Abstract
Aim:
The aim of this study was to investigate whether Gs-Rbl relieves the CoCl2-induced apoptosis of hypoxic neonatal rat cardiomyocytes and in which the role of glucose transporter-4 (GLUT-4).
Methods:
Gs-Rbl (0, 10, 50, 100, 200, 400, and 500 μmol/L), adenine 9-β-D-arabinofuranoside (ara A, 500 μmol/L; AMPK inhibitor) and wortmannin (0.5 μmol/L; PI3K inhibitor) only in combination with 200 μmol/L Gs-Rbl were administered in hypoxic cardiomyocytes, which were induced by 500 μmol/L CoCl2 for 12 h. Then, the apoptotic rate (AR), 2-[3H]-deoxy-D-glucose (2-[3H]-DG) uptake, and the expression of GLUT-4 (including in plasma membrane, PM), phospho-AMPKα (Thr172), AMPKα and Akt in cells were assayed.
Results:
Compared with simple hypoxia (0 μmol/L Gs-Rbl), Gs-Rb1 greater than 10 μmol/L significantly decreased the apoptotic rate (P<0.01) and significantly increased 2-[3H]-DG uptake (P<0.01), GLUT-4 content in cells and PM (P<0.01), AMPK activity (P<0.01) and Akt (P<0.01) levels in a dose-dependent manner. AMPK activity was completely suppressed by ara-A, just as Akt was suppressed by wortmannin. The AR, glucose uptake and GLUT-4 levels in cells and PM were partly down-regulated by ara-A or wortmannin.
Conclusion:
Gs-Rb1 may protect neonatal rat cardiomyocytes from apoptosis induced by CoCl2. The anti-apoptotic effect of Gs-Rb1 may occur by improving glucose uptake, in which GLUT-4 translocation and expression played a key role. Both the AMPK and the PI3K/Akt pathways may take part in the anti-hypoxic efficacy of Gs-Rb1.
Keywords: neonatal rat cardiomyocytes, hypoxia, apoptosis, ginsenosides-Rbl, glucose transporter-4
Introduction
Myocardial ischemia plunges heart muscle into an energy-deficient state, which does not satisfy the energy requirement of cardiomyocytes. Ischemia and hypoxia, if not corrected, can lead to cardiomyocyte “hibernation,” apoptosis and even necrosis. Hibernating myocardium, an adaptive response to ischemia and/or metabolic imbalance1, 2, 3, 4, 5, 6, may result in characteristic ultrastructural changes in cardiomyocytes, such as myolysis and subsequent glycogen accumulation in the myolytic areas3, 4, 5, 6. Although it is reversible, cardiomyocyte “hibernation” and apoptosis will damage heart function to an extreme extent. Therefore, a method for improving cardiomyocytes' anti-hypoxic response has become an important area of cardiovascular research.
In cardiomyocytes, glucose is the major metabolic substrate during ischemia and hypoxia. Its facilitative uptake is dependent on the activity of glucose transporters (GLUT) on the cell surface, in particular, glucose transporter-4 (GLUT-4)7, 8. The increase in glucose uptake is mainly dependent on GLUT-4 translocation via AMP-activated protein kinase (AMPK)9, 10, 11, phosphatidylinositol-3 kinase (PI3K)12, 13, etc. The increased glucose uptake provides a substrate for glycolysis and then partly compensates for the energy deficit.
Ginsenoside Rbl (Gs-Rbl), which is the main ginsenoside that is extracted from ginseng (the root of Panax ginseng C. A. Meyer, family Araliaceae) as a traditional medicine in Asian countries, contains an aglycone with a dammarane skeleton and has been reported to show various biological activities resisting ischemic reperfusion injury in myocardial infarction14, 15, 16. However, few reports exist regarding whether Gs-Rbl can prevent the apoptosis and death of hypoxic cardiomyocytes. Thus, we explored whether and how Gs-Rbl relieves hypoxia-induced apoptosis of neonatal rat cardiomyocytes ex vivo (ie, what role glucose uptake, GLUT-4 translocation, etc, play in Gs-Rbl-based protection against hypoxia in the present study.
Materials and methods
This study was implemented in accordance with the institutional guidelines for animal care of China Medical University and approved by the local animal research committee.
Cardiomyocyte culture
Ventricles from 1- to 3-day-old Wistar rats [SCXK(Liao) 2003-0009, supplied by the Laboratory Animal Center of China Medical University] were minced and sequentially digested with phosphate-buffered saline (PBS) containing 0.08% trypsin and 0.05% collagenase II (Gibco company) at 37 °C for 10 min under sterile conditions; the procedure was repeated two to three times after collection of the cell suspension. The cell suspension was centrifuged (100×g, 10 min), and then cells were suspended in Dulbecco's modified Eagle's medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 0.1 mmol/L bromodeoxyuridine (to prevent nonmyocardial cells from proliferating) and incubated in 100-mL culture dishes (37 °C, 5% CO2, 95% air; NuAire 8000 Autoflow CO2 Incubator; Thermo Electron Corporation, Franklin, MA) for 90 min to remove fibroblasts. Cardiomyocytes were separated according to their different adherence times, as previously described. Unattached cells (mainly cardiomyocytes) were seeded on culture flasks at a density of 1.0×105 cells per mL and then were incubated for 3 days before being used for experiments. About 85%–95% of cells were identified as cardiomyocytes by immunocytochemical staining with monoclonal antisarcomeric actin clone AC40-CY3 (Sigma).
CoCl2-induced hypoxia and Gs-Rb1 intervention
According to a previous study17 and our preliminary experiments with CoCl2-induced hypoxia, 500 μmol/L CoCl2 was prepared fresh in culture medium. After 500 μmol/L CoCl2 intervention, cardiomyocytes were incubated in L-DMEM supplemented with 10% FBS and different concentrations of fresh Gs-Rb1 (0, 10, 50, 100, 200, 400, and 500 μmol/L) for 12 h. In addition, adenine 9-β-D-arabinofuranoside (ara A, 500 μmol/L; AMPK inhibitor) and wortmannin (0.5 μmol/L; PI3K inhibitor) were administered for 12 h in the presence of 200 μmol/L Gs-Rb1.
