Abstract
Aim:
To evaluate the potential drug-drug interactions between bifendate and cyclosporine, a substrate of CYP3A4, in relation to different CYP3A4*18B genotype groups.
Methods:
Eighteen unrelated healthy subjects (six CYP3A4*1*1, six CYP3A4*1/*18B, and six CYP3A4*18/*18B) were selected for this study. After repeated oral administration of a placebo or bifendate (three times daily for 14 d), the whole-blood level of cyclosporine was measured using high performance liquid chromatography-electrospray mass spectrometry (HPLC/ESI-MS). This study was carried out in a two-phase randomized crossover manner.
Results:
After the treatment with bifendate, the areas under the curve (AUC0–24 and AUC0–∞) decreased significantly by 9.7%±3.7% (P=0.01) and 19.2%±16.8% (P=0.001) in CYP3A4*1/*1 subjects, 11.3%±9.4% (P=0.03) and 10.5%±9.6% (P=0.043) in CYP3A4*1/*18B subjects, and 40.2%±14.7% (P=0.02) and 37.5%±15.8% (P=0.003) in CYP3A4*18B/*18B subjects. Meanwhile, the decreases in the AUC0–24 and AUC0–∞ values in the three groups were significantly different (using one-way analysis of variance, P=0.001 and P=0.001), and the change in the CYP3A4*18B/*18B group was greater than that in the other two groups. The oral clearance of cyclosporine was altered in all the subjects, with substantial increases by 10.2%±4.4% (P=0.004) in CYP3A4*1/*1 subjects, 14.0%±12.0% (P=0.048) in CYP3A4*1/*18B subjects, and 32.4%±21.7% (P=0.013) in CYP3A4*18B/*18B subjects.
Conclusion:
These results suggest that bifendate decreases the plasma concentration of cyclosporine in a CYP3A4 genotype-dependent manner.
Keywords: bifendate, cyclosporine, pharmacokinetics, CYP3A4*18B
Introduction
Bifendate (4,4′-dimethoxy-5,6,5′,6′-bi(methylenedioxy)-2,2′-bicabomethoxybipheny1) is a synthetic intermediate of schisandrin C, which is widely used to lower the levels of alanine transaminase (ALT) for the treatment of chronic hepatitis. Following the intake of bifendate in rats, the drug was observed to improve liver function by increasing the detoxification process, reducing pathological lesions, and accelerating hepatocyte regeneration. Bifendate can also function as a membrane-stabilizing agent to protect the cell from damage. After treatment with bifendate, the protein metabolic processes of hepatitis patients were improved, with increased serum albumin levels and decreased globulin levels1, 2, 3, 4, 5, 6. Bifendate is a potent inducer of cytochrome proteins (CYPs) and can result in clinically significant interactions7, 8. It has been proposed that the increased detoxification capability of bifendate originates from an increase in the level of P450. Bifendate may function as a protecting agent to prevent drug-induced liver dysfunction by increasing the activity of CYP450. Previous research has found that treatment with bifendate may strongly induce the expression of P450, which may result in increased activity of glutathione peroxidase, glutathione reductase, and glutathione-S-transferase9. The effect of bifendate on the activity of erythromycin demethylase may also result from increased activity of CYP3A410. This information strongly indicated that bifendate affects the amount and activity of CYP450.
Cyclosporine is a calcineurin inhibitor that was developed for the treatment of many immune-mediated diseases, in particular, for the prevention of allograft rejection after solid organ transplantation11, 12. Leukocytes and special helper CD4+ lymphocytes are the main therapeutic targets of this drug13, 14. It is regarded as highly effective in preventing acute rejection and is characterized by a narrow therapeutic index and variable pharmacokinetic characteristics15. Irreversible organ failure or acute graft rejection may be caused by prolonged exposure to supra- or subtherapeutic cyclosporine concentrations. Cyclosporine must be dosed according to the whole-blood cyclosporine concentration rather than to body weight to avoid over- or under-immunosuppression. The variable oral bioavailability of cyclosporine is regarded as the main cause of the difference in pharmacokinetics16, 19.
A recent study has made it clear that the activity of the permeability-glycoprotein (P-gp) and cytochrome (CYP) P450 enzyme systems are key factors20, 21 in the variable oral bioavailability of cyclosporine22. The CYP system consists of more than 50 isozymes that metabolize many exogenous agents and drugs23. Expressed in both the liver and small intestine, the CYP3A subfamily has been found to metabolize about 50%–60% of drugs, and these enzymes are the most abundant CYPs. The variations in the oral bioavailability and systemic clearance of CYP3A substrates may result from the great interindividual differences in their activity24, 25. Recently, a new SNP in intron 10 of CYP3A4 (CYP3A4*18B), involving a G-to-A substitution at position 82266, was found by direct sequencing in a Japanese population; it is speculated to be associated with an increase in the level of CYP3A4 activity26.
