Abstract
Home telemonitoring can augment home health care services during a patient's transition from hospital to home. Home health care agencies commonly use telemonitors for patients with heart failure although studies have shown mixed results in the use of telemonitors to reduce rehospitalizations. This randomized trial investigated if older patients with heart failure admitted to home health care following a hospitalization would have a reduction in rehospitalizations and improved health status if they received telemonitoring. Patients were followed up to 180 days post-discharge from home health care services. Results showed no difference in the time to rehospitalizations or emergency visits between those who received a telemonitoring vs. usual care. Older heart failure patients who received telemonitoring had better health status by home health care discharge than those who received usual care. Therefore for older adults with heart failure telemonitoring may be important adjunct to home health care services to improve health status.
Keywords: aging, heart failure, health status, home care, telemonitoring
Introduction
Trials of in-home telemonitoring for patients with heart failure (HF) continue to produce mixed evidence in the rate of rehospitalizations. Recently a large randomized trial found no benefit of telemonitoring over usual care for all-cause mortality or rehospitalization although adherence to monitoring had dropped considerably by the end of the study. (Chaudhry et al.) In contrast, a Cochrane Review and a meta-analysis (structured telephone interventions included) concluded that telemonitoring reduces all-cause mortality and HF-related hospitalizations.(Inglis et al.; Klersy, De Silvestri, Gabutti, Regoli, & Auricchio, 2009) Health status has been less frequently studied as an outcome, but may be where patients glean the most benefit from telemonitoring. Health status is markedly important to older and chronically ill adults in the community. (Gellis et al., 2012)
Despite the variable results for telemonitoring trials, telemonitoring has become a popular service and marketing tool for many home health care agencies (HHC) in the United States. Initially the use of telemonitoring was cost driven. HHC agencies viewed telemonitoring as a “nurse extender” to reduce the number of home visits needed per patient but providing the same patient oversight. HHC have public reporting of processes and outcomes of care on the Medicare website. While there are a number of important processes and outcomes of care, HHC agencies pay particular attention to rates of acute care hospitalization. Telemonitoring is seen as one way to control or reduce acute care hospitalization. HHC agencies perceive telemonitoring as desirable since it may allow for early recognition of HF decompensation and reduce the clinician response time allowing for earlier intervention.
The Ohio Telecare study was a pragmatic randomized trial of telemonitoring (TM) vs. usual care (UC) in older HHC patients with HF to evaluate the effectiveness of TM on rehospitalizations and health status. An important goal was to capture HF patients in the community who are known to have multiple comorbid conditions and impaired functional status.(Madigan, 2008) In contrast to other trials, patients were already referred for HHC as part of their hospital discharge plan. This assured that the study patients truly reflected the type of patient who would receive HHC in the community. In addition, the research team did not control the monitoring patterns nor interfere or dictate the response to TM data. Rather, the HHC agency nurses managed their HF patients according to each agency's policies and procedures. This allowed study of routine HHC for HF in present day practice and as close to routine operating procedures as possible. We hypothesized that those who received a telemonitor would have fewer rehospitalizations and improved health status compared to those who received usual care.
Methods
Seven Ohio-based HHC agencies participated. One agency had a dedicated HF program which included a medical director and dedicated HF nurses. The Visiting Nurse Association of Cleveland supported the trial, but had no access to the trial data or analysis. Each agency received payment to support staff education and 5 free telemonitors from Honeywell HomMed. Honeywell HomMed representative had no active role in the trial, nor did they have access to the data or the evaluation of the outcomes. To cover all the home care agencies, IRB approval was obtained from University Hospitals/Case Medical Center, Ohio Health and Genesis Health Care. The authors are solely responsible for the design and conduct of this study, all study analyses and drafting and editing of the paper.
Education and Preparation of Home Health Care Agency Nursing Staff
HHC staff were given an intensive 8 hour course adapted from the National Heart Failure Training Program (www.nheft.org). The educational program was designed to teach all the nurses about HF management and provide tools for standardized patient education on HF self-management.
Telemonitoring
The TM was installed in the patient's home post-hospitalization and prompted the patient to measure blood pressure, pulse, oxygen saturation and weight daily at a pre-specified time. Data were transmitted through the patient's telephone line to a central monitoring station at each HHC agency. A trained nurse reviewed the data within a few hours. The study protocol did not provide instructions on when a physician should be contacted or how to respond to abnormal data. This was performed according to the HHC agency's protocol. Nurses did not have the authority to change medications without a physician's order.
Usual Care
Patients in UC received the same HF education from their HHC nurse as the TM group. All patients were taught to follow their own weights and symptoms. Visits to the home were based on each individual agency's protocol and the needs of the patient.