Flow cytometric analysis of cardiomyocyte apoptosis
The above-described cells were adjusted to 1.0×106/mL per sample and then analyzed by flow cytometry (FCM) according to the Annexin V FITC/PI kit (Boehringer Mannheim Biological Technology Ltd). Briefly, cardiomyocytes were harvested, washed twice in cold PBS and then fixed with 70% ethanol at 4 °C for 12 h. After being washed twice with cold PBS, the fixed cells were suspended in PBS containing RNase A (1 mg/mL, Sigma-Aldrich) at 37 °C for 30 min and centrifuged (1000×g for 5 min). The cells were then resuspended in PBS containing Annexin V FITC/PI (50 μg/mL) and stained in the dark for 30 min. A 488 nm wavelength was used for excitation; a bandpass filter at a wavelength of 515 nm detected FITC fluorescence, and a bandpass filter at a wavelength of 560 nm detected PI fluorescence. More than 99% of the non-specific fluorescence of control pipe was attributable to background and shown on a 2D point lattice map. In a double variance scatter plot, the left inferior quadrant represented living cells (FITC−/PI−), the right inferior quadrant represented apoptotic cells (FITC+/PI−), and the right superior quadrant represented necrosis cells (FITC+/PI+). Data were obtained using a FACSCalibur (Becton Dickinson) and analyzed with ModFit LT 3.0 software (Variety Software House).
2-[3H]-Deoxy-D-glucose uptake
The cardiomyocyte suspensions (1.0×105/mL, 2 mL per sample) were washed three times with 0.5 mL PBS and then placed immediately in 0.5 mL PBS containing 2 mmol/L glucose and trace amounts (1 μCi/mL) of 2-[3H]deoxy-D-glucose (2-[3H]-DG, Amersham Biosciences, Arlington Heights, IL) for 15 min at 37 °C. Next, cells were washed four times with 0.5 mL cold PBS and then transferred to counting vials with 3.5 mL of scintillation fluid. In the 3H window of a Tricarb scintillation counter, 2-[3H]-DG were counted, and then glucose uptake (pmol/mg protein per 10 min) was determined. Net specific uptake was calculated as the difference between the total and nonspecific values, which was determined in the presence of excess D-glucose (100 mmol/L).
Plasma membrane preparation for GLUT-4 translocation assay
Cardiomyocytes were washed (as indicated above), centrifuged at 600×g for 5 min and then homogenized in buffer A with protease inhibitors (Cell Fractionation Kit; Biovision, Mountain View, CA) for 50 min. After centrifugation at 20 000×g for 15 min, the pellet was resuspended and centrifuged at 110 000×g for 60min on a linear sucrose gradient with a density ranging from 1.05 to 1.25 g/mL in order to isolate the plasma membrane (PM), which are assayed for GLUT-4 protein. All centrifugations were carried out at 4 °C.
Immunocytochemistry
The coverslips were removed from culture dishes and washed three times with PBS for 5 min per rinse; the coverslips were then fixed in PBS (pH 7.2) with 4% paraformaldehyde (PFA) for 20 min at 4 °C and washed twice with PBS for 15 min per rinse. According to the instructions for the GLUT-4 (Sigma) and Akt (Sigma) kits, primary antibodies against GLUT-4(1:200) and Akt (1:200) were incubated overnight at room temperature (with PBS as a negative control), and secondary antibodies (goat anti-rabbit lgG) conjugated to either HRP or Cy3 (Invitrogen) were employed, respectively. The cells were photographed. Buff-colored or red fluorescent grains on the cell membrane and/or in the cytoplasm suggested that the cells were positive; otherwise, we assumed that the cells were negative.
Western blotting
The cardiomyocytes (1.0×107) used for Western blotting were immediately frozen in liquid nitrogen and stored at −80 °C until use. Total protein was extracted with ice-cold lysis buffer and centrifugation (4 °C, 12 000×g, 10 min). The protein (20 μL per sample) was subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were blotted with primary rabbit anti-rat antibodies to GLUT-4 (1:1000), phospho-AMPKα (Thr172, p-AMPKα), AMPKα, Akt (1:1000) and β-actin (1:1000). After incubation overnight at 4 °C, blots were rinsed in TTBS for 30 min and incubated in HRP-conjugated secondary antibody (goat anti-rabbit IgG, 1:2000); bands were visualized using chemiluminescence (Pierce, Rockford, IL). All of the above primary antibodies were purchased from Santa Cruz Biotechnology. Relevant band intensities were quantified after normalization to the amount of β-actin protein.
AMPK activity assay
AMPK activity was measured as previously described. Briefly, both phospho-AMPKα (Thr172) and AMPKα were acquired by Western blot. AMPK activity was expressed as the ratio of p-AMPK relative to the total amount of AMPK (ie, defined as the optimal ratio of p-AMPKα to AMPKα).
Data analysis
All the experiments were repeated three times. All data in this study are presented as means±SD. Statistical analysis was performed using one-way analysis of variance (ANOVA). P values <0.05 were considered significant.