In China, the combined therapy of bifendate with cyclosporine in patients with renal transplants is used to decrease the hepatotoxic side effects induced by cyclosporine. The use of bifendate may be influenced by the potential for pharmacokinetic drug-drug interaction, which resulted from the induction of CYPs and P-gp by bifendate. Current research indicates that the combination of bifendate and cyclosporine may cause the variability of plasma cyclosporine concentrations and lead to irreversible organ failure and even graft failure.
The aim of this study was to assess the effect of bifendate on cyclosporine pharmacokinetics, especially in healthy volunteers with different CYP3A4*18 genotypes.
Methods
Subjects
Eighteen healthy subjects (six CYP3A4*1/*1, six CYP3A4*1/*18B, and six CYP3A4*18B/*18B) were recruited for this study from 218 male healthy Chinese volunteers who had been genotyped for CYP3A4*18B. After confirmation of good health by medical histories, physical examinations, electrocardiograms, blood chemistry, hematology, and urine analyses conducted for this study, all subjects gave written informed consent to participate. The participants aged 18−25 years, with standard body mass indexes between 18 and 30 kg/m2. Subjects were not allowed to smoke or consume coffee or alcohol and were instructed to abstain from any products that contain grapefruit juice, herbal dietary supplements, or CYP3A4 inducers or inhibitors for at least one week before taking part in this trial. This study was approved by the Ethics Committee Board of Central South University, Changsha, China.
PCR-RFLP for CYP3A4*18B genotyping
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analyses for CYP3A4*18B were carried out. Briefly, 100 ng of genomic DNA was used in a total reaction volume of 25 μL, containing 2.5 μL of 10×PCR buffer, 0.2 mmol/L of each dNTP, 1.5 U Taq DNA polymerase, and 40 pmol/L each of the forward (5′-caccctgatgtccagcagaaact-3′) and reverse (5′-aatagaaagcagatgaaccagagcc-3′) primers. PCR conditions were as follows: 7 min at 94 °C; 35 cycles of 30 s at 94 °C, 1 min at 62 °C, and 1 min at 72 °C; and finally 5 min at 72 °C. The PCR product (10 μL) was digested with RsaI in a total volume of 20 μL at 37 °C for 4 h and subsequently analyzed by electrophoresis on a 3% agarose gel in the presence of ethidium bromide. The wild-type DNA contains one restriction site, and RsaI digestion yields 217- and 70-bp fragments.
Study protocol
The study was performed in a two-phase, randomized and crossover manner with a 14-d washout period between phases. During the treatment period, the 18 volunteers were orally administered a placebo or 15 mg bifendate drop pill (Wan Bang pharmaceutical, Zhejiang, China; BN: 20061228) together with 200 mL tap water, three times daily for 14 d. Forearm venous blood samples were collected before and 0.33, 0.66, 1, 1.33, 1.66, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 14, and 24 h after ingestion of a 4 mg/kg cyclosporine capsule (Neoral, Novartis Pharmaceutical, R.P. Scherer GmbH, Germany) on the 15th day. All volunteers had fasted overnight before the cyclosporine administration. Standardized meals were allowed at 8:00 AM, 12:00 PM, and 8:00 PM on day 15. All blood samples were stored in EDTA-containing tubes at −80 °C until the quantitative analysis.
Analytic assay for cyclosporine
Whole-blood concentrations of cyclosporine were qualified by high-performance liquid chromatography-electrospray mass spectrometry (HPLC/ESI-MS). A mixture of acetonitrile and 10 ammonium formate in double distilled water (70/30, v/v) was applied for the mobile phase. The flow rate was 0.2 mL/min, and the column temperature was maintained at 50 °C. Cyclosporine G mixed with 200 μL was used as an internal standard. The mass transitions were monitored at mass-to-charge ratios (m/z) of 1203–1225 for cyclosporine and 1217–1239 for cyclosporine G. A standard curve was constructed by plotting the peak area ratios against the theoretical whole-blood concentrations of cyclosporine. The lower limit of detection was 1 ng/mL. Concentrations were linear over the range of 25.5−5100 ng/mL. The intra- and inter-day precision values for the 25.5, 2500 and 5100 ng/mL concentrations were all <8.0%, and the accuracy ranged from 92.9% to 108.5% of the nominal value.
