Abstract
Aim:
We have investigated the effects of lysophosphatidylcholine (LPC), a product of lipid peroxidation, on Aβ1–42-induced SH-SY5Y cell apoptosis.
Methods:
The viability of cultured SH-SY5Y cells was measured using a CCK-8 kit. Apoptosis was determined by Chip-based flow cytometric assay. The mRNA transcription of Bcl-2, Bax, and caspase-3 were detected by using reverse transcription and real-time quantitative PCR and the protein levels of Bax and caspase-3 were analyzed by Western blotting. The cytosolic calcium concentration of SH-SY5Y cells was tested by calcium influx assay. G2A expression in SH-SY5Y cells was silenced by small interfering RNA.
Results:
Long-term exposure of SH-SY5Y cells to LPC augmented the neurotoxicity of Aβ1–42. Furthermore, after LPC treatment, the Bax/Bcl-xL ratio and the expression levels, as well as the activity of caspase-3 were, elevated, whereas the expression level of TRAF1 was reduced. Because LPC was reported to be a specific ligand for the orphan G-protein coupled receptor, G2A, we investigated LPC-mediated changes in calcium levels in SH-SY5Y cells. Our results demonstrated that LPC can enhance the Aβ1–42-induced elevation of intracellular calcium. Interestingly, Aβ1–42 significantly increased the expression of G2A in SH-SY5Y cells, whereas knockdown of G2A using siRNA reduced the effects of LPC on Aβ1–42-induced neurotoxicity.
Conclusion:
The effects of LPC on Aβ1–42-induced apoptosis may occur through the signal pathways of the orphan G-protein coupled receptor.
Keywords: amyloid beta (1–42) peptide, lysophosphatidylcholine, neuronal apoptosis
Introduction
Alzheimer's disease (AD) is a degenerative disorder of the central nervous system that causes mental deterioration and progressive dementia. Neuropathologic lesions, including senile plaques in the brains of AD patients, accompany AD. The β-amyloid protein (Aβ), a hydrophobic 39 to 43 residue peptide, is the major component of senile plaques. Much accumulated evidence shows that Aβ fibrillar deposition in senile plaques correlates with the progression of cognitive dysfunction in AD patients. Furthermore, Aβ exhibits neurotoxicity both in vitro and in vivo1, 2. Recent studies have demonstrated that the exposure of cultured neurons to Aβ results in neuronal apoptosis3, 4.
Abnormal brain phospholipid metabolism is a feature of AD5, 6. Products of lipid peroxidation occur early in the progression of AD. Phosphatidylcholine (PC) is the major phospholipid of eukaryotic membranes7. The pathological breakdown of phospholipids by oxidation leads to the production of lysophosphatidylcholine (LPC). It has been reported that Aβ1–42 causes lipid peroxidation8. Current evidence supports the notion that Aβ-induced lipid peroxidation may in part account for neurodegeneration in the AD brain.
The biological effects and signaling properties of LPC have been extensively studied in atherosclerosis-related cells9. LPC can induce neuronal sheath demyelination, axonal degeneration and neuronal apoptosis10, 11, which are common pathological characteristics of neurodegenerative diseases12, 13. However, the physiological role of LPC in neuronal cells is not yet fully understood. Because LPC was recently shown to be the agonist for an orphan G protein-coupled receptor (GPCR)14, G2A, it will be interesting to determine whether the effects of LPC on Aβ1–42-induced neuronal apoptosis occur through signal pathways of the orphan GPCR.
In this study, we evaluated the effects of LPC on the neurotoxicity of Aβ1–42 and examined the expression of several apoptosis-related genes in SH-SY5Y cells. In addition, we analyzed second messenger systems, including intracellular concentrations of calcium, in SH-SY5Y cells after LPC treatment. We found that the orphan G-protein receptor G2A may be involved in LPC effects on neuronal apoptosis. We discuss possible molecular mechanisms underlying the effects of LPC on Aβ1–42-induced neuronal apoptosis.
Materials and methods
Cell culture and drug treatment
Human neuroblastoma SH-SY5Y cells (ATCC, USA) were routinely cultured in DMEM medium with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin at 37 °C. All media and sera were purchased from Invitrogen Inc. Aβ1–42 peptide (CPC Scientific Inc, USA) was dissolved in distilled water at a concentration of 1 mmol/L and stored at -20 °C. The peptide stock solution was diluted to the desired concentrations and maintained for 3 days at 37 °C before use. Lysophosphatidylcholine (LPC) was purchased from Avanti Polar Lipids (Alabaster, AL) and dissolved in DMSO to make a 20 mmol/L stock solution. SH-SY5Y cells were treated with different concentrations of Aβ1–42 and LPC, as indicated in the figure legends.