Study Patients
Patients referred to a participating HHC agency by their discharging hospital were offered participation. Inclusion criteria included: HF diagnosis (primary or secondary), NYHA(New York Heart Association) class II- IV. Exclusions criteria included: inability to stand on a scale, weight over 500 pounds, unable to hear and/or see, no working phone line, unstable angina, MI(myocardial infarction) and/or CABG(coronary artery bypass graft) in the previous 6 weeks, severe uncorrected valvular disease, home inotropes, oxygen-dependent chronic lung disease, active cancer, uncorrected thyroid disease, AIDS, or end stage renal disease on dialysis. Study duration was for as long as the patient received HHC services and was dictated by the HHC agency's clinical protocols.
Patients were consented by the HHC nurse and randomized within each HHC site to either UC or TM following an unconstrained randomization scheme with 50% chance of allocation to each arm. Patients agreed to accept the randomization assignment prior to randomization as part of the consenting process. The randomization sequence was held by an investigative team member who was responsible for following the randomization protocol at each HHC site and who was un-involved with the consenting process or the patient's care. All records were kept as confidential.
Data Collected
Data on patient demographics, HHC visits, and all-cause re-hospitalizations were collected using the HHC chart and the Outcome and Assessment Information Set (OASIS) dataset. OASIS is the standardized data collection instrument used by HHC agencies for adult patients' insured by Medicare or Medicaid.
The length of HHC services was dependent on clinical status and whether the patient met HHC goals for self-care. HHC payment episodes are 60 days in length but if the HHC goals are not met additional 60 day intervals are provided. If HHC goals are met prior to 60 days, the agency may discharge the patient earlier. Telephone follow-up was performed at 90 and 180 days post-HHC discharge to document rehospitalizations, ED and urgent care visits, and death during the post-intervention period.
Combined Outcome
The primary outcome was the combined outcome of all-cause rehospitalizations, all –cause emergency department/urgent care visits, or death. Once a patient reached one of these endpoints and was still alive, we did not continue to follow them for additional rehospitalizations or emergency department visits. However, the patients who reached an endpoint remained in the study in order to collect their health status questionnaire at the time of HHC discharge.
Health Status
For the purpose of measuring health status, patients remained in the study for as long as they received HHC. Since rehospitalized patients are not officially discharged from HHC during rehospitalization, the health status questionnaire was collected at the time of patient discharge from HHC. Once the patient is discharged from the hospital, HHC is resumed until the patient meets the preset HHC goals. At the HHC discharge time point, the HHC team has deemed the patient “independent” and able to take over their own care. Collecting health status at this time point allowed for the most uniformity of patient function for their follow-up.
The Kansas City Cardiomyopathy Questionnaire (KCCQ) (Green, Porter, Bresnahan, & Spertus, 2000; Spertus et al., 2005) was self-administered at the first HHC visit and at the last visit before discharge. The HHC nurse was permitted to assist the patient if needed due to poor comprehension or eyesight. The KCCQ was followed according to protocol and patients reflected on the prior 2 weeks to answer the questions. Scoring for the KCCQ is 0 to 100. Higher scores indicate better health status.
Physician-Nurse Contacts
Nurses assessed if the patient medication list adhered to the HF Guidelines. Nurses faxed the medication list with Guideline recommendations to the physician overseeing the HHC services to prompt adherence to HF medication guidelines.
The number of calls from the nurse to the physician per patient was collected from the clinical chart. These data were collected to assess if TM impacted how frequently the nurse and physician would need to discuss a patient and/or change the orders. Physician responses, when required, were also collected. Calls were categorized as HF-related or not HF-related. Calls that required a physician response such as a request for a medication change, or request for an order regarding signs and symptoms were counted.
Statistical Analysis
Summary statistics described baseline characteristics. The time to combined endpoint is interval censored because for subjects who had the combined endpoint after the HHC period the exact date of the combined endpoint is not known. Therefore, time to combined endpoint is analyzed with a non-parametric maximum likelihood method, Turnball's estimate(Turnbull, 1976). Crude analyses of the effect of TM on KCCQ scores were carried out using an ANCOVA model with group and baseline KCCQ as the covariates. The impact of adjustment for various potential confounders on the effect of TM on KCCQ scores was assessed with a progressive, pre-specified set of ANCOVA models. The simplest model included only group and baseline KCCQ in modeling follow-up KCCQ. Subsequent models added the effects of age and race followed by baseline NYHA class and agency and finally magnitude of HHC services (length of HHC services, number of HHC visits). Length of stay and the number of HHC visits are highly correlated, so they were assessed jointly. Crude comparisons between groups for the physician call data were made with chi-square and t-tests. Regression models were considered to adjust for the subject's time in the study (results not shown).
