Abstract
Hyperpolarization-activated, cyclic nucleotide-gated channels (HCN channels) are expressed widely in the brain and invovled in various neuronal activities, including the control of neuronal rhythmic activity, setting the resting membrane potential, as well as dendritic integration. HCN channels also participate in the regulation of spontaneous activity of midbrain dopamine (DA) neurons to some extent. In slice preparations of midbrain, a hyperpolarization-activated non-selective cation current (Ih) mediated by the channels has been proposed as an electrophysiological marker to identify DA neurons. Recent evidence, however, shows that the functional roles of HCN channels in midbrain DA neurons are obviously underestimated. Here, we review the recent advances in the studies of the functional roles of Ih in midbrain DA neurons and further, their involvement in drug addiction and Parkinson's disease.
Keywords: dopamine, drug addiction, hyperpolarization-activated, nucleotide-gated channels (HCN channels), midbrain, Parkinson's disease
Introduction
Since the discovery of dopamine (DA) as a neurotransmitter in central nervous system (CNS) fifty years ago, central DA system has been under intensive studies because of its importance in brain physiology and diseases. Altered DA system is associated with various neurological and mental disorders such as Parkinson's disease (PD), schizophrenia and drug abuse. In midbrain slices and cultured midbrain neurons, one of the electrophysiological characteristics to identify DA neurons is the presence of hyperpolarization-activated non-selective cation current (Ih), which is mediated by the hyperpolarization-activated, nucleotide-gated channels (HCN channels), in response to membrane hyperpolarization. Recent information indicates that HCN channels play important roles in the functional modulation of DA physiology and pathophysiology of related diseases. In this article, we will review the recent progress in functional implications and regulatory roles of HCN channels in midbrain DA neurons.
Midbrain DA system
Midbrain DA neurons originating from substantial nigral par compacta (SNc) and ventral tegmental areas (VTA) innervate wide and discrete brain areas through three major pathways: (1) Nigrostriatal DA pathway that connects SNc and dorsal striatum and participates in motor control 1. The degeneration of this dopaminergic projection has been implicated in pathogenesis of Parkinson's disease (PD)2. (2) Mesolimbic DA pathway is the dopaminergic projections from VTA to limbic structures3 and has been proposed to be the major mediator for behavioral responses to rewarding and reinforcement and also served as the main target for psychostimulants4. (3) Mesocortical DA pathway which arises from VTA DA neurons, and mainly projects to the frontal cortex3. The pathway is speculated to be essential in the control of executive functions5 and its abnormality has been proposed to be closely associated with the pathophysiology of mental disorders such as schizophrenia6.
Electrophysiological characteristics of DA neurons
The midbrain DA neurons in vivo have been characterized with extracellular and intracellular recording techniques combining with histochemical fluorescence methods during 1970s7, 8, 9. DA neurons could be identified by in vivo extracellular recording technique according to their unique electrophysiological and pharmacological properties, such as a relatively low spontaneous firing rate (<10 Hz), long duration of action potentials (>3.0 ms) with biphasic or triphasic waveforms, and their sensitivity to D2-like receptor agonists/antagonists.
Two firing patterns have been observed in midbrain DA neurons in vivo: single-spike firing and short cluster-spike of burst firing. The two distinct firing patterns lead to different modes of DA release in brain regions innervated by their fiber terminals. The irregular single-spike firing maintains the tonic DA level in extracellular space, while burst firing induced by the afferent stimuli causes robust and transient phasic DA release in synaptic cleft10, which would be rapidly removed by DA transporters11. Synaptic transmission is mainly mediated by the transient phasic DA release, while the tonic DA release determines the basic stimulation level of both autoreceptors and postsynaptic receptors, further influencing the intensity of phasic DA response. Therefore, extracellular tonic DA level, to some degree, influences the efficacy of the synaptic information transmission associated with the phasic DA release11. Because of the distinct functional consequences between the two firing modes, the mechanism underlying the transition from single-spike firing to burst firing has attracted wide attention in understanding the functional regulation of DA neuron12.
In contrast, DA neurons in VTA or SNc slice preparations display only regular pacemaker like single-spike firing without bursting. The loss of burst spiking in deafferented preparations has been attributed to the loss of synaptic input from other brain areas, which is essential for the burst firing of DA neurons10, 12. Moreover, DA neurons in vitro display a large amplitude, slowly activated current (Ih) without time-dependent inactivation in response to prolonged membrane hyperpolarization. Lacey et al carried out a pioneering study to identify the cell types in SNc slices two decades ago13, since then Ih has been advocated as a hallmark of DA neurons in SNc and VTA slices, although this standard has recently been challenged14.