Proliferation analysis of SH-SY5Y cells
SH-SY5Y cells were grown overnight in a 96-well plate (5×104 cells/mL). Different concentrations of Aβ1–42 and LPC in Opti-MEM I medium were added to the cells and incubated for 48 h. Cell viability was measured using a CCK-8 kit according to the manufacturer's instruction (Dojindo Laboratories, Japan). Briefly, 10 μL of CCK-8 solution was added to each well and incubated for 30 min at 37 °C. The absorbance was measured at 450 nm with a GENios microplate reader (Tecan, Austria).
Chip-based flow cytometric assay
SH-SY5Y cells were treated with 10 μmol/L Aβ1–42 in Opti-MEM I medium for 48 h and then harvested to the density of 1×106 cells/mL. An Annexin V FITC Apoptosis Detection Kit (EMD Chemicals Inc, Germany) was used to measure apoptosis according to the manufacturer's protocol. Briefly, 1.25 μL of Annexin V was added in a 500 μL cell suspension and incubated for 10 min at room temperature. The cells were centrifuged and resuspended in 500 μL of 1×binding buffer containing 2 μg/mL Fluorolink-Cy5 streptavidin (Amersham Biosciences, UK) and 1 μmol/L Calcein-AM (Invitrogen Inc., USA). The samples were incubated for another 10 min at room temperature. After centrifugation, the cells were resuspended again in 100 μL of cell buffer (in Cell Fluorescence LabChip Kit) by gentle pipetting. The cell suspension (10 μL) was added into the cell chip and analyzed by the Agilent 2100 Bioanalyzer.
Reverse transcription and real-time quantitative PCR
SH-SY5Y cells (1×105) were treated with 5 or 10 μmol/L Aβ1–42 and 10 μmol/L LPC in Opti-MEM I medium for 48 h in a 6-well plate. Total RNA was extracted with a Trizol reagent (Invitrogen Inc., USA) based on the manufacturer's instructions. RNA was eluted in 20 μL of nuclease-free water and stored at −70 °C before use. cDNA was synthesized using the M-MLV Reverse Transcriptase (Invitrogen Inc, USA) and oligo(dT) primers (Takara Bio Inc, Shiga, Japan). Three μL of mRNA was incubated with 1 μL of oligo(dT) primer, 1 μL of 10 mmol/L dNTPs (Promega, CA, USA) and 7 μL of nuclease-free water for 5 min at 65 °C. The reaction tubes were immediately placed on ice and a mixture solution (4 μL of 5×first-strand buffer, 2 μL DTT, 1 μL of RNAase-out) was added. The reverse transcription was continued by the addition of 1 μL of M-MLV reverse transcriptase and incubation at 37 °C for 50 min. Finally, the reaction was incubated at 70 °C for 15 min. For fluorescence real-time quantitative RT-PCR, β-actin was used as the reference gene. Primers were designed using Primerexpress software and synthesized by Invitrogen Inc (Table 1). Real-time PCR was carried out in 2 μL of PCR buffer, 0.4 μL of 10 μmol/L sense and antisense primers, 0.4 μL of 10 mmol/L deoxyribonucleotides (dNTPs), 0.5 μL of first-strand cDNA, 0.2 μL of SYBR Green and Taq polymerase (Promega, CA, USA); distilled water was added to a total volume of 20 μL. The reaction was incubated at 94 °C for 5 min, followed by 34 cycles at 94 °C for 1 min; 52 °C for 30 s; and 72 °C for 30 s and finally kept at 72 °C for 5 min using the DNA Engine Opticon 2 system (Bio-Rad, USA). At the end of the reaction, a melting curve (disassociation curve) was run to ensure that only a single specific product was amplified. All reactions were performed in triplicate. Relative transcript quantities were calculated and presented with 2-ΔΔCt values.
Table 1. The primers used for quantitative PCR to detect the expression of apoptosis-related genes and orphan GPCR G2A.
