Abstract
Endothelial cells release various substances to control the tone of the underlying vascular smooth muscle. Nitric oxide (NO) is the best defined endothelium-derived relaxing factor (EDRF). Endothelial cells can also increase vascular tone by releasing endothelium-derived contracting factors (EDCF). The over-production of EDCF contributes to the endothelial dysfunctions which accompanies various vascular diseases. The present review summarizes and discusses the mechanisms leading to the release of EDCFs derived from the metabolism of arachidonic acid. This release can be triggered by agonists such as acetylcholine, adenosine nucleotides or by stretch. All these stimuli are able to induce calcium influx into the endothelial cells, an effect which can be mimicked by calcium ionophores. The augmentation in intracellular calcium ion concentration initiates the release of EDCF. Downstream processes include activation of phospholipase A2 (PLA2), cyclooxygenases (COX) and the production of reactive oxygen species (ROS) and vasoconstrictor prostanoids (endoperoxides, prostacyclin, thromboxane A2 and other prostaglandins) which subsequently diffuse to, and activate thromboxane-prostanoid (TP) receptors on the vascular smooth muscle cells leading to contraction.
Keywords: cyclooxygenase, EDCF, endothelium, gap junctions, phospholipase A2, prostanoids, reactive oxygen species, TP-receptors
Introduction
Following the first report by Furchgott and Zawadzki (1980)1 that in response to acetylcholine, endothelial cells release a vasodilator substance [endothelium-derived relaxing factor (EDRF)] later identified as nitric oxide (NO), a number of other inhibitory endothelial signals [endothelium-derived hyperpolarizing factors (EDHF)] have been shown to contribute to relaxations of the underlying vascular smooth muscle cells2, 3, 4, 5, 6, 7, 8, 9. In addition, it soon became apparent that under certain circumstances the endothelium can also produce diffusible substances [endothelium-derived contracting factors (EDCF)] which activate the contractile process in the underlying vascular smooth muscle cells10. Besides receptors-mediated agonists such as thrombin, acetylcholine and adenosine nucleotides (ADP and ATP)11, 12, 13, stretch can also elicit endothelium-dependent contractions, at least in canine cerebral arteries14. The early observation that such endothelium-dependent contractions could be prevented by inhibitors of cyclooxygenase suggested that down-stream products of this enzyme, ie prostanoids, were likely candidates as EDCF12, 15, 16, 17, 18. Although endothelial cells can produce vasoconstrictors including endothelin-1 and angiotensin II, there is lack of convincing evidence showing a direct link between these substances and instantaneous changes in tension that can be attributed to the release of EDCF. Thus, the present article focuses on the mechanisms leading to the production of endothelial and cyclooxygenase-derived vasoconstrictors, and updates earlier reviews on this topic19, 20, 21.
Endothelial calcium concentration
An increase in intracellular calcium concentration in the endothelial cells is the triggering event leading to the release of EDCF. This conclusion is based on the following observations: (a) Activation of cell membrane receptors by agonists such as acetylcholine [activating endothelial M3-muscarinic receptors22], ADP and ATP [activating purinoceptors11, 23, which are known to induce the release of calcium from the sarcoplasmic reticulum24, initiate the production of EDCF; (b) Reduction in the extracellular calcium concentration decreases endothelium-dependent contractions25; (c) Calcium ionophores such as A23187 elicit endothelium-dependent contractions13, 26, 27, 28, 29; (d) Endothelium-dependent contractions induced by acetylcholine in the rat aorta are accompanied by an increase in cytosolic endothelial calcium concentration26, 27 and this increment is greater in preparations of spontaneously hypertensive rats (SHR) compared to those of age-matched normotensive Wistar-Kyoto rats (WKY), in line with the larger EDCF-mediated responses in the former12, 15, 27. On the other hand, no significant difference in the increase of calcium concentration in the two strains was observed if the aortae were exposed to A2318727.