Pharmacokinetic analysis
The maximum plasma concentration (Cmax) and the maximum time to Cmax (Tmax) were taken directly from the serum concentration-time curves. The area under the blood concentration-time curves (AUC) from 0 to 24 h of cyclosporine was calculated using the linear trapezoidal rule, where K represents the slope of the linear terminal part of the concentration versus time curve after semilogarithmic transformation. The oral clearance after oral administration was estimated by dividing the dose by the AUC.
Statistical analysis
The SPSS software for Windows (version 11, SPSS, Chicago) was used for statistical analyses. The normality test was applied for all pharmacokinetic parameters. For the AUC, t1/2, and CL/F values, which were normally distributed, the paired Student's t-test was carried out. For the Cmax and tmax values, which were not normally distributed, the Wilcoxon signed test was performed. We compared the differences among the CYP3A4 genotype groups using one-way analysis of variance. For all data, both mean and SD were used, and P values less than 0.05 were considered statistically significant.
Results
The allelic frequency of CYP3A4*18B was determined in a group of 218 Chinese healthy males, of whom 111 were CYP3A4*1/*1 and 91 were CYP3A4*18B, with 16 homozygous for the mutation. The CYP3A4*18B allele was in Hardy-Weinberg equilibrium (P=0.90).
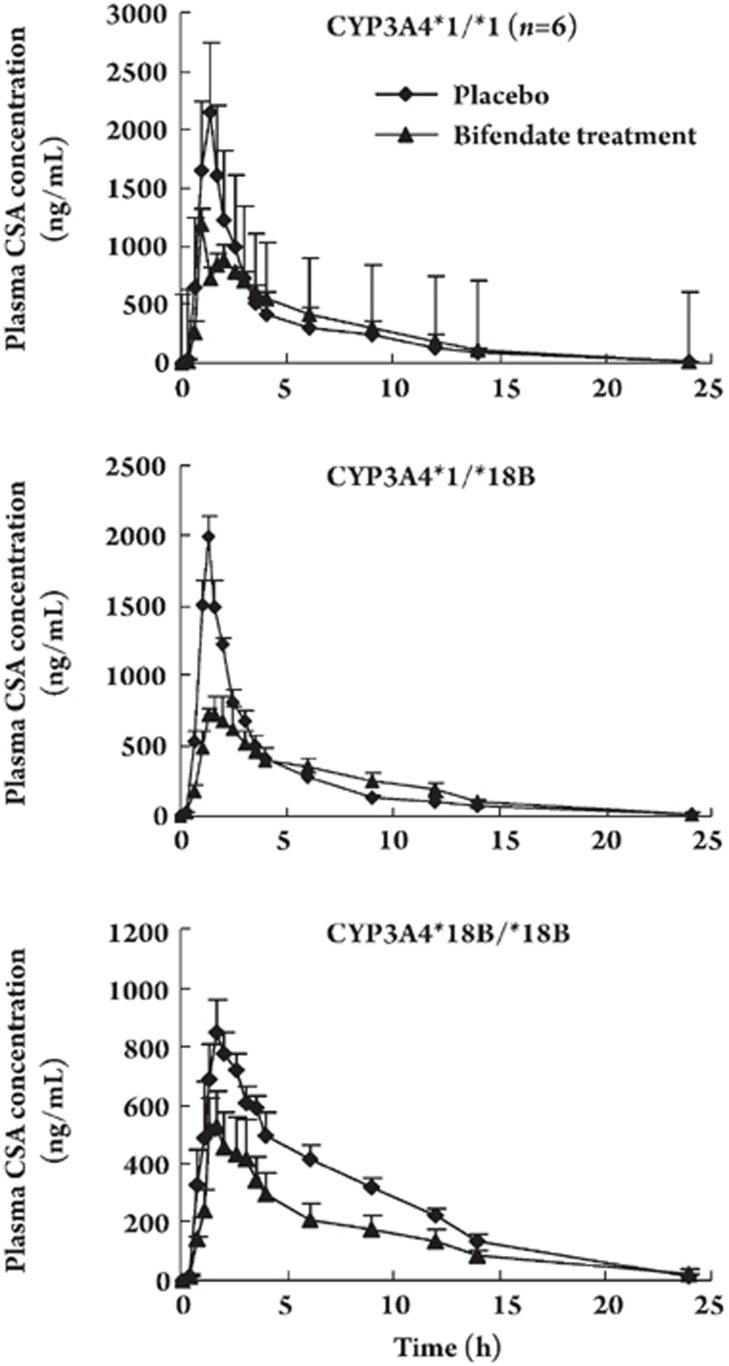
Table 1 and Figure 1 present the main pharmacokinetic parameters of cyclosporine after treatment with placebo or bifendate for 14 d. After administration of bifendate for 14 d, the cyclosporine areas under the curve (AUC0–24 and AUC0–∞) decreased significantly by 9.7%±3.7% (P=0.01) and 19.2%±16.8% (P=0.001) in the CYP3A4*1/*1 group, 11.3%±9.4% (P=0.03) and 10.5%±9.6% (P=0.043) in the CYP3A4*/*18B group, and 40.2%±14.7% (P=0.02) and 37.5%±15.8% (P=0.003) in CYP3A4*18B/*18B group. The bifendate treatment decreased the Cmax of cyclosporine by 61.3%±10.2% (P=0.001) in the CYP3A4*1/*1 group, 60.2%±4.3% (P=0.01) in the CYP3A4*1/*18B group, and 37.6% ± 10.2% (P=0.01) in the CYP3A4*18B/*18B group.
