Abstract
Family and twin studies suggest that a range of neurocognitive traits index the inherited liability to ADHD; however, the utility of such measures as endophenotypes in molecular genetic studies remains largely untested. The current paper examined whether the inclusion of neurocognitive measures in a genomewide linkage analysis of ADHD could aid in identifying QTL linked to the behavioral symptoms of the condition. Data were from an affected sibling pair linkage study of DSM-IV ADHD conducted at Massachusetts General Hospital. The sample included 1,212 individuals from 271 families. ADHD symptoms were assessed with the K-SADS-E. The neurocognitive battery included Wechsler Intelligence Scales subtests, the Stroop, the Wisconsin Card Sorting Test (WCST), the Rey Osterreith Complex Figure, a working memory CPT, the CVLT and WRAT-III subscales. Evidence for linkage was assessed using a simulation-based method that combines information from univariate analyses into the equivalent of a multivariate test. After correction for multiple trait testing, a region on chromosome 3q13 showed suggestive linkage to all neurocognitive traits examined and inattention symptoms of ADHD. The second highest peak occurred on 22q12 but showed linkage to a single subscale of the WCST. In univariate analysis, this region retained criteria for suggestive linkage to this measure after correction for multiple trait testing. Our primary findings raise the possibility that one or more genes on 3q13 influence neurocognitive functions and behavioral symptoms of inattention. Overall, these data support the utility of neurocognitive traits as ADHD endophenotypes, but also highlight their limited genetic overlap with the disorder.
Keywords: ADHD, multivariate linkage, endophenotype, cognition
INTRODUCTION
Attention deficit-hyperactivity disorder (ADHD) is a condition characterized by developmentally inappropriate levels of inattention and/or hyperactivity/impulsivity that onset in early childhood and cause impairment in multiple settings (American Psychiatric Association 1994). Evidence for substantial genetic influence on the disorder has been accumulating since the 1960's (Lopez 1965), with twin studies yielding consistent evidence of high heritability (ranging from .6 to.9 (Faraone and others 2005; Waldman and Gizer 2006)). Yet, progress in revealing the genetic architecture of ADHD has been slow.
Several genes that are good biological candidates for the disorder have been implicated in meta-analyses of association studies (Faraone and others 2005), but polymorphisms in these genes explain only a small proportion of the variance in liability. Results from the handful of published linkage studies (Arcos-Burgos and others ; Asherson and others 2008; Bakker and others 2003a; Fisher and others 2002; Ogdie and others 2002; Romanos and others 2008), show some degree of overlap for regions on chromosomes 5p, 9q, 16q and 17p if nominally significant findings are considered; however, no regions have achieved genomewide significance using strict criteria (Lander and Kruglyak 1995). This pattern of findings may, in part, reflect the low power of standard linkage studies to identify variants with modest effect sizes in complex phenotypes (Suarez and others 1994).
Gaining a better understanding of the etiology of ADHD is important from a public heath perspective due to the high prevalence of the disorder worldwide (approximately 8-12% (Faraone and others 2003)) and its association with academic and occupational failure, substance abuse, criminality and driving accidents (Biederman and Faraone 2005). Given the multifactorial nature of the condition, strategies that can improve statistical power to detect risk variants of small to moderate effect are clearly desirable. One such approach that has yielded tangible results in the field of medicine (Borecki and others 1990) is the examination of ‘endophenotypes.’ Although this term has been used in a variety of ways, it predominantly refers to a phenotype that is more proximal to the biological etiology of a clinical disorder than its signs and symptoms, and is influenced by one or more of the same genes as the condition (Almasy and Blangero 2001; Gottesman and Gould 2003; Skuse 2001). Theoretically, the increased power gained from targeting an endophenotype compared to the disorder it underlies derives from a reduction in genetic complexity and error variance due to its greater proximity to gene products and potential to partition potentially heterogeneous pathophysiological deficits.
While the pathophysiology of ADHD has not been determined, there is strong evidence for dysfunction in prefrontal-striatal neural networks (Castellanos 2001; Castellanos and others 2002; Castellanos and Tannock 2002; Faraone and Biederman 2004). Neurocognitive impairments in “executive” functions that reflect dysfunction in these pathways are well-established in ADHD via meta-analyses (Lijffijt and others 2005; Martinussen and others 2005; Willcutt and others 2005), are clinically relevant (Biederman and others 2006; Biederman and others 2007) and are theoretically compelling as core deficits underlying the disorder (Barkley 2006). Recently, they have generated considerable interest as candidate endophenotypes due to evidence of impairments in unaffected siblings (Doyle and others 2005a; Schachar and others 2005) and unaffected co-twins (Bidwell and others 2007) that suggest a relationship to the inherited risk for the disorder.
Despite these supporting data, designing molecular genetics studies of ADHD that include neurocognitive measures is not straightforward. First, recent analyses (Doyle 2006; Willcutt 2006) suggest that the familial/genetic overlap between neurocognitive performance and ADHD symptoms is partial rather than complete. As a result, studies aiming to use executive and other neurocognitive measures to find genes for ADHD must incorporate strategies to distinguish overlapping genetic influences from those that separately influence the cognitive and behavioral phenotypes. Second, prioritizing among the wide range of candidate traits is difficult because a) inconsistencies exist regarding the specific measures of executive functions that show impairments in unaffected relatives (Doyle and others 2005b); b) weaknesses in non-executive neurocognitive traits (including sustained attention, verbal learning, processing speed and intellectual function, as well as reading and math achievement) also show associations with the condition and impairments in relatives (see Table 1); and c) studies estimating the heritability of neurocognitive measures are small in number and predominantly based on small sample sizes (for a review see (Doyle and others 2005b)).
Table 1.
Justification for inclusion of neurocognitive phenotypes
| Construct | Measures | Association w/ADHD | Association w/fronto-striatal networks | Heritability | Familial/genetic link to ADHD |
|---|---|---|---|---|---|
| Verbal Working Memory | Wechsler arithmetic Wechsler digit span; Seidman WM-INT | Martinussen, 2005, Willcutt, 2005; Frazier, 2004 | Seidman, 1998; Gerton 2004 | Kremen 2007; Ando, 2001; Goodman, 1989 | Doyle, 2005; Bidwell, 2007 |
| Abstract Problem-Solving | WCST | Frazier, 2004; Willcutt, 2005 | Moreno-Iniguez 2007 | Pennington, 1996 | Bidwell, 2007 |
| Interference Control | Stroop color-word interference | Frazier, 2004 | Bush, 2003; Cabeza, 2000; Bush, 1999 | Swan, 2002; Stins 2004 | Slaats-Willemse, 2003; Doyle, 2005; Bidwell, 2007 |
| Organization/Planning | Rey Osterreith Copy Organization | Willcutt, 2005 | |||
| Processing Speed | Wechsler coding/digit symbol & symbol search; Stroop color naming | Willcutt, 2005 Chhabildas, 2001; van Mourik, 2005 | Swan, 2002; Stins, 2004 | Doyle, 2005; Bidwell, 2007 | |
| Verbal learning | California Verbal Learning Test | Hervey, 2004; Seidman, 1998 | Greenwood 2007 | Seidman, 2000 | |
| Intellectual Ability | Wechsler vocabulary & block design | Frazier, 2004 | Bouchard, 1998 | Faraone, 1993; Kuntsi, 2004 |
To date, only one published study (Rommelse and others 2008b) has examined candidate endophenotypes in a genomewide search for susceptibility loci for ADHD. This study found significant genomewide linkage to 2q21.1 and 13q12.11 for Motor Timing and Digit Span measures, respectively, and other regions of suggestive linkage for additional traits. Yet, because analyses incorporated ADHD symptoms as covariates, the extent to which QTL across the genome have pleitropic effects on ADHD symptoms and neurocognitive measures remains unknown. Moreover, because corrections for multiple testing of the nine phenotypes were not implemented, replications are needed to rule out the possibility of Type I error.
The current paper aims to address these issues by capitalizing on a recently-developed multivariate linkage approach (Ferreira and others 2005) to examine the utility of candidate neurocognitive traits for understanding the genetic bases of ADHD. By first performing the multiple individual genomewide univariate analyses separately and then combining the results into the equivalent of a multivarate test, our strategy allows us to use all available data to evaluate a wide range of candidate endophenotypes without a priori assumptions about the extent of their genetic relationship to ADHD or one another. In this way, our approach represents a computationally efficient method for identifying quantitative trait loci (QTL) that regulate the expression of a single trait as well as those that contribute to two or more traits. Our overarching hypothesis is that the neurocognitive impairments in ADHD index a latent trait, or traits, that partially overlap with the heritable pathophysiology of the disorder. Thus, although the main analysis of the ADHD phenotype in our data set was negative (Faraone and others 2007), we predict that incorporating neurocognitive traits into a multivariate genomewide linkage analysis will aid in identifying susceptibility loci for the disorder.
MATERIALS AND METHODS
Sample Ascertainment
Our sample consists of 1212 individuals from 271 families enrolled in a genomewide linkage study of ADHD at the Massachusetts General Hospital. Subjects were recruited based on having at least one pair of full biological siblings meeting all diagnostic criteria for the disorder or with one member of the sibling pair meeting full and the other meeting subthreshold criteria. Full diagnoses required all DSM-IV diagnostic criteria, including age of onset prior to seven, and the presence of impairment in more than one setting. Subthreshold diagnoses were assigned when one of the following exceptions occurred: only two thirds of the number of required symptoms, age of onset greater than seven or impairment in only a single setting.