| Gene name | Genebank cDNA accession number | Primer sequence |
|---|---|---|
| Bax | NM_004324 | Forward: 5′ TTTGCTTCAGGGTTTCATCC 3′ |
| Reverse: 5′ GCCACTCGGAAAAAGACCTC 3′ | ||
| Bcl-xL | NM_138578 | Forward: 5′ ATGAACTCTTCCGGGATGG 3′ |
| Reverse: 5′ TGGATCCAAGGCTCTAGGTG 3′ | ||
| Caspase-3 | NM_001167 | Forward: 5′ TGGAACAAATGGACCTG 3′ |
| Reverse: 5′ ACCACGGCAGGCCTGA 3′ | ||
| TRAF1 | NM_005658 | Forward: 5′ GTGTCGGCTGCTCCTTCAA 3′ |
| Reverse: 5′ CAAACACACGCAGCTTCCC 3′ | ||
| G2A | NM_013345 | Forward: 5′ CGCCAAGAAGTGTCCAGAATC 3′ |
| Reverse: 5′ CCTCAATCAGCCTCTTTGCAG 3′ | ||
| Beta-actin | NM_001101 | Forward: 5′ GGACATCCGCAAAGACCTGTA 3′ |
| Reverse: 5′ ACATCTGCTGGAAGGTGGACA 3′ |
Western blot analysis
SH-SY5Y cells (1×105) were treated with 10 μmol/L Aβ1–42 and 10 μmol/L LPC in Opti-MEM I medium for 48 h in a 6-well plate. Cells were homogenized in a cell lysis buffer (Beyotime, Jiangsu, China). After the proteins were quantified in the homogenates, an equal amount of protein (20 μg/well) from each sample was boiled for 5 min in loading buffer (5% mercaptoethanol, 0.05% bromphenol blue, 75 mmol/L Tris-HCl, pH 6.8, 2% SDS, and 10% glycerol). Proteins were separated by 7.5% SDS-PAGE and transferred to PVDF membranes (Millipore, MA,USA) in a glycine/methanol transfer buffer (20 mmol/L Tris base, 0.15 mol/L glycine, and 20% methanol) using the Trans-Blot SD semidry transfer cell system (BioRad, CA, USA). After being blocked with 5% nonfat dried milk in 1×TBS-T buffer (20 mmol/L Tris-HCl, pH 7.5, 137 mmol/L NaCl and 0.05% Tween-20) for 1 h at room temperature, membranes were probed with primary antibodies (rabbit anti-Bax, rabbit anti-cleaved caspase-3, or rabbit anti-β-actin, Cell Signaling Technology, MA, USA) at a 1:1000 dilution in 1×TBS-T buffer, pH 7.6 at 4 °C overnight. The blots were washed and incubated with anti-rabbit secondary antibodies (1:2000) for 1 h at room temperature. Chemiluminescent detection was performed using an ECL Western blotting Detection kit from Amersham Pharmacia (Buckinghamshire, UK).
Caspase-3 activity assay
Caspase-3 activity was measured using a caspase-3/CPP32 colorimetric assay kit according to the manufacturer's protocol (Calbiochem, Darmstadt, Germany). Briefly, cells were treated with 10 μmol/L Aβ1–42 and 10 μmol/L LPC in Opti-MEM I medium for 48 h in a 6-well plate, washed with PBS and suspended in lysis buffer. The lysates were centrifuged for 5 min at 14000×g and the supernatant was stored at −80 °C before use. Protein concentrations were measured by the BCA method. Lysates (20 μg of protein) were incubated at 37 °C for 2 h in 2 mmol/L Ac-DEVD-pNA substrate and caspase-3 activity was measured using an optimal microplate reader (Tecan GENios, Switzerland), in which the substrate cleavage was monitored at 405 nm.
Calcium influx assay
The cytosolic calcium concentration of SH-SY5Y cells was measured using a Flexstation imaging plate reader (Molecular Devices Corporation, USA). Cells (1.5×105cells/mL) were seeded into 96-well black plates with clear bottoms coated with poly-D-lysine, cultured overnight, and then incubated with a FLIPR Calcium 4 Assay Kit (Molecular Devices Corporation, USA) for 1 h at 37 °C. Fluorescence signals (λEX=488 nm, λEM=540 nm) were measured before and after the addition of Aβ1–42 (10 μmol/L), either alone or together with LPC (10 μmol/L). To examine the source of calcium flux induced by Aβ1–42, cells were treated with 1 μmol/L of thapsigargin (TG) for 1 h before the addition of Aβ1–42. The total fluorescence units within 200 s were calculated.
Reduction of G2A expression in SH-SY5Y cells by small interfering RNA
SH-SY5Y cells grown to 50% confluency were transfected with G2A siRNA duplexes (5′-CAACGUGUCCUUCGAAGAGtt-3′, 5′-CUCUUCGAAGGACACGUUGtt-3′) and scrambled siRNA duplexes (5′-UUCUCCGAACGUGUCACGUtt-3′, 5′-ACGUGACACGUUCGGAGAAtt-3′) (GenePharma, Shanghai, China) using the TransFast™ Transfection Reagent (Promega, CA, USA). Cells were transfected for 48 h with 3.75 μg of each siRNA according to the manufacturer's protocol (Promega, CA, USA). Efficiency of RNA interference was assessed by real-time quantitative PCR. The cultures were then treated with Aβ1–42 (10 μmol/L) and/or LPC (0, 2, 10, and 50 μmol/L) and subjected to cell viability analyses.
Statistical analysis
Data were calculated as the mean±SEM by Sigmaplot 9.0 (Systat Software Inc, USA). A Student's t-test was applied to determine the statistical significance. Statistical significances are shown as bP<0.05 and cP<0.01.