Table 1. Pharmacokinetic characteristics of cyclosporine in healthy subjects with different CYP3A4*18B genotypes after 14 days of treatment with placebo or bifendate. bP<0.05 vs placebo group.
| CYP3A4*1/*1 | CYP3A4*1/*18B | CYP3A4*18B/*18B | ||||
|---|---|---|---|---|---|---|
| (n=6) | (n=6) | (n=6) | ||||
| Placebo | Bifendate | Placebo | Bifendate | Placebo | Bifendate | |
| AUC(0–24) (ng·h/mL) | 6826±521 | 6174±610b | 5810±208 | 5159±651b | 6175±505 | 3669±832b |
| AUC(0–∞) (ng·h/mL) | 6927±510 | 6264±630b | 5927±198 | 5308±642b | 6287±562 | 3891±867b |
| Cmax (ng/mL) | 2181±251 | 928±105b | 1998±140 | 795±108b | 870±77 | 546±118b |
| tmax (h) | 1.44±0.17 | 1.66±0.21 | 1.33±0.0 | 1.5±0.26 | 1.77±0.18 | 1.44±0.17 |
| t1/2 (h) | 4.36±0.47 | 4.13±0.50 | 4.38±0.51 | 4.19±0.35 | 4.91±0.59 | 4.55±0.45 |
| CL/F (1/h) | 36.8±2.88 | 40.6±4.41b | 43.1±1.47 | 49.2±6.59b | 40.7±3.36 | 53.7±8.28b |
Figure 1.
Mean plasma concentration-time profiles of cyclosporine in CYP3A4*1/*1 (A), CYP3A4*1/*18B (B), and CYP3A4*18B/*18B (C) subjects who were administered a single oral dose of cyclosporine (4 mg/kg) after treatment with placebo or bifendate. Data are expressed as mean±SEM.
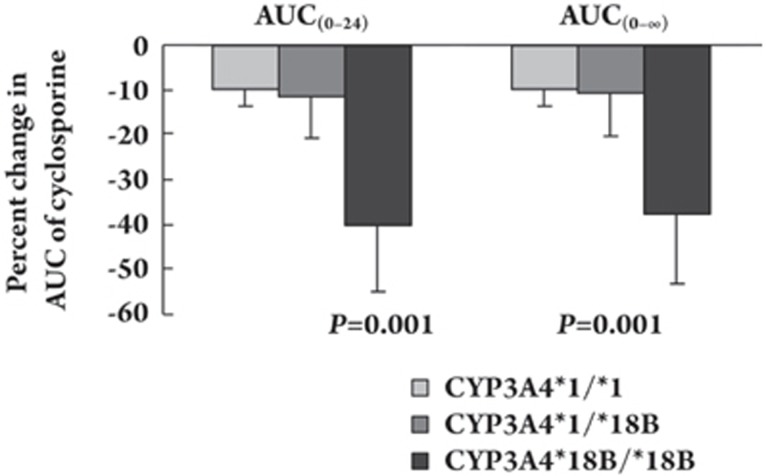
Meanwhile, bifendate administration affected the oral clearance of cyclosporine in all the subjects, with substantial increases by 10.2%±4.4% (P=0.004) in the CYP3A4*1/*1 group, 14.0%±12.0% (P=0.048) in the CYP3A4*1/*18B group, and 32.4±21.7% (P=0.013) in the CYP3A4*18B/*18B group. No significant differences in tmax and t1/2 of cyclosporine were observed between the placebo and bifendate treatments. Figure 2 shows that the time-concentration curves of different plasma concentrations of cyclosporine after the bifendate treatment significantly decreased among the different CYP3A4*18B genotype groups. The decreases in the AUC0–24 and AUC0–∞ values of the three groups were significantly different (using one-way analysis of variance, P=0.001 and P=0.001 respectively), and the change in the CYP3A4*18B/*18B group was greater than that in the other two groups.