Is Ih a hallmark of VTA DA neurons?
In slice preparations of midbrain, Ih has been proposed as an electrophysiological marker to distinguish DA neurons from the non-DA cells15, 16. However, the classic criterion was established based on the data obtained from SNc where up to 95% of neurons contain DA and comprise a relatively distinct layer13. Considering anatomical proximity of VTA and SNc, the criterion is also applied for identification of VTA DA neurons. However, DA-containing neurons compromise only 50%–60% of the total VTA cells and scatter sparsely in a large volume17. It is noted that the criterion for identifying VTA DA neurons has been challenged recently14. Margolis et al concluded that no physiological and pharmacological properties thus far examined were reliable indicators for VTA DA neurons. They also found that display of Ih in VTA slices did not definitely represent a DA neuron, while the absence of Ih always indicated a VTA neuron not being dopaminergic14. Consistently, Lammel et al. reported that a subpopulation of VTA DA neurons projecting to prefrontal cortex did not display pronounced voltage sag in response to hyperpolarizing current injection, indicating a lack of HCN channels expression18. However, it was found in mice brain slices that 98% VTA neurons exhibiting Ih is also TH positive, indicating a high correlation between the presence of Ih and DA containing19. Considering the diversity of VTA neurons17, 20, 21, only immunohistochemical, instead of electrophysiological, pharmacological and morphological characteristics can be used to confirm the recorded neurons in midbrain slices as dopaminergic in nature.
Properties of HCN channels
A family of HCN channels were cloned about ten years ago22. Currents recorded from heterologous expression of HCN channels in HEK293 cells displayed the main properties of native Ih, confirming that HCN channels are the molecular basis underlying Ih current22.
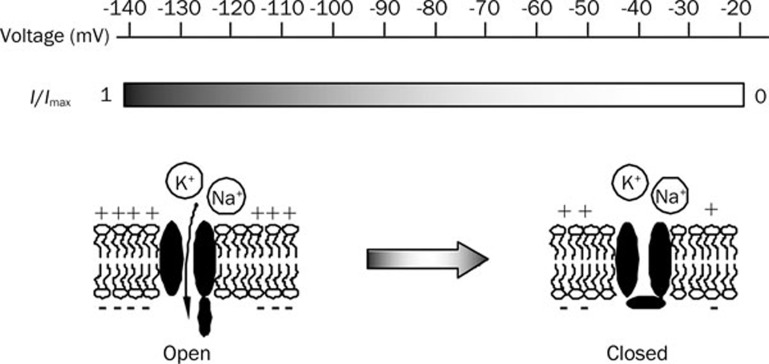
HCN channels are widely expressed in CNS, despite its first discovery in sinoatrial cells23. Under physiological conditions the channels are activated between −70 to −140 mV with slow activation kinetics without time-dependent inactivation (Figure 1). It has been proposed that HCN channels participate in setting and limiting the resting membrane potential (RMP) in a variety of neurons. Activation of HCN channels leads to both K+/Na+ influx (Figure 1) with a permeability ratio range from 3:1 to 5:1, deriving value for reversal potential between −20 to −30 mV. Interestingly, voltage sensor of HCN channels is similar to the voltage sensors of depolarization-activated ion channels, but with opposite gating polarity. The mechanisms underlying this discrepancy remain unclear24, 25.
Figure 1.
Basic properties of HCN channels. Hyperpolarization of the membrane potential (<-70 mV) activates the HCN channels and mediate the K+/Na+ influx non-selectively, whereas membrane depolarization produces the opposite effect. The shaded band (middle) indicates the percentage of the activated HCN channels at a given neuronal membrance potential (top). The bottom cartoon depicts the transition from the open state (left) to the closed state (right) of HCN channels.
