Abstract
Background
The present study explored the hypothesis that adolescent ethanol exposure may cause long lasting changes in ethanol sensitivity by exploring the age-related effects of acute alcohol on intoxication and on event-related potential (ERP) responses to acoustic stimuli in ethanol naïve adolescent and adult male Wistar rats and in adult rats that were exposed to chronic ethanol/control conditions during adolescence.
Methods
Ethanol naïve adolescent (postnatal day 32 (PD32)) and adult male rats (PD99) were included in the first study. In a second study, rats were exposed to 5 weeks of ethanol vapor (Blood ethanol concentrations @ 175 mg%) or air from PD24 to PD59 and allowed to mature until PD90. In both studies rats were implanted with cortical recording electrodes, and the effects of acute ethanol (0.0, 1.5, and 3.0 g/kg) on behavioral and ERP responses were assessed.
Results
Adolescents were found to have higher amplitude and longer latency P3a and P3b components at baseline as compared to adult rats, and ethanol was found to produce a robust dose-dependent increase in the latency of the P3a and P3b components of the auditory ERP recorded in cortical sites in both adolescents and adults. However, ethanol produced significantly larger delays in P3a and P3b latencies in adults as compared to adolescents. Acute ethanol administration was also found to produce a robust dose dependent increase in the latency of the P3a and P3b components in adult animals exposed to ethanol vapor as adolescents and air exposed controls; however, larger acute ethanol-induced increases in P3a and P3b latencies were seen in controls as compared to adolescent vapor exposed rats.
Conclusions
Adolescent rats have a less intense P3 latency response to acute ethanol administration when compared to adult rats. Exposure to chronic ethanol during adolescence can cause “retention” of the adolescent phenotype of reduced P3 latency sensitivity to ethanol.
Keywords: adolescence, alcohol, ethanol, ERPs, tolerance, P300
Introduction
During adolescence the brain undergoes a neural reorganization at the cellular level that includes processes such as synaptic pruning, changes in neurotransmitter levels and neural receptor sensitivity (see Spear, 2013).These developmental alterations in neural plasticity may underlie some of the changes seen in social and emotional behavior that are observed over the course of adolescence (Dahl and Spear, 2004; Spear, 2000; for review).
Ontogenetic differences in brain maturation have also been shown to be associated with changes in the sensitivity and tolerance to alcohol, factors that have been linked to the development of alcohol dependence (Ehlers et al., 2006; Spear, 2000). Studies in animal models have shown that adolescent rats are less sensitive than adult rats to the effects of acute alcohol on hypothermia, motor in-coordination, sedation, as well as electrophysiological effects (Pian et al., 2008a; Silveri and Spear, 1998, 2000). Adolescent rats display greater tolerance to the sedative effects of acute ethanol administration than adult rats, and regain consciousness at higher blood alcohol levels (Pian et al., 2008b; Silveri and Spear, 1998). However, increases in the sensitivity to some of the cognitive impairing effects of ethanol have also been seen in adolescents. For instance, adolescent rats show more memory deficits following an alcohol challenge than adults (White et al., 2000b). Although the mechanisms underlying the developmental differences in sensitivity to ethanol are not entirely clear, Swartzwelder and colleagues have demonstrated that ethanol more significantly disrupts both the induction of long-term potentiation (LTP) (Pyapali et al., 1999; Swartzwelder et al., 1995a), and the n-methyl-D-aspartate (NMDA)receptor-mediated synaptic activity (Swartzwelder et al., 1995b) in hippocampal slices from adolescents, than those from adults. This group has also demonstrated that acute e thanol increases tonic inhibitory current mediated by extrasynaptic GABAA receptors more significantly in the dentate granule cells obtained from adolescent rat brains than adults (Fleming et al., 2007).
The extent of the potentially long lasting effects of adolescent alcohol exposure on sensitivity to alcohol administration during adulthood has also been investigated previously. Exposure to chronic intermittent ethanol during adolescence has been demonstrated to cause a “retention” of the adolescent phenotype of reduced sensitivity to ethanol on both behavioral (Slawecki, 2002; White et al., 2000a,b) and electrophysiological measures in hippocampus (Fleming et al., 2012, 2013; Slawecki, 2002) and in parietal cortex (Slawecki, 2002). Some authors have suggested that adolescent ethanol exposure may “lock-in” adolescent sensitivity to ethanol and then sustain it into adulthood (Fleming et al., 2012). Since a number of studies in a variety of human populations suggest that sensitivity or “level of response” to alcohol may be one of the best biologically-based risk factors for the development of alcohol use disorders (Schuckit and Smith, 1996), it is reasonable to suggest that the “locked-in” adolescent phenotype for response to alcohol may, at least theoretically, put them at higher risk for heavy drinking and its consequences.
While mechanistic studies have provided important clues to the nature of adolescent alcohol sensitivity it may be difficult to translate such studies directly to humans. We have previously demonstrated that event-related potentials (ERPs) are effective in the neurophysiological assessment of developmental differences following alcohol exposure in both animals and humans thus allowing a more direct translation of findings to the clinical setting (Kaneko et al., 1996; Pian et al., 2008a). One ERP measure that has been intensively studied in human subjects to assay ethanol effects, ethanol-related risks, and vulnerability to several mental disorders is the P300 or P3 component of the ERP (see Porjesz et al., 2005). The P3 component is a positive-going potential with a latency of about 300 ms when it is elicited by auditory stimuli in normal young adults. The component can be identified from averaged electroencephalographic (EEG) waveforms, and although the exact cognitive concomitants of the P3 are not certain, it reliably occurs after “unexpected” or “task-relevant” events (see Polich and Kok, 1995). We have demonstrated that P3 components can be recorded in both rats and mice and that the potentials respond to changes in stimulus characteristics (e.g. frequency, loudness, probability) in a manner very similar to human studies (Ehlers and Somes, 2002; Ehlers et al., 1992, 1994).