Phospholipase A2
The increase in endothelial concentration of the activator ion elicited by agonists such as acetylcholine involves two steps, release of calcium from the sarcoplasmic reticulum followed by influx of extracellular calcium. Acetylcholine binds to the G proteins-coupled muscarinic receptors on the endothelial cell membrane and activates phospholipase C. The latter produces inositol triphosphate which in turn causes the release of calcium from intracellular stores. The resulting calcium-depletion process leads to the production of a messenger termed calcium influx factor [CIF; 30] which displaces the inhibitory calmodulin from the calcium-independent phospholipase A2 [iPLA2; 31, 32, 33, 34]. Activation of iPLA2 is an initiating event in the generation of EDCF induced by acetylcholine in the rat aorta35. Activated iPLA2 produces lysophospholipids which facilitate the opening of store-operated calcium channels (SOCs) leading to the influx of extracellular calcium into the endothelial cells34, 36. This large influx of calcium ions then activates the calcium-dependent phospholipase A2 (cPLA2) which converts membrane phospholipids to arachidonic acids, the precursor of prostanoids (Figure 1). That the calcium-dependent form of phospholipase A2 is crucial for the ultimate production of EDCF is demonstrated by the observation that a specific inhibitor of iPLA2 does not affect A23187-induced endothelium-dependent contractions, while quinacrine, which inhibits both forms of the enzyme, abolishes the response to both acetylcholine and A2318712, 35.
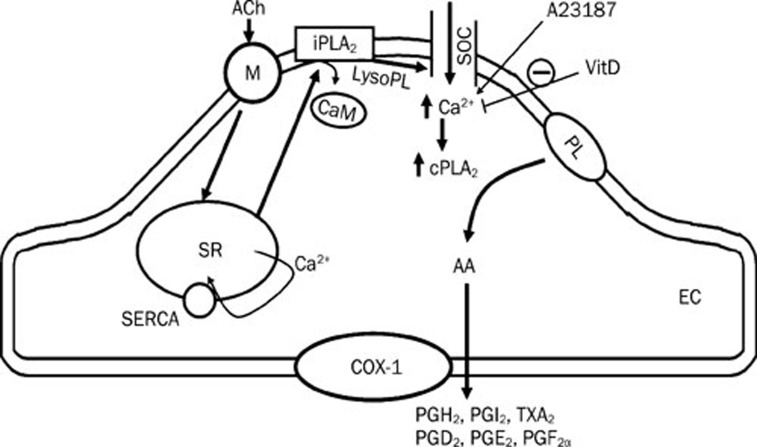
Figure 1.
Acetylcholine (ACh) activates muscarinic receptors (M) on the endothelial cell membrane and triggers the release of calcium from intracellular stores. The resulting calcium-depletion process displaces the inhibitory calmodulin (CaM) from iPLA2. Activated iPLA2 produces lysophospholipids (LysoPL) which in turn open store-operated calcium channels (SOCs) leading to the influx of extracellular calcium into the endothelial cells. This large influx of calcium ions then activates cPLA2 which catalyze the production of arachidonic acids (AA). The later is then metabolized by cyclooxygenase-1 (COX-1) to prostanoids. 1,25-Dihydroxyvitamin D3 (Vit D) acutely reduces endothelium-dependent contraction by inhibiting the calcium surge. cPLA2=calcium dependent phospholipase A2; EC=endothelial cells; iPLA2=calcium independent phospholipase A2; PGD2=prostaglandin D2; PGE2=prostaglandin E2; PGF2α=prostaglandin F2α; PGH2=endoperoxides; PGI2=prostacyclin; PL=phospholipids; SERCA=sarco/endoplasmic reticulum Ca2+-ATPase; SR=sarcoplasmic reticulum; TXA2=thromoboxane A2.
Vitamin D and EDCF
High concentrations of vitamin D appear to have an acute protective effect on endothelial cells by reducing the production of EDCF. Indeed, the in vitro administration of 1,25-dihydroxyvitamin D3 [the most active metabolite of vitamin D37] reduces EDCF-mediated responses induced by acetylcholine but not by the calcium ionophore A23187 in aorta of both SHR and WKY, suggesting that vitamin D acutely reduces EDCF production by an action upstream of the increase in calcium concentration and thus interferes with the calcium surging process (Figure 1)26.