Figure 2.
Comparison of the mean changes in the AUC0–24 and AUC0–∞ of cyclosporine among the CYP3A4*1/*1, CYP3A4*1/*18B, and CYP3A4*18B/*18B groups after administration of bifendate for 14 d compared with placebo. Data are expressed as mean±SEM.
Discussion
This study was designed to investigate a potential drug-drug interaction between bifendate and cyclosporine with regard to CYP3A4 genotypes. This is a study to describe in detail the effect of bifendate on the pharmacokinetic parameters of cyclosporine in different CYP3A4 genotypes. Administration of the usual therapeutic dose of bifendate decreased the plasma concentration of cyclosporine in all subjects, which was accompanied by a marked decrease in the peak plasma concentration (Cmax). The induction of drug metabolism enzymes or transporters involved in the bio-transformation of cyclosporine might be a plausible explanation for this effect, and therefore CYP3A4 was intensively studied. Cyclosporine is metabolized by CYP3A4 primarily in the liver and small intestine27, 28, 29. Bifendate is a synthetic intermediate of schisandrin, which is known to alter the expression of CYP3A via a pregnane X receptor (PXR)-dependent pathway30, 31. Based on these studies, the influence of bifendate treatment on the metabolism of cyclosporine could be attributed to the induction of CYP3A4 in the human body. Bifendate may be regarded as a potential inducer of CYP3A4.
Expressed in both the liver and small intestine, the CYP3A subfamily is responsible for metabolizing 50%−60% of drugs32. To date, periodic monitoring of CYP3A4 substrate serum levels has been used for clinical control of drug dosage adjustment33, 34. For example, decreasing the dosage of cyclosporine is required when CYP3A4 inhibitors, such as grapefruit, were coadministered35. Ketoconazole, identified as a purer type of CYP3A4 inhibitor, may raise the serum level of a CYP3A4 substrate to a toxic level and lead to serious medical consequences36. As an inducer of CYP3A4, comedication with St John's wort may contribute to substantial reduction of the bioavailability of a wide variety of drugs, such as nevirapine, amitriptyline, warfarin, simvastatin, and irinotecan37. As an integral part of drug research, investigations of the CYP3A4 isozymes and drug interactions have already received careful consideration.
CYP3A4 has been identified as a key player in the bio-transformation of cyclosporine, which is widely used for the prevention of acute rejection after organ transplantation. The lower oral bioavailability and a narrow therapeutic index have severely limited its usefulness and resulted in over- and under-immunosuppression38. Co-administration of bifendate and cyclosporine is commonly used in China to decrease the hepatotoxic side effects induced by cyclosporine. Bifendate is a synthetic intermediate of schisandrin, which is also a component of the indigenous Chinese plant Wu Wei Zi. It has been shown that many herbal products have the ability to directly alter hepatic drug metabolizing enzymes, such as CYPs, and interact with co-administered drugs, especially drugs that can function as hepatoprotective, detoxifying, and antioxidative agents39. For example, Gan Cao (licorice root) can increase serum CYP levels and the metabolic rate of imipramine. The pharmacokinetics of warfarin, a substrate of CYP2C, can be altered substantially by the traditional herbal remedy Dan-Shen40, 41. According to the results of this study, the plasma concentration and Cmax of cyclosporine were reduced dramatically when coadministered with bifendate. The changes in the pharmacokinetics parameters could be attributed partly to the increased activity of CYP3A4, which is responsible for the bio-transformation of cyclosporine. This investigation may indicate that bifendate also interacts with other CYP3A4 substrates. We hypothesize that the repeated administration of bifendate may influence the pharmacokinetics of a series of CYP-mediated drugs.
