Abstract
Type І natural killer T cells (NKT cells), a subset of CD1d-restricted T cells with invariant Vαβ TCR, are characterized by prompt production of large amounts of Th1 and/or Th2 cytokines upon primary stimulation through the TCR complex. The rapid release of cytokines implies that type І NKT cells may play a critical role in modulating the upcoming immune responses, such as anti-tumor response, protection against infection, and autoimmunity. As a bridge between innate and adaptive immunity, type І NKT cells differentiate and mature upon stimulations to achieve and maintain a homeostasis. Orchestrating with other arms of adaptive immunity, type І NKT cells show strong cytotoxic effects in response to various tumors in a direct and/or indirect manner(s). This review will focus primarily on type І NKT cell development, homeostasis, and effector functions, especially in anti-tumor immunity, and followed by their potential applications in treatment of cancers.
Keywords: NKT cells, tumor immunity, cell development and homeostasis, Th1/Th2 cytokines
Introduction
Type І natural killer T cells, referring to as invariant NKT cells (thereafter abbreviated as NKT cells), are the most extensively studied due to its unique Vα14-Jα18 TCR in mouse (Vα24-Jα18 in humans)1, 2, while type ІІ NKT cells with a diverse TCR repertoire are less well studied. Unlike conventional T cells, which respond to foreign peptides presented by MHC class І or ІІ molecules through interaction with their αβ TCR, NKT cells recognize antigenic glycolipids presented by the MHC class І-like molecule CD1d3, 4. Mice with deletion of CD1d gene eradicates NKT cells including both type І and type ІІ. Because the positive selection of these two NKT cell subtypes is strictly dependent on CD1d during thymic ontogeny. Exogenous glycolipid α-galactosylceramide (α-GalCer) derived from marine sponge Agelas mauritianus or symbiotic microorganism has commonly been used to identify and activate NKT cells in vitro. Recently, several endogenous mammalian self lipids have been defined as CD1d ligands recognized by NKT cells, including isoglobotrihexosylceramide (iGb3) and other phosphatidyl inositol compounds, but these are still controversial and need to be further confirmed5, 6. However, we found that the infection of Epstein-Barr virus (EBV), but not human T-cell leukemia virus type I (HTLV-1), can profoundly promote EBV-associated CD8+ NKT cell development in humans and human-thymus/liver-SCID (hu-thym/liv-SCID) chimeras, suggesting whether other types of antigen are involved in differentiation and maturation of NKT cells is still unknown7, 8, 9.
Unlike the T cells, unconventional glycolipid-reactive NKT cells that bridge innate and adaptive immunity set the keynote and the tone for the subsequent adaptive immune responses through expression of Th1/Th2 cytokines in response to glycolipid antigens. After activation, NKT cells rapidly produce Th1, Th2 cytokines and various chemokines, as well as up-regulate co-stimulatory molecules to respond to the infections, tumors and autoimmune disorders7, 10, 11, 12. Upon expression of CD4 and CD8 molecules, the NKT cells are divided into two main subpopulations including CD4+ and CD4− NKT cells, and the subpopulation of CD4− NKT cells is further subdivided into CD4−CD8− double negative (DN) and CD8+ single positive (SP) NKT cells, which is limited in human beings. It is widely believed that CD8 is expressed on a minor proportion of human NKT cells, but it is usually acquired after egression from the thymus13. The finding of limited correlation between human thymic CD4+ NKT cells and peripheral CD4− NKT cells, including DN and CD8+ SP, has raised a requisition on the direct evidence for origin of DN and CD8+ NKT cells. Many papers from independent groups have described that CD4+ NKT cells usually produce both Th1 and Th2 cytokines, whereas CD4− NKT cells including human CD8+ NKT cells are skewed more toward Th1 cytokines14. Once it occurs, the initial production of Th1/Th2 cytokines may leads to a corresponding adaptive immunity towards Th1 or Th2 response, thus resulting in activation of various arms of immune system such as dendritic cells (DCs), natural killer (NK) cells, CD8+ cytotoxic T lymphocytes (CTLs), and B lymphocytes15. Recently published data that EBV-induced CD8+ NKT cells display an effector memory phenotype upon TCR stimulation after infection have provided with the direct evidences on effector functions for innate nature of human NKT cells9. Concerning the effector functions, NKT cells can mediate both protective and regulatory immunologic functions, including anti-tumor responses, protection against pathogens, maintenance of transplant tolerance, and inhibition of autoimmunity16. In this review, we will discuss recent advances in NKT cell biology, mainly focusing on their development, homeostasis, and effector function in anti-tumor immunity.
Basic pathway of NKT cell development
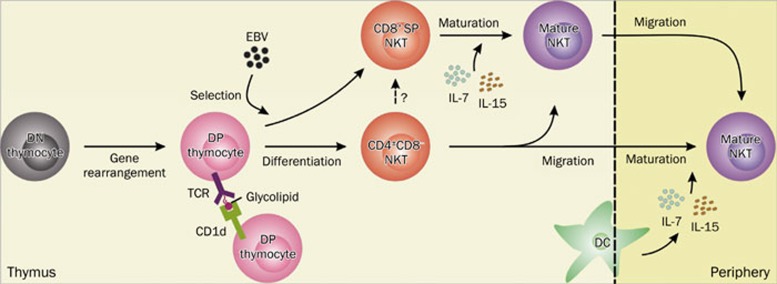
It is clear that the NKT cells originate from a common precursor pool of CD4+CD8+ double positive (DP) thymocytes, which have undergone random TCR gene rearrangement and expression17. Unlike conventional T cells depend on thymic epithelial cells during positive selection, NKT cell precursors are positively selected by CD1d+ DP thymocytes in the thymic cortex18. Once the interaction between TCRs expressed on DP thymocytes and self glycolipids presented by CD1d-bearing DP thymocytes occurs, NKT cell precursors undergo consecutive differentiation and maturation stages, including CD24+CD44loNK1.1− (stage 0), CD24loCD44loNK1.1− (stage 1), CD24−CD44hiNK1.1− (stage 2), and CD24−CD44hiNK1.1+ (stage3), and ultimately develop into mature NKT cells1, 19. The surface CD8 molecule is constantly down-regulated in total NKT cells during initial stages of development, while CD4 is lost in parts of these cells, which results in generation of CD4+ SP and DN NKT cells, but the actual existence of murine CD8+ SP NKT cells is still unknown. However, a fraction of NKT cells expressing CD8 markers, predominantly CD8αα, are present in normal persons and healthy people with latent EBV infection9. It is likely that the unique cellular and molecular composition of the EBV-exposed thymus contributes to the differentiation and development of EBV-induced CD8+NKT cells9. Moreover, expression of co-receptors on NKT cell precursors, which might be more favorable targets, facilitate the selection of NKT cells by invasive pathogens. Thymus appears to be important for generation of intermediate NK1.1− NKT cells in mice, but the majority of NKT cells emigrating from thymus to peripheral organs are NK1.1−, suggesting maturation of NKT cells occurs mainly in the periphery, although the acquisition of the NK receptors NK1.1 (CD161 in humans) or Ly49 is not required for thymic emigration20. Once committed to the NKT cell lineage, numerous transcription factors, cytokines, chemokines, and costimulatory molecules have been documented that are uniquely required for maturation and homeostasis of NKT cells, but dispensable for conventional T cell development. At least two key control points exist in NKT cell development, which govern NKT cell selection to give rise to immature NKT cells branched from the conventional T cell development pathway and NKT cell maturation with a series of phenotypic and functional changes, respectively3, 21. The basic pathway of NKT cell development is illustrated in Figure 1.