The majority of families were ascertained through the Clinical and Research Program in Pediatric Psychopharmacology within the child psychiatry unit at the Massachusetts General Hospital (49%). Remaining families were referred from local private child psychiatry and pediatric practices (24%), newspaper and support group advertisements (18%), the outpatient psychiatry unit at Children's Hospital in Boston (5%) and an outpatient child psychiatry clinic at the University of Nebraska (4%). No ethnic or racial groups were excluded, resulting in a sample that was 95% Caucasian, 4% African American and 1% other. Potential subjects were excluded if they had been adopted, if their nuclear family was not available for study or if they did not wish to provide a blood sample for DNA extraction. Youth with major sensorimotor handicaps, active psychosis or suspected IQ less than 70 were also excluded. After being given the details of the study, parents provided written informed consent for themselves and their children, and children and adolescents provided written assent to participate. The study was approved by the Institutional Review Board of the Massachusetts General Hospital
A two-stage procedure confirmed the diagnosis of the sib-pair. The first stage involved a telephone questionnaire with the primary caregiver that included ADHD criteria and exclusion criteria. The second stage was a clinically-reviewed structured diagnostic interview (see below). Only subjects whose diagnoses were confirmed at both stages were included in the study.
Psychopathology Assessment
For youth ages 6 to 17, lifetime and current DSM-IV diagnoses of ADHD, in addition to a range of internalizing and externalizing Axis I disorders, were assessed using the Schedule for Affective Disorders - Child Epidemiological Version (Kiddie-SADS--E). The KSADS-E is a widely used semi-structured psychiatric diagnostic interview, with established psychometric properties (Orvaschel 1994). For all youth, psychiatric data were collected from the mother or primary caregiver. In addition, those 12 and older were directly interviewed. Children younger than 12 were not interviewed directly because studies suggest limited reliability of interviews in young children (Achenbach and McConaughy 1987; Breton and others 1995; Edelbrock and others 1985; Schwab-Stone and others 1994). Subjects 18 and older received the Structured Clinical Interview for DSM IV (SCID) (First and others 1997) along with a supplement from the K-SADS-E to assess ADHD and other psychiatric disorders that onset in childhood.
Interviewers had Master's or Bachelor's degrees in psychology or a related field. They underwent a three-month training program that included mastery of DSM-IV criteria, observation of experienced raters, and achievement of high levels of inter-rater reliability with senior raters. Kappa coefficients assessed diagnostic agreement between randomly audiotaped interviews and three board certified child and adult psychiatrists for 500 subjects. Kappas for ADHD, bipolar disorder, major depressive disorder, conduct disorder, generalized anxiety disorder and panic disorder were 0.88, 0.95, 1.0, 1.0, 0.95 and 0.95, respectively. Raters were blind to the ascertainment status of subjects because they were conducting comparable psychiatric interviews for other family studies at MGH. Final diagnoses were made after blind review of interview data by psychiatrists and clinical psychologists. The committee made a best estimate diagnosis as described by Leckman et al. (1982). To combine discrepant parent-offspring reports, the most severe diagnosis was used unless it was felt to be unreliable.
Neurocognitive Assessment
Neurocognitive measures were administered to all subjects by psychometricians who received training and ongoing supervision by a team of licensed neuropsychologists (AED, LJS). Raters were blind to the diagnostic status of subjects.
The battery was comprised of well-studied clinical instruments selected based on evidence that they are 1) indirect indices of fronto-striatal systems; 2) associated with ADHD in meta-analyses and/or 3) impaired in unaffected co-twins or first degree relatives of affected youth. It also included an IQ screen and achievement testing. Below, we describe the tests themselves. Table 1 summarizes the data supporting their inclusion in the current analyses as well as the primary constructs they assess, though measures were analyzed individually because of the likelihood that they are influenced by more than one neurocognitive domain (Pennington and Ozonoff 1996).
The Wechsler Intelligence Scales, including the Wechsler Intelligence Scale for Children-Third Edition (Wechsler 1991; Wechsler 1997) for ages 6 to 17, and the Wechsler Adult Intelligence Scale- Third Edition (Wechsler 1991; Wechsler 1997) for ages 18 and older, are standardized, well-normed measures of general and specific cognitive abilities (Wechsler 1991; Wechsler 1997). Measures of overall intellectual ability, verbal working memory and speed of information processing were administered. These included: 1) Vocabulary, which requires the subject to provide a verbal definition of increasingly difficult words; 2) Block Design, which requires the subject to use groups of four and then nine colored blocks to construct patterns of increasing complexity; 3) Digit Span, which requires the subject to repeat a series of orally presented digits of increasing length in forwards and reverse order; 4) Arithmetic, which requires the subject to solve orally presented arithmetic problems without the use of paper and pencil; 5) Coding/ Digit Symbol, which requires the subject to rapidly copy symbols into empty boxes using a key code; and 6) Symbol Search, which requires the subject to visually scan a set of symbols and mark the presence or absence of two target symbols.
The Wisconsin Card Sorting Test (WCST;Computerized Version) (Grant and others 1990; Heaton and others 1993) is a measure of abstract problem solving and perseveration. The task requires subjects to sort cards with differing shapes, numbers and colors to a set of stimulus cards, according to a changing set of principles. The Stroop Color Word Test (Golden 1978), is a measure of interference control, or the ability to carry out a primary task while suppressing a competing response. It requires subjects to read color names, name colors and then name the incongruent color in which a color name is printed. The Rey-Osterreith Complex Figure (Osterrieth 1944), which involves copying a complex design, provides a measure of planning and organization. Performance was assessed using the Copy Organization Score from the Developmental Scoring System (Bernstein and Waber 1996). The Seidman Auditory Continuous Performance Test (Seidman and others 1998) is a measure of working memory that requires subjects to listen to a series of letters read aloud and tap their finger in response to certain rules. In the high working memory load condition with interference (WM-INT), subjects tap their finger after hearing an “A” four letters after a “Q,” in the presence of distractor “Q's” occurring between the stimulus and target. The California Verbal Learning Test -Child and Second Editions (Delis and others 1987; Delis and others 1994) assesses verbal learning. Subjects are presented with a list of words and given five trials to learn and verbally reproduce as many as they can. An interference list of words is also presented. The Wide Range Achievement Test - Third Edition (WRAT-III) (Jastak and Jastak 1993) was used to asses Arithmetic and Reading skills and involves performing mathematical calculations and pronouncing words of increasing difficulty.
Genotyping
Genotyping was completed at the Center for Inherited Disease Research (CIDR) at Johns Hopkins. Markers were derived using Illumina's Linkage IVb SNP-based marker panel based on its BeadArray™ technology on a BeadLab system. This system shows good power for mapping complex phenotypes, with a genomewide average information content of > 97.1%. The panel consisted of 6,008 SNPs across all autosomes and sex chromosomes. Markers cover the genome with an average genetic distance of 0.64 cM and an average 482 Kb gap. The average marker heterozygosity is 0.43 in Caucasians, 0.38 in African Americans and 0.36 in Asians. In the current data set, 1,251 DNA samples were genotyped in 279 nuclear families. 7,433,140 total genotypes were obtained for 5,980 SNPs. Using Illumina's Gentrain software to assess the quality of genotypes, only 28 SNPs were dropped for poor performance. DNA samples from eight individuals were dropped due to poor performance. The missing data rate for 1,243 samples in the family analysis was 0.192%. The overall missing data rate after removing Mendelian inconsistencies was 0.56%. Duplicate concordance was excellent: based on 56 experimental samples genotyped in duplicate, the blinded duplicate error rate was 0.002%. Thus, overall the genotype data are of very high quality. After removal of monozygotic twins and subjects who did not complete phenotypic assessments (N= 24), our total sample was comprised of 1,212 from 271 families. Table 2 shows a breakdown of the number of individuals genotyped per family included in the analysis of each phenotype.
Table 2.
Families with linkage information per phenotype
| Families with linkage information | |||||||
|---|---|---|---|---|---|---|---|
| 2 parents | 1 parent | 0 parents | |||||
| Phenotype | ≥3 siblings | 2 siblings | ≥3 siblings | 2 siblings | ≥3 siblings | 2 siblings | Total |
| Hyperactivity symtoms | 65 | 97 | 29 | 40 | 2 | 12 | 245 |
| Inattention symtoms | 65 | 97 | 29 | 40 | 2 | 12 | 245 |
| Digit Span | 52 | 91 | 26 | 33 | 2 | 11 | 215 |
| Digit Symbol | 51 | 92 | 26 | 34 | 2 | 11 | 216 |
| Arithmetic | 52 | 92 | 25 | 35 | 2 | 11 | 217 |
| Symbol Search | 43 | 81 | 22 | 30 | 2 | 11 | 189 |
| Color Naming | 47 | 90 | 25 | 31 | 2 | 11 | 206 |
| Color Word Interference | 45 | 91 | 25 | 31 | 2 | 11 | 205 |
| Nonperseverative Errors | 42 | 72 | 22 | 31 | 2 | 9 | 178 |
| Perseverative Errors | 42 | 73 | 22 | 31 | 2 | 9 | 179 |
| Rey Copy Organization | 48 | 83 | 24 | 31 | 2 | 11 | 199 |
| Block Design | 52 | 87 | 25 | 34 | 2 | 11 | 211 |
| Vocabulary | 52 | 92 | 26 | 34 | 2 | 11 | 217 |
| WRAT-III Reading | 50 | 88 | 25 | 34 | 2 | 11 | 210 |
| WRAT-III Math | 51 | 93 | 26 | 33 | 2 | 11 | 216 |
| Seidman WM-INT | 12 | 41 | 11 | 16 | 2 | 8 | 90 |
| CVLT | 35 | 64 | 20 | 29 | 2 | 11 | 161 |
To avoid biases in the estimation of identity-by-descent (IBD) probabilities, the dataset was pruned to remove correlated SNPs (r2 > 0.2), resulting in a final set of 4885 markers. Marker physical and genetic positions were obtained from the published CIDR map http://www.cidr.jhmi.edu/snp_marker.html.
Analytic method
Heritability estimates and genetic and environmental cross-trait correlations were calculated using SOLAR version 4.1.5 (Almasy and Blangero 1998). Heritabilities for all traits were determined by maximum likelihood univariate variance component analysis, which decomposed the expected phenotypic covariance into components due to polygenic additive genetic variance and environmental factors not shared between relatives. Given that our sample is comprised of reared together non-twin siblings, the genetic parameter, in actuality, represents the upper bound of heritability because it also includes variance due to environmental factors shared between relatives; nonetheless, it is useful for assessing the suitability of our phenotypes for inclusion in linkage analyses. The effects of age and sex covariates and a correction for proband ascertainment were included in the analyses, and the significance of models was determined by the likelihood ratio chi-square test.