Results
Anti-apoptotic effect of Gs-Rb1 on hypoxic cardiomyocytes
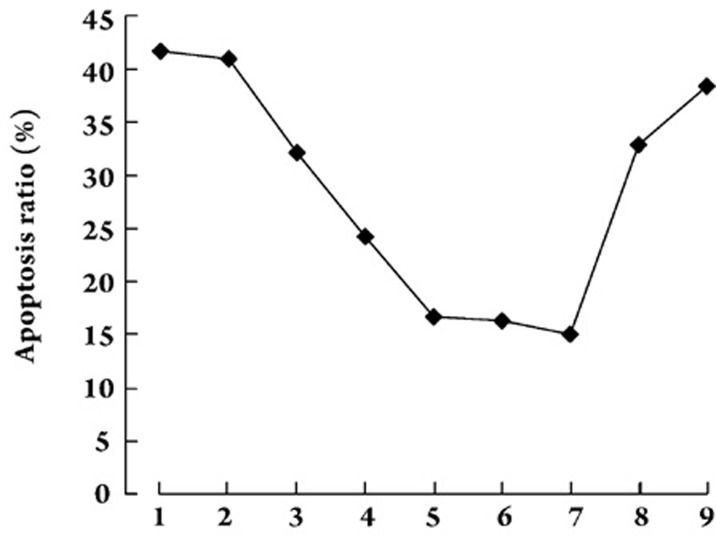
Because single positive (FITC+/PI−) populations are considered early apoptotic or necrotic cells, whereas the double positive (FITC+/PI+) cells exist in the late stage of apoptosis, both FITC+/PI− and FITC+/PI+ cells were classified as apoptotic cells. The apoptosis ratios of CoCl2-induced hypoxic cardiomyocytes were significantly decreased by treatment with Gs-Rb1 at all concentrations except 10 μmol/L (P=0.057), when compared with 0 μmol/L (P<0.001). In addition, there was a significant dose-dependent response from 50−200 μmol/L(P<0.05); this response was no longer seen at concentrations greater than 200 μmol/L (P>0.05). Taken the above results into account, 200 μmol/L Gs-Rb1 may be the optimal concentration, and the following experiments were based on this concentration (Figure 1).
Figure 1.
Anti-apoptotic effect of Gs-Rb1 on hypoxic cardiomyocytes induced by 500 μmol/L CoCl2 ex vivo. The numbers 1, 2, 3, 4, 5, 6, and 7 on the X-axis represent the concentration of Gs-Rb1 in which cells were incubated for 12 h (ie, 0, 10, 50, 100, 200, 400, and 500 μmol/L). Number 8 is 500 μmol/L ara A and number 9 is 0.5 μmol/L wortmannin, both of which were added to cells treated with 200 μmol/L Gs-Rb1 and 500 μmol/L CoCl2. The apoptotic ratio (including both FITC+/PI− and FITC+/PI+cells) in each group was 41.7%±5.44%, 40.9%±3.91%, 32.1%±4.13%, 24.3%±2.84%, 16.7%±1.41%, 16.3%±5.19%, 15.1%±4.67%, 32.9%±4.84%, 38.4%±3.49%, respectively. n=3 for all groups.
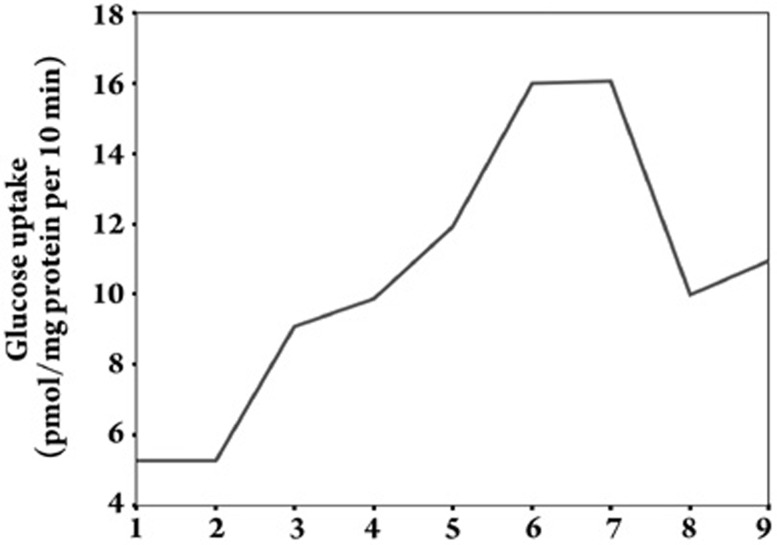
Effect of Gs-Rb1 on glucose uptake (Figure 2)
Figure 2.
Glucose uptake of hypoxic cardiomyocytes treated with different Gs-Rb1 concentrations in vitro. The numbers 1, 2, 3, 4, 5, 6, and 7 on the X-axis refer to the concentration of Gs-Rb1 incubation for 12 h (ie, 0, 10, 50, 100, 200, 400, and 500 μmol/L). Number 8 is 500 μmol/L ara A based on 200 μmol/L Gs-Rb1 and 500 μmol/L CoCl2, and number 9 is 0.5 μmol/L wortmannin based on 200 μmol/L Gs-Rb1 and 500 μmol/L CoCl2. The mean±SD of glucose uptake (pmol/mg protein per 10 min incubation) of cardiomyocytes was 5.263±0.064, 5.277±0.107, 9.070±0.131, 9.890±0.197, 11.900±0.200, 15.933±0.153, 16.033±0.306, 9.971±0.419 and 10.829±0.607, respectively. n=3 for all groups.
The 2-[3H]-DG uptake of cardiomyocytes after CoCl2-induced hypoxia was significantly increased up to 1.72±0.24-fold, 1.88±0.17-fold, 2.26±0.86-fold, 3.02±0.21-fold and 3.04±0.34-fold by treatment with 50, 100, 200, 400, and 500 μmol/L Gs-Rbl compared with hypoxia (P<0.01), respectively; no significant change was observed with 10 μmol/L Gs-Rbl (P=0.85). Significant dose-dependent increases were observed from 50−400 μmol/L; no significant difference was observed between 400 and 500 μmol/L (P=0.315). In order to identify the correlation between glucose uptake and cell apoptosis, we found a strong negative correlation (r=-0.57, P=0.002), which suggested that Gs-Rb1 might prevent hypoxic cardiomyocytes from undergoing apoptosis by improving glucose uptake.