The pioneering work by Magee et al found that the mean current density of Ih at the soma (1.8 pF/μm2) of hippocampal CA1 pyramidal is almost 6-fold lower as compared with a density of 12.5 pF/μm2 recorded at the dendrites located 300–350 μm away from the soma26, indicating a spatial gradient of HCN channels across somatodendritic axis of hippocampal CA1 pyramidal neurons. The passive properties of dendrites tend to attenuate the amplitude of EPSPs and to slow down their kinetics as they propagate from distal dendrites to the soma (the filtering effects of dendrite). Dendritic filtering effects would facilitate the temporal summation of distal EPSPs conducting towards the soma. The density gradient of HCN channels efficiently counteracts the dendritic filtering effects. The rising phase of EPSP leads to rapid deactivation of resting HCN channels, thus reduces the inward Ih and increases effective net outward currents that will hyperpolarize the membrane and accelerate the decay of EPSP. The higher density of HCN channels in the distal dendrites makes distal EPSP decay faster with shorter duration. At the soma, the temporal summation of EPSPs is more dampened for distal than proximal inputs. As a result, the temporal summation of all inputs reaching the soma is nearly equal24. Additionally, the voltage-dependent activation and deactivation of HCN channels always actively opposite to the deviation of the membrane potential away from the resting membrane potential. The unique properties of HCN channels make them a very unique “molecular machine” to modulate the intrinsic neuronal excitability and the responsiveness to afferent information. Together, the physiological functions of HCN channels in CNS are summarized as following: (1) participating in the pacemaker activity of spontaneously activated neurons; (2) setting and controlling neuronal RMP; (3) regulation of membrane input resistance and the synaptic integration; (4) modulation of neurotransmission and synaptic plasticity (see references 24, 25 for more details).
Regulation of HCN channels
Regulation by cyclic neucleotides
HCN channels are tightly regulated by cyclic neucleotides. Both cAMP and cGMP could directly bind to the highly conserved cyclic-neucleotide bindng domain (CNBD) on the C-terminus of the channel, leading to an acceleration of the channel activation and a significant shift of the activation curve (0 to +20 mV) to positive voltage24. Although both cAMP and cGMP enhance the activity of HCN channels in a similar way, the apparent affinities of HCN channels are about 10-fold higher for cAMP (Ka=0.5 μmol/L) than that for cGMP (Ka=6 μmol/L)22. Therefore, neurotransmitters or exogenous compounds (eg drugs) should be able to regulate the activity of HCN channels in a cAMP-dependent manner to fullfil their phyisological or therapeutical actions27, 28, 29, 30, 31, 32, 33, 34, 35. For example, a recent study suggested that HCN channels and α2A-adrenoceptors are co-localized in dendritic spines in PFC and stimulation of postsynaptic α2A-adrenoceptors strengthens working memory through inhibition of cAMP, closing HCN channels and strengthening the functional connectivity of PFC networks35. In contrast to the action of α2A-adrenoceptors, stimulation of D1 receptors, which are also expressed extensively in the spines in superficial PFC36, increase the intracellular cAMP levels. Thus it has been speculated that the activation of D1 receptors, leading to an up-regulation of HCN channels activity, should weaken the functional connectivity of PFC networks. Therefore, activation of D1 receptors on the spines will be accompanied by a selective inhibition of irrelevant afferent information (“noise”) from nonprefered spatial direction35, 37. Whereas the modulation of the activity of HCN channels by D1 receptors in PFC and its physiological significance remain unclear and needs more direct evidences to support this speculation.
Regulation by PIP2
In addition to cyclic neucleotide, phosphatidylinositol 4,5-bisphosphate (PIP2), as a ligand, could also bind directly to HCN channels from the intracellular side and shift the activation curve of HCN channels about +20 mV to positive voltage38, 39. The modulation of HCN channels by PIP2 is distinctly different from that of cAMP since the effets of PIP2 persisted in the channels lacking of CNBD38. Given the extensive voltage shift in the V1/2 of channel activation curve, the gating of HCN channels by PIP2 should exert profoundly impact on the Ih physiology, for example, its role in the neuronal autonomous activity38. However, the exact mechanisms underlying the interaction between PIP2 and HCN channels and the physiological significance for the regulation of HCN channels by PIP2 need to be further investigated.
Regulation by protein kinase
Similar to other voltage-gated ion channels, the phosphorylation/deposphorylation of HCN channels is indeed a modulatory factor to regulate the activity of the channels. It has been demonstrated that Ih could be regulated by tyrosine phosphorylation through Src kinase under physiological conditions in neurons40. Moreover, serine/treonine kinase, p38-MAP kinase is also a strong modulator of HCN channels41. In hippocampal pyramidal neurons, up-regulation of Ih via activation of p38-MAP kinase caused an approximately +11 mV positive shift in V1/2 of Ih activation curve, accompanied by a depolarization of resting potential, and a reduction in temporal summation41. All these studies indicated the control of the phosphorylation status of HCN channels is a pivotal mechanism to modulate the properties of Ih under different physiological or pathophysiological conditions.