Genetic polymorphisms in drug metabolism enzymes contribute to individual variations in responses to clinical medications. As the major enzyme involved in the metabolism of cyclosporine, the expression level of CYP3A4 varies up to 40-fold in the human population, including the inducible and constitutive levels. Differences in CYP3A4 activity could lead to great variations in the pharmacokinetics of CYP3A4 substrates, including cyclosporine42. To date, 22 SNPs of CYP3A have been found in Chinese populations43. CYP3A4*18B was speculated to be associated with an increased level of CYP3A4 activity44. This study investigated the effects of the CYP3A4*18B allele on both the inducible and basal levels of orally administered cyclosporine in healthy subjects. At the basal level, the AUC0–24, AUC0–∞, and Cmax were statistically lower in the mutant homozygous group than those in the wild-type group. Oral clearance was increased in the mutant homozygous individuals compared with the wild-type group. The results of our study are in agreement with previous work, indicating that the naturally occurring polymorphisms in CYP3A4*18B can increase the activity of the enzyme. As a functional SNP of CYP3A4, this clinical study has also suggested that a strong relationship exists between the dose-adjusted concentrations of both C0 and C2 and the homozygosities of CYP3A4*18B. On the inducible level, we found that the decrease in the serum cyclosporine levels of the homozygous CYP3A4*18B group was greater than that of the other two groups. However, the exact mechanism of the significant difference that occurred with the CYP3A4 phenotype has not been elucidated, so it is difficult to explain the variance of the activity of drug metabolism enzymes by just one or a few SNPs. Moreover, cyclosporine is also the substrate of P-gp, which may be affected by bifendate. So, the results of our studies may be partly attributed to the activity of the CYP3A4 variant.
Our study is to explore the relationship between bifendate and cyclosporine in humans. In addition, this drug-drug interaction is very likely relevant to bifendate interactions with other substrates metabolized by CYP3A4. In this investigation, bifendate treatment led to a dramatic decrease in systemic plasma exposure to cyclosporine, especially in subjects with the CYP3A4*18B/*18B genotype.
In summary, bifendate decreased the systemic exposure to cyclosporine in all CYP3A4*18B groups. The results suggest that the decrease in the plasma concentrations of cyclosporine co-administered with bifendate is at least partially mediated by bifendate induction of hepatic cyclosporine bio-transformation by CYP3A4 in a gene dose-dependent manner.
Conflict of interest
None of the authors has any conflict of interest regarding this study.
Author contribution
Yong ZENG and Hong-hao ZHOU designed research; Yong ZENG and Lan FAN Performed research; Yong ZENG, Fu-yuan HE and Lan FAN contributed new analytical tools and reagents; Yong ZENG analyzed data; Yong ZENG, Yi-jing HE and Lan FAN wrote paper.
Acknowledgments
This work was supported by research grants from the National Natural Science Foundation of China (No 30672497), the China Medical Board of New York (grant No 01-755), and the Hunan Health Research Foundation of Traditional Chinese Medicine (grant No 204041).
References
- Liu GT. From the study of Fructus Schizandrae to the discovery of biphenyl dimethyl-dicarboxylate. Yao Xue Xue Bao. 1983;18:714–20. [PubMed] [Google Scholar]
- Liu GT, Wei HL, Song ZY. Further studies on the protective action of biphenyl dimethy-dicarboxylate (BDD) against experimental liver injury in mice. Acta Pharm Sin. 1982;17:101–5. [PubMed] [Google Scholar]
- Guan LP, Nan JX, Jin XJ, Jin QH, Kwak KC, Chai KY, et al. Protective effects of chalcone derivatives for acute liver injury in mice. Arch Pharm Res. 2005;28:81–6. doi: 10.1007/BF02975140. [DOI] [PubMed] [Google Scholar]
- Abdel-salam OM, Sleem AA, Morsy FA. Effect of biphenyldimethyl-dicarboxylate administrate alone or combined with silymarin in the CCl4 model of liver fibrosis in rats. Scientific World Journal. 2007;7:1242–55. doi: 10.1100/tsw.2007.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Hameid NA. Protective role of dimethyl diphenyl bicarboxylate (DDB) against erythromycin induced hepatotoxicity in male rats. Toxicol In Vitro. 2007;21:618–25. doi: 10.1016/j.tiv.2006.12.006. [DOI] [PubMed] [Google Scholar]