The present study characterized the behavioral and P3 event-related potential (ERP) responses following acute and chronic ethanol administration in adolescent and adult Wistar rats. The present study used an auditory ERP paradigm that is currently implemented in our studies in humans (Ehlers et al., 1998a, 2012; Kaneko et al., 1996). This paradigm produces a set of late positive potentials between 250 and 450 msec after the stimuli that have many of the characteristics of the human P3a and P3b components, such as response to stimulus probability and task relevance (see Ehlers et al, 1992, 1994). Late positive components of the ERP (particularly P3b) have been linked to risk for alcoholism in a very large number of studies in humans (Porjesz et al., 2005 for review). They have also been demonstrated to discriminate between rats selectively bred for preference for alcohol drinking (P rats) as compared to those who are non-preferring (NP) (Ehlers et al., 1999) as well as high drinking vs. low drinking mouse lines (Ehlers and Somes, 2002).
The specific aims of the present study were to: (1) determine the age-related effects of acute ethanol on intoxication and on P3a-like and P3b-like event-related potential (ERP) responses to acoustic stimuli in alcohol ethanol naïve adolescent and adult male Wistar rats, and (2) to ascertain whether adolescent rats exposed to 5 weeks of chronic ethanol vapor during adolescence “retained” a low level of response to ethanol, as adults, in their ERP responses.
Materials and Methods
Subjects
Nineteen male adolescent Wistar rats and 19 male adult Wistar rats were used in the first study (study 1) of the effects of acute ethanol administration on ERPs (Charles River, Wilmington, MA). The ethanol naïve adolescent rats were received on postnatal day (PD) 23 and the ethanol naïve adult rats were received on PD90. Upon receipt, the adolescent rats averaged 68.1 ± 5.6 g in weight and the adult rats averaged 370.0 ± 9.3 g in weight. In the second study (study 2), the effects of ethanol vapor/control exposure during adolescence on the response to acute ethanol administration on ERPs was evaluated. For study 2, 44 ethanol naïve male Wistar rats were received on PD23, and those animals averaged 56.8 ± 5.0 g in weight. All the animals were pair-housed in standard plastic cages on a 12 hour light/dark cycle (lights on at 8am) and food and water were provided ad libitum. The experimental procedure described below adheres to the guidelines stipulated in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996) and was reviewed and approved by The Scripps Research Institute's Institutional Animal Care and Use Committee.
Ethanol Vapor Exposure
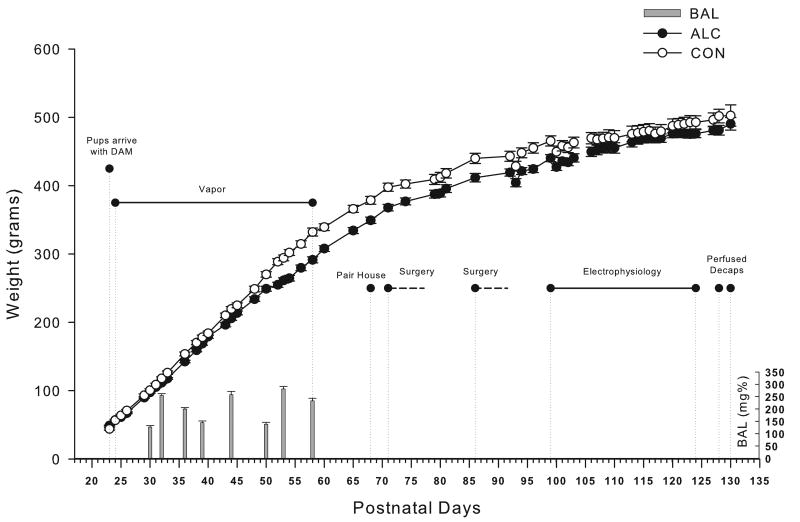
Adolescent ethanol exposure, in study 2, was accomplished through the use of ethanol vapor chambers. Ethanol vapor exposure has been shown to reliably allow for the titration of blood ethanol concentrations (BECs) that are sufficient for inducing ethanol physical dependence. The ethanol vapor inhalation procedure and the chambers used in this study were previously described (Slawecki et al., 2001). Ethanol vapor chambers were calibrated to produce high to moderate BECs between 150-225 mg/dL. In brief, adolescent rats (n = 44) were randomly divided into two groups each (ethanol-exposed group, n = 24; control group, n = 20). Ethanol-exposed rats were housed in sealed chambers, which were infused with vaporized 95% ethanol from 8 p.m. to 10 a.m. For the remaining 10 hrs of the day, ethanol vapor was not infused into the chambers. At the start of the ethanol exposure, adolescent rats were 24 days old and the exposure continued until they were 59 days old. Age-matched controls were handled identically to ethanol-exposed rats. Food and water were always available. Blood samples were collected from the tip of the tail 9 times during the 5 week exposure period in order to assess BECs (average for 5 weeks 189 ± 6.1). Control animals also had blood removed from the tail at the same time points. BECs were determined in the ethanol exposed animals using the Analox micro-statGM7 (Analox Instr. Ltd., Lunenberg, MA). Following the 5 week exposure animals were transferred to standard vivarium cages for the duration of the experiment. Figure 1 shows graphical representation of the timing of the experimental protocol, the animals’ body weights during exposure and their blood ethanol levels.
Figure 1.
Experimental timeline for study 2. Weights and BAL's shown over time for the adolescent vapor exposed rats (N=24) and their controls (N=20). Significant events and durations are shown, all electrophysiological data collected between PD99 and PD124.
Surgical Procedure
In both studies surgical procedures were used to implant recording electrodes to be used for the ERP recordings. For the surgery the rats were anesthetized with isoflurane; atropine (0.03 mL for adolescents; 0.06 mL for adults) was then co-administered subcutaneously in order to minimize respiratory suppression. Surgical coordinates were obtained from the Paxinos and Watson (1986) atlas. Screw electrodes were placed in the skull overlying the frontal cortex (AP: +1.5 mm, ML: ±3.0 mm) and parietal cortex (AP: -4.5mm, ML: ±4.5 mm) in adult rats and the frontal cortex (AP: +1.5mm, ML: ±2.0mm) and parietal cortex (AP: -4.0mm, ML: ±3.5mm) in adolescent rats. Two EMG wire electrodes were also inserted into the rat's neck muscles on the right and left. The stereotaxic coordinates for adolescent rats were based on our previous studies that resulted in placement overlaying the same cortical areas (Pian et al., 2008a). A midline screw electrode was placed posterior to lambda in the skull overlying the cerebellum. Electrode connections were made to an Amphenol five-pin connector (adult rats) or a custom 5-pin cap (adolescent rats), and the assembly was anchored to the skull with dental acrylic and anchor screws. A one-week recovery period was provided before the effects of ethanol were assessed.