Generally, the expression and activity of HCN channels are dynamically regulated by cytolic proteins (eg protein kinase, scaffold proteins) and small molecular factors (eg cAMP, H+, Cl−). Whereas the regulation of HCN channels is not the major topic of the present article, we just give an overview of HCN channel modulators in this section (Please see Reference 24 for intensive discussion about the regulation of HCN channels in CNS).
Expression of HCN channels in midbrain DA neurons
The four cloned HCN subunits thus far display differences in the biophysical properties and the distribution in CNS. For instance, HCN1 subunit shows a fastest kinetics while HCN4 is the slowest one in response to membrane hyperpolarization42, 43. On the other hand, HCN1 is less sensitive to cAMP than HCN2 and HCN444, 45. HCN1 is mainly expressed in neocortex, hippocampus, cerebellar cortex and brain stem. HCN2 subunit is widespread throughout the brain with the highest expression in thalamus and brain stem. HCN3 distributes sparsely with the lowest expression level in CNS, whereas the expression of HCN4 is limited to some brain regions such as thalamus and olfactory bulb43, 46.
In the midbrain DA neurons, only the mRNA of HCN2-HCN4 were detected in SNc DA neurons by using qualitative single-cell RT-mPCR or in situ hybridization43, 47. Consistent with the lack of HCN1 subunit, the activation time constants of native Ih recorded in SNc DA neurons (τ: 0.5 to 5 s) at −80 to −120 mV are dramatically larger than those obtained from neocortical and hippocampal pyramidal neurons (τ: 50 to 500 ms). Moreover, native Ih in SNc DA neurons is also more sensitive to cAMP than its counterparts in hippocampus pyramidal neurons47, which is also in line with the different sensitivity to cAMP between HCN1 and the other HCN subunits. It seems to be convincing that the Ih current in SNc DA neurons is mainly mediated by HCN2-HCN4 subunits.
Given the heterogeneity of VTA DA neurons, the expression and distribution of HCN channels seem to be more complicated than the neighboring SNc. Recent studies indicated that a subpopulation of VTA DA neurons possessed almost no functional HCN channels18, 48, whereas Morgolis EB et al14 indicated that the absence of Ih reliably predicted a VTA neurons was non-dopaminergic. This discrepancy maybe attribute to the different species (rat or mouse) and ages (Postnatal 12–16 days, Postnatal 20–36 days or 3-month-old adult) of the animals as well as the methodological considerations. When the expression pattern of HCN subunits was considered, although there is no direct evidence for the lack of HCN1 in VTA DA neurons, it is most likely that HCN1 is absent in this region according to its overall expression pattern throughout the brain47. Interestingly, studies in entorhinal cortical stellate cells indicated that HCN1 subunits play a pivotal role in setting the resting membrane conductance and low-frequency fluctuations in membrane potential49, the physiological significance of lack of HCN1 subunits in the midbrain dopamine neurons remains unknown and needs to be further addressed. In addition, a recent study implicated that HCN channels are mainly located at the dendrites of VTA DA neurons in comparison to the soma, which has been speculated to preferentially influence the burst firing of VTA DA neurons50. However, there still lack of direct evidences for the somatodendritic spatial gradient of HCN channels in VTA DA neurons and more detailed investigations are needed in the future.