Cyclooxygenase
The two isoforms of cyclooxygenase (COX), COX-1 and COX-2, have a comparable ability to catalyze the transformation of arachidonic acid into prostaglandins (Figure 2)38. Both isoforms can play a key role in the generation of EDCF depending on the species, the blood vessel studied and the health conditions of the donor2, 3, 19, 28, 39, 40, 41, 42. COX-1 is constitutively expressed in most tissues while COX-2 is inducible43, 44. Early studies demonstrated that non-selective COX inhibitors abolish endothelium-dependent contractions13, 17, an observation that has been repeated over the years. Selective inhibitors of COX-1, but not those of COX-2, abrogate endothelium-dependent contractions in the rat aorta15, 45, 46, 47. In that preparation, COX-1 is expressed in both endothelial and vascular smooth muscle cells, but the over-expression of this isoform seen in the SHR aorta is confined to the endothelial cells48. Likewise, bioassay studies demonstrate that only the activation of endothelial COX contributes to the generation of diffusible EDCF in the SHR aorta15. Endothelium-dependent contractions are present in the aorta of COX-2, but not in that of COX-1 knock-out mice49. Taken in conjunction, these findings demonstrated that COX-1 is the preferential constitutive isoform of cyclooxygenase which mediates endothelium-dependent contractions in large arteries of rat and mice. However, with aging or disease, COX-2 can be induced and then contributes to EDCF-mediated responses50, 51, 52, 53. By contrast, constitutively expressed COX-2 plays a dominant role in the endothelium-dependent contraction of the hamster aorta irrespective of age39.
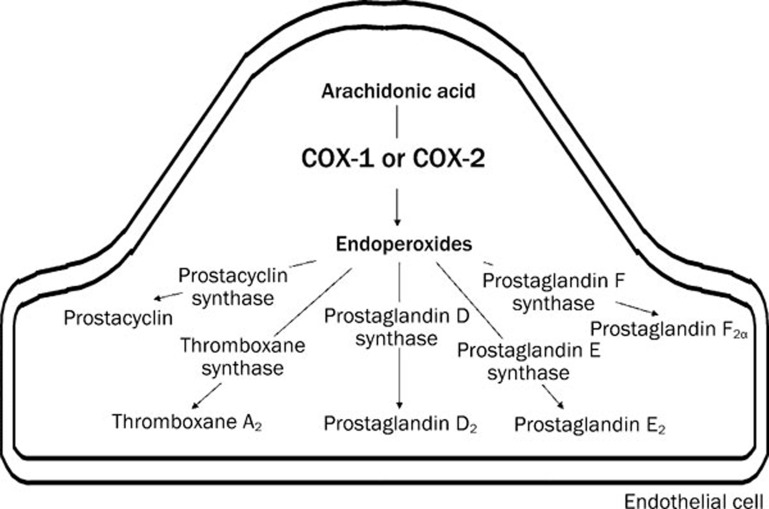
Figure 2.
Metabolism of arachidonic acid into specific prostanoids. Arachidonic acid is converted to endoperoxides by the activity of cyclooxygenase (COX). Endoperoxides are then converted to various prostaglandins by their respective synthase.
Prostanoids
Cyclooxygenase converts arachidonic acid into endoperoxides (PGH2), the intermediate of the prostanoid biosynthesis, which can either act as an EDCF per se47, 54 or be further transformed into prostacyclin (PGI2), thromboxane A2 and various other prostaglandins including prostaglandin D2 (PGD2), prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) by their respective synthases (Figure 2)29, 48, 55, 56.
Although PGH2 has a relatively short half-life and is unstable57, it can be a vasoconstrictor EDCF29, 47, 48, 55 by activating TP receptors of vascular smooth muscle47, 57, 58. This conclusion is supported by two observations: (a) The aorta of SHR releases more PGH2 than that of WKY when exposed to acetylcholine47. (b) Similarly to the acetylcholine-induced EDCF-mediated responses, PGH2-induced contractions in aortae without endothelium are transient and are larger in SHR compared to WKY47, 56. In addition, when tyrosine nitration caused by the local production of peroxynitrite inhibits the activity of prostacyclin synthase59, PGH2 may become even more important in the process.
Prostacyclin is the major cyclooxygenase-derived metabolite of arachidonic acid in endothelial cells60. During endothelium-dependent contractions of rodent aortae in response to acetylcholine, its production is markedly larger than that of other prostaglandins and, together with PGH2, prostacyclin becomes a major EDCF19, 21, 47, 53, 56. This conclusion is in line with the findings that the gene expression of PGI synthase in the rat aortic endothelial cells is greatly augmented by aging and spontaneous hypertension48.