Electrophysiological Recording Procedures
ERPs were elicited in both studies by auditory stimuli that were presented through a small speaker centered approximately 70 cm above the rat's head. Auditory stimuli and ERPs were elicited using an oddball plus “noise” paradigm described previously (Ehlers et al., 1996, 1998b). Each auditory ERP session consisted of 312 trials that lasted approximately 10 minutes and each trial presented one of three randomly generated tones: standard tone (84% probability, 75db, 1000Hz), rare tone (10% probability, 85db, 2000Hz), or startle tone (6% probability, 100db, white noise). Individual trials were 1000ms in duration (200ms pre-stimulus + 800ms post-stimulus) and the interval between tones varied from 750ms to 1500ms. Signals were transferred to a PC and digitized at a rate of 256 Hz. The EEG amplifier input range corresponding to the full range of the 12-bit analog-to-digital converter was about +/- 250 microvolts. Periodic calibration results were used to scale the digitized EEG to microvolts.
Each wave component was quantified on the basis of latency to peak amplitude from stimulus onset, peak amplitude, and polarity. Pre-stimulus baseline activity was determined from average EEG activity 200 msec prior to stimulus onset. Each component was identified with an automated peak detection program and confirmed by visual inspection. Movement artifact (i.e. voltages exceeding ±400 μV) was assessed with an automated computer detection program and eliminated following confirmation by visual analysis. Trials for each tone type were then averaged. ERP components were identified based on the largest amplitude peak within a specified latency range, as previously described (Ehlers et al., 1991). Latency windows used to identify the two ERP components were as follows: P3a, (225-350 ms) P3b. (325-400 ms). One adult and one adolescent animal's individual ERP waveforms are presented in Figure 2 with the identification of the regions where the P3a and P3b peak components are selected.
Figure 2.
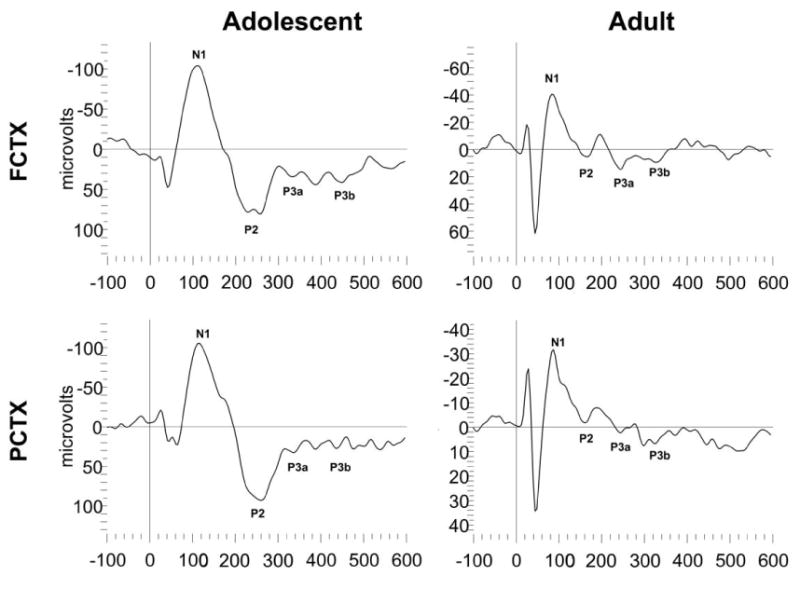
Representative ERP waveforms of an adolescent and adult rat from study 1. Approximate locations for N1, P2, P3a and P3b are indicated on each waveform.
Acute Ethanol Administration
In both studies rats were injected intraperitoneally (I.P.) on a randomized schedule of three injections on three different days: a saline dose, a high (3.0 g/kg) and low (1.5g/kg) dose of 17.5-20% w/v ethanol. Adult rats were between PD99 and PD124 days old and adolescent rats were between P32 and P40 days old at the time of the injections. These moderate-high doses of ethanol were chosen as previous studies have demonstrated that EEG differences in ethanol response between adolescents and adults may be more significant at higher ethanol doses (Slawecki, 2002). The ethanol concentrations were prepared fresh for each day of testing for groups containing three to eight rats each, and the saline doses were given in equal frequency and at equivalent volumes to the ethanol doses. The rats were placed in the electrophysiological recording chamber approximately 15 minutes post-injection. The injection volumes ranged from 0.7 to 9.5 mL based on the subjects’ body weight (134 – 554g). All rats were habituated to the testing procedure before the first experimental recording and the rats were then given at least 72 hours between doses. All rats were scored visually for intoxication by using a modified 5 point scale that was similar to that described by Freund (1969) for mice. Intoxication was rated just before the onset of the EEG/ERP recordings. On that scale a score of 0 indicated no signs of intoxication, 1 indicated that the rat was calm with decreased muscle tone during handling, 2 indicated mild ataxia with a rapid gait, 3 indicated that the gait was impaired with the animal falling to one side, and a score of 4 was given if the subject was immobile with little muscle tone.
Statistical Analysis
Two-way mixed analysis of variance (ANOVA) was used to assess the effects of ethanol on the P3a and P3b latency and amplitude and intoxication score in the two studies. To reduce multiple comparisons only the rare tone was analyzed for the two channels. In the first study, a 2 group (adolescent vs. adult) X 3 doses (saline, 1.5, 3.0 g/kg ethanol) design was used. To increase power in the second study, the adult control animals from study 1 and study 2 were combined. For the behavioral state assessment of intoxication scores, a 2 group (study 1: adolescent vs. adult; study 2: ethanol vapor exposure vs. controls) X 2 ethanol doses (1.5 and 3.0 g/kg) ANOVA design was used. One way repeated measures ANOVA was used to determine post-hoc significance for the two doses of ethanol for the four groups (adolescent, adult, vapor exposed, air exposed) separately.