Results
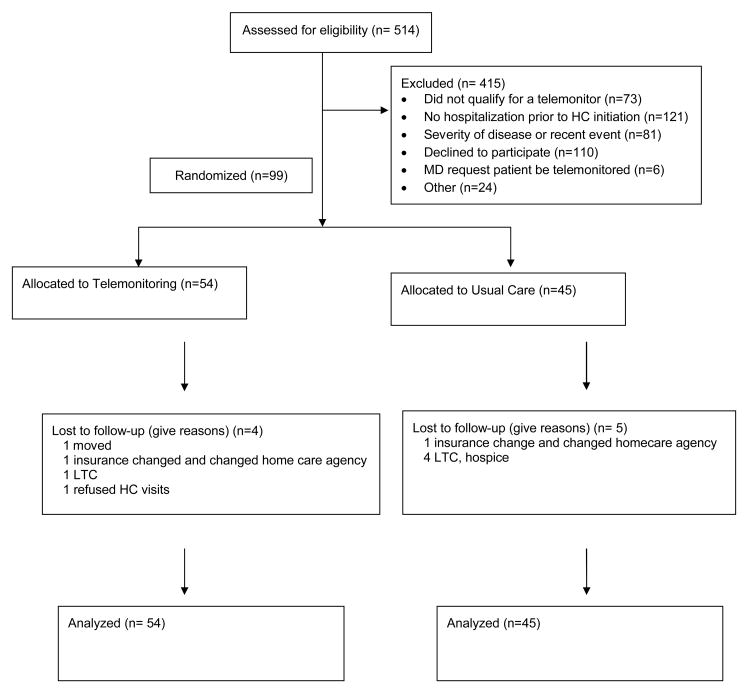
A total of 514 patients were screened and ninety-nine participated (54 TM, 45 UC) from 6 HHC agencies representing 44 counties in Ohio. (see Figure 1). One agency did not enroll any patients. Patients were predominantly female, Caucasian and had HF with preserved ejection fraction. (Table 1). Baseline demographics were balanced except for more diabetics in the usual care group.
Figure 1. Screening, Randomization, and Follow-up of the Study Patients.
Table 1. Baseline Characteristics.
| Variable | Sample sizes (if different) | Usual Care (N=45) | Telemonitor (N=54) |
|---|---|---|---|
| Patient Demographics | |||
| Age (yrs), mean ± SD | 74.7 ± 11.3 | 75.0 ± 12.1 | |
| Female, n (%) | 27 (60.0) | 40 (74.1) | |
| African American Race, n (%) | 12 (26.7) | 9 (16.7) | |
| Ischemic HF etiology, n (%) | 39, 44 | 27 (69.2) | 32 (72.7) |
| Systolic HF type, n (%) | 36, 42 | 11 (30.6) | 20 (47.6) |
| NYHA Class*, n (%) | |||
| NYHA II | 43, 53 | 19 (44.2) | 22 (41.5) |
| NYHA III | 20 (46.5) | 28 (52.8) | |
| NYHA IV | 4 (9.3) | 3 (5.7) | |
| KCCQ, mean ± SD | |||
| Overall Summary Score | 43, 52 | 36.4 ± 18.0 | 36.0 ± 21.7 |
| Clinical Summary Score | 43, 52 | 37.7 ± 20.7 | 35.4 ± 22.0 |
| Comorbidities, n (%) | |||
| Diabetes** | 28 (62.2) | 23 (42.6) | |
| Anemia | 4 (8.9) | 6 (11.1) | |
| Hypertension | 24 (53.3) | 25 (46.3) | |
| CAD | 15 (33.3) | 17 (31.5) | |
| AFib | 15 (33.3) | 27 (50.0) | |
| COPD | 15 (33.3) | 18 (33.3) | |
| Acute infection(UTI, Pneumonia) | 6 (13.3) | 3 (5.6) | |
| Chronic Kidney Disease | 7 (15.6) | 5 (9.3) | |
| Chronic Skin Ulcer | 3 (6.7) | 4 (7.4) | |
| Gen Muscle Weak/Fatigue | 20 (44.4) | 22 (40.7) | |
| Medications and Dosage‡ | |||
| Enalapril eq., n (%) | 14 (31.1) | 23 (42.6) | |
| Daily dose, mg | 25.7 (2.5, 20, 80) | 20.3 (2.5, 10, 100) | |
| Valsartan eq., n (%) | 13 (28.9) | 13 (24.1) | |
| Daily dose, mg | 240 (80, 320, 320) | 194 (40, 160, 640) | |
| Metoprolol eq., n (%) | 37 (82.2) | 42 (77.8) | |
| Daily dose, mg | 93.9 (12.5,100,320) | 92.0 (12.5, 87.5, 400) | |
| Furosemide eq., n (%) | 40 (88.9) | 43 (79.6) | |
| Daily dose, mg | 104.5 (20, 80, 480) | 60.9 (20, 40, 320) |
NYHA- New York Heart Association Class
Class I – Asymptomatic
Class II – Symptomatic with usual activity
Class III – Symptomatic with minimal activity
Class IV – Symptomatic at rest
difference in number of diabetics between groups p=0.05
Medication daily doses are mean (min, median, max).