Effect of stimulants on GLUT-4 protein levels
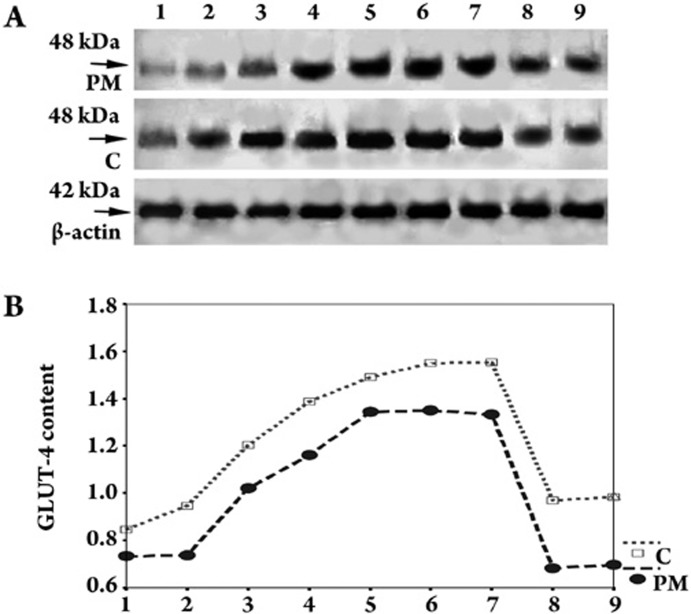
Despite hypoxia or Gs-Rb1 intervention, the GLUT-4 protein was expressed on the cell membranes and/or the cytoplasm of cardiomyocytes (Figure 3). To address GLUT-4 expression patterns in cells and PM, we evaluated it in treated cardiomyocytes. As shown in Figure 4, GLUT-4 protein content in cells was significantly up-regulated up to 1.54±0.11-fold at 50 μmol/L, 2.29±0.20-fold at 100 μmol/L, 2.49±0.19-fold at 200 μmol/L, 2.50±0.14-fold at 400 μmol/L and 2.49±0.23-fold at 500 μmol/L when Gs-Rb1 was applied (10 μmol/L had no effect, P=0.148) compared with hypoxia. There was a significant dose-dependent increase from 50 to 200 μmol/L Gs-Rb1. Similar to GLUT-4 protein content in cells, Gs-Rb1 induced the expression of GLUT-4 to 1.66±0.10- (50 μmol/L), 1.84±0.07- (100 μmol/L), 2.01±0.14- (200 μmol/L), 2.07±0.13- (400 μmol/L), and 2.05±0.22-fold (500 μmol/L) increases (P<0.001) over the hypoxia in PM (except 10 μmol/L, P=0.899); these effects were also dose-dependent from 50 to 200 μmol/L, with only marginal differences with doses greater than 200 μmol/L (P>0.05).
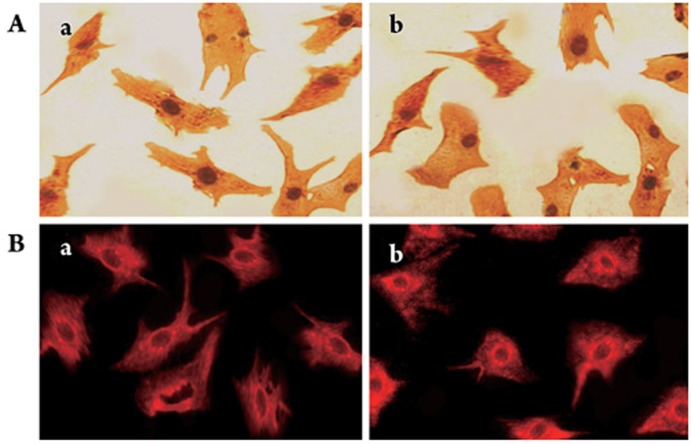
Figure 3.
GLUT-4 and Akt expression in hypoxic cardiomyocytes. A: immunocytochemistry for GLUT-4 (400×); buff-colored appear on the cell plasma membrane and/or in the cytoplasm, suggesting that these cells were positive. B: fluorescent immunocytochemistry for Akt (200×); red fluorescent grains on the cell plasma membrane and/or in the cytoplasm suggested that they were positive. a: 500 μmol/L CoCl2-induced hypoxia without Gs-Rb1 incubation for 12 h; b: 200 μmol/L Gs-Rb1 incubation after hypoxia induced with 500 μmol/L CoCl2.
Figure 4.
Expression of GLUT-4 contents in cells (C) and cell plasma membrane (PM) of cardiomyocytes using Western blot analysis. A shows a representative Western blot and B shows its polygram. Lane 1: 0 μmol/L Gs-Rb1, 2: 10 μmol/L Gs-Rb1, 3: 50 μmol/L Gs-Rb1, 4: 100 μmol/L Gs-Rb1, 5: 200 μmol/L Gs-Rb1, 6: 400 μmol/L Gs-Rb1, 7: 500 μmol/L Gs-Rb1, 8: 500 μmol/L ara A+200 μmol/L Gs-Rb1 and 9: 0.5 μmol/L wortmannin +200 μmol/L Gs-Rb1; all lanes contain 200 μmol/L CoCl2. β-actin served as an internal control. n=3 for all groups.