Results
Effects of LPC on Aβ1–42-induced neuronal cell death
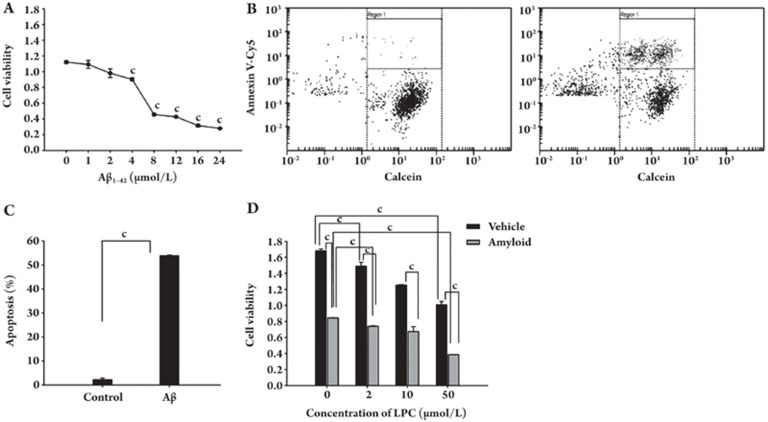
SH-SY5Y cells were treated with Aβ1–42 and cell viability was determined by the CCK-8 assay. Figure 1A shows that Aβ1–42 treatment significantly reduced the viability of SH-SY5Y cells in a dose-dependent manner. Annexin V, a calcium-dependent phospholipid-binding protein, has a high affinity for phosphatidylserine (PS) and binds to PS exposed on the surface of apoptotic cells. We applied the chip-based flow cytometric assay to analyze PS externalization induced by Aβ1–42 in SH-SY5Y cells. Annexin V-Cy5 and Calcein-AM staining showed that the number of apoptotic cells increased significantly after Aβ1–42 treatment (Figure 1B–1D). To examine the effect of LPC on Aβ1–42-induced cell death in SH-SY5Y cells, we treated SH-SY5Y cells with LPC at different concentrations. Our results showed that LPC inhibited the growth rate of SH-SY5Y cells in a dose-dependent manner. Furthermore, LPC greatly enhanced Aβ1–42-induced growth inhibition (Figure 1E).
Figure 1.
Effects of LPC on Aβ1–42 neurotoxicity in SH-SY5Y cells. (A) Aβ1–42 induced growth inhibition in a dose-dependent manner. (B–D) Aβ1–42 mediated apoptosis in SH-SY5Y cells. (B) Apoptosis measured by Annexin V-Cy5/Calcein staining, dot plot of control SH-SY5Y cells; (C) Apoptosis measured by Annexin V-Cy5/Calcein staining, dot plot of SH-SY5Y cells after exposure to 10 μmol/L Aβ1–42 for 48 h; (D) The percentage of Annexin V-Cy5-stained cells was calculated with respect to the total number of Calcein stained cells in a field. (E) LPC enhancement on Aβ1–42-induced growth inhibition. SH-SY5Y cells were treated with different concentrations of LPC (0, 2, 10, and 50 μmol/L) and Aβ1–42 (10 μmol/L) for 48 h. Cell viability was measured as described under Materials and Methods. Data are expressed as means±SEM from three independent experiments.
Regulation of gene expression via the Aβ1–42 pathway
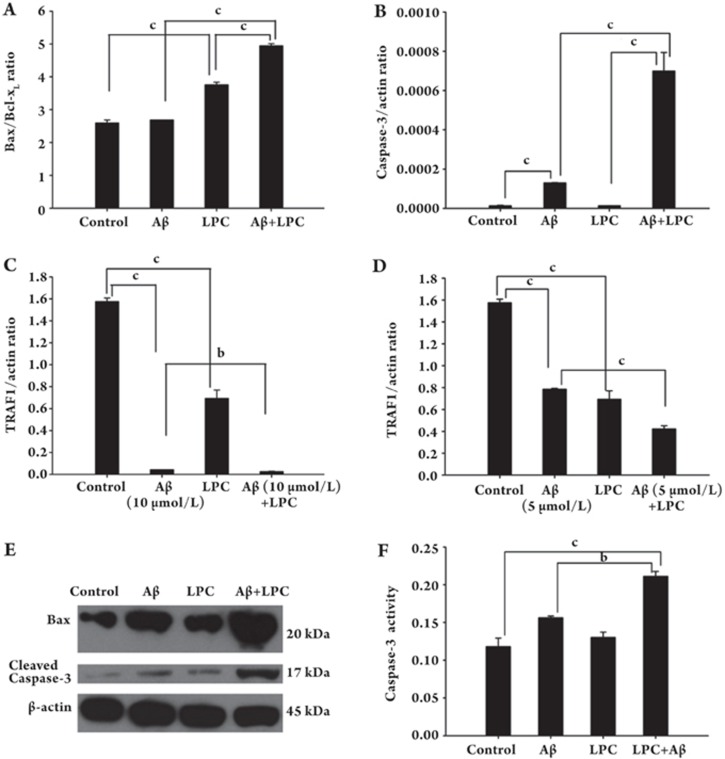
To understand the molecular mechanisms involved in Aβ1–42-induced neuronal apoptosis, we analyzed the expression of a number of apoptosis-related genes in SH-SY5Y cells with or without Aβ1–42 treatment. First, we examined the mRNA levels of apoptosis associated genes using a quantitative PCR method. Our results showed that Aβ1–42 increased the ratio of Bax/Bcl-xL (Figure 2A) mRNAs and the expression of caspase-3 (Figure 2B), whereas it decreased the expression of TRAF1 in SH-SY5Y cells after Aβ1–42 treatment (Figure 2C and 2D).