Combined endpoint (all-cause rehospitalizations, emergency department visits, and death)
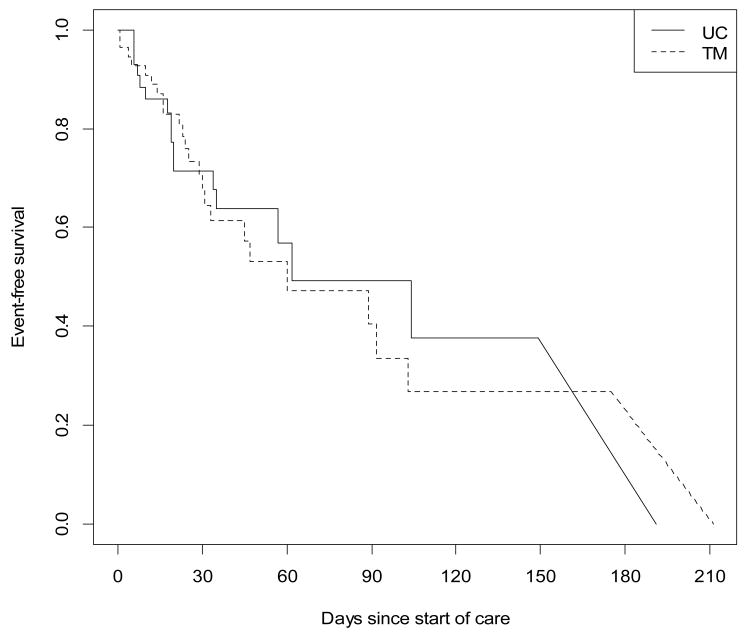
The median time to combined endpoint was 62 days in the UC group compared to 60 days demonstrating that there no difference between the two groups (p=0.5) (Figure 2).
Figure 2. Time to Combined Endpoint.
Median Time to CE Control 62 days, TM 60 days, p=.50. Diagonal lines are used to indicate ranges of time at which the survival estimate is indeterminate due to interval censored data. Combined Endpoint: hospitalization or ED visit or urgent care visit or death. HHC = Home Health Care.
Health Status
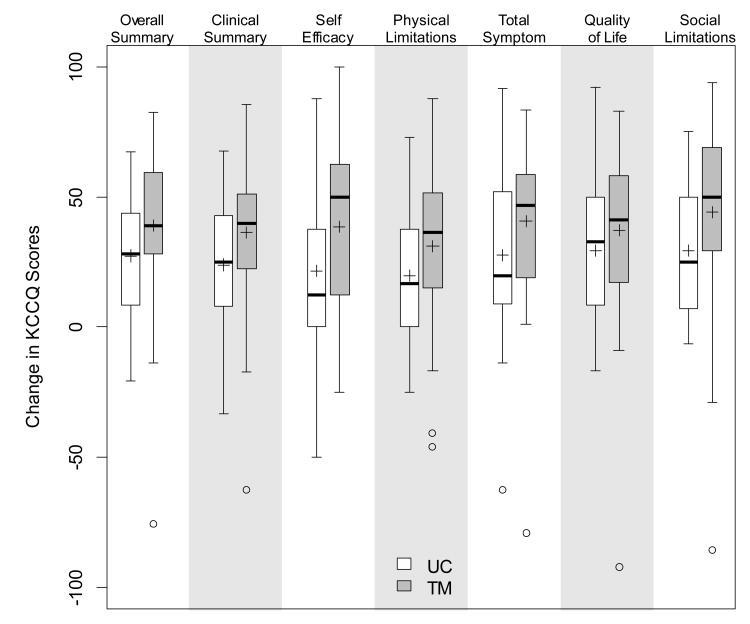
The KCCQ scores were low at baseline in all domains. The Overall Summary Score was 37.7 ± 20.7 for the TM group and 36.4 ± 18.0 for the UC group and increased to 72 ± 20.2 for the TM group and 64.7± 26.8 for the UC group. (Figure 3) There was no significant difference in any baseline characteristics (including KCCQ scores) between those who did not have a second KCCQ (n=18) compared with those who completed follow up (n=81). Reasons for the missing follow up KCCQ: 5-entered long term care facility, 5- missing, 3-death, 2-patient or family requested immediate discharge, 1-withdrew from the study.
Figure 3. Change in the KCCQ: Usual Care vs. Telemonitor.
Boxplots show median, IQR, and range of the data up to the “potential outliers” which are individually marked. Means are appended as +. For all domains, mean scores for TM had a larger positive change in KCCQ than mean scores in UC. All p-values < 0.05 except Quality of Life subscale (p=.21).
When controlling for baseline KCCQ, age, race, baseline NYHA class, agency A (indicator variable for the one agency with a well-established and more comprehensive HF program), study duration and the number of HHC visits, the KCCQ improvement remained significantly higher in the TM group. The association between TM and a greater increase in KCCQ Overall Summary Score persisted in all models (Table 2). The corresponding sets of models were also fit for the other KCCQ subscales: clinical summary (treatment effect=10.4, p=0.04), physical limitation (treatment effect=11.3, p=0.016), quality of life (treatment effect=10.6, p=0.05), and social limitation (treatment effect=15.1, p=0.02); all showed a significant effect of TM, whereas self-efficacy (treatment effect=7.3, p=.16) and total symptom score did not (treatment effect=8.3, p=0.1) (results from the full model, detailed model results not shown).