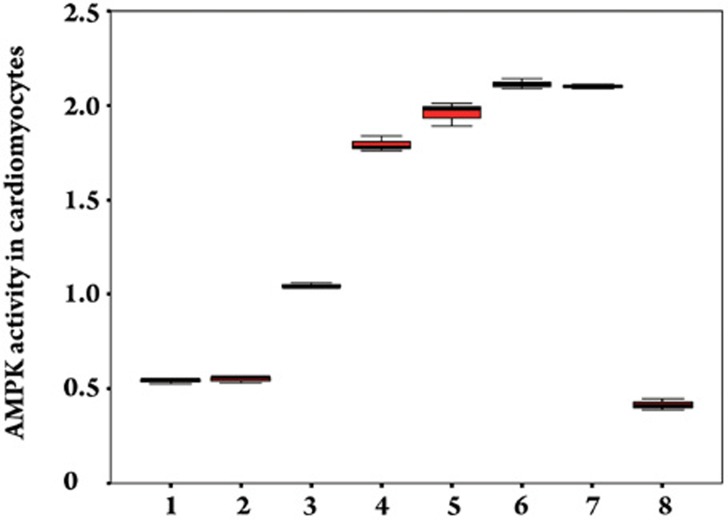
Effect of Gs-Rb1 on AMPK activity
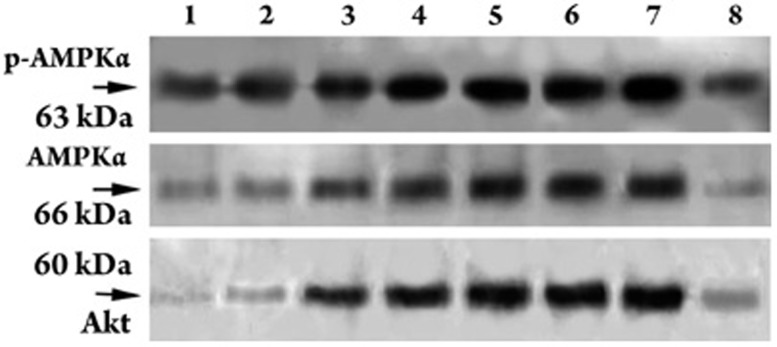
Cardiomyocytes expressed very low levels of AMPK activity in response to 500 μmol/L CoCl2-induced hypoxia. Compared with hypoxia, Gs-Rb1 significantly increased AMPK activity (P<0.01) by 1.93±0.13- (50 μmol/L), 3.30±0.29- (100 μmol/L), 3.61±0.34- (200 μmol/L), 3.90±0.26- (400 μmol/L), and 3.87±0.12-fold (500 μmol/L) except for Gs-Rb1 10 μmol/L (1.02±0.15-fold, P=0.501) (Figures 5 and 6). In accordance with glucose uptake and GLUT-4 expression, there was a significant dose-dependent increase in AMPK activity between 50 and 200 μmol/L (P<0.01) and no significant difference between 400 and 500 μmol/L Gs-Rb1 (P=0.573). To further determine whether AMPK activity mediated the above-mentioned role of Gs-Rb1 on CoCl2-induced hypoxia, ara-A, a competitive inhibitor of AMPK, was administered. Cardiomyocytes were randomized to either the ara-A group (incubated simultaneously for 12 h with 500 μmol/L CoCl2, 200 μmol/L Gs-Rb1 and 500 μmol/L ara-A) or the non-ara-A group (incubated synchronously for 12 h with 500 μmol/L CoCl2 and 200 μmol/L Gs-Rb1). AMPK activity was completely suppressed (Figure 6), GLUT-4 translocation and expression (Figure 4B) and glucose uptake (Figure 2) were significantly down-regulated, and the apoptosis ratio (Figure 1) was up-regulated when the ara-A group was compared with the non-ara-A group.
Figure 5.
p-AMPKα (Thr172), AMPKα, and Akt (Ser473) expression levels in cardiomyocytes using Western blot analysis. Lanes 1, 2, 3, 4, 5, 6, and 7 represent 0, 10, 50, 100 , 200 , 400, and 500 μmol/L Gs-Rb1. In lane 8, bands p-AMPKα (Thr172) and AMPKα were treated with 500 μmol/L ara-A and 200 μmol/L Gs-Rb1; band Akt in lane 8 was treated with 0.5 μmol/L wortmannin and 200 μmol/L Gs-Rb1. All lanes were treated with 200 μmol/L CoCl2 for 12 h. n=3 for all groups.
Figure 6.
Effect of Gs-Rb1 on AMPK activity in cardiomyocytes. 1, 2, 3, 4, 5, 6, and 7 represented 0, 10, 50, 100, 200, 400, and 500 μmol/L Gs-Rb1 incubation based on 200 μmol/L CoCl2 for 12 h. Number 8 represented 500 μmol/L ara-A based on 200 μmol/L Gs-Rb1 and 200 μmol/L CoCl2 together. n=3 for all groups.
Effect of stimulants on Akt protein levels
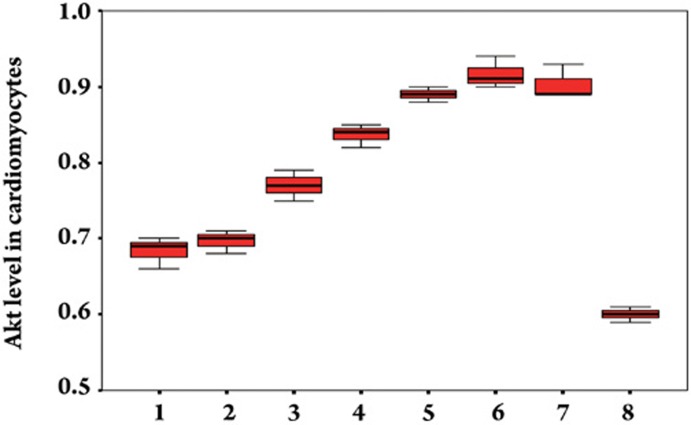
To ascertain the role of the PI3K/Akt pathway during Gs-Rb1 protection of cardiomyocytes from apoptosis, we further assayed whether Gs-Rb1 promoted Akt expression and whether the effect of Gs-Rb1 could be inhibited by wortmannin. Cells were randomized to the wortmannin group (incubated simultaneously with 500 μmol/L CoCl2, 200 μmol/L Gs-Rb1 and 0.5 μmol/L wortmannin for 12 h) or the non-wortmannin group (incubated simultaneously with 500 μmol/L CoCl2 and 200 μmol/L Gs-Rb1 for 12 h). As shown in Figures 5 and 7, stimulation with Gs-Rb1 produced a 1.05±0.24- (50 μmol/L), 1.21±0.19- (100 μmol/L), 1.27±0.17- (200 μmol/L), 1.26±0.21- (400 μmol/L), and 1.27±0.09-fold (500 μmol/L) increase (except for Gs-Rb1 10 μmol/L, P=0.714) of Akt (P<0.01) compared with hypoxia in a significant, dose-dependent manner from 50 to 400 μmol/L. Wortmannin completely inhibited Gs-Rb1-mediated Akt expression (P<0.01, Figures 5 and 7) and partly down-regulated both translocation and expression of GLUT-4 (Figure 4B) and glucose uptake (Figure 2) and up-regulated the apoptosis ratio (Figure 1).
Figure 7.