Figure 2.
Effects of LPC and Aβ1–42 on the expression of Bax, caspase-3 and TRAF1, as well as the enzymatic activity of caspase-3. SH-SY5Y cells were treated with Aβ1–42 (5 or 10 μmol/L) and/or LPC (10 μmol/L) for 48 h. Quantitative RT-PCR analysis was performed to examine the mRNA expression of Bax, caspase-3 and TRAF1 (A–D). (E) Western blotting for determination of levels of Bax and caspase-3 using various antibodies as described under Materials and Methods. (F) caspase-3 activity in Aβ1–42 and LPC treated SH-SY5Y cells. Caspase-3 activity was determined as described under Materials and Methods. Data were expressed as means±SEM from three independent experiments.
Alterations in the protein levels of Bax and caspase-3 were analyzed by Western blotting (Figure 2E) using antibodies against Bax and cleaved caspase-3 (the latter antibody recognizes the p17 large/active subunit of caspase-3). As expected, treatment with Aβ1–42 or LPC increased Bax expression. In addition, LPC enhanced the effects of Aβ1–42 on the expression of Bax and the p17 cleavage product of caspase-3 (Figure 2E).
To assess whether the observed increase in the cleavage of caspase-3 correlated with an increase in its activity, the cleavage of Ac-DEVD-pNA, a colorimetric peptide substrate specific for caspase-3, was measured. As shown in Figure 2F, caspase-3 cleavage activity was induced in SH-SY5Y cells in the presence of Aβ, and the addition of LPC further increased this activity.
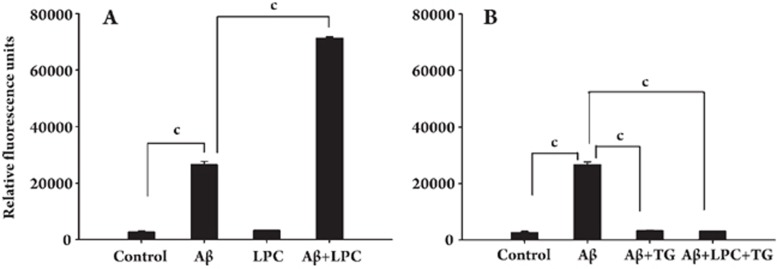
Effects of Aβ1–42 and LPC on calcium flux
Activation of a G2A receptor with LPC can induce calcium mobilization15. We investigated whether LPC affects Aβ1–42-induced calcium flux in SH-SY5Y cells. Although LPC alone had no effect on calcium influx, it significantly enhanced the Aβ1–42-induced elevation of intracellular calcium in SH-SY5Y cells (Figure 3A). We also found that Aβ1–42 treatment alone could induce calcium influx in SH-SY5Y cells and that this activity was blocked by pretreatment of cells with 1 μmol/L of TG, a Ca2+-ATPase inhibitor (Figure 3B).
Figure 3.
Effects of LPC and Aβ1–42 on calcium levels in SH-SY5Y cells. Calcium levels were measured as described under Materials and Methods. (A) LPC (10 μmol/L) augmented the effect of Aβ1–42 (10 μmol/L) on calcium levels. (B) After depletion of the Ca2+ store by the addition of TG (1 μmol/L) for 1 h, Aβ1–42 could not change the cytosolic calcium concentration. Data were expressed as means±SEM from three independent experiments.