Figure 1.
Basic pathway of NKT cell development. In the thymus, NKT precursors originate from a common precursor pool of CD4+CD8+ (DP) thymocytes, which most likely have undergone random gene rearrangement and expression of TCR from CD4−CD8− (DN) thymocytes. Upon the stimulation of glycolipids presented by CD1d-bearing DP thymocytes, NKT precursors undergo a series of differentiation steps and ultimately generate immature NKT cells. Particularly, human CD8+ SP NKT cells can be induced by EBV challenge from NKT cell precursors, but did not exclude the possibility that CD8+ NKT cells develop from intermediate CD4−CD8− NKT cells or CD4+CD8− NKT cells through up-regulation of CD8 and/or down-regulation of CD4. Most immature NKT cells gain functional and mature phenotypes in thymus in the support of IL-7 and IL-15 secreted from DCs, and then some emigrate from the thymus and others remaining reside in the thymus. Another pathway suggests that some mature thymic NKT cells also migrate to the periphery. NKT, natural killer T cell; DC, dendritic cell; EBV, Epstein-Barr virus; DP, CD4+CD8+ double positive; DN, CD4−CD8− double negative; SP, single positive.
The earliest NKT precursors may also be susceptible to negative selection in the presence of strong agonist ligands. A dose- and time-dependent deletion of NKT cells is induced by the injection of high dose of the glycolipid agonist α-GalCer to fetal thymic organ culture (FTOC) or persistent injection to young adult mice22. Similarly, the intrathymic NKT cell development is specifically blocked by the TCR-mediated selective signals, which strongly suggests NKT cells are subject to negative selection if they are highly reactive to self-glycolipid ligands during their development23. However, whether negative selection of NKT cells really exists in vivo during inherent cell development is still unclear, because α-GalCer is not considered to be a natural mammalian product. Furthermore, over-expression of CD1d resulted in a thymic deletion of high-affinity CD1d-restricted NKT cells in transgenic mice, as well drastically reduced frequency of NKT cells in FTOC, suggesting negative selection of NKT cells may be influenced by ligand expressing cells in the thymus through altering ligand density. Particularly, CD1d expressing thymic stromal cells, mainly DCs, rather than DP thymocytes mediated negative selection of NKT cells, and CD4+ NKT cells formed the majority of these deleted NKT cells22. Rarity in humans, even deficiency in mice, of CD8+ SP NKT cells suggests a possible mechanism of CD8-mediated negative selection during CD8+SP NKT cell development. However, Engel and colleagues believe that the exclusion of CD8+SP NKT cells from murine NKT cells is a by-product of enhanced expression of CD4 molecules on NKT cells, which is regulated by the transcription factor Th, Poxviruses and Zinc-finger (POZ), and Krüppel family (Th-POK)24.
Controls of NKT cell development and homeostasis
Complete absence of NKT cells in mice deficient in recombinase subunits RAG-1 and RAG-2 or the TCR Jα18 segment demonstrated that successful rearrangement of TCRα gene segments Vα14 to Jα18 is absolutely necessary to the subsequent selection of DP thymocytes25, 26. The signaling and transcription factors mentioned below act as master regulators on the NKT cell lineage, but distinct from conventional T cells19. Retinoic acid-related orphan receptor-γt (ROR-γt), a transcription factor that induces the expression of antiapoptotic molecule Bcl-xL, allows for rearrangement and expression of distal segments in DP thymocytes, which require multiple excisions dependent on an extended lifespan of DP thymocytes27, 28. The normal canonical Vα14 to Jα18 rearrangement in DP thymocytes but with reduced numbers of NKT cells in the absence of the transcription factor Runx1, suggests that Runx1 is quite likely involved in NKT cell selection or subsequent expansion17. Invariant NKT cell precursors are selectively blocked at an immature CD44loNK1.1− stage (stage 0 or 1) in conditional ablation of c-Myc in DP thymocytes, but without any perturbation in the development of conventional T cells. However, transgenic expression of BCL-2 to support cell survival did not rescue NKT cell development in c-Myc knockout (KO) mice unlike that in Runx1 KO mice29, 30. These findings imply a c-Myc-mediated intrathymic proliferation wave in immediately early stage of NKT cell development in response to selected signals involving the Slam/Sap/FynT signaling axis30. The T box transcription factor T-bet is essential to generation of fully mature Vα14 NKT cells at the transit of stage 2 to 3, which may be associated with some cytokines such as IL-12, IL-15, and IL-18 to promote NKT cell maturation31, 32. NF-κB family members are also implicated in NKT cell development downstream of SLAM-Sap-Fyn signaling cascade and/or TCR-mediated signals. The NIK-mediated activation of RelB in thymic stroma is a unique contribution to intact generation of immature NKT cell precursors. Blocking NK-κB signaling in precursors leads to an impaired expansion of NKT cells predominantly at the transition of stage 2 to 333. The deficiency of E protein transcription factor HEB seriously blocked the development of NKT cells at the stage 0 but made no difference to conventional T cells because it failed to regulate thymocyte survival or distal rearrangements of the TCRα chain34. Promyelocytic leukemia zinc finger transcription factor (PLZF) is responsible for the early stages (stage 1 to 2) of NKT cell maturation and production of the functional cytokines, even in the absence of SLAM-SAP-Fyn signaling35, 36. Recently, the identification of selective function for the transcription factor early growth response 2 (Egr2) in maturation and homeostasis of NKT cells also emphasizes the importance of the calcineurin-NFAT-Egr2 pathway in the development of NKT cells. A majority of NKT cells assembled in the liver may be attributable to Id2, which regulates the expression of chemokine receptor CXCR6 and anti-apoptotic molecules to determine the maturation and localization of NKT cells37.