Effect of Gs-Rb1 on Akt expression in cardiomyocytes. The numbers 1, 2, 3, 4, 5, 6, and 7 represent 0, 10, 50, 100, 200, 400, and 500 μmol/L Gs-Rb1 incubation based on 200 μmol/L CoCl2 for 12 h. Number 8 represents 0.5 μmol/L wortmannin based on 200 μmol/L Gs-Rb1 and 200 μmol/L CoCl2 together. n=3 for all groups.
Discussion
Because hypoxia, a pathological state, may induce cardiomyocyte apoptosis, which is an important mode of cell death in various heart diseases, many scholars in the cardiovascular field have dedicated themselves to improve the anti-hypoxic capability of cardiomyocytes. Although ginseng is frequently used as a crude substance taken orally in many countries, its major component, Gs-Rb1, plays a role in improving ischemic reperfusion injury14, 15, 16; however, the anti-hypoxic effect of Gs-Rb1 has rarely been analyzed. Because an effective dose of Gs-Rb1 has not been reported, we tested different Gs-Rb1 concentrations (0, 10, 50, 100, 200, 400, and 500 μmol/L), and we found that Gs-Rb1 could significantly decrease the apoptotic ratio of hypoxic cardiomyocytes induced by CoCl2. Of the doses tested, 200 μmol/L of Gs-Rb1 may be the optimal anti-hypoxic concentration. Our data suggested that Gs-Rb1 may effectively protect hypoxic cardiomyocytes from apoptosis in vitro. For a better understanding of how Gs-Rb1 exerts its anti-apoptotic effect, we investigated glucose uptake and GLUT-4, among other potential mechanisms.
The predominant substrate for cardiac energy metabolism during ischemia/hypoxia is glucose; therefore, increasing glucose uptake may prevent cardiomyocytes from undergoing apoptosis once they are hypoxic. Treatment of cells with 500 μmol/L CoCl2 significantly increases the apoptotic ratio and inhibits glucose uptake; thus, Gs-Rb1 was administered to hypoxic cardiomyocytes (induced with 500 μmol/L CoCl2) ex vivo in order to determine both the efficacy and the mechanism of Gs-Rb1. Our results, in which Gs-Rb1 significantly increased glucose uptake in a dose-dependent manner compared with hypoxic cells and a strong negative correlation existed between glucose uptake and apoptosis, suggested that Gs-Rb1 may prevent the apoptosis of hypoxic cardiomyocytes as a result of improved glucose uptake.
Glucose enters cardiomyocytes by facilitative transport, a process that is mainly mediated by GLUT-4 proteins7, 8. The sustained increase in glucose uptake in cardiomyocytes mainly depended on both translocation and expression of GLUT-4, and translocation (ie, constitutively residing at the cell surface to optimize cell exposure to extracellular glucose) is particularly important18. In the physiological state of the cells, GLUT-4 is found on cell membranes and in the intracellular compartment; once the cell encounters stress, such as hypoxia19, ischemia20, etc.21, 22, 23, GLUT-4 can be rapidly translocated to the cell surface, allowing the cell to transiently increase its access to extracellular glucose24, 25. Cardiac glucose uptake and glycolysis are protective in the ischemic heart26, and glucose transporter deficiency decreases ischemic tolerance27. In the present study, we have shown that GLUT-4 was expressed at higher levels and readily exchanged with the cell surface and presumably endosomes in hypoxic cardiomyocytes after exposure to Gs-Rb1. Thus, it is likely that both GLUT-4 translocation and expression play key roles in Gs-Rb1-induced, persistent, increased glucose uptake in hypoxic cardiomyocytes.
A growing number of in-vivo28, 29, 30, 31 and in-vitro32 studies have indicated that AMPK activation may directly regulate GLUT-4 expression11, 28, 29, 30, 31, 32, 33 and induce a significant increase in glucose uptake9, 10, 11, 28, 29, 30, 31, 32, 33, 34 in cultured skeletal muscle cells. In cardiomyocytes, hypoxia may increase AMPK-mediated glucose uptake and GLUT-4 translocation35, which is not sufficient to increase glucose uptake36. In the present study, Gs-Rb1 significantly increased AMPK activity in a dose-dependent manner up to a threshold concentration. AMPK phosphorylation can be regulated by its physiological regulators, including upstream kinases (Ca2+/calmodulin-dependent protein kinase kinases) and LKB1 and the cellular AMP/ATP ratio. Whether the efficacy of Gs-Rb1 on AMPK activity was also mediated by these regulators requires further investigation. To further determine the role of AMPK in Gs-Rb1 anti-hypoxia, ara-A (a competitive inhibitor of AMPK) was also employed in our present study. The results showed that AMPK activity was wholly inhibited, both glucose uptake and GLUT-4 translocation (including expression) were partly inhibited, and the apoptotic ratio was up-regulated by ara-A. These findings suggested that Gs-Rb1 may protect cardiomyocytes from apoptosis by other mechanisms beside AMPK. To summarize, AMPK activity was an important regulator of Gs-Rb1's anti-hypoxic effect on cardiomyocytes, which may be part of a signaling cascade, such as, both myocardial glucose uptake and glycolysis are activated, followed by an increase in fatty acid oxidation.
In this study of how Gs-Rb1 protects cardiomyocytes from apoptosis, we found that Gs-Rb1 could also significantly up-regulate Akt expression and that this expression was entirely abrogated by preincubation with wortmannin, a competitive inhibitor of phosphatidylinositol 3-kinase (PI3K) activity. Thus, it appeared that PI3K activity was necessary for the Akt-mediated effects of Gs-Rb1. Akt, a serine/threonine kinase that acts downstream of PI3K, is activated by a dual mechanism involving the binding of phosphatidylinositol 3,4,5-trisphosphate (PIP3)37 to the Akt PH domain38, as well as phosphorylation of either phosphatidylinositol 3,4-bisphosphate (PIP2) or serine/threonine by one or more Akt kinases. It was possible that PI3K pathway is the main downstream pathway of Gs-Rb1. Many studies have demonstrated that PI3K/Akt may be necessary for GLUT-4 translocation12, 13, 39, 40 and glucose uptake. Consistent with these findings, the present results — in which the effects of Gs-Rb1, were partly inhibited by wortmannin without Akt activation — indicated that PI3K was partly responsible for the efficacy of Gs-Rb1. In addition, PI3K also phosphorylates the insulin receptor substrate-1 (IRS-1), an in vitro substrate for this activity41, 42, and exhibits both lipid and protein kinase activities, all of which may play an important role in the effects of Gs-Rb1. In the mechanism through which Gs-Rb1 protects cardiomyocytes from apoptosis, it is important to determine whether PI3K can affect Gs-Rb1 via pathways beside Akt. Briefly, Gs-Rb1 may allow cardiomyocytes to survive under hypoxic conditions through the activation of Akt as a PI3K-dependent upstream activator of GLUT-4 translocation.