Results
Behavioral State Assessment
Subjective visual inspection of adolescent and adult rats preceding ethanol administration showed normal exploratory and grooming behaviors in both groups. Acute administration of ethanol produced dose-dependent effects on rats’ behavior. In study 1, 1.5 g/kg of ethanol primarily produced some mild ataxia with a rapid gait 15 minutes post-injection (mean intoxication score: adolescents 2.17 ± 0.20; adults 2.71 ± 0.21), whereas the 3.0 g/kg ethanol dose impaired gait with rats often falling to one side as well as immobility in some animals (mean intoxication score: adolescents 3.78 ± 0.12; adults 3.71 ± 0.12). A significant main effect of ethanol administration was seen on intoxication score (F=69.56, df=1,33, p<0.00001). No significant main effects of group or ethanol x group interactions were observed.
In study 2, 1.5 g/kg of ethanol was also found to primarily produce some mild ataxia with a rapid gait, 15 minutes post-injection (mean intoxication score: controls 2.59 ± 0.14; adolescent vapor exposed: 2.17 ± 0.20), whereas the 3.0 g/kg ethanol dose impaired gait with rats often falling to one side as well as immobility in some animals (mean intoxication score: controls 3.74 ± 0.08; adolescent vapor exposed 3.78 ± 0.11). A significant main effect of ethanol administration was seen on intoxication score (F=97.22, df=1,50, p<0.000001). No significant main effects of adolescent ethanol exposure or acute dose x exposure interactions were observed.
ERP Assessment
Frontal and Parietal Cortical ERP amplitudes and latencies following acute ethanol challenge in ethanol naïve adolescent and adult rats
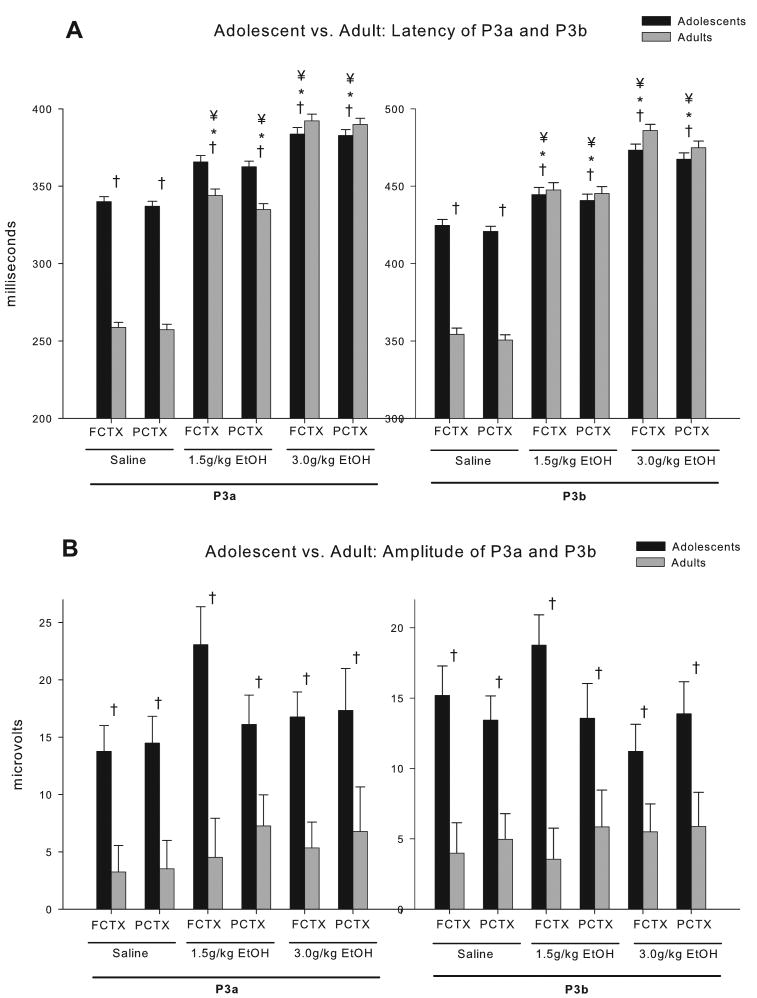
ERPs to the auditory stimuli were recorded approximately 20 minutes following I.P. injections of saline, 1.5 or 3.0 g/kg ethanol. Thirty-five (35) rats survived all procedures in good health and had technically adequate recordings for all three doses (<20% artifact/noise, mean 0.6%). Grand averages of the ERP waveforms from those animals, at each dose, are provided in Figure 3. Calculation of the latency and amplitude of the P3a and P3b waveforms in the two electrode locations (frontal cortex [FCTX], parietal cortex [PCTX]) revealed in 2 (adolescent vs. adult) X 3 (saline, 1.5, 3.0 g/kg) ANOVAs that significant age effects, acute ethanol dose effects, and significant age X dose interactions could be observed. As reported previously (Ehlers et al., 1994), a comparison of the amplitudes of the P3b component recorded following the infrequent tone to the amplitudes following the frequent tone for the saline condition revealed that the amplitude for the infrequent tone was significantly higher than the frequent tone (FCTX F=8.6, df=1,74, p=0.004; PCTX F=9.07, df=1,72, p=0.004). As seen in Figure 4, adolescents had significantly longer latency P3a and P3b components and significantly larger component amplitudes than adults in both electrode locations (F's=27.1–141.6; df's=1, 32 or 33, p's all<0.001). Significant main effects of the doses of ethanol were also seen. Ethanol produced a robust dose dependent increase in the latency of both the P3a and P3b components in both electrode locations (F's=229.3–277.8; df's=2, 31 or 32, p's all<0.001). No significant main effects of the doses of ethanol were seen on the P3a or P3b component amplitudes. Several group (adolescent vs. adult) X ethanol dose (saline, 1.5, 3.0 g/kg) interactions were also observed. Most notably ethanol produced larger increases in P3a and P3b latencies in adults as compared to adolescents (F's=57.6–65.6; df's=2, 31 or 32, p's all<0.001) (all post hoc analyses p<0.015). In the adolescents the 1.5 g/kg dose produced an average increase in latency of 7% for the P3a latency and 5% for the P3b latency and the 3.0 g/kg dose produced and average increase in latency of 12% for the P3a latency and 10% for the P3b latency. Whereas in the adults the 1.5 g/kg dose produced an average increase in latency of 24% for the P3a latency and 21% for the P3b latency and the 3.0 g/kg dose produced an average increase in latency of 34% for the P3a latency and 27% for the P3b latency. Only one significant interaction was seen in P3b amplitude in the FCTX (F=3.79, df=2,32, p<0.03) (post hoc analyses, NS); no significant interactions were seen in P3a amplitude. There was a significant age effect on both P3a and P3b amplitude with adolescents having significantly higher amplitudes for both components and seen in Figure 4.