- El-Beshbishy HA. The effect of dimethyl dimethoxy biphenyl dicarboxylate (DDB) against tamoxifen-induced liver injury in rats: DDB use is curative or protective. J Biochem Mol Biol. 2005;38:300–6. doi: 10.5483/bmbrep.2005.38.3.300. [DOI] [PubMed] [Google Scholar]
- Liu KT, Cresteil T, Le Provost E, Lesca P. Specific evidence that schizandrins induce a phenobarbital-like cytochrome P-450 form separated from rat liver. Biochem Biophys Res Commun. 1981;103:1131–7. doi: 10.1016/0006-291x(81)90240-0. [DOI] [PubMed] [Google Scholar]
- Liu ZY, Wang YL, Zhou ZH. Effect of bifendate on pharmacokinetics of cyclosporine A in rabbits. Chin Hosp Pharm J. 2002;(22):8. [Google Scholar]
- el-Sawy SA, el-Shafey AM, el-Bahrawy HA. Effect of dimethyl diphenyl bicarboxylate on normal and chemically-injured liver. East Mediterr Health J. 2002;8:95–104. [PubMed] [Google Scholar]
- Lu H, Li Y. Effects of dimenthyl diphenyl bicarboxylate on the metabolism and hepatotoxicity of aflatoxin B1 in rats. Acta Pharm Sin. 2002;37:753–7. [PubMed] [Google Scholar]
- Awni WM. Pharmacodynamic monitoring of cyclosporine. Clin Pharmacokinet. 1992;23:428–48. doi: 10.2165/00003088-199223060-00004. [DOI] [PubMed] [Google Scholar]
- Blanchet B. Therapeutic monitoring of immunosuppressive drugs: interest of calcineurin activity assessment in liver transplantation. Ann Pharm Franc. 2008;66:96–101. doi: 10.1016/j.pharma.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Lin CS, Boltz RZ, Siekierka JJ, Sigal NH. FK-506 and cyclosporine A inhibit highly similar signal transduction pathways in human T lymphocytes. Cell Immunol. 1991;133:269–84. doi: 10.1016/0008-8749(91)90103-i. [DOI] [PubMed] [Google Scholar]
- Sehajpal PK, Sharma VK, Ingulli E, Stenzel KH, Suthanthiran AN. Synergism between the CD3 antigen- and CD2 antigen-derived signals: exploration at the level of induction of DNA-binding proteins and characterization of the inhibitory activity of cyclosporine. Transplantation. 1993;55:1118–24. doi: 10.1097/00007890-199305000-00035. [DOI] [PubMed] [Google Scholar]
- Buchler M, Johnston A. Seeking optimal prescription of cyclosporine ME. Ther Drug Monit. 2005;27:3–6. doi: 10.1097/00007691-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Ozbay A, Karamperis N, Jorqensen KA. A review of the immunosuppressive activity of cyclosporine metabolites: new insights into an old issue. Curr Clin Pharmacol. 2007;2:244–8. doi: 10.2174/157488407781668758. [DOI] [PubMed] [Google Scholar]
- Brunet M, Crespo M, Millan O, Seron D, Torreqrisa V, Jimenez O, et al. Pharmacokinetics and pharmacodynamics in renal transplant recipients under treatment with cyclosporine and Myfortic. Transplant Proc. 2007;39:2160–2. doi: 10.1016/j.transproceed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kahan BD. Cyclosporine. N Engl J Med. 1989;(321):1725–38. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Dunn CJ, Wagstaff AJ, Perry CM, Plosker GL, Goa KL. Cyclosporine: an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (neoral) 1 in organ transplantation. Drugs. 2001;61:1957–2016. doi: 10.2165/00003495-200161130-00006. [DOI] [PubMed] [Google Scholar]
- Chowbay B, Cumaraswamy S, Cheung YB, Zhou Q, Lee EJ. Genetic polymorphisms in MDR1 and CYP3A4 genes in Asians and the influence of MDR1 haplotypes on cyclosporine disposition in heart transplant recipients. Pharmacogenetics. 2003;13:89–95. doi: 10.1097/00008571-200302000-00005. [DOI] [PubMed] [Google Scholar]
- Hesselink DK, Van Schaik RH, Van der Heiden IP, Van der Werf M, Greqoor PJ, Lindemans J, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–54. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Hebert MF. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev. 2997;27:201–14. doi: 10.1016/s0169-409x(97)00043-4. [DOI] [PubMed] [Google Scholar]
- Pachecka J, Tomaszewski P, Kubiak-Tomaszewska G. Cytochrome P450 polymorphism-molecular, metabolic and pharmacogenetic aspects. I. Mechanisms of activity of cytochrome P450 monoxygenases. Acta Pol Pharm. 2008;65:303–6. [PubMed] [Google Scholar]