Human CD4− NKT cells predominantly response to IL-15, whereas CD4+ NKT cells are more responsive to IL-7 by coupling with up-regulated receptors, which is consistent with cytokine signaling pathways initiated by IL-15/IL-15R and IL-7/IL-7R. These pathways play a central role in proliferation and survival of NKT cells, but not conventional T cells13, 38. Mice with a deficiency in several genes, including IL-2Rβ (CD122), interferon-regulatory factor 1 (IRF-1), Fyn, Ets, lymphotoxin (LT) and the hypomorphphic allele of the NIK located in alymphoplasia (aly/aly), leads to a unique reduction in NKT cell number due to close link between these genes and IL-15/IL-15R signaling pathway. NK-like phenotype is acquired by human NKT cells in thymus and does not require peripheral expansion, which suggests that final maturation of NKT cells is completed mainly in thymus, although it does not exclude that CD161− NKT thymic emigrants may acquire CD161 in periphery as well13. In our study, mouse fetal and neonatal NKT cells homogenously express IL-7Rα (CD127) and respond vigorously to IL-7 in vitro by proliferating and differentiating into mature CD161+ effector cells. Similarly, the maturation of human CD8+ SP NKT cells is also affected, which suggests that IL-7/IL-7R signaling is responsible for the expansion and maturation of NKT cells during fetal and postnatal lifecycles9, 39. Mutation of signaling lymphocytic activation molecule-associated protein (SAP), which is responsible for recruitment and activation of the Src kinase Fyn, leads to a deficiency of NKT cell development and results in X-linked lymphoproliferative (XLP) syndrome in humans40. Small microRNAs (miRNAs) regulate basic functions such as cellular proliferation, apoptosis, lineage commitment, and differentiation in the immune system as regulatory elements41. So the deletion of dicer in DP thymocytes resulted in a dramatic NKT cell-autonomous defect in both thymic and peripheral compartments, supporting that dicer-dependent miRNAs play a critical role in differentiation and homeostasis of NKT cells42. Although the influence of costimulatory signaling, including CD28/B7 and CD40/CD40L, on NKT cell stimulation is controversial, reduced frequency of NKT cells in liver and spleen resulted from the blockage of CD28/B7 signaling pathway in vivo through transgenic CTLA-4Ig expression. This may point to a possibility that these signaling pathways are critical in periphery expansion of NKT cells, though, this remains to be formally investigated43.
Polarization of Th1 and Th2 cytokines in NKT cells
The predominant characteristic of NKT cells is rapid production of various cytokines, particularly Th1 and Th2 cytokines. The ready mRNA cytokines promise a swift production of copious amounts of Th1 and/or Th2 cytokines following stimulation15. Human cord blood exhibits a stronger Th2 response after polyclonal stimulation compared to adult NKT cells. However, neonatal NKT cells could be shifted towards a Th1 response under more polarizing conditions, while adult NKT cells are more resistant to shift in cytokine production44. Liver-derived DN NKT cells seem more protective than the ones from spleen and thymus. This suggests that different effects of NKT cells on tumor growth also depend on their tissue of origin45. This shift or switch between Th1 and Th2 cytokines produced by NKT cells is possibly a requirement of development, or even a byproduct of maturation. Yuling et al have reported that EBV-induced CD8+ NKT cells are prone to express more IFN-γ under the stimulation of α-GalCer, whereas CD4− NKT cells bias towards secretion of IL-4 and IL-10, which is in accord with the finding that CD4+ NKT cells have the superior capacity to produce Th2 cytokines over CD4− NKT cells including CD8+ NKT cells in humans, which preferentially induce Th1 cytokines production7, 8. This provides an explanation for separate immunoregulatory responses of individual subset of NKT cells, which could significantly affect the direction of an immune reaction. The detailed information on differences of three distinct functional subpopulations of NKT cells is listed in Table 1.
Table 1. Comparison of distinct functional subpopulations of NKT cells.