During ADP- and A23187-induced endothelium-dependent contractions, the release of thromboxane A2 is augmented and an inhibitor of thromboxane A2 can reduce these contractions, unlike those to acetylcholine29, 55, 57. Therefore, thromboxane A2 can be regarded as a key EDCF during the EDCF-mediated responses elicited by these agents. Likewise, in certain blood vessels (hamster aorta) or with aging and disease (such as diabetes), an augmented contribution of PGE2 and PGF2α to EDCF-mediated contractions may become obvious39, 61. This can be explained best by the increased generation of these prostaglandins under conditions of enhanced oxidative stress62, in particular as a consequence of the augmented formation of peroxynitrite which inhibits PGI synthase59, 63 and diverts arachidonic acid towards PGE2 and PGF2α synthases56. Obviously, the involvement of individual prostanoids in EDCF-mediated responses varies depending on the species, the blood vessels studied, the endothelium-dependent agonist used, and the age and disease state of the donor.
Reactive oxygen species
Reactive oxygen species (ROS) are generated during a number of normal metabolic activities, but their overproduction leads to oxidative stress which is commonly observed in hypertension, diabetes and atherosclerosis20, 64, 65. During the generation of prostanoids by COX, ROS are formed as by-products. ROS of relevance for endothelium-dependent responses include superoxide anions (O2−), hydroxyl radicals (·OH) and hydrogen peroxide (H2O2) (Figure 3). ROS either directly act as EDCF66, 67 or indirectly potentiate EDCF-mediated responses by reducing the bioavailability of NO68, 69, 70 and activating COX in the vascular smooth muscle cells16, 18, 71. This conclusion is based on the following observations: (a) An increased ROS production accompanies acetylcholine- or A23187-induced endothelium-dependent contractions27; (b) Tiron (which scavenges superoxide anions intracellularly) or catalase (which converts hydrogen peroxide to water and oxygen) plus deferoxamine (which prevents the formation of hydroxyl radicals) reduce endothelium-dependent contractions in the SHR aorta72 and the femoral artery of diabetic rats64, suggesting that superoxide anions and hydrogen peroxide augment or even mediate part of the response; (c) ROS formed by the xanthine plus xanthine oxidase reaction elicit contractions of SHR aortae without endothelium which are prevented by both COX inhibitors and TP receptor antagonists, suggesting that the oxygen-derived free radicals stimulate COX in the vascular smooth muscle to produce prostanoids which in turn activate their TP receptors18, 72. (d) ROS increase the degradation of nitric oxide68, 69; (d) Peroxynitrite, a strong cytosolic oxidant generated by the reaction of the superoxide anions and nitric oxide, inactivate PGI synthase59, 63 and shifts the production of prostacyclin to that of other vasoconstrictor prostanoids56, 73. (e) In canine basilar arteries, superoxide dismutase (SOD) plus catalase abolish the A23187 induced endothelium-dependent contractions but not the production of prostaglandins and thromboxane A2 indicating that ROS rather than COX-derived prostanoids are the EDCF in this particular artery66; (f) In the rat pulmonary artery, ROS induce contraction involving the activity of protein kinase C in the vascular smooth muscle74; (g) In vascular smooth muscle of the rat aorta, the ROS-induced calcium sensitization is mediated through the activation of Rho and a subsequent increase in Rho kinase activity75, and the latter is crucial in the response to EDCF76; and (h) ROS directly depolarize vascular smooth muscle by inhibiting ATP-sensitive potassium channel (KATP), voltage-activated potassium channel (Kv) and large conductance calcium-activated potassium channel (BKCa)77, 78, 79.
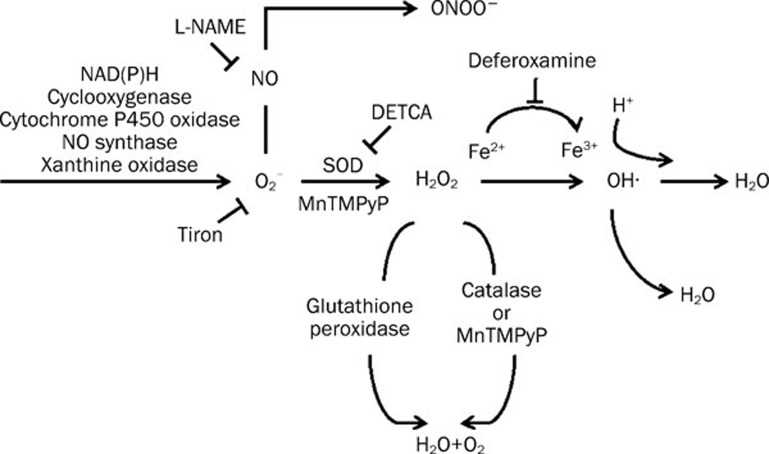
Figure 3.