Figure 3.
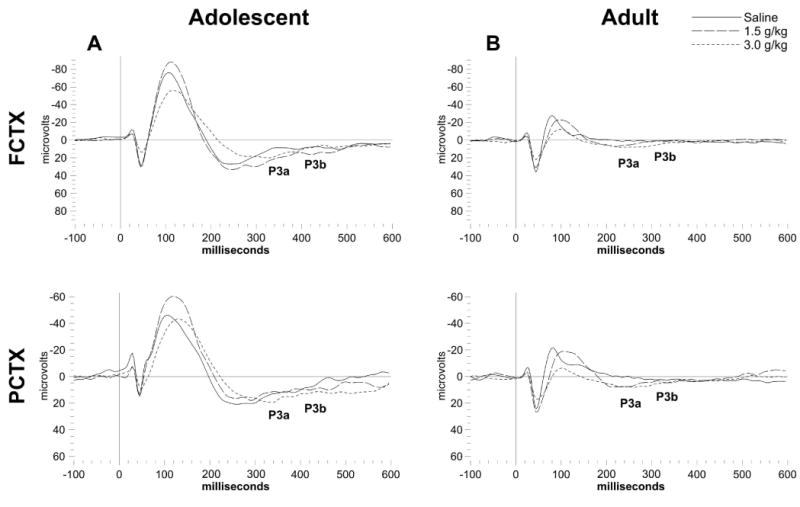
Grand averages of ERP waveforms in the FCTX and PCTX for adolescent (N=18) and adult (N=17) rats from study 1. Each acute ethanol dose is overlaid for each channel and approximate locations for P3a and P3b indicated for the saline dose. (A) Adolescent rats have much greater amplitude overall compared to (B) adults, while dose dependent changes in latency can be seen in both age groups.
Figure 4.
P3a and P3b component analysis between adolescent (N=18) and adult (N=17) rats given acute ethanol. (A) Latency of the P3a and P3b component was observed to be significantly longer in adolescents compared to adults, and while both showed an ethanol dose dependent increase in latency, adults produced larger increases by dose compared to adolescents in both electrode locations. Post-hoc analyses showed that the 1.5 g/kg and 3.0 g/kg ethanol dose were significant in P3a and P3b at both electrode locations. (B) P3a and P3b amplitudes were significantly higher in adolescent rats compared to adults, but no group x dose effects were seen. * = dose effect; † = group effect; ¥ = group x dose interaction.
Frontal and Parietal Cortical ERP amplitudes and latencies following acute ethanol challenge in adult rats exposed to ethanol vapor during adolescence and their controls
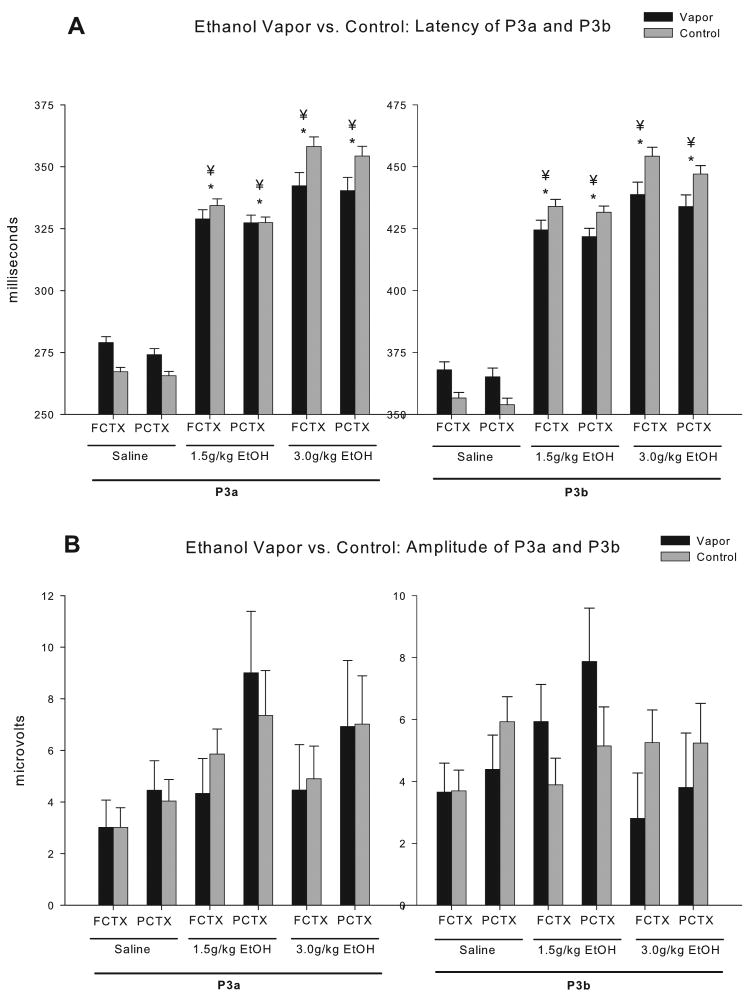
Fifty-two (52) rats survived all surgical and vapor exposure procedures in good health and had technically adequate recordings for all three doses of ethanol (<23% artifact/noise, mean 0.5%). Grand averages of the ERP waveforms from those animals, at each dose, are provided in Figure 5. Body weights of adolescent vapor exposed animals were significantly higher than controls (control= 425 + 8.2, vapor= 451.3 + 6.9; F=4.46, df=1,50, p<0.05), therefore, the 2 group (ethanol vapor exposure vs. controls) x 3 dose (saline, 1.5, 3.0 g/kg ethanol) design was co-varied by body weight. Calculation of the latency and amplitude of the P3a and P3b waveforms in the two electrode locations (frontal cortex [FCTX], parietal cortex [PCTX]) revealed in 2 (adolescent vapor exposed vs. control) X 3 (saline, 1.5, 3.0 g/kg) ANOVAs, co-varied by body weight, that significant acute ethanol dose effects and significant vapor exposure X dose interactions could be observed. As seen in Figure 6, significant main effects of the doses of ethanol were observed for latency. Ethanol produced a robust dose dependent increase in the latency of both the P3a and P3b components in both electrode locations (F's=29.5-56.2; df's=2, 47 or 48, p's all<0.001). No significant main effects of the doses of ethanol were seen in the P3a or P3b amplitudes. Several group (adolescent vapor exposed vs. control) X ethanol dose (saline, 1.5, 3.0 g/kg) interactions were also observed. Most notably ethanol produced larger increases in P3a and P3b latencies in controls as compared to adolescent vapor exposed rats in both electrode locations (F's=5.9-7.9; df's=2,47 or 48, p's all<0.008). (all post hoc analyses p<0.01). In the control rats the 1.5 g/kg dose produced an average increase in latency of 20% for the P3a latency and 18% for the P3b latency and the 3.0 g/kg dose produced an average increase in latency of 26% for the P3a latency and 22% for the P3b latency. Whereas in the vapor treated rats the 1.5 g/kg dose produced an average increase in latency of 15% for the P3a latency and 13% for the P3b latency and the 3.0 g/kg dose produced an average increase in latency of 17% for the P3a latency and 15% for the P3b latency. No significant interactions were seen in P3a or P3b amplitudes.