| Feature | CD4+ NKT | CD4−CD8− | CD8+ NKT | References | ||
|---|---|---|---|---|---|---|
| Frequency | Human | High frequency | Low frequency | Low frequency (nomal), or high frequency (specific stimulation) | 7, 8, 9, 13, 45, 59, 61, 69, 70, 76, 80 | |
| Mouse | High frequency | Low frequency | Undetectable | 16, 20, 24, 53, 71, 72 | ||
| Distribution | 70%–80% of hepatic NKT cells; 55%–75% of splenetic and thymic NKT cells; ∼60% of myeloid and lymphatic NKT cells (individual variations) | ∼25% of hepatic NKT cells; 25%–35% of splenetic and thymic NKT cells; 35%–45% of myeloid NKT cells (individual variations) | Very few in Thymus, Liver, Spleen, bone marrow, Lymph nodes (normal); ∼25% of thymic NKT cells and hepatic NKT cells (EBV challenge) | 7, 8, 9, 11, 12, 13, 14, 18, 20, 44, 45, 61, 70, 71, 72 | ||
| Development | Thymus-dependent origin, maturate in thymus or in periphery, thymic proliferation and output | Thymus-dependent origin, maturate in thymus or in periphery, thymic proliferation and output | Thymus-dependent origin, maturate mainly in thymus and thymic output, peripheral expansion | 7, 8, 9, 13, 17, 18, 20, 22–24, 30 | ||
| Cytokines | Th1 | High levels, but depending on conditions | High levels | High levels | 7, 8, 9, 11, 13, 14, 44, 45, 59, 61, 80 | |
| Th2 | High levels, but depending on conditions | Very low levels | Undetectable level | 11, 12, 13, 14, 44, 45, 53, 59, 61, 80 | ||
| Cytotoxic molecules | FasL(CD95L), TRAIL, Perforin/granzyme (depending on conditions) | Perforin/granzyme, TRAIL (depending on conditions), FasL (expressed on very few or no cells) | Perforin/granzyme | 7, 8, 9, 12, 45, 69 | ||
| Associated diseases | Autoimmunity diseases (type I diabetes, allergic asthma, SLE), infections, transplantation associated diseases (GVHD and allograft tolerance), some tumor types | Some tumors, transplantation associated diseases (GVHD and allograft tolerance), infections | Some tumors (EBV-associated malignancies), infections | 7, 8, 9, 11–14, 16, 45, 53, 59, 61, 80 | ||
Invariant NKT cells could be induced under different conditions to mediate opposite effects by altering the balance of cytokine profiles4. Activation of NKT cells after α-GalCer treatment can alter the balance of secreted cytokines from a Th2-bias to a Th1-bias46. The activation/up-regulation of T-bet and GATA-3 is responsible for the induction of chromatin remodeling Th1 and Th2 cytokine genes locus and expression of Th1 and Th2 cytokines, respectively31, 47. IFN-γ-deficient mice exhibited an increased incidence of carcinoma, suggesting that IFN-γ-dependent immune responses might be effective in the promotion phase of carcinogenesis48. Reconstitution of NKT cells from ICOS KO mice to NKT-deficient mice failed to develop airway hyperreactivity, because the ICOS/ICOSL interaction contributes to peripheral homeostasis of CD4+ NKT cells and production of Th2 cytokines such as IL-4 and IL-1349. The Th1 and Th2 effects of periphery NKT cells were abolished in CD28-deficient mice under the stimulation of α-GalCer, while Th1-like function was suppressed in CD40-deficient mice but with rather enhanced Th2 effect, which suggests that CD28- and CD40-mediated costimulatory signals differentially contribute to the regulation of Th1 and Th2 functions of NKT cells50. Polarization of NKT cells is based on intrinsic DC-mediated modulation of the Th1/Th2 balance, which is mainly dependent on IL-12 or IL-4 produced by mature DCs in vivo. To achieve the Th1/Th2 cytokine balance, a negative feedback loop selectively inhibits prolonged Th1 or Th2 responses of NKT cells through counter-regulation of Th2 or Th1 cytokines by mature DCs in vivo51, 52. In turn, immature DCs up-regulate MHC class ІІ and costimulatory molecules in response to the stimulation of CD40 expressed on NKT cells, and activate NKT cells by autocrine IL-12 to express more IFN-γ.
It is clear that NKT cells that are protective against tumors are skewed toward Th1 cytokines, while type ІІ NKT cells suppress tumor immunosurveillance based on their production of Th2 cytokines53. The potentially opposite effect of these two subsets of NKT cells was also determined in CD1d KO mice lacking both subtypes of NKT cells with decreased production of Th1 and Th2 cytokines but Jα18 KO mice lacking only NKT cells with reduced IFN-γ during murine schistosomiasis54. Jα18 KO mice are more susceptible to tumorigenesis and lack anti-tumor CTLs-mediated responses at the early time point, which demonstrates that NKT cells contribute to the natural anti-tumor immunosurveillance through Th1 cytokines during early tumor growth. However, the stimulation of type ІІ NKT cells surpresses anti-tumor immunosurveillance by Th2 cytokines secretion55. Type І and type ІІ NKT cells define a new immunoregulatory axis of opposing forces analogous to Th1 and Th2 cells since they not only have opposing functions but also counter-regulate each other, and any alterations of this axis may result in infectious diseases, autoimmune diseases and tumors. However, the detailed mechanism of suppressing anti-tumor immunosurveillance of type II NKT cells is not possible to define completely. Because it lacks specific markers to determine the selective development of type II NKT cells alone. Importantly, this immunoregulatory axis between type І and type ІІ NKT cells could be a major determinant in the subsequent immune response to any skewing.
NKT cells in tumor immunity
Understanding of cross talk between various arms, cells and molecules of the immune system is instrumental in achieving novel immunotherapy protocols for cancers. Type І NKT cells mediate both protective and regulatory immune functions, including anti-tumor responses, protection against pathogens, maintenance of transplant tolerance and inhibition of autoimmunity, whereas type ІІ NKT cells suppress anti-tumor surveillance in certain model systems16, 56. A large mount of studies demonstrated the anti-tumor activity of NKT cells stimulated by α-GalCer or by IL-12 in vivo57, 58. Invariant NKT cells induce the so called adjuvant effect on anti-tumor immunity by activating other anti-tumor cytolytic cells mainly through the Th1 cytokine cascades, although NKT cells have their own lytic activity and cause direct lysis of various tumor cells59, 60. The numerical and functional alteration of circulating Vα24+Vβ11+ NKT cells has arisen in patients with different types of tumor. The number of circulating Vα24 NKT cells significantly decreased in patients with colon cancer, head and neck cancer, breast cancer, renal cell cancer, and melanoma. IFN-γ producing ability of single NKT cell was preserved, whereas the responsiveness of Vα24+Vβ11+ human NKT cells was still low in patients with lung cancer, advanced prostate cancer or other undefined advanced cancers except glioma, even after stimulation with α-GalCer or granulocyte-colony stimulating factor (G-CSF)61, 62, 63, 64. Significantly enriched NKT cells in surgically resected specimens of lung tumor and colorectal tumor may explain the decrease of circulating NKT cells in quantity65, 66. Some lipid components derived from tumor cell membranes have been demonstrated to bond with CD1d and stimulate NKT hybridoma cells, so the dysfunction of NKT cells is possibly attributable to tumor-derived ligands67.