Table 2. Multivariable models for KCCQ Overall Summary Score at Follow-Up.
| Variable | Model 1 Crude Estimate | Model 2 adds Demographics | Model 3 adds HF Measures | Model 4 adds Time and Visits | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| Monitored | 9.61 | 0.05 | 9.58 | 0.05 | 11.76 | 0.01 | 11.12 | 0.03 |
| Base Overall | 0.31 | 0.01 | 0.29 | 0.02 | 0.20 | 0.12 | 0.21 | 0.11 |
| Age | 0.16 | 0.45 | 0.24 | 0.30 | 0.22 | 0.34 | ||
| Non-white | 1.74 | 0.78 | 13.86 | 0.05 | 10.87 | 0.17 | ||
| NYHA > II | -5.60 | 0.39 | -5.18 | 0.44 | ||||
| Agency A | 14.58 | 0.02 | 16.62 | 0.02 | ||||
| Length of Time with HHC | 0.04 | 0.70 | ||||||
| # of HC visits | 0.01 | |||||||
Medication Management for Study Participants with Systolic Failure
Medication use was evaluated for adherence to HF Guidelines (Hunt et al., 2005) in patients with HF due to systolic dysfunction. A minority of patients were on ACE inhibitors, angiotensin receptor blockers, and/or beta blockers. (Table 3) Although physicians were prompted to increase the use of these medications, and up-titrate doses to Guideline recommendations, there was little change in medications from baseline to follow-up.
Table 3. Guideline Medication Use for Participants with Systolic Failure at Baseline and at Follow-Up.
| Baseline | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
| Usual Care (N=11) |
Telemonitor (N=20) |
Usual Care (N=11) |
Telemonitor (N=20) |
|||||
| Medication | n (%) | Mean, median Dose (Range) | n (%) | Mean, median Dose (Range) | n (%) | Mean, median Dose (Range) | n (%) | Mean, median Dose (Range) |
| Enalapril eq. | 2 (18.2) |
22.5, 22.5 (20, 25) |
11 (55.0) |
18.5, 10 (3.3, 40) |
2 (18.2) |
22.5, 22.5 (20, 25) |
11 (55.0) |
22.1, 20 (3.3, 40) |
| Valsartan eq. | 2 (18.2) |
240, 240 (160, 320) |
6 (30.0) |
247, 200 (40, 640) |
2 (18.2) |
240, 240 (160, 320) |
5 (25.0) |
136, 80 (40, 320) |
| Metoprolol eq. | 11 (100) |
105, 50 (12.5, 320) |
12 (60.0) |
77.1, 75 (25, 200) |
10 (90.9) |
95.8, 50 (12.5, 320) |
14 (70.0) |
83.9, 100 (12.5, 320) |
| Furosemide eq. | 10 (90.9) |
128, 80 (20, 480) |
15 (75.0) |
58.7, 60 (20, 80) |
10 (90.9) |
128, 80 (20, 480) |
16 (80.0) |
81.3, 80 (20, 400) |
Dosage of different medications is converted to the equivalents as follows:
Enalapril equivalent = Captopril/7.5 or Quinapril*2 or Ramipril*2
Valsartan equivalent = Candesartan*10 or Irbesartan*1.066 or Losartan*3.2 or Olmesartan*8
Metoprolol equivalent = Carvedilol*4 or Nebivolol*10
Furosemide equivalent = Bumetanide*40 or Torsemide*2
Duration of HHC Services
The duration in which the subjects received HHC was shorter in UC compared to TM (49.6±64.4 days vs. 72.5 ±69.9 days; p=.09). The number of home nursing visits was significantly different with 9.4±4.1 visits in UC vs. 12.8±7.9 visits in TM (p=.008), reflective of the longer HHC duration for TM patients.
HHC Agencies
Patient outcomes were evaluated by HHC agency because one agency (Agency A) had an established HF team with a physician director and dedicated HF nurses. The time to combined endpoint for patients from this agency was not statistically significantly different compared with the other agencies regardless of TM (p=0.7). This agency had a shorter mean duration of HHC services for their patients 27.8 ± 14.5 days (median=26.5) vs. 91.8 ± 81.4 days (median=59.0) (p<0.001). Despite the shorter duration, the average number of HHC visits were not significantly different 10.4 ± 4.2 visits vs. 11.9 ± 8.2 visits (p=0.24) indicating frontloading of visits (provision of more visits earlier in the home health care episode).