- Thummel KE, Wilkinson GR. In vitro and in vivo interactions involving human CYP3A. Annu Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- Ozdemir V, Kalow W, Tang BK, Paterson AD, Walker SE, Endrenyi L, et al. Evaluation of the genetic component of variability in CYP3A4 activity: a repeated drug administration method. Pharmacogenetics. 2000;10:373–88. doi: 10.1097/00008571-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Fukushima-uesaka H, Saito Y, Watanabe H, Shiseki K, Saeki M, Nakamura T, et al. Haplotypes of CYP3A4 and their close linkage with CYP3A5 haplotypes in a Japanese population. Hum Mutat. 2004;23:100. doi: 10.1002/humu.9210. [DOI] [PubMed] [Google Scholar]
- Kronbach T, Fischer V, Meyer UA. Cyclosporine metabolism in human liver: identification of a cytochrome P-450 III gene family as the major cyclosporine-metabolizing enzyme explains interactions of cyclosporine with other drugs. Clin Pharmacol Ther. 1988;43:630–5. doi: 10.1038/clpt.1988.87. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Yamano S, Waxman DJ, Lapenson DP, Meyer UA, Fischer V, et al. Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem. 1989;264:10388–95. [PubMed] [Google Scholar]
- Utecht KN, Hiles JJ, Kolesar J. Effect of genetic polymorphisms on the pharmacokinetics of calcineurin inhibitors. Am J Health Syst Pharm. 2006;63:2340–8. doi: 10.2146/ajhp060080. [DOI] [PubMed] [Google Scholar]
- Zhu M, Yeung RY, Lin KF, Li RC. Improvement of phase I drug metabolism with Schisandra chinensis against CCL4 hepatotoxicity in a rat model. Planta Med. 2000;66:521–5. doi: 10.1055/s-2000-11202. [DOI] [PubMed] [Google Scholar]
- Mu Y, Zhang J, Zhang S, Zhou HH, Toma D, Ren S, et al. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J Pharmacol Exp Ther. 2006;316:1369–77. doi: 10.1124/jpet.105.094342. [DOI] [PubMed] [Google Scholar]
- Kato M. Intestinal first-pass metabolism of CYP3A4 substrates. Drug Metab Pharmacokinet. 2008;23:87–94. doi: 10.2133/dmpk.23.87. [DOI] [PubMed] [Google Scholar]
- Wang SM, Lai MK, Chueh SC, Tai HC, Chung SD. Optimal C2 concentration of cyclosporine corrected with good efficacy and safety in Asian kidney transplant recipients. Transplant Proc. 2008;40:2243–4. doi: 10.1016/j.transproceed.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Morris RG. Cyclosporine therapeutic drug monitoring — an established service revisited. Clin Biochem Rev. 2003;24:33–46. [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Altman H. Food-drug interaction: grapefruit juice augments drug bioavailability-mechanism, extent and relevance. Eur J Clin Nut. 2004;58:1–9. doi: 10.1038/sj.ejcn.1601736. [DOI] [PubMed] [Google Scholar]
- Lorusso P, Heath EI, McGreivy J, Sun YN, Melara R, Yan L, et al. Effect of coadministration of ketoconazole, a strong CYP3A4 inhibitor, on pharmacokinetics and tolerability of motesanib diphosphate (AMG 706) in patients with advanced solid tumors. Invest New Drugs. 2008;26:455–62. doi: 10.1007/s10637-008-9144-1. [DOI] [PubMed] [Google Scholar]
- Singh YN. Potential for interaction of kava and St. John's wort with drugs. J Ethnopharmacol. 100:108–13. doi: 10.1016/j.jep.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Pollard SG. Pharmacologic monitoring and outcomes of cyclosporine. Transplant Proc. 2004;36:404–7. doi: 10.1016/j.transproceed.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Venkataramanan R, Komoroski B, Strom S. In vitro and in vivo assessment of herb drug interactions. Life Sci. 2006;78:2105–15. doi: 10.1016/j.lfs.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Hu WY, Li YW, Hou YN, He K, Chen JF, But PP, et al. The induction of liver microsomal cytochrom P450 by Glycyrrhiza uralensis and glycyrrhetinic acid in mice. Biomed Environ Sci. 1999;12:10–4. [PubMed] [Google Scholar]
- Lin G, Nnane IP, Cheng TY. The effects of pretreatment with glycyrrhizin and glycyrrhetinic acid on the retrorsine-induced hepatotoxicity in rats. Toxicon. 1999;37:1259–70. doi: 10.1016/s0041-0101(98)00263-3. [DOI] [PubMed] [Google Scholar]
- Thummel KE, Wilkinson GR. In vivo and in vitro drug interactions involving human CYP3A. Annu Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- Hsieh KP, Lin YY, Cheng CL, Lai ML, Lin MS, Siest JP, et al. Novel mutations of CYP3A4 in Chinese. Drug Metab Dispos. 2001;29:268–73. [PubMed] [Google Scholar]
- Fukushima-Uesaka H, Saito Y, Watanabe H, Shiseki K, Saeki M, Nakamura T, et al. Haplotypes of CYP3A4 and their close linkage with CYP3A5 haplotypes in a Japanese population. Hum Mutat. 2004;23:100. doi: 10.1002/humu.9210. [DOI] [PubMed] [Google Scholar]