As the first line responder, NKT cells promptly secrete large amounts of inflammatory cytokines to promote cytotoxic effects in response to antigens in a direct and/or indirect manner. Activated NK cells have been well characterized in their ability to directly mediate anti-tumor responses through the delivery of cytotoxic effector molecules such as granzyme and perforin68. In our study, EBV-induced CD8+ NKT cells produced, remarkably, more perforin than their counterpart CD4+ NKT cells, which suggests a direct tumor cell-killing ability of NKT cells in vivo9. NKT cells directly eradicate target cells through cell death inducing ligands FasL and TNF-related apoptotic induced ligand (TRAIL)69. The recruitment of leukocytes into tissues is dependent on a series of adhesive and activation steps mediated by adhesion molecules and interaction of chemokine and chemokine receptors, which restrict their tissue and microenvironmental distribution70, 71, 72. In this regard, NKT cells resemble effector T cells because CD4+, CD8+, and DN subsets uniformly express non-lymphoid-tissue-homing chemokine receptors, such as CCR2, CCR5, and CXCR370. The production of RANTES by NKT cells is important for the recruitment of APCs and the induction of regulatory T cells. It suggests that NKT cells can influence immune responses by the regulation of chemokines expression, which is likely to shape the ensuing immune responses73, 74. In addition, NKT cells can enhance antigen specific B cell response to secrete elevated levels of IgG, which leads to antibody-dependent cellular cytotoxity (ADCC) to lyse cancer cells mediated by NK cells75. Moreover, NKT cells facilitate proper DCs maturation and also improve migration of more mature DCs towards tumor sites from lymph nodes76, 77. Interaction of chemokines and its receptors facilitate licensed DCs for cross-priming naïve CTLs in a helper T cell-dependent way78. Activated NKT cells also induce DCs to up-regulate CCL17 acting with its receptor CCR4 expressed on CTLs, which is distinct from classical interaction of CCR5 and its three ligands, and might permit an additional level of selectivity in CTL attraction for NKT cell-licensed cross-priming79, 80. A semi-permeable transwell membrane co-culture system prevented the interaction between syngeneic spleen CD3+ T cells and NKT cells, which strongly suggests the synergy of NKT cells is direct cell-cell contact dependent11. In turn, murine hepatic NKT cells show negative regulatory activities on recruitment, activation and effector functions of intrahepatic γδT cells dependent on TLR3 signaling pathway81.
It is important to note that potency of anti-tumor mediated by NKT cells may also be influenced by the type, complexity, and composition of tumor microenvironment in which they interact with neoplastic cells and other immune cells, although NKT cells may not infiltrate all tumors. Inflammatory mediators and effector molecules possibly released by tumor-infiltrating immunocytes are a contributing factor in suppression or promotion of angiogenesis and tumor growth. Expansion and activation of other immune effector cells are associated with activation of NKT cells in vivo. Immature DCs are activated by NKT cells to secrete IL-12 and IL-15, which are responsible for the activation of NK cells along with IFN-γ production. Moreover, activated DCs induced by NKT cells present tumor-derived antigens to activate conventional CD4+ Tregs and CD8+ CTLs, which enriches the adjuvant effect of NKT cells82. Th1 cytokines from activated NKT cells responded to α-GalCer promote cell-mediated cytotoxity and maturation of DCs, which are involved in the development of cognate T cell responses83. The EBV-induced human CD8+ NKT cells cooperated with syngenic CD3+T cells to kill or suppress EBV-associated tumors upon induction of Th1-bias, and the synergy is enhanced by addition of CD4+ NKT cells, which secrete high levels of IL-2 to maintain the transferred cells7, 8. Thymic CD8+ NKT cells induced by EBV could significantly drive CD3+ T cells to produce high levels of IFN-γ combined with autocrine Th1 cytokines to resist tumors, which is consistent with IFN-γ-mediated anti-tumor activity of NKT cells on α-GalCer stimulation84, 85. Additionally, the synergistic effect of EBV-exposed thymic CD4+ and CD8+NKT cells also significantly suppressed EBV-associated malignancies and prolonged animal survival7.
In clinical trails, therapeutic strategies mainly focus on the reconstitution of an adequate number of functional NKT cells either by active immunization or adoptive transfer of NKT cells activated in vitro86. With identification of glycolipids with high specificity to NKT cells and following high responsiveness to tumors, α-GalCer (KRN700) and its variants have been continuously identified and tested as powerful anti-tumor immunotherapeutic agents87. Exogenous glycolipid α-GalCer specially promotes the peripheral expansion of human CD8+ NKT cells from EBV infected human thymus transplanted SCID chemeric mouse, whereas the moderate cell proliferation for CD4+NKT cells is noted9. Administration of α-GalCer-pulsed DCs activate murine NKT cells and eradicate established metastatic tumor foci in models of the mouse liver and lung metastasis. They may exert a greater anti-tumor activity than α-GalCer alone57. It possibly ascribes this difference to dysfunction of APCs in patients with cancer along with numerical and functional deficits of NKT cells, or free α-GalCer anergizes NKT cells after initial activation88. Autologous in vitro expanded and activated NKT cells were adoptively transferred to patients with cancers as a potential immunotherapeutic strategy. Endogenous murine NKT cells were also activated and expanded to inhibit tumor metastasis through intravenous injection of α-GalCer-pulsed DCs at intervals. Faster and more robust secondary immune activation points to the existence of NKT cells activation and/or memory induction89. Moreover, in vitro expansion and polarization methods of circulating NKT cells facilitate the production of activated NKT cells and improve therapies based on NKT cells90. Some clinical trials with α-GalCer-pulsed DCs as a phase I study show obvious tumor regression without severe adverse immunological and clinical responses91. Although adoptive transfer of in vitro activated circulating Vα24+Vβ11+ human NKT cells with α-GalCer seems to makes up for the deficiency of number and/or function of endogenous target NKT cells, refusion of expanded and activated NKT cells in vitro is accompanied with potential problems including in vivo premature clearance of activated NKT cells and dysfunction of APCs92. In contrast to therapeutic efficiency in animals, few clinical trials to date has succeeded in achieving significant efficacy against human tumors in vivo, which reflects a species-related difference in NKT cell activity or a planning difference to treat advanced cancer patients and adoptively tumor-transplanted animals. Thus immunotherapy based on NKT cells to bolster anti-tumor immunity might be a potent new tool for modulating immune responses against malignancies. Overall, these events that occur in tumor-immunity by NKT cells are illustrated in Figure 2. Since a limited space, detailed description of other important functions of NKT cells, such as protection against infection, maintenance of transplant tolerance, and inhibition of autoimmunity were not included in this review.