Nurse-Physician Phone Contacts
There were significantly more calls for patients in the TM group compared to UC for the number of calls and the number of HF-related calls from nurse to physician. However, once controlled for the amount of time in the study, there were no significant differences in the number of calls. The rate of physician response to nurse calls was the similar regardless of group. (Table 4)
Table 4. Telephone Contacts from Nurse to Physician and Physician Response.
| Among all subjects | Overall (n=99) |
Not monitored (n=45) |
Telemonitored (n=54) |
p-value |
|---|---|---|---|---|
| Number of total calls per subject | 4.9 ± 4.2 | 3.9 ± 3.3 | 5.6 ± 4.8 | 0.04 |
| Number of HF related calls per subject | 2.4 ± 3.0 | 1.6 ± 1.9 | 3.1 ± 3.6 | 0.01 |
| Subjects with at least 1 HF call requiring a response | 58 (58.6) | 24 (53.3) | 34 (63.0) | 0.3 |
| Study length (days) | 62.1 ± 68.1 | 49.6 ± 64.4 | 72.5 ± 69.9 | 0.09 |
| Among subjects with at least 1 HF related call requiring a response | Overall (n=58) |
Not monitored (n=24) |
Telemonitored (n=34) |
p-value |
|---|---|---|---|---|
| Number of HF related calls (requiring response) per subject | 2.7 ± 2.3 | 2.3 ± 1.8 | 3.1 ± 2.6 | 0.2 |
| % calls responded to per subject | 79.5 ± 33.5 | 76.3 ± 38.0 | 81.7 ± 30.3 | 0.6 |
Discussion
In this study of HHC with telemonitoring, the health status of patients randomized to the TM, significantly improved compared to those in a UC group. However, patients received HHC longer if they were in the TM group. This may indicate that TM results in closer attention to a patient's physiologic status leading to additional interventions. This premise is supported by the fact that those with TM received HHC longer than those without TM. Additionally, there is evidence of an important relationship between an agency's ability to manage patients with HF. The HHC agency with a structured HF program including dedicated HF nurses and a medical director had a significantly shorter duration of HHC services compared to the other agencies. This observation suggests that telemonitoring may result in longer HHC duration when there is not a HF team in place to manage the patients and along with the telemoniring data. The role of a HF team in managing telemonitored home-bound HF patients requires further investigation.
There may be reasons why those in the TM group had improved health status which are unrelated to the telemonitor. The act of daily monitoring could make people feel better about their health, i.e., enhanced surveillance as a placebo effect. The nurses frequently call the telemonitored patients to discuss results and to recommend that they retest if data are out of range. Although the nurses were instructed and provided tools to educate their patients regardless of group, the increased contact with the HHC nurse and structured patient self-monitoring may be the driving effect. Regardless if the benefit in health status is from the telemonitor or the increase in human contact, being part of a HHC program with telemonitoring improved perceived health status including quality of life.
There is marked heterogeneity between trials of TM post-acute care including patient selection, differences in data acquisition and the how and who responds to TM data. These results are similar to those reported by others. (Chaudhry et al.; Goldberg et al., 2003; Koehler et al., 2011; Soran et al., 2008) Health status, however, has not been consistently collected in other home monitoring trials which have primarily focused on mortality and hospitalizations. The improvement in KCCQ that was seen with TM was >10 points in each domain. This is substantially better than the needed minimum to be clinically meaningful; (≥5 points). (Green et al., 2000; Spertus et al., 2005) For older adults, particularly those who are home-bound and with multiple comorbid diseases, health status is important because quality of life is often paramount to longevity. TM trials which have evaluated the effect of TM on health status have been diverse with some recruiting only patients with systolic failure (Goldberg et al., 2003) or found no difference in health status between groups.(Dar et al., 2009; Schwarz, Mion, Hudock, & Litman, 2008) Our study included HF patients regardless of EF and therefore captured a generalizable sample of older HF patients. Over 50% of older adults with HF have preserved systolic function and are predominantly women, which is consistent with our cohort of patients.(Miller & Pina, 2009) Our sample was also representative of a true HHC population who can use a telemonitor in that they had multiple comorbidities. Another possible explanation for differences in results in the health status assessment tool in our study was the use of the KCCQ while others used the Minnesota Living with HF Questionnaire.(Dar et al., 2009; Schwarz et al., 2008)
Telemonitoring was independently associated with improvement in health status in regression modeling. Additionally, one HHC agency with a dedicated HF program also was independently associated with better health status for those who were TM. This likely reflects an increase in knowledge and specialty focus of staff which is known to be important in the multidisciplinary approach to HF.(Hunt et al., 2005) Our findings suggest that TM without the addition of a multidisciplinary specialty team may be less effective. Of note, this agency had lower event rates regardless of randomization group while providing fewer HHC visits. Thus, this agency did reduce HHC visits while having better patient outcomes.