Figure 5.
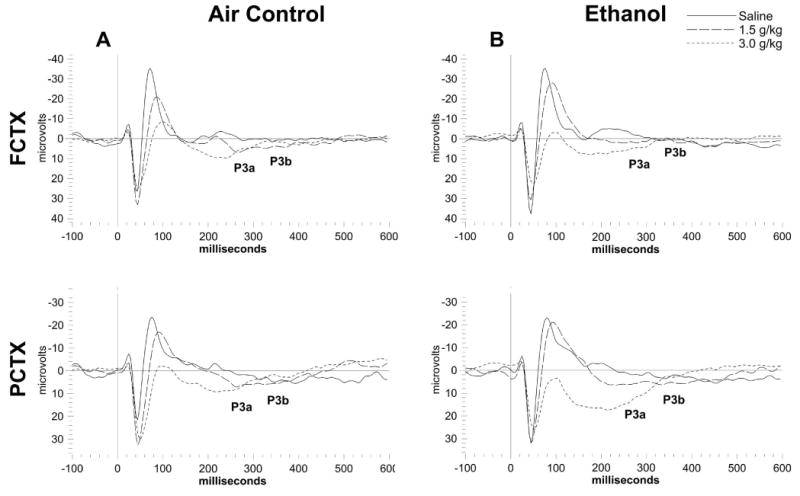
Grand averages of ERP waveforms in the FCTX and PCTX for adolescent vapor exposed rats (N=18) and their air exposed controls (N=17) rats from study 2. Each acute ethanol dose is overlaid for each channel and approximate locations for P3a and P3b indicated for the saline dose. Ethanol dose dependent effects can be seen in both (A) adolescent vapor exposed rats and their (B) controls.
Figure 6.
P3a and P3b component analysis between adolescent vapor exposed rats (N=18) and air exposed controls (N=34) rats given acute ethanol. Dose dependant increases can be seen in (A) latency for both the P3a and P3b components in both electrode locations. Additionally, treatment x dose interaction show control rats have greater increases in latency compared to rats exposed to vapor during adolescence. Post-hoc analyses showed that the 1.5 g/kg and 3.0 g/kg ethanol dose were significant in P3a and P3b at both electrode locations. (B) No significant effects were seen in amplitude between adolescent vapor exposed rats and their controls. * = dose effect; ¥ = dose x treatment interaction.
Discussion
We have previously shown that alcohol vapor exposure during adolescence can lead to deficits in prepulse startle, increases in behavioral measures of disinhibition, increased alcohol drinking, reductions in hippocampal size and measures of neurogenesis, as well as loss of cholinergic neurons in the basal forebrain (Criado and Ehlers, 2010a,b, 2013; Criado et al., 2011; Ehlers and Criado, 2010; Ehlers et al., 2011, 2013a,b; Pian et al., 2008a,b). The findings from the present study extend those findings to the evaluation of ERP responses to acute ethanol challenge in adolescents and adults and in animals exposed to ethanol vapor during adolescence. In the present study, ethanol was found to produce a robust dose dependent increase in the latency of both the P3a and P3b components of the auditory ERP recorded in cortical sites. Small non-significant effects of ethanol were observed on the P3a and P3b component amplitudes. Ethanol produced significantly larger increases in P3a and P3b latencies in adults as compared to adolescents in study 1. Significant findings were also found in study 2 that evaluated acute response to ethanol in adult rats that had been exposed to ethanol vapor/control conditions as adolescents. Ethanol was found to produce a robust dose dependent increase in the latency of both the P3a and P3b components in controls and adolescent vapor exposed animals; however, larger acute ethanol-induced increases in P3a and P3b latencies were seen in controls as compared to adolescent vapor exposed rats. These studies demonstrate that exposure to chronic ethanol during adolescence can cause a “retention” of the adolescent phenotype, of reduced P3 latency sensitivity to ethanol, in adulthood.