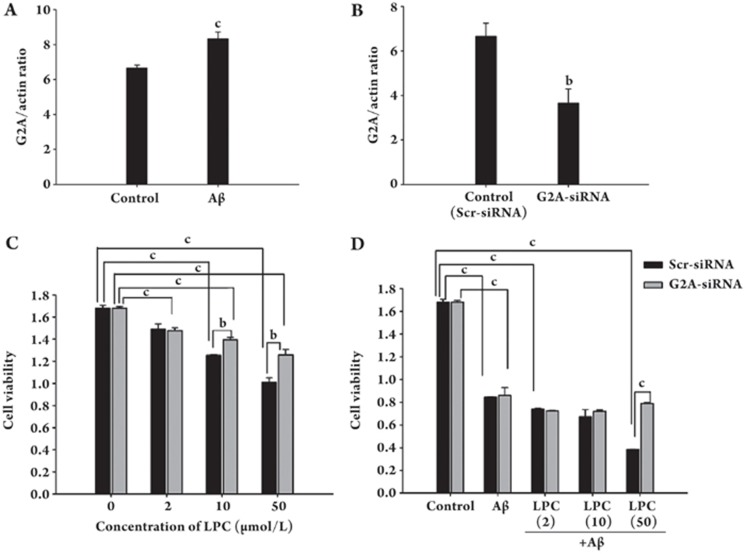
Reduction of G2A inhibits the effects of LPC on Aβ1–42-induced neurotoxicity
Because LPC enhanced Aβ1–42-induced neuronal apoptosis and was the specific ligand for an orphan GPCR, G2A, we analyzed the expression of this receptor. Our results demonstrated that LPC significantly induced expression of G2A (Figure 4A). To further examine the involvement of G2A in LPC effects on Aβ1–42-induced neuronal apoptosis, we used siRNA to reduce the expression of the orphan GPCR. Transfection of SH-SY5Y cells with G2A-specific siRNA resulted in a 55% inhibition of G2A expression (Figure 4B). Furthermore, the down-regulation of G2A expression reduced the effects of LPC on Aβ-induced neurotoxicity (Figures 4C and 4D).
Figure 4.
Reduction of G2A expression blocked LPC effects on Aβ1–42-induced neurotoxicity. (A) G2A mRNA expression in SH-SY5Y cells after the treatment with Aβ1–42 (10 μmol/L). (B) G2A mRNA expression in SH-SY5Y cells after transfected with siRNA specific for G2A (“G2A-siRNA”) or scrambled siRNA (“Scr-siRNA”) as the control. Expression of G2A was normalized to β-actin in each sample. Relative G2A expression was calculated as described under Materials and Methods. (C) The reduction of cell viability in SH-SY5Y cells exposed to LPC after transfection with siRNA. Following transfection with siRNA, the cells were incubated with different concentrations of LPC (0, 2, 10, and 50 μmol/L) for 48 h and cell viability was determined using a CCK-8 kit. (D) The cell viability in SH-SY5Y cells exposed to LPC and/or Aβ1–42 after transfection with siRNA. Following transfection with siRNA, the cells were incubated with different concentrations of LPC (0, 2, 10, and 50 μmol/L) and Aβ1–42 (10 μmol/L) for 48 h. Cell viability was determined using a CCK-8 kit. Data are expressed as means±SEM from three independent experiments.
Discussion
Aβ peptide, the main component of senile plaque, plays a central role in AD pathogenesis17. Oxidative stress increased the production of Aβ peptides in neuronal cell lines and in AD animal models18, 19, 20. Genetic and biochemical analysis has demonstrated that Aβ may be a causative factor both in neuronal apoptosis and in the cognitive impairments seen in AD patients21, 22. Furthermore, much recent evidence indicates that Aβ may act as an initiating factor, inducing specific signaling pathways leading to neuronal apoptosis23, 24, 25, 26, 27, 28. However, the molecular targets of Aβ effects remain unidentified.
Lipid peroxidation is a predominant manifestation of oxidative stress in the central nervous system (CNS)16. Accumulating data suggests that increased lipid peroxidation is an early event in the pathogenesis of AD. In this study, we used SH-SY5Y cells as a cellular model to examine the molecular basis of Aβ1–42-induced neuronal apoptosis and to address a possible role for lipid peroxidation in amyloid neurotoxicity. For the latter, we examined the effect of LPC, a product of lipid peroxidation, on Aβ1–42-induced apoptosis. As reported, Aβ1–42 decreased cell viability in a dose-dependent manner and effectively induced apoptosis in SH-SY5Y cells. We further found that Aβ1–42 regulated the expression of a number of apoptosis associated genes and that treatment of SH-SY5Y cells with LPC not only inhibited the proliferation of SH-SY5Y cells, but also enhanced Aβ1–42-induced neuronal apoptosis.