As in other studies of older HF patients, there was a gap between practice and evidence-based care. There remains hesitancy to medicate older adults to Guideline doses in the community.(Saczynski et al., 2009) This may be due to perceived side effects or intolerance. Telemonitoring did not have a significant impact on medication use compared to UC. Prompting physicians to make changes had no measurable effect. We did not study physician reasons for not making medication changes and it is not clear the extent to which HHC nurses are comfortable with this kind of medication advocacy (not wanting to overstep their boundaries with physicians) or how the physicians would respond to more extensive input and advocacy by HHC nurses.
An unanticipated finding was that patients with TM tended to receive HHC services longer than those in UC. TM may identify instability in a patient's condition or ongoing need for treatment which qualifies for ongoing home care services. Once recertified, patients gain an additional 60 days of services. The TM may also provide a sense of security for the patient. The substantial increase in health status with TM supports this assertion. However, it is not known the extent to which patients benefit from TM or the ideal length of time for TM, particularly in light of the lack of reduction on the combined endpoint.
Since the trial was designed to assess practice in the community setting, we were able to collect data on the “burden” of calls made to physicians from nurses. Phone calls from nurse to physician were equal in both groups (when controlling for time). Physicians responded to HF related calls approximately 80% of the time in both groups. Since telemonitoring collects data in real time and has the potential to identify rapidly decompensating patients, the nurse-physician relationship is vital to intervening in a timely fashion. Physicians and nurses may perceive increased burden of work that telemonitoring creates since the technology generates a huge amount of data that often may require a response or intervention. While our results suggest that there is not an increased number of telephone calls, the communication between nurses and physicians and burden of work surrounding telemonitoring requires further study.
Studies of telemonitoring demonstrate a range of variability in patient characteristics and telemonitoring systems and processes which makes comparisons difficult. While telemonitoring is being widely implemented in HHC agencies, the best practices for integration of the technology with the existing services have not been determined.
Interventions which benefit health status in older adults should not be under-valued. Since the cost-savings of improving health status are difficult to there is a risk of de-emphasizing interventions which benefit health status. In patients with HF, however, better health status has been associated with reduced costs compared to those with worse health status (Chan et al., 2009). In addition, health status has been associated with other clinical outcomes such as hospitalizations and mortality. (Heidenreich et al., 2006; Sullivan, Levy, Russo, Crane, & Spertus, 2007) The relationship between health status and telemonitoring for older adults receiving HHC requires more study to further understand the potential long lasting health impact and cost effectiveness.
Study Limitations
We recognize several limitations that exist in this study. First, a large number of individuals were screened for the study to obtain the current number of patients. This is not unexpected, but it highlights two important issues: 1. The majority of patients who were referred to home care and screened in the community had severe chronic illness and were disabled. 2. The patient/family/or physician's perceived benefit with TM and therefore were unwilling to be randomized to usual care. Despite the lack of convincing evidence that telemonitoring improves outcomes, marketing campaigns and advertising by home care agencies may have already sold the health care industry and consumers on the benefits of this technology. In addition, there were a few physicians who did not want their patients in the study because of the perceived volume of calls that the data generated and review of transmitted data. Some patients and caregivers felt that monitoring was burdensome with frequent nursing calls, and requests for re-testing.
Second, since this study was not blinded, the improvement of health status could be influenced by the Hawthorne effect. Regardless of the reason for improvement in health status with telemonitoring, patients may feel better and thus rate their health better even without a change in outcomes.
One agency was unable to recruit due to staff turnover and leadership issues. Therefore, the results of our study may be generalizable only to patients in agencies that would be capable of successfully implementing a telemonitoring program. Using HHC and telemonitoring may provide the most benefit with the commitment of a team including: nurse, physician, agency and patient/caregiver.
Third, there was an imbalance in randomized sample sizes between groups due to the unconstrained randomization scheme for the trial, i.e. there was no “blocking” to ensure group balance at specified recruitment levels throughout the trial. In a sequence of 99 independent trials, an imbalance as large as or larger than was observed in this trial would be expected 42% of the time in repeated sampling. Therefore, the observed group imbalance is in keeping with what would reasonably be expected given the randomization scheme.
The nurses had no autonomy to alter medication therapy without the express orders of a physician. Evaluating the effectiveness of HHC nurse driven medication titration protocols (with physician oversight) in conjunction with telemonitoring will be an important next step for further study.
Conclusion
Decreasing rehospitalizations and improving outcomes are national issues which are complex. The simple addition of TM to UC may have unanticipated benefits for health status in older adults. Our experience with the agency that had the most comprehensive and multidisciplinary program, which includes a physician, suggests that TM is a tool that works best when integrated into systems that provide a comprehensive approach to patient care.
Acknowledgments
This work was supported by the Visiting Nurse Association of Cleveland. Dr. Boxer was supported by the KL2RR024990 and made possible by the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the NIH and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH).
Contributor Information
Elizabeth Madigan, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA.
Brian J. Schmotzer, Center for Clinical Investigation, Case Western Reserve University, Cleveland, Ohio, USA.
Cynthia J. Struk, Home Health and Hospice, Summa Health System, Akron, Ohio, USA.