To evaluate the appropriateness of our set of traits for a multivariate genetic analysis, we estimated the genetic and environmental correlations between all pairs of phenotypes using bivariate maximum likelihood variance components analyses. The genetic correlation was modeled as the correlation between the latent additive genetic factors influencing the traits and the environmental correlation as the correlation between latent environmental influences not shared between relatives. Here again, the genetic component of the correlation represents an upper-bound estimate. For 17 traits, there are 136 possible two-trait combinations and therefore 136 bivariate analyses were performed. As above, significance testing relied on likelihood ratio chi-square test as above, using age and sex covariates and proband ascertainment correction. To correct for multiple testing the threshold for significance of these parameters was set at P = 0.05/136 = 0.00036. Phenotypic correlations attributable to genetic and environmental factors, which are also calculated based on these models, are included in a supplementary table.
Our multivariate linkage approach, which combines results from univariate analyses into a multivariate test, has been described previously (Ferreira and others 2006; Ferreira and others 2005). Similar strategies have been used or suggested by others for multivariate linkage (Buil and others 2005; Daniels and others 1996; de Andrade and others 1997) and association analyses (Dudbridge and Koeleman 2004; Hoh and others 2001). Briefly, we first performed multipoint univariate linkage analyses for each of the 17 individual traits (the two ADHD symptom dimensions and the fifteen neurocognitive traits) using MERLIN-regress (Abecasis and others 2002), a powerful regression-based method that is robust to ascertainment. All analyses were adjusted for age and sex.
Second, for each trait, LOD scores were converted to empirical pointwise P-values (-log10P) through the analysis of 1,000 genome-scan datasets simulated under the null hypothesis of no linkage using Merlin. This step standardized the results of the individual univariate analyses, adjusting for possible biases induced by factors such normality violations, outliers or non-random missing data.
Third, at each marker, the empirical -log10P for the 17 traits were sorted and then sequentially added to represent a summary statistic (Sk) that assessed the evidence for linkage across all k traits. The significance of each of these Sk statistics was then determined empirically using the simulated datasets described above. Finally, at each marker, the Sk statistic with the minimum P-value was identified (denoted Pmin) and its significance corrected for the number of Sk statistics computed using the simulated datasets. The significance of Pmin represents our multivariate test for linkage, while the traits that were included in the corresponding Sk statistic represent the traits with evidence for linkage to that particular marker. Suggestive and significant levels of significance were calculated using the 1,000 simulated genome-scans based on recognized guidelines {Kruglyak, 1995 #11075}.
RESULTS
Offspring were 57.6% male and had a mean age of 15.2 (SD 9.1) and a mean IQ of 107.3 (SD 14.5). ADHD diagnoses in siblings included the following proportion of DSM-IV subtypes: 56% Combined Type, 36% Predominantly Inattentive Type and 8% Predominantly Hyperactive/Impulsive Type. Rates of comorbid disorders in the offspring were as follows: oppositional defiant disorder (37.2%), conduct disorder (14.9 %), major depressive disorder (13.7%), bipolar disorder (2.6%), generalized anxiety disorder (14.7%) and panic disorder (6.46%).
Table 3 shows the genetic and environmental correlations for all pairs of phenotypes. Measures in our neurocognitive battery generally show moderate to high genetic correlations with one another, suggesting that the traits are well-suited for our multivariate linkage approach. On the other hand, the data show low to moderate genetic correlations between the ADHD symptoms and neurocognitive measures. Although these did not reach significance after a strict correction for multiple testing, it is possible that the variability of our ADHD-acertained sample and/or our sample size may have limited power to determine the significance of these low-level correlations. Yet, given that many of the genetic correlations between ADHD symptoms and neurcognitive traits fall in the moderate range, which is consistent with some degree of genetic overlap, we proceeded with planned multivariate analyses of all traits.
Table 3.
Genetic and environmental correlation calculated using SOLAR
| Hyper- activity |
Inattention | Digit Span |
Coding | Arith- metic |
Sym Search |
Stroop CN |
Stroop CWI |
WCST NPE |
WCST PE |
Rey Copy |
Block Desi |
Vocab- ulary |
WRAT Read |
WRAT Math |
WM-INT | CVLT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Note: Bold = p< 0.00036. Lower diagonal shows correlations between latent additive genetic factor; upper diagonal shows correlations between latent environmental factors not shared between relatives; | |||||||||||||||||
| CN = Color Naming; CWI = Color Word Interference; WCST= Wisconsin Card Sorting Test; NPE= Non-perseverative errors; PE = Perseverative errors; WRAT= Wide Range Achievement Test - Third Edition; WM-INT = Seidman Working Memory - Interference Test; CVLT = California Verbal Learning test - Second or Child Editions | |||||||||||||||||
| Hyperactivity | 0.660 | −0.074 | −0.141 | −0.086 | 0.038 | −0.117 | −0.202 | 0.033 | 0.132 | −0.160 | 0.061 | −0.097 | 0.041 | −0.120 | 0.057 | 0.205 | |
| Inattention | 0.814 | −0.172 | −0.252 | −0.145 | −0.178 | −0.195 | −0.262 | 0.025 | 0.161 | −0.132 | −0.021 | −0.256 | −0.122 | −0.180 | −0.010 | 0.204 | |
| Digit Span | −0.098 | −0.064 | 0.257 | 0.194 | 0.201 | 0.098 | 0.100 | 0.140 | −0.070 | 0.119 | 0.221 | 0.178 | 0.219 | 0.237 | 0.185 | −0.033 | |
| Coding | −0.311 | −0.175 | 0.126 | 0.163 | 0.333 | 0.436 | 0.327 | −0.017 | −0.108 | 0.018 | 0.121 | 0.145 | 0.042 | 0.199 | 0.360 | −0.048 | |
| Arithmetic | −0.116 | 0.026 | 0.755 | 0.479 | 0.142 | 0.031 | 0.091 | 0.059 | 0.068 | 0.113 | 0.237 | 0.188 | 0.274 | 0.435 | 0.316 | 0.183 | |
| Symbol Search | −0.462 | −0.223 | 0.270 | 0.883 | 0.494 | 0.278 | 0.141 | −0.021 | 0.007 | −0.017 | 0.190 | 0.224 | 0.156 | 0.195 | 0.228 | 0.138 | |
| Stroop CN | −0.041 | 0.122 | 0.684 | 0.449 | 0.505 | 0.654 | 0.565 | −0.108 | −0.229 | 0.062 | 0.136 | 0.063 | 0.063 | 0.097 | 0.256 | −0.025 | |
| Stroop CWI | 0.041 | 0.172 | 0.664 | 0.510 | 0.684 | 0.782 | 0.793 | −0.065 | −0.181 | 0.065 | 0.082 | 0.087 | 0.027 | 0.008 | 0.188 | −0.110 | |
| WCST NPE | −0.356 | −0.367 | 0.379 | 0.234 | 0.642 | 0.452 | 0.330 | 0.460 | 0.627 | 0.026 | 0.078 | 0.045 | 0.052 | −0.006 | 0.049 | 0.029 | |
| WCST PE | −0.515 | −0.623 | 0.742 | 0.453 | 0.750 | 0.444 | 0.495 | 0.637 | 0.948 | −0.121 | 0.004 | −0.076 | 0.131 | 0.030 | 0.012 | 0.087 | |
| Rey Copy | 0.158 | 0.114 | 0.415 | 0.336 | 0.601 | 0.404 | 0.333 | 0.499 | 0.629 | 1.000 | 0.105 | 0.102 | 0.032 | 0.027 | 0.110 | −0.041 | |
| Block Design | −0.315 | −0.116 | 0.430 | 0.603 | 0.740 | 0.639 | 0.388 | 0.519 | 0.767 | 0.835 | 0.722 | 0.186 | 0.083 | 0.207 | 0.248 | 0.114 | |
| Vocabulary | −0.062 | 0.201 | 0.531 | 0.371 | 0.835 | 0.350 | 0.433 | 0.582 | 0.478 | 0.521 | 0.482 | 0.573 | 0.315 | 0.159 | 0.137 | −0.079 | |
| WRAT Read | −0.376 | −0.108 | 0.664 | 0.602 | 0.705 | 0.544 | 0.502 | 0.716 | 0.478 | 0.387 | 0.604 | 0.593 | 0.860 | 0.386 | 0.054 | 0.168 | |
| WRAT Math | −0.220 | 0.000 | 0.614 | 0.569 | 0.979 | 0.469 | 0.578 | 0.761 | 0.718 | 0.709 | 0.707 | 0.722 | 0.788 | 0.697 | 0.252 | 0.226 | |
| WM-INT | −0.457 | −0.528 | 0.547 | 0.451 | 0.458 | 0.691 | 0.450 | 0.597 | 0.726 | 0.678 | 0.656 | 0.699 | 0.475 | 0.712 | 0.468 | 0.146 | |
| CVLT | −0.581 | −0.654 | 0.815 | 0.721 | 0.707 | 0.321 | 0.768 | 0.842 | 0.557 | 0.594 | 0.486 | 0.355 | 0.942 | 0.721 | 0.579 | 0.591 | |
Table 4 lists the heritability estimates for all traits. Highest heritability (0.6) emerged for the Wechsler vocabulary subtest, which is the best proxy for full scale IQ {Sattler, 1988 #12419}. Measures of executive functions, attention and verbal learning showed heritability estimates spanning the low to moderate range (.17-.47), and measures of reading and math achievement showed moderate level estimates (approximately .5). Although the above estimates are consistent with the literature, the heritability of inattention and hyperactivity/impulsivity symptom dimensions were lower than expected, which may suggest that the correction used did not fully account for the selection bias introduced by the ascertainment through ADHD diagnosis.
Table 4.