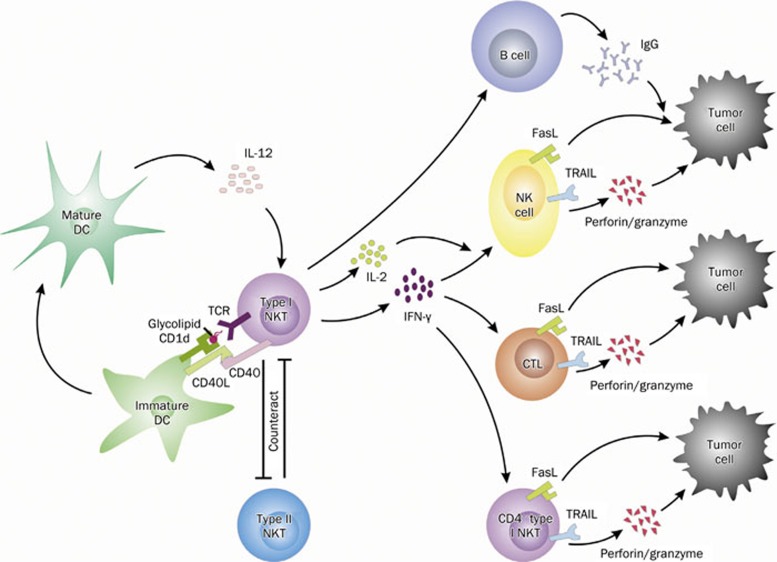
Figure 2.
NKT cells in tumor immunity. Upon the stimulation with glycolipids presented by CD1d-bearing DCs, activated type I NKT cells up-regulate the expression of CD40, which interacts with CD40L expressed on immature DCs and then leads to the generation of mature DCs. Under the promotion by IL-12 from mature DCs, NKT cells express higher levels of IFN-γ and IL-2, which induce NK cells and CTLs to kill tumor cells through perforin/granzyme, Fas/FasL, and TRAIL pathway. Likewise, mature CD4− type I NKT cells, including CD4−CD8− NKT cells and CD8+ NKT cells limited in humans, also display strong anti-tumor activity other than the adjuvant effect on activating other anti-tumor cytolytic cells. Additionally, NKT cells help antigen specific B cells to secrete elevated levels of IgG, which possibly leads to antibody-dependent cellular cytotoxity (ADCC) to lyse tumor cells mediated by NK cells. To achieve a balance, the protective type I and suppressive type II NKT cells counteract and cross-regulate each other, defining a novel immunoregulatory axis in tumor immunity. NKT, natural killer T cell; DC, dendritic cell; NK, natural killer cell; CTL, cytotoxic T lymphocyte; TRAIL, TNF-related apoptotic induced ligand.
Conclusion remarks
Since NKT cells were discovered nearly three decades ago, there have been great advances in the understanding of NKT cell development, homeostasis, effector function, and therapeutic applications. However, there are still some perplexities that have not yet been completely resolved in spite of the progress made. Why is there so much individual variation between individuals, even congenic mouse strains and identical twins? In contrast to the mouse, very few CD8+ NKT cells reside in normal person and a significantly increased number of CD8+ NKT cells is found in healthy persons with latent EBV infection. This raises the question what the CD8+ NKT cell origin and development pathways might be. How could the homeostasis of NKT cells and their production of Th1/Th2 cytokines be properly regulated or controlled in vivo? Another challenge in the field is to properly manipulate this balance of functional distinctive type I and type II NKT cells in the treatment of pathogenic states, particularly, in tumor therapy. Furthermore, understanding how to maintain this balance within the normal immune system is vitally important. Immunotherapeutic approaches targeting NKT cells should break tolerance and prove to be useful in the induction of more effective immune responses for the improved treatment of cancers, infections and autoimmune diseases. A major paradox is the ability of NKT cells to synchronously promote and suppress immune responses since functionally distinct subpopulations of NKT cells and the communication with other immune arms exist. In order to completely understand the role of NKT cells, significant markers should be identified to differential diagnosis type ІІ NKT cells and more work needs to be done to determine their functions other than the suppressive activity. In conclusion, an important point is that various clinical applications based on NKT cells to treat malignancies, even combined with other effectors and tools, possibly become potential immunotherapy for cancers.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (30730054, 30572119, 30670937, 30971279, 30901363), the Hi-tech Research and Development Program of China from Ministry of Science and Technology (2007AA02Z120), the Ministry of Education (20060486008, 20090141120011), Provincial Department of Science and Technology of Hubei (2007ABC010), Provincial Department of Health of Hubei (JX4B14), Innovation Program of Wuhan University for Young Scholars (WU3082007), National Innovation Experiment Program for College Students (WU2007061), China, and Chang Jiang Scholars Program from Ministry of Education, China and Li Ka Shing Foundation, Hong Kong, China (Chang Jiang Scholar TJ).