The balance between anti-apoptotic and pro-apoptotic proteins43, 44, the release of cytochrome c from mitochondria, caspase activation, and the activation of protein kinase C isozymes may reduce myocardial apoptosis. Whether Gs-Rb1 decreases pro-apoptotic Bax, increases the anti-apoptotic Bcl-2 proteins, resulting in an altered Bcl-2/Bax ratio, attenuates cytochrome c release from mitochondria or inhibits caspase activity must still be demonstrated.
In conclusion, our findings present direct evidence supporting the role of Gs-Rb1 in protecting hypoxic cardiomyocytes from apoptosis. In addition, the stimulation of GLUT-4 translocation and glucose transport activity and the lack of hypoxia-induced apoptosis inhibition by either ara-A or wortmannin suggested that the effect of Gs-Rb1 may be the result of several pathways in addition to AMPK and PI3K. Nevertheless, these results suggested that both AMPK and PI3K played an important role in the determination of overall glucose utilization. However, future experiments will be necessary to determine the precise mechanism of Gs-Rb1 in mediating this efficacy.
Author contribution
Hong-liang KONG designed research; Hong-liang KONG and Jian-ping WANG performed research; Hong-liang KONG, Zhan-quan LI and Shu-mei ZHAO contributed new analytical tools and reagents; Hong-liang KONG and Jing DONG analyzed data; Hong-liang KONG, Shu-mei ZHAO, and Wei-wei ZHANG wrote the paper.
Acknowledgments
We thank Prof Hai-peng ZHANG (Department of Pathophysiology, China Medical University) for providing reagents.
References
- Rahimtoola SH. The hibernating myocardium. Am Heart J. 1989;117:211–21. doi: 10.1016/0002-8703(89)90685-6. [DOI] [PubMed] [Google Scholar]
- Ausma J, Thoné F, Dispersyn GD, Flameng W, Vanoverschelde JL, Ramaekers FC, et al. Dedifferentiated cardiomyocytes from chronic hibernating myocardium are ischemia-tolerant. Mol Cell Biochem. 1998;186:159–68. [PubMed] [Google Scholar]
- Freude B, Masters TN, Kostin S, Robicsek F, Schaper J. Cardiomyocyte apoptosis in acute and chronic conditions. Basic Res Cardiol. 1998;93:85–9. doi: 10.1007/s003950050066. [DOI] [PubMed] [Google Scholar]
- Dispersyn GD, Ausma J, Thoné F, Flameng W, Vanoverschelde JL, Allessie MA, et al. Cardiomyocyte remodelling during myocardial hibernation and atrial fibrillation: prelude to apoptosis. Cardiovasc Res. 1999;43:947–57. doi: 10.1016/s0008-6363(99)00096-6. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Fallavollita JA, Suzuki G, Borgers M, Canty JM., Jr Dissociation of regional adaptations to ischemia and global myolysis in an accelerated swine model of chronic hibernating myocardium. Circ Res. 2002;91:970–7. doi: 10.1161/01.res.0000040396.79379.77. [DOI] [PubMed] [Google Scholar]
- Ausma J, Wijffels M, van Eys G, Koide M, Ramaekers F, Allessie M, et al. Dedifferentiation of atrial cardiomyocytes as a result of chronic atrial fibrillation. Am J Pathol. 1997;151:985–97. [PMC free article] [PubMed] [Google Scholar]
- Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–8. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- Burant CF, Sivitz WI, Fukumoto H, Kayano T, Nagamatsu S, Seino S, et al. Mammalian glucose transporters: structure and molecular regulation. Recent Prog Horm Res. 1991;47:349–87. doi: 10.1016/b978-0-12-571147-0.50015-9. [DOI] [PubMed] [Google Scholar]
- Chen J, Hudson E, Chi MM, Chang AS, Moley KH, Hardie DG, et al. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev Biol. 2006;291:227–38. doi: 10.1016/j.ydbio.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, et al. Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–34. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, et al. Lucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–7. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- Martin SS, Haruta T, Morris AJ, Klippel A, Williams LT, Olefsky JM. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem. 1996;271:17605–8. doi: 10.1074/jbc.271.30.17605. [DOI] [PubMed] [Google Scholar]
- Fischer Y, Kamp J, Thomas J, Pöpping S, Rose H, Carpéné C, et al. Signals mediating stimulation of cardiomyocyte glucose transport by the alpha-adrenergic agonist phenylephrine. Am J Physiol. 1996;270 4 Pt 1:C1211–20. doi: 10.1152/ajpcell.1996.270.4.C1211. [DOI] [PubMed] [Google Scholar]
- Liu YF, Liu SW, Liu ZX. Effects of ginsenoside Rb1 on blood vessel regeneration after ischemia and reperfusion in rats. Chin J Histochem Cytochem. 2008;17:39–44. [Google Scholar]
- Zeng HS, Liu ZX. Influence of ginsenoside Re on cardiomyocyte apoptosis and Fas expression after acute ischemia-reperfusion in rats. J Huazhong Univ Sci Tech [Health Sci] 2004;33:286–8. [Google Scholar]
- Guan L, Li WZ, Liu ZX. Effect of ginsenoside-Rbl on cardiomyocyte apoptosis after ischemia and reperfusion in rats. J Huazhong Univ Sci Tech[Med Sci] 2002;22:212–5. doi: 10.1007/BF02828182. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y, Miyauchi T, Yuki K, Murakoshi N, Goto K, Yamaguchi I. Mitochondrial dysfunction of cardiomyocytes causing an impairment of cellular energy metabolism induces apoptosis, which is accompanied by an increase in cardiac ET-1 expression. J Cardiovasc Pharmacol. 2000;36:S201–4. doi: 10.1097/00005344-200036051-00061. [DOI] [PubMed] [Google Scholar]