Heritability calculated using SOLAR with age and sex as covariates and correction for ascertainment by ADHD
| Trait | Heritability |
|---|---|
| CN = Color Naming; CWI = Color Word Interference; WCST= Wisconsin Card Sorting Test; NPE= Non-perseverative errors; PE = Perseverative errors; WRAT= Wide Range Achievement Test - Third Edition; WM-INT = Seidman Working Memory - Interference Test; CVLT = California Verbal Learning test - Second or Child Editions | |
| Hyperactivity | 0.234 |
| Inattention | 0.161 |
| Digit Span | 0.370 |
| Digit Symbol | 0.319 |
| Arithmetic | 0.463 |
| Symbol | |
| Search | 0.393 |
| Stroop CN | 0.348 |
| Stroop CWI | 0.451 |
| WCST NPE | 0.233 |
| WCST PE | 0.179 |
| Rey Copy | 0.271 |
| Block Design | 0.475 |
| Vocabulary | 0.607 |
| WRAT Read | 0.450 |
| WRAT Math | 0.479 |
| WM-INT | 0.289 |
| CVLT | 0.206 |
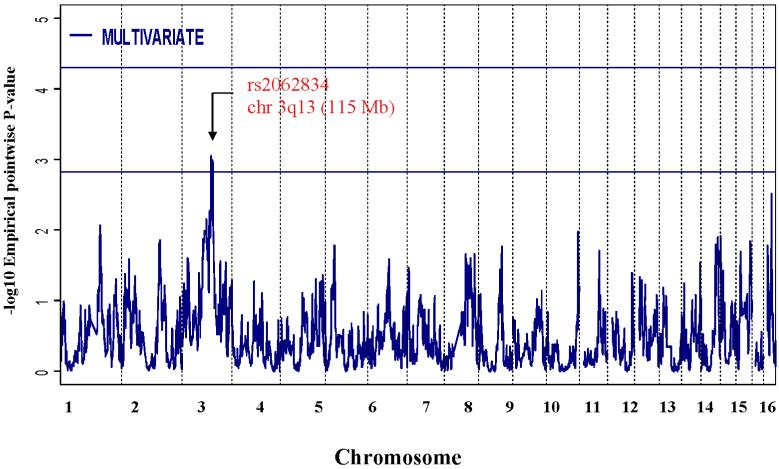
Findings from the genomewide multivariate analysis are shown in Figure 1. Although no regions of the genome surpassed the criteria for significant linkage (empirical P < 0.00005), one region on chromosome 3q13 surpassed the threshold for suggestive linkage of P <.0015 (Table 5).
Figure 1.
Results of genomewide multivariate linkage test of ADHD symptoms and neurocogntitive measures (17 phenotypes). One region on 3q13 surpassed the empirically derived multivariate genomewide threshold for suggestive linkage (-log10 empirical pointwise p value (2.822, P=.0015). No regions surpassed the empirically derived multivariate genomewide threshold for significant linkage (-log10 empirical pointwise p value (4.303, P=.00005).
Table 5.
Detail of multivariate linkage results at 3q13 peak marker (cM115.433; rs 2062834)
| Rank (k) | Trait | P | -log10P | Cumulative -log10P (Sk) | P(Sk) |
|---|---|---|---|---|---|
| CN = Color Naming; CWI = Color Word Interference; WCST= Wisconsin Card Sorting Test; NPE= Non-perseverative errors; PE = Perseverative errors; WRAT= Wide Range Achievement Test - Third Edition; WM-INT = Seidman Working Memory - Interference Test; CVLT = California Verbal Learning test - Second or Child Editions | |||||
| 1 | Symbol Search | 0.00288 | 2.541 | 2.541 | 0.03563 |
| 2 | Stroop CWI | 0.00465 | 2.332 | 4.873 | 0.01188 |
| 3 | Digit Span | 0.00887 | 2.052 | 6.925 | 0.00474 |
| 4 | WRAT Math | 0.01655 | 1.781 | 8.706 | 0.00242 |
| 5 | WCST PE | 0.02870 | 1.542 | 10.248 | 0.00152 |
| 6 | WCST NPE | 0.03783 | 1.422 | 11.671 | 0.00102 |
| 7 | Coding | 0.07769 | 1.110 | 12.780 | 0.00088 |
| 8 | Arithmetic | 0.09669 | 1.015 | 13.795 | 0.00075 |
| 9 | WRAT Read | 0.12599 | 0.900 | 14.695 | 0.00064 |
| 10 | Stroop CN | 0.24576 | 0.609 | 15.304 | 0.00065 |
| 11 | Inattention Sxs | 0.24594 | 0.609 | 15.913 | 0.00060 |
| 12 | Voabulary | 0.24664 | 0.608 | 16.521 | 0.00052 |
| 13 | Rey Copy Org | 0.34639 | 0.460 | 16.982 | 0.00047 |
| 14 | WM-INT | 0.35147 | 0.454 | 17.436 | 0.00040 |
| 15 | CVLT | 0.44223 | 0.354 | 17.790 | 0.00035 |
| 16 | Block Design Hyperactivity | 0.44754 | 0.349 | 18.139 | 0.00028 |
| 17 | Sxs | 1.00000 | 0.000 | 18.139 | 0.00028 |
Multiple traits were individually linked to the peak marker (rs2062834) at 3q13. When considering only the best trait (the Wechsler Symbol Search test, uncorrected univariate P = 0.0028), the overall evidence for linkage after correcting for the number of, and correlation between traits was P = 0.0356. However, as additional traits were included in the multivariate Sk sum statistic, the overall evidence for linkage to the peak marker improved, reaching a minimum of P = 0.00028 when considering the top-ranked 16 traits. After correcting for the number of Sk statistics computed, the multivariate evidence for linkage at rs2062834 was P = .00091 (equivalent to a χ2=11.0 or LOD=2.39), surpassing the threshold for genomewide suggestive linkage (P <.0015). Based on a one LOD score drop-off from this marker, this linkage region was ∼16Mb long, from 108,452 to 124,567 Mb.
The second highest peak in the multivariate analysis was located on chromosome 22q12 (rs762883). Unlike the 3q13 region, the evidence for linkage at 22q12 was largely restricted to one trait (the WCST nonperseverative errors, uncorrected univariate P=.001, corrected P=.003), and did not improve when considering the cumulative evidence for linkage across multiple traits.
To facilitate comparisons with other studies, Table 6 summarizes the results from the univariate linkage analyses of each trait. After correction for multiple trait testing, only the linkage between WCST nonperseverative errors and chromosome 22q12 (uncorrected P=.000073, corrected P=.002) exceeded the respective threshold for genome-wide suggestive linkage.
Table 6.
Results of univariate linkage analyses that form the basis of the multivariate test shown to facilitate replication
| -log10 Empirical pointwise p-value | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| chr | Position, Mb |
Peak SNP |
Hyperactivity | Digit Span |
Digit Symbol |
Arithmetic | Symbol Search |
Stroop CN |
Stroop CWI |
WCST NPE |
WCST PE |
Vocabulary | WRAT Read |
WRAT Math |
CVLT |
| Note: Only traits with at least one region of suggestive linkage are shown. P values shown are not corrected for multiple trait testing. | |||||||||||||||
| 1 | 157.5 - 168.07 | rs1891931 | - | - | - | - | 3.376 | - | - | - | - | - | - | - | - |
| 2 | 19.51 - 35.12 | rs1868071 | - | - | - | - | - | 2.937 | - | - | - | - | - | - | - |
| 2 | 169.66 - 174.51 | rs17664 | - | - | 2.748 | - | - | - | - | - | - | - | - | - | - |
| 3 | 20.36 - 29.32 | rs2076993 | - | - | - | - | - | - | - | - | - | - | - | - | 3.147 |
| 3 | 78.52 - 103.85 | rs2048861 | - | - | - | - | - | - | - | - | - | - | - | 3.457 | - |
| 3 | 113.54 - 124.57 | rs4258973 | - | - | - | - | 2.990 | - | - | - | - | - | - | - | - |
| 3 | 133.02 - 141.10 | rs719300 | - | - | - | - | - | - | - | - | - | - | - | - | 2.498 |
| 3 | 162.85 - 175.77 | rs1285082 | - | - | - | - | - | - | 3.107 | - | - | - | - | - | - |
| 3 | 194.13 - 198.80 | rs9343 | - | - | - | - | - | 2.829 | - | - | - | - | - | - | - |
| 5 | 158.49 - 173.47 | rs9216 | - | 2.782 | - | - | - | - | - | - | - | - | - | - | - |
| 6 | 24.20 - 41.39 | rs2213661 | - | - | - | - | - | - | - | - | 3.338 | - | - | - | - |
| 8 | 3.47 - 6.90 | rs1920469 | - | - | - | 3.027 | - | - | - | - | - | - | - | - | - |
| 9 | 85.50 - 92.35 | rs729958 | - | - | - | - | - | - | - | - | - | - | - | 3.065 | - |
| 9 | 91.81 - 105.52 | rs894673 | - | - | - | - | 3.042 | - | - | - | - | - | - | - | - |
| 10 | 80.77 - 93.34 | rs1857586 | - | 3.307 | - | - | - | - | - | - | - | - | - | - | - |
| 12 | 127.93 - 129.26 | rs1543045 | - | - | - | - | - | - | 3.343 | - | - | - | - | - | - |
| 13 | 75.83 - 85.35 | rs768218 | - | - | - | - | - | - | - | - | - | - | - | - | 3.256 |
| 15 | 20.37 - 22.99 | rs898368 | - | - | - | - | - | - | - | - | - | - | 2.869 | - | - |
| 17 | 50.05 - 69.05 | rs1881441 | - | - | - | - | - | - | - | - | - | - | - | - | 2.498 |
| 17 | 73.65 - 76.49 | rs894976 | - | - | - | 2.818 | - | - | - | - | - | - | - | - | - |
| 18 | 56.32 - 69.8 | rs624821 | - | - | - | - | - | 3.182 | - | - | - | - | - | - | - |
| 19 | 0 - 2.5 | rs351111 | - | 3.002 | - | - | - | - | - | - | - | - | - | - | - |
| 19 | 10.33 - 18.59 | rs1529729 | - | - | - | - | - | - | - | - | - | 2.970 | - | - | - |
| 20 | 13.6 - 47.15 | rs2077147 | - | - | - | - | - | - | - | - | - | 2.863 | - | - | - |
| 20 | 52.26 - 59.49 | rs186659 | - | - | - | - | - | - | - | - | 2.919 | - | - | - | - |
| 22 | 16.21 - 19.14 | rs387399 | 3.335 | - | - | - | - | - | - | - | - | - | - | - | - |
| 22 | 29.53 - 32.28 | rs762883 | - | - | - | - | - | - | - | 4.139 | - | - | - | - | - |
| Genomewide significance thresholds | |||||||||||||||
| Suggestive linkag | 2.715 | 2.499 | 2.689 | 2.528 | 2.541 | 2.691 | 2.681 | 2.690 | 2.699 | 2.677 | 2.477 | 2.526 | 2.498 | ||
| Significant linkag | 4.225 | 4.094 | 4.192 | 4.316 | 4.096 | 4.225 | 4.064 | 4.248 | 4.039 | 4.071 | 4.132 | 4.065 | 4.114 | ||
Finally, for discussion purposes, Table 7 highlights results from our multivariate and univariate analyses that overlap with the handful of genomewide linkage and other relevant studies of ADHD and neurocognition in the literature.