References
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–88. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapin L. iNKT cell autoreactivity: what is 'self' and how is it recognized. Nat Rev Immunol. 2010;10:272–7. doi: 10.1038/nri2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Pellicci DG, Patel O, Kjer-Nielsen L, McCluskey J, Rossjohn J. Antigen recognition by CD1d-restricted NKT T cell receptors. Semin Immunol. 2010;22:61–7. doi: 10.1016/j.smim.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Yuling H, Ruijing X, Li L, Xiang J, Rui Z, Yujuan W, et al. EBV-induced human CD8+ NKT cells suppress tumorigenesis by EBV-associated malignancies. Cancer Res. 2009;69:7935–44. doi: 10.1158/0008-5472.CAN-09-0828. [DOI] [PubMed] [Google Scholar]
- Xiao W, Li L, Zhou R, Xiao R, Wang Y, Ji X, et al. EBV-induced human CD8+ NKT cells synergise CD4+ NKT cells suppressing EBV-associated tumours upon induction of Th1-bias. Cell Mol Immunol. 2009;6:367–79. doi: 10.1038/cmi.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuling H, Ruijing X, Xiang J, Li L, Lang C, Jie X, et al. EBV promotes human CD8+ NKT cell development. PLoS Pathog. 2010;6:e1000915. doi: 10.1371/annotation/1730f52d-2ba9-4f08-b330-47d71b31ae4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- Sen Y, Yongyi B, Yuling H, Luokun X, Li H, Jie X, et al. V alpha 24-invariant NKT cells from patients with allergic asthma express CCR9 at high frequency and induce Th2 bias of CD3+ T cells upon CD226 engagement. J Immunol. 2005;175:4914–26. doi: 10.4049/jimmunol.175.8.4914. [DOI] [PubMed] [Google Scholar]
- Tao D, Shangwu L, Qun W, Yan L, Wei J, Junyan L, et al. CD226 expression deficiency causes high sensitivity to apoptosis in NK T cells from patients with systemic lupus erythematosus. J Immunol. 2005;174:1281–90. doi: 10.4049/jimmunol.174.3.1281. [DOI] [PubMed] [Google Scholar]
- Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, Weinberg KI, et al. Distinct homeostatic requirements of CD4+ and CD4− subsets of Valpha24-invariant natural killer T cells in humans. Blood. 2004;104:4150–6. doi: 10.1182/blood-2004-04-1629. [DOI] [PubMed] [Google Scholar]
- Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–41. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, et al. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–16. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Li Y, Thapa P, Hawke D, Kondo Y, Furukawa K, Hsu FF, et al. Immunologic glycosphingolipidomics and NKT cell development in mouse thymus. J Proteome Res. 2009;8:2740–51. doi: 10.1021/pr801040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz LM, Yang CY, Goldrath AW. Transcriptional regulation of NKT cell development and homeostasis. Curr Opin Immunol. 2010;22:199–205. doi: 10.1016/j.coi.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(–)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195:835–44. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–18. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- Chun T, Page MJ, Gapin L, Matsuda JL, Xu H, Nguyen H, et al. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med. 2003;197:907–18. doi: 10.1084/jem.20021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicci DG, Uldrich AP, Kyparissoudis K, Crowe NY, Brooks AG, Hammond KJ, et al. Intrathymic NKT cell development is blocked by the presence of alpha-galactosylceramide. Eur J Immunol. 2003;33:1816–23. doi: 10.1002/eji.200323894. [DOI] [PubMed] [Google Scholar]
- Engel I, Hammond K, Sullivan BA, He X, Taniuchi I, Kappes D, et al. Co-receptor choice by V{alpha}14i NKT cells is driven by Th-POK expression rather than avoidance of CD8-mediated negative selection. J Exp Med. 2010;207:1015–29. doi: 10.1084/jem.20090557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrella V, Poliani PL, Casati A, Rucci F, Frascoli L, Gougeon ML, et al. A hypomorphic R229Q Rag2 mouse mutant recapitulates human Omenn syndrome. J Clin Invest. 2007;117:1260–9. doi: 10.1172/JCI30928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai H, Rybouchkin A, Hongo N, Nagata Y, Sakata S, Sekine E, et al. Generation of functional NKT cells in vitro from embryonic stem cells bearing rearranged invariant Valpha14-Jalpha18 TCRalpha gene. Blood. 2010;115:230–7. doi: 10.1182/blood-2009-04-217729. [DOI] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–73. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Guo J, Hawwari A, Li H, Sun Z, Mahanta SK, Littman DR, et al. Regulation of the TCRalpha repertoire by the survival window of CD4(+)CD8(+) thymocytes. Nat Immunol. 2002;3:469–76. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- Mycko MP, Ferrero I, Wilson A, Jiang W, Bianchi T, Trumpp A, et al. Selective requirement for c-Myc at an early stage of V(alpha)14i NKT cell development. J Immunol. 2009;182:4641–8. doi: 10.4049/jimmunol.0803394. [DOI] [PubMed] [Google Scholar]
- Dose M, Sleckman BP, Han J, Bredemeyer AL, Bendelac A, Gounari F. Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc. Proc Natl Acad Sci USA. 2009;106:8641–6. doi: 10.1073/pnas.0812255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Zhang Q, Ndonye R, Richardson SK, Howell AR, Gapin L. T-bet concomitantly controls migration, survival, and effector functions during the development of Valpha14i NKT cells. Blood. 2006;107:2797–805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–94. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197:1613–21. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz LM, Knell J, Fujimoto JK, Goldrath AW. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat Immunol. 2010;11:240–9. doi: 10.1038/ni.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–64. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Yang Y, Knell J, D'Cruz LM, Cannarile MA, Engel I, et al. Transcriptional regulator Id2 controls survival of hepatic NKT cells. Proc Natl Acad Sci U S A. 2009;106:19461–6. doi: 10.1073/pnas.0908249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Fedeli M, Napolitano A, Wong MP, Marcais A, de Lalla C, Colucci F, et al. Dicer-dependent microRNA pathway controls invariant NKT cell development. J Immunol. 2009;183:2506–12. doi: 10.4049/jimmunol.0901361. [DOI] [PubMed] [Google Scholar]
- Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N, Antonenko S, Ho S, Rissoan MC, Soumelis V, Porcelli SA, et al. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med. 2001;193:1221–6. doi: 10.1084/jem.193.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–88. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru W, Peijie C. Modulation of NKT cells and Th1/Th2 imbalance after alpha-GalCer treatment in progressive load-trained rats. Int J Biol Sci. 2009;5:338–43. doi: 10.7150/ijbs.5.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PJ, Pai SY, Brigl M, Besra GS, Gumperz J, Ho IC. GATA-3 regulates the development and function of invariant NKT cells. J Immunol. 2006;177:6650–9. doi: 10.4049/jimmunol.177.10.6650. [DOI] [PubMed] [Google Scholar]