Functional roles of HCN channels in the modulation of DA neuron activity
HCN channels and autonomous activity of DA neurons
The neuronal resting membrane potential is determined by the balance of the membrane background conductance to permeable ions, such as the resting K+ background conductance through K2P channels51 and Na+ background conductance through the recently characterized NALCN channel52. HCN channel, which is permeable to both K+/Na+, starts to open at a membrane potential of -70 mV and also participates in the setting of neuronal resting membrane potentials. The resting membrane potential of DA neurons is usually between −55 to −40 mV7, 53, 54, 55. Therefore, the role of HCN channels in setting of resting membrane potential and the spontaneous firing of DA neurons is rather complicated. It has been reported that substantial reduction of Ih by extracellularly application of 1 mmol/L Cs+ did not affect the spontaneous activity of DA neurons significantly53. However, recent studies show that blockade of Ih by the specific HCN channel blocker ZD7288 (10 to 30 μmol/L) or inhibitor of PIP2 synthesis wortmannin (10 μmol/L) reduced the spontaneous firing activity in a subpopulation of DA neurons34, 38, 48, 56. Whereas there is one study implicated a small but significant increase in the firing rate of VTA DA neurons57. The discrepancy might be ascribed to: (1) different agents used to block HCN channels (nonspecific Cs+ vs specific blocker ZD7288) and recording methods used (extracelluar recording or whole-cell patch clamp). (2) different ages and species of animals used since that age-dependent increase of functional HCN channels has been reported in mouse hippocampal pyramidal neurons58. Additionally, HCN channels together with persistent Na+ channels participated in the pacemaker activity of SNc DA neurons only in the first three weeks after birth, thereafter the importance of HCN channels in pacemaker activity of SNc DA neurons was progressively replaced by Cav1.3 channels59. (3) The heterogeneity of midbrain DA system: midbrain DA neurons are not a single population of neurons but distinct neuronal populations with diverse neurochemical and functional properties21.
HCN channels and AHP
In all subtypes of midbrain dopamine neurons, HCN channels regulate the amplitude and duration of the afterhyperpolarization (AHP) that follows action potentials18, 19, 34, 48. During AHP after a burst firing, the membrane potential is likely to be more negative than −70 mV and thus activates HCN channels, which leads to a reduction in amplitude and duration of AHP. It has been reported in VTA DA neurons that neurotransmitters such as serotonin60 and norepinephrine50 reduced Ih, leading to an enhancement of the amplitude and duration of AHP after bursting and a reduction of neuronal excitability. The activation of HCN channels and the down-regulation of DA neuron excitability would filter off lots of synaptic inputs and further inhibit the burst firing DA neurons that depends on the excitatory afferent inputs12.
Recently, HCN channels were suggested to participate in chemical transmission between dopaminergic pairs through coupling with D2 autoreceptors61. It was shown that the bidirectional interaction between DA neurons pairs is mainly mediated by D2-like receptors and HCN channels, and the authors propose that there is a subpopulation of synaptic D2-like receptors coupled with HCN channels, rather than with certain potassium conductance to mediate the response of chemical transmission between DA pairs. However, this hypothesis is hampered because of the lack of morphological or other more direct evidences on molecular level to support the inference.
HCN channels as modulatory targets for the activity modulation of DA neurons
HCN channels are targets for many neurotransmitters and drugs for modulating the activity of DA cells34, 50, 60, 62, 63. As discussed in the previous section, the activity of HCN channels is sensitive to many factors, such as cAMP, PIP2 and protein kinase, thus any alteration in the activity of postsynaptic receptors are very likely to induce changes in the function of HCN channels, further influencing the activity of DA neurons. For example, serotonin dramatically inhibited HCN channels in VTA DA cells through activation of 5-HT2A receptors and such an effect may be involved in the serotonin-induced potentiation of auto-inhibition of the spontaneous firing rate of VTA DA neurons60. In addition, activation of D2-like receptors in DA neurons would dramatically reduce Ih , which is secondary to the activation of potassium conductance50, 62, 64. For instance, the modulation of Ih by norepinephrine in VTA DA neurons has been attributed to its non-specific stimulation of D2-like receptors. Activation of D2-like receptors opens G-protein coupled inward rectifier potassium (GIRK) channels and increases the membrane conductance, which leads to a poor voltage clamp of the sites distant from recording electrode. Thus norepinephrine-induced suppression of Ih in VTA DA neurons appears to result from the inefficient space clamp of neurons50.
The role of DA neuron HCN channels in neurological diseases
HCN channels and drug addiction
The mesolimbic DA system plays a critical role in mediating the rewarding effect and reinforcement in drug addiction. Although HCN channels express extensively in VTA DA neurons, its role in drug abuse is largely unknown.