Table 7.
Suggestive linkage peaks: overlap with regions of interest in genomewide linkage studies of ADHD and neurocognitive traits
| Current Study. | Overlapping Study | Distance to our peak marker (kb) | |||
|---|---|---|---|---|---|
| Chromosome | Phenotype | Study | Phenotype | Peak Marker | |
| WRAT Math = Wide Range Achievement Test - Third Edition, arithmetic subtest | |||||
| 2q31.1 | Digit Symbol | Posthuma 2006 | Performance IQ | D2S2330 | 6,571 |
| 2p23.1 | Color Naming | Rommelse 2008 | Motor Timing; | rs1309 | 19,436 |
| Digit Span | rs1079417 | 24,055 | |||
| 3q13.3 | Multivariate | Bakker 2003 | ADHD | D3S2460 | 3,351 |
| 5q33.3 | Digit Span | Arcos-Burgos | ADHD | D5S1480 | 15,484 |
| 9q21.3 | WRAT-Math | Asherson 2008 | ADHD | rs7043803 | 1,015 |
| 9q22.3 | Symbol Search | Hebebrand 2005 | ADHD | D9S1851 | 940 |
| 15q11.2 | WRAT Math | Romanos 2008 | ADHD | rs4310812-3850097 | 9,477 |
| 20q13 | Perseverative errors | Ogdie 2004 | ADHD | D20S1106 | 5,707 |
DISCUSSION
The current study represents the first genomewide linkage analysis to combine ADHD symptoms and candidate endophenotypes into a multivariate test to search for QTL for the disorder. Although our previous linkage scans of quantitative and dichotomous ADHD phenotypes were largely negative (Faraone and others 2007), this multivariate approach revealed a region on 3q13 showing suggestive linkage to symptoms of inattention as well as to a wide range of neurocognitive traits that are associated with the condition. The second highest multivariate peak occurred on 22q12 for a subtest of the Wisconsin Card Sorting Test. Overall, our findings indicate that neurocognitive measures may assist in the genetic dissection of ADHD and that one or more genes on 3q13 may influence variation in behavioral inattention and a wide range of higher order neurocognitive functions.
Endophenotypes for psychiatric disorders, though widely discussed, have rarely been used in practice, and strategies for their incorporation into molecular genetic analyses are still being developed. As Allison and colleagues point out (Allison and others 1998), there are a variety of approaches to handling multiple phenotypes in general in linkage analyses, each of which has advantages and disadvantages. Conducting separate univariate analyses of each measure (Grigorenko and others 1997) promotes interpretability and ease of replication, but results in a loss of power after correction for multiple testing to avoid Type I error. Using biometrical modeling to extract a single factor representing the common genetic variance of the neurocognitive measures and ADHD (Sham and others 2000) is parsimonious but results in a loss of opportunity to search for QTL that influence only a subset or a single trait. Formal multivariate variance components analysis (Marlow and others 2003), although theoretically desirable, is computationally intensive with more than a small number of traits. This latter approach also presents challenges to the assessment of empirical significance and is not guaranteed to provide greater power than corresponding univariate analyses when the traits are moderately correlated (Amos and others 2001; Ferreira and others 2006).
Our chosen strategy (Ferreira and others 2006; Ferreira and others 2005) combined a series of univariate analyses into the equivalent of a multivariate test and assessed their significance by simulation. The approach capitalizes on increased statistical power from the shared covariance of the behavioral and neurocognitive phenotypes when it exists, while still allowing for identification of QTL that contribute solely to individual traits or a subset of traits. Additionally, it preserves information pertaining to individual measures thereby facilitating replication in other data sets.
This approach highlighted a region on chromosome 3q13 showing suggestive linkage to ADHD inattention symptoms and all 15 neurocognitive traits examined. This region was not previously identified in the main analysis of the dichotomous ADHD phenotype (Faraone and others 2007) and would not have reached suggestive linkage had we only performed individual univariate analyses. Therefore, provided that this result can be subsequently replicated, this finding underscores the potential of using multiple correlated endophenotypes and a multivariate approach to identify susceptibility loci for ADHD.
Although our results require replication, the area under our 3q13 peak is worthy of further investigation given that it is also a region of interest in other relevant studies. One of the nine peaks with a LOD score >1 in the Dutch (Bakker and others 2003b) genomewide ADHD linkage scan mapped to 3q13.32 approximately 3 megabases from our peak marker (D3S2460; LOD 1.36). Additionally, Petryshen et al (2005) identified a QTL for prepulse inhibition (PPI) of acoustic startle on a segment of mouse chromosome 16 that is syntenic with this region. PPI is thought to tap aspects of inhibitory control, attention and efficiency of information processing (Bitsios and Giakoumaki 2005) and has been associated with prefrontal-striatal circuits in rats (Schneider and Koch 2005) and performance on measures of executive functions in humans (Bitsios and Giakoumaki 2005; Bitsios and others 2006). Linkage studies of autism (Allen-Brady and others 2008; Schellenberg and others 2006), which is characterized by neurocognitive impairments in executive, attentional and verbal domains, have also highlighted this 3q13 region. Finally, our area overlaps with a region identified for reading disability (Nopola-Hemmi and others 2001) which, consistent with our results, has been shown to have a genetic link to ADHD that is mediated by inattention (Willcutt and others 2007).
Several genes in this 3q13 region are promising biological candidates for regulating the behavioral symptoms of attention and higher order neurocognition. Most notably, the Dopamine D3 receptor (DRD3) resides within 52 kb of our peak snp. Both animal models of ADHD (Russell 2007) and the efficacy of psychostimulants (Biederman and Spencer 2008) have implicated the dysregulation of dopamine (DA) in the pathophysiology of the disorder, and four DA-related genes (DRD4, DRD5, SLC6A3 and DBH), have previously shown association with ADHD in a meta-analysis (Faraone and others 2005). Although the handful of studies examining the association of DRD3 and the dichotomous diagnosis of ADHD have yielded largely negative findings for common, functional polymorphisms (Barr and others 2000; Muglia and others 2002; Payton and others 2001), none of these studies tagged the full haplotype block structure of the gene.
The distribution of DA receptors in the brain and studies of non-human primates also support its role in neurocognition (Brown and others 1979). Recently, Bombin et al (2008) found that DRD3 Gly/Gly homozygotes performed more poorly on a combined measure of executive functions in healthy individuals and those with first episode psychosis; however, Rybakowski et al (2005) found no association between DRD3 and the WCST in patients with schizophrenia. Together with our data, these findings raise the possibility that DRD3 influences the piece of the etiological puzzle of ADHD that is shared with neurocognitive traits but may have a negligible effect when the disorder is considered as a whole and/or that the specific variant in DRD3 that regulates ADHD inattention symptoms and neurocognitive traits has yet to be identified.
Genes at 3q13 that have not previously been investigated for ADHD are also targets for further study because of their potential involvement in neural development or their expression in the brain. Promising candidates at or near our peak snp include: 1) LSAMP (limbic system associated protein), which plays a role in the formation of limbic circuits and axonal growth (Pimenta and others 1998); and 2) ZNF80, a cDNA clone that codes for a zinc finger protein domain which can be part of regulatory proteins and transcription factors involved in early neural development (Di Cristofano and others 1995). Also within the one- LOD score drop-off region is CBLB, a proto-oncogene that, in mice, is expressed in the brain and plays a role in the synaptic mechanisms underlying long-term memory (Tan and others 2006). More work is needed to evaluate whether these or other genes show true association to the phenotypes linked to this region.
If our results are replicated, it is intriguing to consider the mechanism by which such a wide range of phenotypes may share a genetic etiology. Although, we had anticipated partial overlap of multiple neurocognitive traits and at least one ADHD symptom dimension, our findings indicate overlap at a single locus between all 15 neurocognitive phenotypes examined and the behavioral symptoms of inattention. These findings are consistent with a growing behavioral genetics literature showing significant genetic correlations, despite low to moderate phenotypic correlations, between an extensive variety of neurocognitive traits (Butcher and others 2006) including but not limited to correlations with IQ measures (Pedersen and others 1994). Based on this literature, Plomin and colleagues (Butcher and others 2006; Kovas and Plomin 2006) have hypothesized the existence of ‘generalist genes’ that influence a very diverse array of functional neurocognitive processes. Although not discounting genetic influences specific to individual traits, the proposition highlights commonalities across functions that may derive from the heritable organization of fundamental neural systems supporting cognition (Butcher and others). The existence of one or more generalist genes at 3q13 offers a parsimonious explanation for our results, and our linkage of the behavioral manifestations of inattention to this region raises the interesting possibility that attentional processes represent a functional component of the commonalities across measures.