- Wakita D, Chamoto K, Ohkuri T, Narita Y, Ashino S, Sumida K, et al. IFN-gamma-dependent type 1 immunity is crucial for immunosurveillance against squamous cell carcinoma in a novel mouse carcinogenesis model. Carcinogenesis. 2009;30:1408–15. doi: 10.1093/carcin/bgp144. [DOI] [PubMed] [Google Scholar]
- Akbari O, Stock P, Meyer EH, Freeman GJ, Sharpe AH, Umetsu DT, et al. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. J Immunol. 2008;180:5448–56. doi: 10.4049/jimmunol.180.8.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Lumsden JM, Yu X, Feigenbaum L, Zhang J, Steinberg SM, et al. Regulation of thymic NKT cell development by the B7-CD28 costimulatory pathway. J Immunol. 2008;181:907–17. doi: 10.4049/jimmunol.181.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Yanagawa Y, Iwabuchi K, Shinohara N, Harabayashi T, Nonomura K, et al. Negative feedback regulation of T helper type 1 (Th1)/Th2 cytokine balance via dendritic cell and natural killer T cell interactions. Blood. 2005;106:1685–93. doi: 10.1182/blood-2004-12-4738. [DOI] [PubMed] [Google Scholar]
- Onoe K, Yanagawa Y, Minami K, Iijima N, Iwabuchi K. Th1 or Th2 balance regulated by interaction between dendritic cells and NKT cells. Immunol Res. 2007;38:319–32. doi: 10.1007/s12026-007-0011-5. [DOI] [PubMed] [Google Scholar]
- Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. Int J Cancer. 2005;114:80–7. doi: 10.1002/ijc.20669. [DOI] [PubMed] [Google Scholar]
- Mallevaey T, Fontaine J, Breuilh L, Paget C, Castro-Keller A, Vendeville C, et al. Invariant and noninvariant natural killer T cells exert opposite regulatory functions on the immune response during murine schistosomiasis. Infect Immun. 2007;75:2171–80. doi: 10.1128/IAI.01178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179:5126–36. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–33. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toura I, Kawano T, Akutsu Y, Nakayama T, Ochiai T, Taniguchi M. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with alpha-galactosylceramide. J Immunol. 1999;163:2387–91. [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, et al. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–22. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molling JW, Kolgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–50. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Seino K, Ishikawa Y, Nozue M, Todoroki T, Fukao K. Impaired proliferative response of V alpha 24 NKT cells from cancer patients against alpha-galactosylceramide. J Immunol. 2002;168:6494–9. doi: 10.4049/jimmunol.168.12.6494. [DOI] [PubMed] [Google Scholar]
- Dhodapkar KM, Cirignano B, Chamian F, Zagzag D, Miller DC, Finlay JL, et al. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int J Cancer. 2004;109:893–9. doi: 10.1002/ijc.20050. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, et al. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–36. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–76. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Nieda M, Nicol A, Koezuka Y, Kikuchi A, Lapteva N, Tanaka Y, et al. TRAIL expression by activated human CD4(+)V alpha 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood. 2001;97:2067–74. doi: 10.1182/blood.v97.7.2067. [DOI] [PubMed] [Google Scholar]
- Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11–6. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- Cullen R, Germanov E, Shimaoka T, Johnston B. Enhanced tumor metastasis in response to blockade of the chemokine receptor CXCR6 is overcome by NKT cell activation. J Immunol. 2009;183:5807–15. doi: 10.4049/jimmunol.0803520. [DOI] [PubMed] [Google Scholar]
- Germanov E, Veinotte L, Cullen R, Chamberlain E, Butcher EC, Johnston B. Critical role for the chemokine receptor CXCR6 in homeostasis and activation of CD1d-restricted NKT cells. J Immunol. 2008;181:81–91. doi: 10.4049/jimmunol.181.1.81. [DOI] [PubMed] [Google Scholar]
- Faunce DE, Stein-Streilein J. NKT cell-derived RANTES recruits APCs and CD8+ T cells to the spleen during the generation of regulatory T cells in tolerance. J Immunol. 2002;169:31–8. doi: 10.4049/jimmunol.169.1.31. [DOI] [PubMed] [Google Scholar]
- Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104:3984–9. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya CJ, Jie HB, Al-Harthi L, Mulder C, Patino PJ, Rugeles MT, et al. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell-mediated cross-talk with myeloid dendritic cells. J Immunol. 2006;177:1028–39. doi: 10.4049/jimmunol.177.2.1028. [DOI] [PubMed] [Google Scholar]
- Vuylsteke RJ, Molenkamp BG, van Leeuwen PA, Meijer S, Wijnands PG, Haanen JB, et al. Tumor-specific CD8+ T cell reactivity in the sentinel lymph node of GM-CSF-treated stage I melanoma patients is associated with high myeloid dendritic cell content. Clin Cancer Res. 2006;12:2826–33. doi: 10.1158/1078-0432.CCR-05-2431. [DOI] [PubMed] [Google Scholar]
- Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–5. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol. 2010;11:313–20. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- Moreno M, Molling JW, von Mensdorff-Pouilly S, Verheijen RH, Hooijberg E, Kramer D, et al. IFN-gamma-producing human invariant NKT cells promote tumor-associated antigen-specific cytotoxic T cell responses. J Immunol. 2008;181:2446–54. doi: 10.4049/jimmunol.181.4.2446. [DOI] [PubMed] [Google Scholar]
- Gardner TR, Chen Q, Jin Y, Ajuebor MN. Toll-like receptor 3 ligand dampens liver inflammation by stimulating Valpha 14 invariant natural killer T cells to negatively regulate gammadeltaT cells. Am J Pathol. 2010;176:1779–89. doi: 10.2353/ajpath.2010.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Idoyaga J, Charalambous A, Fujii S, Bonito A, Mordoh J, et al. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J Exp Med. 2005;202:1507–16. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, et al. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259–66. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–27. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi S, Nakayama T. Clinical applications of natural killer T cell-based immunotherapy for cancer. Cancer Sci. 2008;99:638–45. doi: 10.1111/j.1349-7006.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci USA. 2005;102:3383–8. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–74. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–9. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- van der Vliet HJ, Molling JW, Nishi N, Masterson AJ, Kolgen W, Porcelli SA, et al. Polarization of Valpha24+ Vbeta11+ natural killer T cells of healthy volunteers and cancer patients using alpha-galactosylceramide-loaded and environmentally instructed dendritic cells. Cancer Res. 2003;63:4101–6. [PubMed] [Google Scholar]
- Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–17. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnara D, Ibatici A, Corselli M, Sessarego N, Tenca C, De Santanna A, et al. Adoptive immunotherapy med iated by ex vivo expanded natural killer T cells against CD1d-expressing lymphoid neoplasms. Haematologica. 2009;94:967–74. doi: 10.3324/haematol.2008.001339. [DOI] [PMC free article] [PubMed] [Google Scholar]