Dramatic changes in the neurophysiology of the brain have been noted during adolescent development in humans. Over the course of adolescence, the P3 component of the ERP is modified, with P3 latency decreasing with age, and P3 amplitude tending to become larger in clinical studies (McIsaac and Polich, 1992). The present study is the first to evaluate P3 amplitude to an auditory task in rodents during different developmental epochs. We found, like the studies in humans, that P3 latency decreased with age; however, we also found that the P3 amplitude in the adolescents was larger than the adults.
The present study evaluated two different components of auditory ERPs (i.e. P3a, P3b). The P3 component can, in some paradigms, consist of two separate positive subcomponents, P3a and P3b, or early and late P300 (for a review, see Polich and Criado, 2006). The earlier P3a component (in the 300 ms range in visual tasks) has a frontocentral distribution and has been associated with the “novelty” of a stimulus and the redirection of attention monitoring. In contrast, the late P3b component (400 ms range in a visual task) has temporoparietal distribution and has been associated with attention and may index memory updating (for a review, see Polich and Criado, 2006). In human young adults a history of binge drinking during adolescence has been found to affect both the latency of a P3a component and the amplitude of a P3b component of an ERP generated by a facial recognition task (Ehlers et al., 2007). However, multivariate analyses revealed that the effects on the earlier component seemed to be due to adolescent alcohol exposure whereas the effects on the later component appeared to be a combined result of predisposing factors such as family history of alcoholism and the presence of other externalizing diagnoses. These data suggest that ethanol exposure may more selectively alter responses to the early processing of stimulus recognition and or novelty detection rather than context or memory updating.
P3a-like and P3b-like components have also been described to occur in rodents using a variety of tasks including those that require active discrimination between stimuli (Ehlers et al., 1994, 1998b; Robledo et al., 1999). The two components recorded in the present study, although not necessarily analogous to those recorded in the human condition, were found to be sensitive to the effects of ethanol. Ethanol produced delays in P3a and P3b latencies, in adolescents and adults; however, larger acute ethanol-induced increases in latencies were seen in adults vs. adolescents and in controls as compared to adolescent vapor exposed rats, suggesting that exposure to chronic ethanol during adolescence can cause “retention” of the adolescent phenotype, of reduced P3 latency sensitivity to ethanol, in adulthood.
These studies confirm and extend previous studies in our laboratory that have shown that acute administration of ethanol produces differential effects in adults as compared to adolescents in the spectral characteristics of the EEG (Pian et al., 2008b; Ehlers et al., in submission). These findings are also consistent with previous studies that have shown that adolescent rats are less sensitive than adults to the acute administration of alcohol on several behavioral measures including: alcohol-induced motor in-coordination, sedation, prepulse startle and hypothermia (Pian et al., 2008a; Silveri and Spear, 1998, 2000; Varlinskaya and Spear, 2002).
One potential source of differences in response to alcohol between adolescents and adults is alcohol metabolism. Although blood alcohol levels were not routinely run in this study following acute alcohol administration, previous studies in our lab have demonstrated that adolescents do not differ from adults in blood alcohol levels at the doses used in this study within the time frames studied (Walker and Ehlers, 2009) Additionally, in the present study no significant differences between adults and adolescents were found on visual scoring of their level of acute behavioral intoxication. However, the doses used in the present study were lower than what has been reported previously for sedation (see Silveri and Spear, 1998), and measures of visual scoring of intoxication appear to be less sensitive than other more quantitative measures of behavioral intoxication, such as geotaxis, that have been used in some studies (Ramirez and Spear, 2010). Additionally, Wistar rats were used in the present study and Sprague-Dawleys were tested in the studies by Spear and colleagues. These data further suggest that measures of sensory processing are more sensitive to the differential effects of alcohol on adults and adolescents than visually scored measures of intoxication.
A number of studies have sought to characterize the cellular and molecular mechanisms underlying the enhanced vulnerability to ethanol exposure during adolescence (see Alfonso-Loeches and Guerri, 2011). Many studies have specifically focused on the effects of alcohol exposure on glutamatergic neurotransmission (see Crews et al., 2002; Fadda and Rossetti, 1998), and in particular the N-methyl-D-aspartate (NMDA) type of glutamate receptor. Substantial loss of synapses, especially the excitatory glutamatergic inputs to the forebrain, occurs during adolescence (see Spear, 2013) and may be vulnerable to ethanol exposure. In rats treated with the NMDA receptor blocker MK-801, during postnatal day 7, a reduction in volume and neuronal number within the hippocampus as well as altered hippocampal NMDA receptor (NR1 subunit) expression and PPI deficits(in females) were seen when the animals reached adulthood (Harris et al., 2003). We have also demonstrated, previously, that two weeks of ethanol vapor exposure during adolescence as compared to adulthood results in decreased NR1 and NR2A subunit expression in hippocampus in the adolescents whereas no such effects were seen in the alcohol exposed adults (Pian et al., 2010). In addition we have found that administration of low doses of MK-801 essentially eliminated P3 components of the ERP recorded in hippocampus and amygdala (Ehlers et al., 1992). Further research is needed to characterize the exact neurochemical mechanisms that modulate ethanol's effects on sensory and cognitive processing and how they are developmentally regulated.
Acknowledgments
This work was supported by grants U01 AA019969; R01 AA006059 to Cindy Ehlers from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The authors thank JP Cheveney for technical assistance, Phil Lau for assistance in statistical analyses and Shirley Sanchez for assistance in editing the manuscript. Dr. James Havstad developed the software used for the ERPs assessments.