An early study using intracellular recording in brain slices reported that ethanol (20–320 mmol/L) increased the firing rate of VTA DA neuron in a concentration-dependent manner and decreased the amplitude of AHP as well as the neuronal input resistance via an augmentation of Ih in VTA DA neurons65. Consistently, a recent study shows that HCN channels in VTA DA neurons may be the target of ethanol in mice. Ethanol-induced increase in the firing rate of DA neurons was blocked by ZD728834. And ethanol not only increased the amplitude of VTA DA Ih current, but also accelerated the activation of Ih as well as shifted the V1/2 of activation curve of Ih about +3 mV to positive potentials, indicating an augmentation of Ih by ethanol. Moreover, chronic ethanol exposure reduced the density of Ih in VTA DA neurons 34. It has been proposed that down-regulation of HCN channels would enhance auto-inhibitory effects of DA through D2-like receptors in vivo60 and alter dendritic membrane resistance, further influencing neuronal dendritic information integration. Based on these data, Okamoto et al proposed that Ih may serve as an important cellular mechanism in the rewarding effects of ethanol34. This is supported by the study of Migliore et al in which they showed that ethanol, through its effects on Ih, could produce dramatic alterations in the temporal pattern of the firing of DA neurons, and lead to a significant changes in dopamine release in specific brain regions66. It should be noted that ethanol-induced stimulation of the firing of VTA DA neurons is not entirely mediated by Ih since ZD7288 (30 μmol/L) failed to abolish the excitatory effects of ethanol in a minor population of the neurons recorded34. Indeed, there are studies reported that ethanol-induced excitattion may involve other mechanisms such as multiple types of K+ channels67.
An important characteristic for the cellular substrate of drug addiction is that it should be shared by different drugs. Whether HCN channels are the common target of different abused substances such as cocaine and morphine? To answer the question, further research involved in different kinds of psychostimulants needs to be conducted.
HCN channels and Parkinson's disease
Progressive degeneration of SNc DA neurons is the main pathology of PD. Thus, developing neuroprotective agents that can prevent or delay the progressive loss of SNc DA neurons become an attractive therapeutic strategy for PD.
SNc DA neurons are autonomous pacemakers and their spontaneous activity is responsible for the sustained DA release to maintain extracellular tonic DA level, which plays an important role in the normal functions of target regions such as striatum and the limbic system11. Based on the facts that dihydropyridines slowed down or stopped pacemaking68, 69, 70, 71, it was suggested that pacemaker activity in SNc DA neurons depends on the voltage-gated L-type Ca2+ channels. This viewpoint, however, was challenged by recent studies59, 72. It was shown that neither pharmacological blockade of L-type Ca2+ channels nor knockout of Cav1.3 affected the pacemaker activity of SNc DA neurons. Furthermore, after the functional down-regulation of Cav1.3 channels, its role in pacemaking firing was progressively switched to HCN channels and persistent Na+ channels, which were used in early life (within first 3 weeks after birth)59. Therefore, the L-type Ca2+ channels appears to participate in the pacemaking of SNc DA neurons but not essential to its autonomous activity.
The transition of pacemaker current from L-type Ca2+ channels to HCN channels and persistent Na+ channels may imply a significant neuroprotective effect. It has been proposed that Ca2+, which influxes through the L-type Ca2+ channels during its broad action potentials of SNc DA neurons, promotes the selective death of neuron in this region73. Accordingly, when the L-type Ca2+ channels were blocked by dihydropyridines, SNc DA neurons exhibited reduced sensitivity to toxins (MTTP, 6-OH-DA)59. Moreover, clinical data also show that patients who use dihydropyridines to control hypertension exhibited a 30% to 50% lower incidence of PD74. Taken together, HCN channels, plus persistent Na+ channels, as a part of pacemaking mechanisms of SNc DA neurons, may play a critical role in the prevention and therapy of PD.
Conclusion
Although critical functional roles of HCN channels are well known in other brain regions, their importance in the regulation of midbrain DA system remains to be explored. The dysfunction of midbrain DA system is involved in PD, drug addiction, as well as in schizophrenia. Given the importance of HCN channels in neuronal passive and active membrane properties and its roles in dendritic integration, synaptic transmission, long-term potentiation, neuronal oscillation, motor learning, and working memory24, it is conceivable that the alteration of HCN channels in DA system may elicit important functional consequences associated with PD, schizophrenia and drug addiction. Studies of regulatory mechanisms of HCN channel in DA neurons may not only provide new insights to the midbrain DA system in physiology and pathology, but also implicate a novel target of drug discovery and therapeutic strategy for related neurological disorders.
Acknowledgments
We thank Dr Guo-yuan HU for his insight discussion and critical reading of the manuscript. This work was supported from National Natural Science Foundation of China (No 30770662, 30825042, 20872153) and National Basic Research Program of the Ministry of Science and Technology of China (No 2007AA02z163, 2009CB522201).
