector system durably restores imatinib sensitivity in imatinib-resistant CML cell lines. Exp Hematol. 2005;33:767–75. doi: 10.1016/j.exphem.2005.03.014. [DOI] [PubMed] [Google Scholar]