References
- Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci. 2011;48:19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- Crews FT, Rudolph JG, Chandler LJ. Glutamate and alcohol-induced neurotoxicity. In: Herman BH, Frankenheim J, Litten RZ, Sheridan PH, Weight FF, Zukin SR, editors. Glutamate and addiction. Humana Press; Totowa, N.J.: 2002. pp. 357–374. [Google Scholar]
- Criado JR, Ehlers CL. Effects of adolescent ethanol exposure on event-related oscillations (EROs) in the hippocampus of adult rats. Behav Brain Res. 2010a;210:164–170. doi: 10.1016/j.bbr.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Event-related oscillations in the parietal cortex of adult alcohol-preferring (P) and alcohol-nonpreferring rats (NP) Alcohol. 2010b;44:335–342. doi: 10.1016/j.alcohol.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Liu T, Ehlers CL, Mathe AA. Prolonged chronic ethanol exposure alters neuropeptide Y and corticotropin-releasing factor levels in the brain of adult Wistar rats. Pharmacol Biochem Behav. 2011;99:104–111. doi: 10.1016/j.pbb.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Effects of adolescent onset voluntary drinking followed by ethanol vapor exposure on subsequent ethanol consumption during protracted withdrawal in adult Wistar rats. Pharmacol Biochem Behav. 2013;103:622–630. doi: 10.1016/j.pbb.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. The New York Academy of Sciences; New York, N.Y: 2004. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Chaplin RI. Long latency event-related potentials in rats: effects of dopaminergic and serotonergic depletions. Pharmacol Biochem Behav. 1991;38:789–793. doi: 10.1016/0091-3057(91)90243-u. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kaneko WM, Wall TL, Chaplin RI. Effects of dizocilpine (MK-801) and ethanol on the EEG and event-related potentials (ERPs) in rats. Neuropharmacology. 1992;31:369–378. doi: 10.1016/0028-3908(92)90069-2. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kaneko WM, Robledo P, Lopez AL. Long-latency event-related potentials in rats: effects of task and stimulus parameters. Neuroscience. 1994;62:759–769. doi: 10.1016/0306-4522(94)90474-x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Parry BL. Electrophysiological findings during the menstrual cycle in women with and without late luteal phase dysphoric disorder: relationship to risk for alcoholism. Biol Psychiatry. 1996;39:720–732. doi: 10.1016/0006-3223(95)00183-2. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Garcia-Andrade C, Wall TL, Sobel DF, Phillips E. Determinants of P3 amplitude and response to alcohol in Native American Mission Indians. Neuropsychopharmacology. 1998a;18:282–292. doi: 10.1016/S0893-133X(97)00160-7. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Lopez AL, Robledo P. Long latency event-related potentials in rats: response of amygdala, nucleus accumbens, dorsal hippocampus and frontal cortex to changes in reward characteristics of conditioned stimuli. Brain Res. 1998b;780:138–142. doi: 10.1016/s0006-8993(97)01294-8. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Li TK, Lumeng L, Kinkead B, Owens MJ, Nemeroff CB. Neurontensin studies in alcohol naive, preferring and non-preferring rats. Neuroscience. 1999;93:227–236. doi: 10.1016/s0306-4522(99)00113-x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C. Long latency event-related potentials in mice: effects of stimulus characteristics and strain. Brain Res. 2002;957:117–128. doi: 10.1016/s0006-8993(02)03612-0. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin Exp Res. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Finnerman G, Gilder D, Lau P, Criado J. P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicol Teratol. 2007;29:153–163. doi: 10.1016/j.ntt.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR. Adolescent ethanol exposure: does it produce long-lasting electrophysiological effects. Alcohol. 2010;44:27–37. doi: 10.1016/j.alcohol.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Havstad J. Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Res. 2012;1450:67–79. doi: 10.1016/j.brainres.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Oguz I, Budin F, Wills DN, Crews FT. Peri-adolescent ethanol vapor exposure produces reductions in hippocampal volume that are correlated with deficits in prepulse inhibition of the startle. Alcohol Clin Exp Res. 2013a;37:1466–1475. doi: 10.1111/acer.12125. Epub 2013 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013b;244C:1–15. doi: 10.1016/j.neuroscience.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007;97:3806–3811. doi: 10.1152/jn.00101.2007. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcohol Clin Exp Res. 2012;36:279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Li Q, Risher ML, Sexton HG, Moore SD, Wilson WA, Acheson SK, Swartzwelder HS. Binge-Pattern Ethanol Exposure During Adolescence, but Not Adulthood, Causes Persistent Changes in GABA(A) Receptor-Mediated Tonic Inhibition in Dentate Granule Cells. Alcohol Clin Exp Res. 2013;37:1154–1160. doi: 10.1111/acer.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund G. Alcohol withdrawal syndrome in mice. Arch Neurol. 1969;21:315–320. doi: 10.1001/archneur.1969.00480150105013. [DOI] [PubMed] [Google Scholar]
- Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur J Neurosci. 2003;18:1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- Kaneko WM, Ehlers CL, Philips EL, Riley EP. Auditory event-related potentials in fetal alcohol syndrome and Down's syndrome children. Alcohol Clin Exp Res. 1996;20:35–42. doi: 10.1111/j.1530-0277.1996.tb01040.x. [DOI] [PubMed] [Google Scholar]
- McIsaac H, Polich J. Comparison of infant and adult P300 from auditory stimuli. J Exp Child Psychol. 1992;53:115–128. doi: 10.1016/0022-0965(92)90044-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd. Academic Press; Sydney, Australia: 1986. [Google Scholar]
- Pian JP, Criado JR, Ehlers CL. Differential effects of acute alcohol on prepulse inhibition and event-related potentials in adolescent and adult Wistar rats. Alcohol Clin Exp Res. 2008a;32:2062–2073. doi: 10.1111/j.1530-0277.2008.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008b;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-d-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–654. doi: 10.1016/j.neuroscience.2010.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neuropathol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Ramirez RL, Spear LP. Ontogeny of ethanol-induced motor impairment following acute ethanol: assessment via the negative geotaxis reflex in adolescent and adult rats. Pharmacol Biochem Behav. 2010;95:242–248. doi: 10.1016/j.pbb.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Somes C, Winkler J, Thal LJ, Ehlers CL. Long latency event-related potentials in rats: effects of nucleus basalis magnocellularis lesions. Int J Neurosci. 1999;96:23–44. [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2002;26:246–254. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent Neurodevelopment. J Adolesc. Health. 2013;52:S7–S13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995a;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-medicated synaptic potentials to ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995b;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol Biochem Behav. 2009;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000a;24:1251–1256. [PubMed] [Google Scholar]
- White AM, Matthews DB, Best PJ. Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus. 2000b;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]