Kovas and Plomin (Kovas and Plomin 2006) provide several simplified examples of how a given gene may exert pleiotropic effects on neurocognitive traits, including: 1) a gene influences a specific brain area, which in turn influences a range of neurocognitive processes; 2) a gene influences multiple brain regions, each of which affects a specific neurocognitive process; or 3) a gene influences multiple brain regions, each of which impact multiple cognitive processes. It is important to note that our data cannot distinguish between these possibilities, despite the fact that our neurocognitive measures were selected based on their potential as mediators of the relationship to ADHD (i.e. endophenotypes) and with heavy emphasis on measures tapping prefrontal-striatal network. Rather, further investigation of this region is needed to determine the underlying neurobiological mechanisms in play.
Several QTL showing suggestive linkage to individual traits also warrant further investigation, although some are likely to be false positives given multiple trait testing. Most striking is the signal on 22q12.3 for the WCST non-perseverative errors that fell just short of genomewide significance and survived correction for multiple trait testing. Previously, this region has shown linkage to bipolar disorder and schizophrenia (Badner and Gershon 2002), both of which show a familial relationship to impairments on the WCST (Savitz and others 2005), and other executive measures. Among the genes in this region, synapsin III, located at the site of our peak SNP, is a compelling candidate for further study because it plays a role in neural development and neurotransmitter release (Kao and others 2008).
Although not significant after correction for multiple trait testing, other univariate findings merit consideration as well. Hyperactivity symptoms showed suggestive linkage to 22q11.21. This region is well-known to exhibit genomic instability, with a 3 Mb hemizygous deletion resulting in velo-cardio-facial (VCFS) syndrome, a multi-system disorder that shows a high prevalence of ADHD and behavioral dysregulation (Antshel and others 2007). Thus, our data lend further support to the hypothesis that one or more variants in this region influences behavioral hyperactivity.
Finally, as shown in Table 7, several neurocognitive measures showed suggestive linkage in regions overlapping with the handful of previous genomewide linkage studies of ADHD and neurocognition. Some of these and other QTL from Table 6 also overlap with regions of interest from linkage studies of other psychiatric and neurodevelopmental disorders characterized by neurocognitive dysfunction, e.g. autism (2q31) (Romano and others 2005) and bipolar disorder (12q24) (Berrettini 2001). Determining whether these QTL have true effects on neurocognition and possibly pleiotropic effects on other disorders will likely require larger samples since our data suggest that effects are not large and that the neurocognitive measures may themselves be complex phenotypes.
Of note is that our current findings show only limited overlap with Rommelse et al. (Rommelse and others 2008a), which is the only genomewide linkage study to date to examine neurocognitive traits in an ADHD sample. Yet the lack of similarity to their results is understandable given that our analysis aimed to find QTL with pleiotropic effects on behavioral symptoms of ADHD and neurocognition. Thus, if our 3q13 finding is indeed real, it may not have emerged in their study because they covaried out the effects of ADHD on the neurocognitive measures before conducting their analyses. Additionally, given that their primary analyses of eight candidate endophenotypic traits and one composite measure were not corrected for multiple trait testing, Type I error cannot be ruled out. Yet, our univariate data do show a signal for color naming/processing speed on 2p in the broad vicinity of their secondary signals for motor timing and digit span. More work is needed to explore the validity of a QTL in this region for these traits.
The current analyses should be considered in light of their limitations. As discussed, formal multivariate variance components analysis (Marlow and others 2003) is theoretically desirable but not practical with our large number of traits and the non-normal distribution of the ADHD phenotypes. Nonetheless, we believe our approach provides a practical heuristic to combine results across individual analyses into a more powerful test, as highlighted by others (Buil and others 2005; Daniels and others 1996; de Andrade and others 1997). Additionally, the current method allowed us to use all available data for each trait, whereas data reduction via factor analysis would have required excluding traits and or individuals with missing information. Second, although our array of neurocognitive measures was chosen based on our evaluation of the empirical literature, several interesting candidate endophenotypes for ADHD were not investigated, including problems with time estimation (Rubia and others 2003; Smith and others 2002; Toplak and others 2003); 2) variability of reaction time (RT) (Castellanos and Tannock 2002); and 3) preference for immediate small rewards over larger delayed ones (Kuntsi and others 2001; Solanto and others 2001; Sonuga-Barke 2003; Sonuga-Barke and others 1992). Molecular genetic studies including these constructs would offer important data to the field. Third, the low correlations between ADHD symptom dimensions and the neurocognitive phenotypes in both our study and the literature suggest that ascertainment through ADHD probands is unlikely to have biased the univariate results for individual neurocognitive traits; however, replication of findings for neurocognitive measures in non-ADHD samples is also needed to confirm the 3q13 finding and determine whether our ascertainment by ADHD could have reduced power to detect signals from other QTL that jointly influence inattention and/or hyperactivity and higher order neurocognitive traits.
In conclusion, we have identified a region on chromosome 3q13 which appears to influence ADHD inattention symptoms and multiple neurocognitive measures. Overall, our results support recent twin and family studies indicating that neurocognitive measures show genetic influences that are shared with and unique from ADHD and justify their further, though cautious use in molecular genetic studies of the disorder. Yet, our findings also highlight the lack of complete genetic overlap of these behavioral and neurocognitive phenotypes and illustrate that genetic influences on associated neurocognitive traits cannot be presumed to influence ADHD. Nonetheless, revealing the genetic influences on executive and other higher order neurocognitive impairments is of considerable public health importance given the lack of available treatments and their association with poor functional outcome in the psychiatric disorders such as ADHD, schizophrenia and bipolar disorder with which they co-occur (Biederman and others 2005; Green 1996; Martinez-Aran and others 2004).
ACKNOWLEDGEMENTS
This work was funded by NIH grants R21MH080730 to Dr. Doyle and R01HD37694 to Dr. Faraone. Portions of these data were presented in October 2007 at the Annual Meetings of the American Academy of Child and Adolescent Psychiatry in Boston and the International Society for Psychiatric Genetics in New York. The authors wish to thank Kimbery Mullen, Hannah-Lise Schofield and Kristy Klein with their help with data collection.
REFERENCES
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30(1):97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, McConaughy SH. In: Empirically Based Assessment of Child and Adolescent Psychopathology:Practical Applications. Kazdin AE, editor. Sage Publications, Inc.; Newbury Park: 1987. p. 167. [Google Scholar]
- Allen-Brady K, Miller J, Matsunami N, Stevens J, Block H, Farley M, Krasny L, Pingree C, Lainhart J, Leppert M. A high-density SNP genome-wide linkage scan in a large autism extended pedigree. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.14. and others. [DOI] [PubMed] [Google Scholar]
- Allison DB, Thiel B, Jean P, Elston RC, Infante MC, Schork NJ. Multiple phenotype modeling in gene-mapping studies of quantitative traits: power advantages. American Journal of Human Genetics. 1998;63:1190–1201. doi: 10.1086/302038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. American Journal of Human Genetics. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: rationale and study design. Am J Med Genet. 2001;105(1):42–4. [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Amos C, de Andrade M, Zhu D. Comparison of multivariate tests for genetic linkage. Hum Hered. 2001;51(3):133–44. doi: 10.1159/000053334. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Faraone SV, Fremont W, Monuteaux MC, Kates WR, Doyle AE, Mick E, Biederman J. Comparing ADHD in velocardiofacial syndrome to idiopathic ADHD: A preliminary study. Journal of Attention Disorders. 2007;11(1):64–73. doi: 10.1177/1087054707299397. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Castellanos FX, Konecki D, Lopera F, Pineda D, Palacio JD, Rapoport JL, Berg K, Bailey-Wilson J, Muenke M. Pedigree disequilibrium test (PDT) replicates association and linkage between DRD4 and ADHD in multigenerational and extended pedigrees from a genetic isolate. Mol Psychiatry. 2004;9(3):252–9. doi: 10.1038/sj.mp.4001396. [DOI] [PubMed] [Google Scholar]
- Asherson P, Zhou K, Anney RJ, Franke B, Buitelaar J, Ebstein R, Gill M, Altink M, Arnold R, Boer F. A high-density SNP linkage scan with 142 combined subtype ADHD sib pairs identifies linkage regions on chromosomes 9 and 16. Mol Psychiatry. 2008;13(5):514–21. doi: 10.1038/sj.mp.4002140. and others. [DOI] [PubMed] [Google Scholar]
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7(4):405–11. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Bakker S, van der Meulen E, Buitelaar J, Sandkuijl L, Pauls D, Monsuur A, van 't Slot R, Minderaa R, Gunning W, Pearson P. A whole-genome scan in 164 Dutch sib pairs with attention-deficit/hyperactivity disorder: suggestive evidence for linkage on chromosomes 7p and 15q. American Journal of Human Genetics. 2003a;72(5):1251–60. doi: 10.1086/375143. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker SC, van der Meulen EM, Buitelaar JK, Sandkuijl LA, Pauls DL, Monsuur AJ, van 't Slot R, Minderaa RB, Gunning WB, Pearson PL. A whole-genome scan in 164 Dutch sib pairs with attention-deficit/hyperactivity disorder: suggestive evidence for linkage on chromosomes 7p and 15q. Am J Hum Genet. 2003b;72(5):1251–1260. doi: 10.1086/375143. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Attention Deficit-Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. Guilford Press; New York: 2006. [Google Scholar]