- James DE, Piper RC. Insulin resistance, diabetes, and the insulin-regulated trafficking of GLUT-4. J Cell Biol. 1994;126:1123–6. doi: 10.1083/jcb.126.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Nguyen N, DeGrado TR, Schwaiger M, Brosius FC., 3rd Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation. 1994;89:793–8. doi: 10.1161/01.cir.89.2.793. [DOI] [PubMed] [Google Scholar]
- Young LH, Renfu Y, Russell R, Hu X, Caplan M, Ren J, et al. Low-flow ischemia leads to translocation of canine heart GLUT-4 and GLUT-1 glucose transporters to the sarcolemma in vivo. Circulation. 1997;95:415–22. doi: 10.1161/01.cir.95.2.415. [DOI] [PubMed] [Google Scholar]
- Coven DL, Hu X, Cong L, Bergeron R, Shulman GI, Hardie DG, et al. Physiological role of AMP-activated protein kinase in the heart: graded activation during exercise. Am J Physiol Endocrinol Metab. 2003;285:E629–36. doi: 10.1152/ajpendo.00171.2003. [DOI] [PubMed] [Google Scholar]
- Fischer Y, Thomas J, Sevilla L, Muñoz P, Becker C, Holman G, et al. Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes. Evidence of the existence of different intracellular GLUT4 vesicle populations. J Biol Chem. 1997;272:7085–92. doi: 10.1074/jbc.272.11.7085. [DOI] [PubMed] [Google Scholar]
- Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest. 1988;82:2017–25. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–62. [PubMed] [Google Scholar]
- Suzuki K, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA. 1980;77:2542–5. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie LH, Sack MN. Metabolic plasticity and the promotion of cardiac protection in ischemia and ischemic preconditioning. J Mol Cell Cardiol. 2002;34:1077–89. doi: 10.1006/jmcc.2002.2066. [DOI] [PubMed] [Google Scholar]
- Tian R, Abel ED. Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation. 2001;103:2961–6. doi: 10.1161/01.cir.103.24.2961. [DOI] [PubMed] [Google Scholar]
- Buhl ES, Jessen N, Schmitz O, Pedersen SB, Pedersen O, Holman GD, et al. Chronic treatment with 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner. Diabetes. 2001;50:12–7. doi: 10.2337/diabetes.50.1.12. [DOI] [PubMed] [Google Scholar]
- Jessen N, Pold R, Buhl ES, Jensen LS, Schmitz O, Lund S. Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J Appl Physiol. 2003;94:1373–9. doi: 10.1152/japplphysiol.00250.2002. [DOI] [PubMed] [Google Scholar]
- Jørgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, et al. Role of AMPKalpha2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–9. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- Suwa M, Egashira T, Nakano H, Sasaki H, Kumagai S. Metformin increases the PGC-1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J Appl Physiol. 2006;101:1685–92. doi: 10.1152/japplphysiol.00255.2006. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Nolte LA, Holloszy JO. Increased expression of GLUT-4 and hexokinase in rat epitrochlearis muscles exposed to AICAR in vitro. J Appl Physiol. 2000;88:1072–5. doi: 10.1152/jappl.2000.88.3.1072. [DOI] [PubMed] [Google Scholar]
- Holmes BF, Sparling DP, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT4 enhancer factor and myocyte enhancer factor 2 by AMP-activated protein kinase. Am J Physiol Endocrinol Metab. 2005;289:E1071–6. doi: 10.1152/ajpendo.00606.2004. [DOI] [PubMed] [Google Scholar]
- Fryer LG, Foufelle F, Barnes K, Baldwin SA, Woods A, Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J. 2002;363:167–74. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan F, Yu B, Qi GX, Hu J, Zeng DY, Luo J. Chemical hypoxia-induced glucose transporter-4 translocation in neonatal rat cardiomyocytes. Arch Med Res. 2008;39:52–60. doi: 10.1016/j.arcmed.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Omar MA, Fraser H, Clanachan AS. Ischemia-induced activation of AMPK does not increase glucose uptake in glycogen-replete isolated working rat hearts. Am J Physiol Heart Circ Physiol. 2008;294:H1266–73. doi: 10.1152/ajpheart.01087.2007. [DOI] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses. J Cell Sci. 2001;114:2903–10. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Downes CP. The role of PI3-kinase in insulin action. Biochim Biophys Acta. 1998;1436:151–64. doi: 10.1016/s0005-2760(98)00133-7. [DOI] [PubMed] [Google Scholar]
- Tanti JF, Grillo S, Grémeaux T, Coffer PJ, Van Obberghen E, Le Marchand-Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–10. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–8. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Lam K, Carpenter CL, Ruderman NB, Friel JC, Kelly KL. The phosphatidylinositol 3-kinase serine kinase phosphorylates IRS-1. Stimulation by insulin and inhibition by wortmannin. J Biol Chem. 1994;269:20648–52. [PubMed] [Google Scholar]
- Tanti JF, Grémeaux T, Van Obberghen E, Le Marchand-Brustel Y. Insulin receptor substrate 1 is phosphorylated by the serine kinase activity of phosphatidylinositol 3-kinase. Biochem J. 1994;304 Pt 1:17–21. doi: 10.1042/bj3040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik N, Yoshida T, Das DK. Regulation of cardiomyocyte apoptosis in ischemic reperfused mouse heart by glutathione peroxidase. Mol Cell Biochem. 1999;196:13–21. doi: 10.1007/978-1-4615-5097-6_2. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Bossy-Wetzel E, Burns K, Fadel MP, Lozyk M, Goping IS, et al. Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J Cell Biol. 2000;150:731–40. doi: 10.1083/jcb.150.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]