References
- Grace AA, Lodge DJ, Buffalari DM.Dopamine — CNS pathways and neurophysiology. In: Encyclopedia of neuroscienceOxford: Academic Press; 2009. p549–555.
- Hornykiewicz O. Parkinson's disease: from brain homogenate to treatment. Fed Proc. 1973;32:183–90. [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–65. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse. Neurosci Biobehav Rev. 2006;30:215–38. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–85. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9:404–16. doi: 10.1177/1073858403252674. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons — 1. Identification and characterization. Neuroscience. 1983;10:301–15. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons — 2. Action potential generating mechanisms and morphological correlates. Neuroscience. 1983;10:317–31. doi: 10.1016/0306-4522(83)90136-7. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–71. [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–34. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–7. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9:1233–41. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons. J Physiol. 2006;577:907–24. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–97. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–81. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–73. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–70. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–18. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Heterogeneity of ventral tegmental area neurons: single-unit recording and iontophoresis in awake, unrestrained rats. Neuroscience. 1998;85:1285–309. doi: 10.1016/s0306-4522(98)00054-2. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–91. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981;314:359–76. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–85. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization–activated cation currents: From Molecules to Physiological Function. Annu Rev Physiol. 2003;65:453–80. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic Hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–24. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobker DH, Williams JT. Serotonin augments the cationic current Ih in central neurons. Neuron. 1989;2:1535–40. doi: 10.1016/0896-6273(89)90041-x. [DOI] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–8. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990;431:319–42. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Williams JT. Opioid inhibition of Ih via adenylyl cyclase. Neuron. 1994;13:179–86. doi: 10.1016/0896-6273(94)90468-5. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3:133–41. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5:767–74. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- Frere SG, Luthi A. Pacemaker channels in mouse thalamocortical neurones are regulated by distinct pathways of cAMP synthesis. J Physiol. 2004;554:111–25. doi: 10.1113/jphysiol.2003.050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) Is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–26. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. α2A-Adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA. 1994;91:5720–4. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–84. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, et al. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron. 2006;52:1027–36. doi: 10.1016/j.neuron.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Pian P, Bucchi A, Robinson RB, Siegelbaum SA. Regulation of gating and rundown of hcn hyperpolarization-activated channels by exogenous and endogenous PIP2. J Gen Physiol. 2006;128:593–604. doi: 10.1085/jgp.200609648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X, Eckert C, Yuan H, Wahl-Schott C, Abicht H, Fang L, et al. A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J Biol Chem. 2005;280:34224–32. doi: 10.1074/jbc.M506544200. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Bullis JB, Roth MK. Modulation of h-channels in hippocampal pyramidal neurons by p38 mitogen-activated protein kinase. J Neurosci. 2006;26:7995–8003. doi: 10.1523/JNEUROSCI.2069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii TM, Takano M, Ohmori H. Determinants of activation kinetics in mammalian hyperpolarization-activated cation channels. J Physiol. 2001;537:93–100. doi: 10.1111/j.1469-7793.2001.0093k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, et al. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–75. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen S, Siegelbaum SA. Regulation of hyperpolarization-activated HCN channel gating and cAMP modulation due to interactions of COOH terminus and core transmembrane regions. J Gen Physiol. 2001;118:237–50. doi: 10.1085/jgp.118.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 2001;411:805–10. doi: 10.1038/35081088. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–76. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Franz O, Liss B, Neu A, Roeper J. Single-cell mRNA expression of HCN1 correlates with a fast gating phenotype of hyperpolarization-activated cyclic nucleotide-gated ion channels (Ih) in central neurons. Eur J Neurosci. 2000;12:2685–93. doi: 10.1046/j.1460-9568.2000.00151.x. [DOI] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. Ih channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Dudman JT, Dodson PD, Santoro B. HCN1 channels control resting and active integrative properties of stellate cells from layer II of the entorhinal cortex. J Neurosci. 2007;27:12440–51. doi: 10.1523/JNEUROSCI.2358-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arencibia-Albite F, Paladini C, Williams JT, Jimenez-Rivera CA. Noradrenergic modulation of the hyperpolarization-activated cation current (Ih) in dopamine neurons of the ventral tegmental area. Neuroscience. 2007;149:303–14. doi: 10.1016/j.neuroscience.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Goldstein SAN, Larry RS.Two-P-Domain (K2P) potassium channels: leak conductance regulators of excitability. In: Encyclopedia of NeuroscienceOxford: Academic Press; 2009. p1207–20.