- Barr CL, Wigg KG, Wu J, Zai C, Bloom S, Tannock R, Roberts W, Malone M, Schachar R, Kennedy JL. Linkage study of two polymorphisms at the dopamine D3 receptor gene and attention-deficit hyperactivity disorder. American Journal of Medical Genetics. 2000;96(1):114–117. [PubMed] [Google Scholar]
- Bernstein JH, Waber DP. Developmental scoring system for the Rey-Osterreith Complex Figure [manual] Psychological Assessment Resources, Inc.; Odessa, Florida: 1996. p. 87. [Google Scholar]
- Berrettini WH. Molecular linkage studies of bipolar disorders. Bipolar Disord. 2001;3(6):276–83. doi: 10.1034/j.1399-5618.2001.30603.x. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Willcutt EG, Defries JC, Pennington BF. Testing for Neuropsychological Endophenotypes in Siblings Discordant for Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention Deficit Hyperactivity Disorder. Lancet. 2005;366(9481):237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty C, Fried R, Fontanella J, Doyle AE, Seidman L, Faraone SV. Impact of Psychometrically-Defined Executive Function Deficits in Adults with ADHD. American Journal of Psychiatry. 2006;163(10):1730–8. doi: 10.1176/ajp.2006.163.10.1730. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty C, Fried R, Fontanella J, Doyle AE, Seidman LJ, Faraone SV. Functional outcomes associated with self-reported executive function deficits in adults with ADHD. AACAP/CACAP; Toronto, Canada: Oct 18-23, 2005. 2005. [Google Scholar]
- Biederman J, Petty CR, Fried R, Doyle AE, Spencer T, Seidman LJ, Gross L, Poetzl K, Faraone SV. Stability of executive function deficits into young adult years: a prospective longitudinal follow-up study of grown up males with ADHD. Acta Psychiatr Scand. 2007;116(2):129–36. doi: 10.1111/j.1600-0447.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Spencer TJ. Psychopharmacological interventions. Child Adolesc Psychiatr Clin N Am. 2008;17(2):439–58. doi: 10.1016/j.chc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG. Relationship of prepulse inhibition of the startle reflex to attentional and executive mechanisms in man. Int J Psychophysiol. 2005;55(2):229–41. doi: 10.1016/j.ijpsycho.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG, Theou K, Frangou S. Increased prepulse inhibition of the acoustic startle response is associated with better strategy formation and execution times in healthy males. Neuropsychologia. 2006;44(12):2494–9. doi: 10.1016/j.neuropsychologia.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Bombin I, Arango C, Mayoral M, Castro-Fornieles J, Gonzalez-Pinto A, Gonzalez-Gomez C, Moreno D, Parellada M, Baeza I, Graell M. DRD3, but not COMT or DRD2, genotype affects executive functions in healthy and first-episode psychosis adolescents. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30710. and others. [DOI] [PubMed] [Google Scholar]
- Borecki IB, Rao DC, Yaouanq J, Lalouel JM. Serum ferritin as a marker of affection for genetic hemochromatosis. Human Heredity. 1990;40(3):159–66. doi: 10.1159/000153924. [DOI] [PubMed] [Google Scholar]
- Breton J, Bergeron L, Valla J, Lepine S, Houde L, Gaudet N. Do children aged 9 through 11 years understand the DISC version 2.25 questions? Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(7):946–956. doi: 10.1097/00004583-199507000-00019. [DOI] [PubMed] [Google Scholar]
- Brown RM, Crane AM, Goldman PS. Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: concentrations and in vivo synthesis rates. Brain Res. 1979;168(1):133–50. doi: 10.1016/0006-8993(79)90132-x. [DOI] [PubMed] [Google Scholar]
- Buil A, Dyer TD, Almasy L, Blangero J. Smoothing of the bivariate LOD score for non-normal quantitative traits. BMC Genet. 2005;6(Suppl 1):S111. doi: 10.1186/1471-2156-6-S1-S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher LM, Kennedy JK, Plomin R. Generalist genes and cognitive neuroscience. Curr Opin Neurobiol. 2006;16(2):145–51. doi: 10.1016/j.conb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Castellanos FX. Neuroimaging studies of ADHD. In: Solanto MV, Arnsten AF, Castellanos FX, editors. Stimulant drugs and ADHD Basic and Clinical Neuroscience. Oxford University Press; Oxford: 2001. pp. 243–258. [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Jama. 2002;288(14):1740–8. doi: 10.1001/jama.288.14.1740. and others. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Daniels SE, Bhattacharrya S, James A, Leaves NI, Young A, Hill MR, Faux JA, Ryan GF, le Souef PN, Lathrop GM. A genome-wide search for quantitative trait loci underlying asthma. Nature. 1996;383(6597):247–50. doi: 10.1038/383247a0. and others. [DOI] [PubMed] [Google Scholar]
- de Andrade M, Thiel TJ, Yu L, Amos CI. Assessing linkage on chromosome 5 using components of variance approach: univariate versus multivariate. Genet Epidemiol. 1997;14(6):773–8. doi: 10.1002/(SICI)1098-2272(1997)14:6<773::AID-GEPI35>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test-adult version. The Psychological Corporation; New York: 1987. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test for Children. The Psychological Corp.; San Antonio, TX: 1994. [Google Scholar]
- Di Cristofano A, Strazzullo M, Longo L, La Mantia G. Characterization and genomic mapping of the ZNF80 locus: expression of this zinc-finger gene is driven by a solitary LTR of ERV9 endogenous retroviral family. Nucleic Acids Res. 1995;23(15):2823–30. doi: 10.1093/nar/23.15.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AE. Familial aggregation of neurocognitive impairments and ADHD symtom dimensions. Boston, MA: 2006. [Google Scholar]
- Doyle AE, Biederman J, Seidman L, Reske-Nielsen J, Faraone SV. Neuropsychological functioning in relatives of girls with and without ADHD. Psychological Medicine. 2005a;35(8):1121–32. doi: 10.1017/s0033291705004496. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Faraone SV, Seidman LJ, Willcutt EG, Nigg JT, Waldman ID, Pennington BF, Peart J, Biederman J. Are endophenotypes based on measures of executive functions useful for molecular genetic studes of ADHD? Journal of Child Psychology and Psychiatry. 2005b;46(7):774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Dudbridge F, Koeleman BP. Efficient computation of significance levels for multiple associations in large studies of correlated data, including genomewide association studies. Am J Hum Genet. 2004;75(3):424–35. doi: 10.1086/423738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelbrock C, Costello AJ, Dulcan MK, Kalas R, Conover NC. Age differences in the reliability of the psychiatric interview of the child. Child Development. 1985;56:265–275. [PubMed] [Google Scholar]
- Faraone SV, Biederman J. Neurobiology of Attention Deficit Hyperactivity Disorder. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. Second Edition Oxford University Press; New York, NY: 2004. [Google Scholar]
- Faraone SV, Doyle AE, Lasky-Su J, Sklar PB, D'Angelo E, Gonzalez-Heydrich J, Kratochvil C, Mick E, Klein K, Rezac AJ. Linkage analysis of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2007 doi: 10.1002/ajmg.b.30631. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick J, Holmgren MA, Sklar P. Molecular genetics of attention deficit hyperactivity disorder. Biological Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Sergeant J, Gillberg C, Biederman J. The Worldwide Prevalence of ADHD: Is it an American Condition? World Psychiatry. 2003;2(2):104–113. [PMC free article] [PubMed] [Google Scholar]
- Ferreira MA, O'Gorman L, Le Souef P, Burton PR, Toelle BG, Robertson CF, Martin NG, Duffy DL. Variance components analyses of multiple asthma traits in a large sample of Australian families ascertained through a twin proband. Allergy. 2006;61(2):245–53. doi: 10.1111/j.1398-9995.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, O'Gorman L, Le Souef P, Burton PR, Toelle BG, Robertson CF, Visscher PM, Martin NG, Duffy DL. Robust estimation of experimentwise P values applied to a genome scan of multiple asthma traits identifies a new region of significant linkage on chromosome 20q13. Am J Hum Genet. 2005;77(6):1075–85. doi: 10.1086/497997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSMIV Axis I Disorders-Clinician Version (SCID-CV) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Fisher SE, Francks C, McCracken JT, McGough JJ, Marlow AJ, MacPhie IL, Newbury DF, Crawford LR, Palmer CG, Woodward JA. A genomewide scan for loci involved in attention-deficit/hyperactivity disorder. Am J Hum Genet. 2002;70(5):1183–96. doi: 10.1086/340112. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Use. Stoelting, Co.; Chicago: 1978. [Google Scholar]
- Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grant ML, Ilai D, Nussbaum NL, Bigler ED. The relationship between continuous performance tasks and neuropsychological tests in children with attention-deficit hyperactivity disorder. Perceptual and Motor Skills. 1990;70:435–445. doi: 10.2466/pms.1990.70.2.435. [DOI] [PubMed] [Google Scholar]
- Green M. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Grigorenko EL, Wood FB, Meyer MS, Hart LA, Speed WC, Shuster A, Pauls DL. Susceptibility loci for distinct components of developmental dyslexia on chromosomes 6 and 15. American Journal of Human Genetics. 1997;60:27–39. [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sort Test Manual: Revised and Expanded. Psychological Assessment Resources, Inc.; Odessa, FL: 1993. [Google Scholar]
- Hoh J, Wille A, Ott J. Trimming, weighting, and grouping SNPs in human case-control association studies. Genome Res. 2001;11(12):2115–9. doi: 10.1101/gr.204001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastak J, Jastak S. Wide Range Achievement Test-Third Edition. Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Kao HT, Li P, Chao HM, Janoschka S, Pham K, Feng J, McEwen BS, Greengard P, Pieribone VA, Porton B. Early involvement of synapsin III in neural progenitor cell development in the adult hippocampus. J Comp Neurol. 2008;507(6):1860–70. doi: 10.1002/cne.21643. [DOI] [PubMed] [Google Scholar]