George Kikano, School of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
Ileana L. Piña, Albert Einstein College of Medicine, Montefiore Medical Center, New York, New York, USA.
Rebecca S. Boxer, School of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
References
- Chan PS, Soto G, Jones PG, Nallamothu BK, Zhang Z, Weintraub WS, Spertus JA. Patient health status and costs in heart failure: insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS) Circulation. 2009;119(3):398–407. doi: 10.1161/CIRCULATIONAHA.108.820472. Comparative Study Multicenter Study Randomized Controlled Trial. [DOI] [PubMed] [Google Scholar]
- Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Krumholz HM. Telemonitoring in Patients with Heart Failure. N Engl J Med. 363(24):2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar O, Riley J, Chapman C, Dubrey SW, Morris S, Rosen SD, Cowie MR. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: results of the Home-HF study. Eur J Heart Fail. 2009;11(3):319–325. doi: 10.1093/eurjhf/hfn050. hfn050 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellis ZD, Kenaley B, McGinty J, Bardelli E, Davitt J, Ten Have T. Outcomes of a Telehealth Intervention for Homebound Older Adults With Heart or Chronic Respiratory Failure: A Randomized Controlled Trial. Gerontologist. 2012 doi: 10.1093/geront/gnr134. [DOI] [PubMed] [Google Scholar]
- Goldberg LR, Piette JD, Walsh MN, Frank TA, Jaski BE, Smith AL, Investigators W. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J. 2003;146(4):705–712. doi: 10.1016/S0002-8703(03)00393-4S0002870303003934. [pii] [DOI] [PubMed] [Google Scholar]
- Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. S0735-1097(00)00531-3 [pii] [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Williams RE. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47(4):752–756. doi: 10.1016/j.jacc.2005.11.021. Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- Inglis SC, Clark RA, McAlister FA, Ball J, Lewinter C, Cullington D, Cleland JG. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev. (8):CD007228. doi: 10.1002/14651858.CD007228.pub2. [DOI] [PubMed] [Google Scholar]
- Klersy C, De Silvestri A, Gabutti G, Regoli F, Auricchio A. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol. 2009;54(18):1683–1694. doi: 10.1016/j.jacc.2009.08.017. S0735-1097(09)02921-0 [pii] [DOI] [PubMed] [Google Scholar]
- Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M on behalf of the Telemedical Interventional Monitoring in Heart Failure, I. Impact of Remote Telemedical Management on Mortality and Hospitalizations in Ambulatory Patients With Chronic Heart Failure: The Telemedical Interventional Monitoring in Heart Failure Study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. CIRCULATIONAHA.111.018473 [pii] [DOI] [PubMed] [Google Scholar]
- Madigan EA. People with heart failure and home health care resource use and outcomes. J Clin Nurs. 2008;17(7B):253–259. doi: 10.1111/j.1365-2702.2008.02334.x. JCN2334 [pii] [DOI] [PubMed] [Google Scholar]
- Miller AB, Pina IL. Understanding heart failure with preserved ejection fraction: clinical importance and future outlook. Congest Heart Fail. 2009;15(4):186–192. doi: 10.1111/j.1751-7133.200900063.x. CHF063 [pii] [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Darling CE, Spencer FA, Lessard D, Gore JM, Goldberg RJ. Clinical Features, Treatment Practices, and Hospital and Long-Term Outcomes of Older Patients Hospitalized with Decompensated Heart Failure: The Worcester Heart Failure Study. J Am Geriatr Soc. 2009 doi: 10.1111/j.1532-5415.2009.02407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz KA, Mion LC, Hudock D, Litman G. Telemonitoring of heart failure patients and their caregivers: a pilot randomized controlled trial. Prog Cardiovasc Nurs. 2008;23(1):18–26. doi: 10.1111/j.1751-7117.2008.06611.x. [DOI] [PubMed] [Google Scholar]
- Soran OZ, Pina IL, Lamas GA, Kelsey SF, Selzer F, Pilotte J, Feldman AM. A randomized clinical trial of the clinical effects of enhanced heart failure monitoring using a computer-based telephonic monitoring system in older minorities and women. J Card Fail. 2008;14(9):711–717. doi: 10.1016/j.cardfail.2008.06.448. S1071-9164(08)00594-0 [pii] [DOI] [PubMed] [Google Scholar]
- Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P Cardiovascular Outcomes Research, C. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–715. doi: 10.1016/j.ahj.2004.12.010. S0002-8703(04)00906-8 [pii] [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Levy WC, Russo JE, Crane B, Spertus JA. Summary health status measures in advanced heart failure: relationship to clinical variables and outcome. J Card Fail. 2007;13(7):560–568. doi: 10.1016/j.cardfail.2007.04.001. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Turnbull B. The empirical distribution function with arbitrarily grouped, censored and truncated data. Journal of the Royal Statistical Society Series B Methodological. 1976;38(3):290–295. [Google Scholar]