- Lu B, Su Y, Das S, Liu J, Xia J, Ren D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129:371–83. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur J Neurosci. 1995;7:462–9. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–90. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seutin V, Massotte L, Renette MF, Dresse A. Evidence for a modulatory role of Ih on the firing of a subgroup of midbrain dopamine neurons. Neuroreport. 2001;12:255–8. doi: 10.1097/00001756-200102120-00015. [DOI] [PubMed] [Google Scholar]
- McDaid J, McElvain MA, Brodie MS. Ethanol effects on dopaminergic ventral tegmental area neurons during block of ih: involvement of barium–sensitive potassium currents. J Neurophysiol. 2008;100:1202–10. doi: 10.1152/jn.00994.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyev DV, Barish ME. Postnatal Development of the hyperpolarization-activated excitatory current ih in mouse hippocampal pyramidal neurons. J Neurosci. 2002;22:8992–9004. doi: 10.1523/JNEUROSCI.22-20-08992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, et al. 'Rejuvenation' protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–6. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Liu Z, Bunney EB, Appel SB, Brodie MS. Serotonin reduces the hyperpolarization-activated current (Ih) in ventral tegmental area dopamine neurons: involvement of 5-HT2 receptors and protein kinase C. J Neurophysiol. 2003;90:3201–12. doi: 10.1152/jn.00281.2003. [DOI] [PubMed] [Google Scholar]
- Vandecasteele M, Glowinski J, Deniau JM, Venance L. Chemical transmission between dopaminergic neuron pairs. Proc Natl Acad Sci USA. 2008;105:4904–9. doi: 10.1073/pnas.0703121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala L, Paupardin-Tritsch D. Effect of catecholamines on the hyperpolarization–activated cationic Ih and the inwardly rectifying potassium IKir currents in the rat substantia nigra pars compacta. Eur J Neurosci. 1999;11:398–406. doi: 10.1046/j.1460-9568.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- Cathala L, Paupardin-Tritsch D. Neurotensin inhibition of the hyperpolarization-activated cation current (Ih) in the rat substantia nigra pars compacta implicates the protein kinase C pathway. J Physiol. 1997;503:87–97. doi: 10.1111/j.1469-7793.1997.087bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AE, Williams JT, Henderson G. Baclofen inhibition of the hyperpolarization-activated cation current, Ih, in rat substantia nigra zona compacta neurons may be secondary to potassium current activation. J Neurophysiol. 1996;76:2262–70. doi: 10.1152/jn.1996.76.4.2262. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res. 1998;22:236–44. [PubMed] [Google Scholar]
- Migliore M, Cannia C, Canavier CC. A modeling study suggesting a possible pharmacological target to mitigate the effects of ethanol on reward-related dopaminergic signaling. J Neurophysiol. 2008;99:2703–7. doi: 10.1152/jn.00024.2008. [DOI] [PubMed] [Google Scholar]
- Appel SB, Liu Z, McElvain MA, Brodie MS. Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J Pharmacol Exp Ther. 2003;306:437–46. doi: 10.1124/jpet.103.050963. [DOI] [PubMed] [Google Scholar]
- Puopolo M, Raviola E, Bean BP. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J Neurosci. 2007;27:645–56. doi: 10.1523/JNEUROSCI.4341-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini B, Clark JW Jr, Canavier CC. Calcium dynamics underlying pacemaker-like and burst firing oscillations in midbrain dopaminergic neurons: a computational study. J Neurophysiol. 1999;82:2249–61. doi: 10.1152/jn.1999.82.5.2249. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stratta F, Stefani A, Bernardi G. Effects of dihydropyridine calcium antagonists on rat midbrain dopaminergic neurones. Br J Pharmacol. 1994;113:831–8. doi: 10.1111/j.1476-5381.1994.tb17068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard S, Flatman JA, Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J Physiol. 1993;466:727–47. [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, Chan CS, Surmeier DJ. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci. 2009;29:11011–9. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, et al. Interplay between cytosolic dopamine, calcium, and alpha — synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–29. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnitzky RL. Can calcium antagonists provide a neuroprotective effect in Parkinson's disease. Drugs. 1999;57:845–9. doi: 10.2165/00003495-199957060-00001. [DOI] [PubMed] [Google Scholar]