- Kovas Y, Plomin R. Generalist genes: implications for the cognitive sciences. Trends Cogn Sci. 2006;10(5):198–203. doi: 10.1016/j.tics.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? Journal of Child Psychology and Psychiatry. 2001;42(2):199–210. [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nature Genetics. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson D, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: A methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol. 2005;114(2):216–22. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Lopez RE. Hyperactivity in twins. Canadien Psychiatric Association Journal. 1965;10(4):421–426. doi: 10.1177/070674376501000516. [DOI] [PubMed] [Google Scholar]
- Marlow AJ, Fisher SE, Francks C, MacPhie IL, Cherny SS, Richardson AJ, Talcott JB, Stein JF, Monaco AP, Cardon LR. Use of multivariate linkage analysis for dissection of a complex cognitive trait. Am J Hum Genet. 2003;72(3):561–70. doi: 10.1086/368201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M. Cognitive Function Across Manic or Hypomanic, Depressed, and Euthymic States in Bipolar Disorder. American Journal of Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(4):377–84. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Muglia P, Jain U, Kennedy JL. A transmission disequilibrium test of the Ser9/Gly dopamine D3 receptor gene polymorphism in adult attention-deficit hyperactivity disorder. Behav Brain Res. 2002;130(1-2):91–5. doi: 10.1016/s0166-4328(01)00438-7. [DOI] [PubMed] [Google Scholar]
- Nopola-Hemmi J, Myllyluoma B, Haltia T, Taipale M, Ollikainen V, Ahonen T, Voutilainen A, Kere J, Widen E. A dominant gene for developmental dyslexia on chromosome 3. J Med Genet. 2001;38(10):658–64. doi: 10.1136/jmg.38.10.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogdie M, Macphie I, Minassian S, Yang M, Fisher S, Francks C, Cantor R, McCracken J, McGough J, Nelson S. A genomewide scan for attention-deficit/hyperactivity disorder in an extended sample: suggestive linkage on 17p11. American Journal of Human Genetics. 2002;72(5):1268–79. doi: 10.1086/375139. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvaschel H. Schedule for Affective Disorder and Schizophrenia for School-Age Children Epidemiologic Version. Nova Southeastern University, Center for Psychological Studies; Ft. Lauderdale: 1994. [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe. Archives de Psychologie. 1944;30:206–256. [Google Scholar]
- Payton A, Holmes J, Barrett JH, Hever T, Fitzpatrick H, Trumper AL, Harrington R, McGuffin P, O'Donovan M, Owen M. Examining for association between candidate gene polymorphisms in the dopamine pathway and attention-deficit hyperactivity disorder: A family- based study. American Journal of Medical Genetics. 2001;105(5):464–70. doi: 10.1002/ajmg.1407. and others. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, McClearn GE. Is there G beyond g? (Is there genetic influence on specific cognitive abilities independent of genetic influence on general cognitive ability?) Intelligence. 1994;18:133–143. [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Kirby A, Hammer RP, Jr., Purcell S, O'Leary SB, Singer JB, Hill AE, Nadeau JH, Daly MJ, Sklar P. Two quantitative trait loci for prepulse inhibition of startle identified on mouse chromosome 16 using chromosome substitution strains. Genetics. 2005;171(4):1895–904. doi: 10.1534/genetics.105.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta AF, Tsui LC, Heng HH, Levitt P. Assignment of the gene encoding the limbic system-associated membrane protein (LAMP) to mouse chromosome 16B5 and human chromosome 3q13.2-q21. Genomics. 1998;49(3):472–4. doi: 10.1006/geno.1998.5280. [DOI] [PubMed] [Google Scholar]
- Romano V, Cali F, Seidita G, Mirisola M, D'Anna RP, Gambino G, Schinocca P, Romano S, Ayala GF, Canziani F. Suggestive evidence for association of D2S2188 marker (2q31.1) with autism in 143 Sicilian (Italian) TRIO families. Psychiatr Genet. 2005;15(2):149–50. doi: 10.1097/00041444-200506000-00013. and others. [DOI] [PubMed] [Google Scholar]
- Romanos M, Freitag C, Jacob C, Craig DW, Dempfle A, Nguyen TT, Halperin R, Walitza S, Renner TJ, Seitz C. Genome-wide linkage analysis of ADHD using high-density SNP arrays: novel loci at 5q13.1 and 14q12. Mol Psychiatry. 2008;13(5):522–30. doi: 10.1038/mp.2008.12. and others. [DOI] [PubMed] [Google Scholar]
- Rommelse N, Altink ME, Martin NC, Buschgens CJ, Faraone SV, Buitelaar JK, Sergeant JA, Oosterlaan J. Relationship between endophenotype and phenotype in ADHD. Behav Brain Funct. 2008a;4:4. doi: 10.1186/1744-9081-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NN, Arias-Vasquez A, Altink ME, Buschgens CJ, Fliers E, Asherson P, Faraone SV, Buitelaar JK, Sergeant JA, Oosterlaan J. Neuropsychological endophenotype approach to genome-wide linkage analysis identifies susceptibility loci for ADHD on 2q21.1 and 13q12.11. Am J Hum Genet. 2008b;83(1):99–105. doi: 10.1016/j.ajhg.2008.06.006. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Noorloos J, Smith A, Gunning B, Sergeant J. Motor timing deficits in community and clinical boys with hyperartive behavior: The effect of methylphenidate on motor timing. J Abnorm Child Psychol. 2003;31(3):301–313. doi: 10.1023/a:1023233630774. [DOI] [PubMed] [Google Scholar]
- Russell VA. Reprint of “Neurobiology of animal models of attention-deficit hyperactivity disorder”. J Neurosci Methods. 2007;166(2):I–XIV. doi: 10.1016/j.jneumeth.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Kapelski P, Dmitrzak-Weglarz M, Hauser J. An association study of dopamine receptors polymorphisms and the Wisconsin Card Sorting Test in schizophrenia. J Neural Transm. 2005;112(11):1575–82. doi: 10.1007/s00702-005-0292-6. [DOI] [PubMed] [Google Scholar]
- Savitz JB, Solms M, Ramesar RS. Neurocognitive function as an endophenotype for genetic studies of bipolar affective disorder. Neuromolecular Med. 2005;7(4):275–86. doi: 10.1385/NMM:7:4:275. [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Crosbie J, Barr CL, Ornstein TJ, Kennedy J, Malone M, Roberts W, Ickowicz A, Tannock R, Chen S. Inhibition of motor responses in siblings concordant and discordant for attention deficit hyperactivity disorder. Am J Psychiatry. 2005;162(6):1076–82. doi: 10.1176/appi.ajp.162.6.1076. and others. [DOI] [PubMed] [Google Scholar]
- Schellenberg GD, Dawson G, Sung YJ, Estes A, Munson J, Rosenthal E, Rothstein J, Flodman P, Smith M, Coon H. Evidence for multiple loci from a genome scan of autism kindreds. Mol Psychiatry. 2006;11(11):1049–60. 979. doi: 10.1038/sj.mp.4001874. and others. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Behavioral and morphological alterations following neonatal excitotoxic lesions of the medial prefrontal cortex in rats. Exp Neurol. 2005;195(1):185–98. doi: 10.1016/j.expneurol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Schwab-Stone M, Fallon T, Briggs M, Crowther B. Reliability of diagnostic reporting for children aged 6-11 years: a test-retest study of the Diagnostic Interview Schedule for Children- Revised. Am J Psychiatry. 1994;151(7):1048–54. doi: 10.1176/ajp.151.7.1048. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Breiter HC, Goodman JM, Goldstein JM, Woodruff PW, O'Craven K, Savoy R, Tsuang MT, Rosen BR. A functional magnetic resonance imaging study of auditory vigilance with low and high information processing demands. Neuropsychology. 1998;12(4):505–18. doi: 10.1037//0894-4105.12.4.505. [DOI] [PubMed] [Google Scholar]
- Sham PC, Sterne A, Purcell S, Cherny S, Webster M, Rijsdijk F, Asherson P, Ball D, Craig I, Eley T. GENESiS: creating a composite index of the vulnerability to anxiety and depression in a community-based sample of siblings. Twin Res. 2000;3(4):316–22. doi: 10.1375/136905200320565292. and others. [DOI] [PubMed] [Google Scholar]
- Skuse DH. Endophenotypes and child psychiatry. Br J Psychiatry. 2001;178:395–6. doi: 10.1192/bjp.178.5.395. [DOI] [PubMed] [Google Scholar]
- Smith A, Taylor E, Rogers JW, Newman S, Rubia K. Evidence for a pure time perception deficit in children with ADHD. J Child Psychol Psychiatry. 2002;43(4):529–542. doi: 10.1111/1469-7610.00043. [DOI] [PubMed] [Google Scholar]
- Solanto M, Abikoff H, Sonuga-Barke E, Schachar R, Logan G, Wigal T, Hechtman L, Hinshaw S, Turkel E. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. Journal of Abnormal Child Psychology. 2001;29(3):215–28. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 2003;27(7):593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion--I. The effect of delay on choice. Journal of Child Psychology and Psychiatry. 1992;33(2):387–98. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Suarez BK, Hampe CL, Van Eerdewegh P. Problems of replicating linkage claims in psychiatry. In: Gershon ES, Cloninger CR, Barrett JE, editors. Genetic Approaches in Mental Disorders. American Psychiatric Press; Washington, D.C.: 1994. pp. 23–46. [Google Scholar]
- Tan DP, Liu QY, Koshiya N, Gu H, Alkon D. Enhancement of long-term memory retention and short-term synaptic plasticity in cbl-b null mice. Proc Natl Acad Sci U S A. 2006;103(13):5125–30. doi: 10.1073/pnas.0601043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak ME, Rucklidge JJ, Hetherington R, C. JS, Tannock R. Time perception deficits in attention-deficit/hyperactivity disorder and comorbid reading difficulties in child and adolescent samples. J Child Psychol Psychiatry. 2003;44(6):888–903. doi: 10.1111/1469-7610.00173. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Gizer IR. The genetics of attention deficit hyperactivity disorder. Clin Psychol Rev. 2006 doi: 10.1016/j.cpr.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children - Third Edition. The Psychological Corporation, Harcourt Brace Jovanovich, Inc.; San Antonio: 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale III [manual] The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Willcutt EG. Bivariate heritability of ADHD and executive functions. Boston, MA: 2006. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of ADHD: A meta-analytic review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Defries JC. Understanding comorbidity: A twin study of reading disability and attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2007 doi: 10.1002/ajmg.b.30310. [DOI] [PubMed] [Google Scholar]