Apoptosis is a tightly regulated process involving changes in the expression of a distinct set of genes29. A tumor necrosis factor death receptor (TNF) signaling cascade is required for Aβ-induced neuronal death30. TNF receptor-associated factor 1 (TRAF1) participates in the inhibition of apoptosis, possibly via recruitment of anti-apoptotic factors, such as cIAP1 and cIAP2, into death receptor signaling complexes31. Interestingly, we found that both Aβ1–42 and LPC could decrease the expression of TRAF1 in SH-SY5Y cells and that LPC enhanced the effect of Aβ1–42 on TRAF1 mRNA expression. Caspases, including caspase-132, caspase-223, caspase-333, caspase-834, and caspase-1224, are involved in the pathogenesis of Aβ toxicity in vitro. Because caspase-3 is a critical downstream protease in the apoptotic cascade, we examined its expression in SH-SY5Y cells after Aβ1–42 treatment. Our results demonstrated that Aβ1–42 significantly increased the expression of caspase-3. Neuronal apoptosis induced by Aβ is related to Bax, a proapoptotic Bcl-2 family member, or Bcl-xL, an antiapoptotic analogue35, 36. Aβ1–42 also increased the mitochondrial ratio of Bax to Bcl-xL in SH-SY5Y cells, and LPC augmented the Aβ1–42-induced elevation of caspase-3 and elevated Bax/Bcl-xL ratio at both the mRNA and the protein levels. In agreement with the expression analysis results, LPC also increased the effect of Aβ1–42 on the enzymatic activity of caspase-3.
One possible explanation for the effects of LPC on neuronal apoptosis is that LPC binds to its specific receptor and performs its physiological and pathological functions via a downstream second messenger system. Many studies have demonstrated that LPC is a high-affinity ligand for G2A15. Interestingly, we found that Aβ1–42 treatment significantly induced the expression of G2A in SH-SY5Y cells. Deregulation of intracellular calcium signaling is implicated in the pathogenesis of Alzheimer's disease37. The activation of a G2A receptor through LPC could induce calcium mobilization15. Indeed, our observation that the elevation in intracellular calcium levels caused by Aβ1–42 could be blocked by TG suggests that the elevation of calcium levels was from intracellular calcium stores. Our results further showed that LPC treatment alone had no effect on intracellular calcium concentration, but could enhance the elevation of Aβ1–42-induced calcium concentration in SH-SY5Y cells. Furthermore, our observation that G2A siRNA blocks the LPC effect suggests that the orphan GPCR may be involved in calcium elevation and Aβ1–42-induced neurotoxicity. These results provide evidence that LPC may exert its neurotoxic effects through orphan GPCR signal pathways. We propose further studies to help elucidate the roles of G2A in neurodegenerative phenotypes in the AD brain.
Author contribution
Ying-he HU and Zhen-xia QIN designed research and wrote the paper; Zhen-xia QIN performed research and analyzed data; Hui-yan ZHU contributed new analytical tools and reagents.
Acknowledgments
This work was supported by grants from Ministry of Science and Technology of China (“973 project” 2003CB716601; “863 project” 2007AA02Z163), the Shanghai Association for Science and Technology, and the Shanghai Municipal Education Commission.
References
- Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA. Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat Med. 1998;4:827–31. doi: 10.1038/nm0798-827. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, et al. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–84. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Bushnell AF, Lee CM, Perlmutter LS, Wong SK. Beta-amyloid induces apoptosis in human-derived neurotypic SH-SY5Y cells. Brain Res. 1996;738:196–204. doi: 10.1016/s0006-8993(96)00733-0. [DOI] [PubMed] [Google Scholar]
- Estus S, Tucker HM, van Rooyen C, Wright S, Brigham EF, Wogulis M, et al. Aggregated amyloid-beta protein induces cortical neuronal apoptosis and concomitant “apoptotic” pattern of gene induction. J Neurosci. 1997;17:7736–45. doi: 10.1523/JNEUROSCI.17-20-07736.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdon JH, Wurtman RJ. Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci USA. 1992;89:1671–5. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusztajn JK, Lopez Gonzalez-Coviella I, Logue M, Growdon JH, Wurtman RJ. Levels of phospholipid catabolic intermediates, glycerophosphocholine and glycerophosphoethanolamine, are elevated in brains of Alzheimer's disease but not of Down's syndrome patients. Brain Res. 1990;536:240–4. doi: 10.1016/0006-8993(90)90030-f. [DOI] [PubMed] [Google Scholar]
- Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm. 2000;107:1027–63. doi: 10.1007/s007020070051. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–64. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kobayashi T, Kamata K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem. 2007;14:3209–20. doi: 10.2174/092986707782793899. [DOI] [PubMed] [Google Scholar]
- Hall SM. The effect of injections of lysophosphatidyl choline into white matter of the adult mouse spinal cord. J Cell Sci. 1972;10:535–46. doi: 10.1242/jcs.10.2.535. [DOI] [PubMed] [Google Scholar]
- Jean I, Allamargot C, Barthelaix-Pouplard A, Fressinaud C. Axonal lesions and PDGF-enhanced remyelination in the rat corpus callosum after lysolecithin demyelination. Neuroreport. 2002;13:627–31. doi: 10.1097/00001756-200204160-00018. [DOI] [PubMed] [Google Scholar]
- Kostrzewa RM, Segura-Aguilar J. Novel mechanisms and approaches in the study of neurodegeneration and neuroprotection. a review. Neurotox Res. 2003;5:375–83. doi: 10.1007/BF03033166. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Alonso Adel C, El-Akkad E, Gong CX, Haque N, Khatoon S, et al. Alzheimer neurofibrillary degeneration: therapeutic targets and high-throughput assays. J Mol Neurosci. 2003;20:425–9. doi: 10.1385/jmn:20:3:425. [DOI] [PubMed] [Google Scholar]
- Lin P, Ye RD. The lysophospholipid receptor G2A activates a specific combination of G proteins and promotes apoptosis. J Biol Chem. 2003;278:14379–86. doi: 10.1074/jbc.M209101200. [DOI] [PubMed] [Google Scholar]
- Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–5. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Misonou H, Morishima-Kawashima M, Ihara Y. Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochemistry. 2000;39:6951–9. doi: 10.1021/bi000169p. [DOI] [PubMed] [Google Scholar]
- Paola D, Domenicotti C, Nitti M, Vitali A, Borghi R, Cottalasso D, et al. Oxidative stress induces increase in intracellular amyloid beta-protein production and selective activation of betaI and betaII PKCs in NT2 cells. Biochem Biophys Res Commun. 2000;268:642–6. doi: 10.1006/bbrc.2000.2164. [DOI] [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, et al. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem. 2004;89:1308–12. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genotypes, phenotypes, and treatments. Science. 1997;275:630–1. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–7. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Friedman WJ, Frappier TF, Brown K, Shelanski ML. Caspase-2 mediates neuronal cell death induced by beta-amyloid. J Neurosci. 2000;20:1386–92. doi: 10.1523/JNEUROSCI.20-04-01386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- LeBlanc A, Liu H, Goodyer C, Bergeron C, Hammond J. Caspase-6 role in apoptosis of human neurons, amyloidogenesis, and Alzheimer's disease. J Biol Chem. 1999;274:23426–36. doi: 10.1074/jbc.274.33.23426. [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Ivins JK, Sharp JP, Cotman CW. Multiple pathways of apoptosis in PC12 cells. CrmA inhibits apoptosis induced by beta-amyloid. J Biol Chem. 1999;274:2107–12. doi: 10.1074/jbc.274.4.2107. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Tinkle BT, Bieberich CJ, Haudenschild CC, Jay G. The Alzheimer's A beta peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat Genet. 1995;9:21–30. doi: 10.1038/ng0195-21. [DOI] [PubMed] [Google Scholar]
- Watt JA, Pike CJ, Walencewicz-Wasserman AJ, Cotman CW. Ultrastructural analysis of beta-amyloid-induced apoptosis in cultured hippocampal neurons. Brain Res. 1994;661:147–56. doi: 10.1016/0006-8993(94)91191-6. [DOI] [PubMed] [Google Scholar]
- Jang JH, Aruoma OI, Jen LS, Chung HY, Surh YJ. Ergothioneine rescues PC12 cells from beta-amyloid-induced apoptotic death. Free Radic Biol Med. 2004;36:288–99. doi: 10.1016/j.freeradbiomed.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Li R, Yang L, Lindholm K, Konishi Y, Yue X, Hampel H, et al. Tumor necrosis factor death receptor signaling cascade is required for amyloid-beta protein-induced neuron death. J Neurosci. 2004;24:1760–71. doi: 10.1523/JNEUROSCI.4580-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–3. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Jordan J, Galindo MF, Miller RJ. Role of calpain- and interleukin-1 beta converting enzyme-like proteases in the beta-amyloid-induced death of rat hippocampal neurons in culture. J Neurochem. 1997;68:1612–21. doi: 10.1046/j.1471-4159.1997.68041612.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Partin J, Begley JG. Amyloid beta-peptide induces apoptosis-related events in synapses and dendrites. Brain Res. 1998;807:167–76. doi: 10.1016/s0006-8993(98)00763-x. [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Thornton PL, Rohn TT, Cotman CW. Neuronal apoptosis induced by beta-amyloid is mediated by caspase-8. Neurobiol Dis. 1999;6:440–9. doi: 10.1006/nbdi.1999.0268. [DOI] [PubMed] [Google Scholar]
- Qin Z, Sun Z, Huang J, Hu Y, Wu Z, Mei B. Mutated recombinant human glucagon-like peptide-1 protects SH-SY5Y cells from apoptosis induced by amyloid-beta peptide (1–42) Neurosci Lett. 2008;444:217–21. doi: 10.1016/j.neulet.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Placzek A, Kundtz A, Yu H, Mullan M. Bcl-XL inhibits apoptosis and necrosis produced by Alzheimer's beta-amyloid1-40 peptide in PC12 cells. Neurosci Lett. 1999;272:5–8. doi: 10.1016/s0304-3940(99)00525-x. [DOI] [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002;3:862–72. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]