Formation of oxygen-derived free radicals of relevance for endothelium-dependent responses, and pharmacological agents commonly used to determine their importance. Superoxide anions (O2−) can be generated from molecular oxygen by the actions of various enzymes. O2− can react with NO to form peroxynitrite (ONOO−). It can also be converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). H2O2 can be transformed to hydroxyl radicals by ferrous ions or converted to H2O by catalase and glutathione. Tiron scavenges O2− inside cells. DETCA inhibits SOD. Deferoxamine is an iron chelator that scavenges hydroxyl radicals. L-NAME inhibits NO synthase. MnTMPyP mimics the combined effect of SOD and catalase. DETCA=diethyldithiocarbamic acid; GSH=glutathione; GSSG=glutathione disulphide; L-NAME=Nω-nitro-L-arginine methyl ester hydrochloride; MnTMPyP=Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride; NO=nitric oxide; tiron=4,5-dihydroxy-1,3-benzenedisulphonic acid. (Adapted from Shi et al 2007, by permission)Arachidonic acid is converted to endoperoxides by the activity of cyclooxygenase (COX). Endoperoxides are then converted to various prostaglandins by their respective synthase.
Gap junctions
The contact between endothelial and vascular smooth muscle cells is important in the genesis of endothelium-dependent contractions. This conclusion is supported by the observation that the endothelium-dependent contractile response to acetylcholine of layered bioassay (“sandwich”) preparations of SHR aortae is much smaller than that of intact aortic rings72. The contraction in the “sandwich preparation” is caused by prostanoids which diffuse across the intracellular gap between the donor (containing endothelial cells) and the recipient strip (without endothelium, responsible for the contraction). Under bioassay conditions, superoxide dismutase plus catalase (both compounds with poor cell permeability) can reduce the acetylcholine-induced endothelium-dependent contractions while they have no effect in intact rings in which tiron inhibits EDCF-mediated responses15, 18. These observations imply that in intact rings, ROS exert their facilitatory effect by either acting in the endothelial cells or being transported from the latter to the vascular smooth muscle cells via preferential channels not accessible to superoxide dismutase. One possible route would be the myoendothelial gap junctions (Figure 4), since the gap junction inhibitor carbenoxolone reduces endothelium-dependent contractions to acetylcholine and the calcium ionophore A2318780.
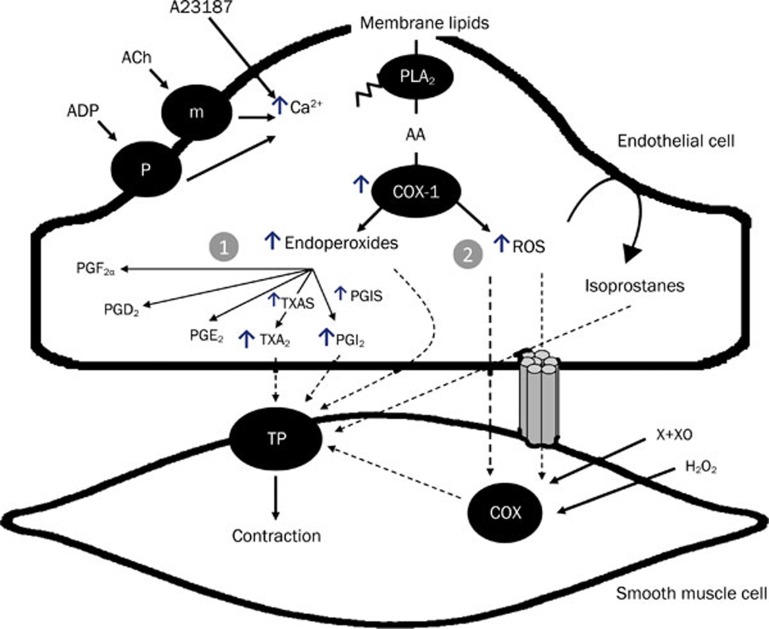
Figure 4.
Endothelium-dependent contraction is likely to be comprised of two components: generation of prostanoids and ROS. Each component depends on the activity of endothelial COX-1 and the stimulation of the TP receptors located on the smooth muscle to evoke contraction. In the SHR aorta, there is an increased expression of COX-1 and EP3 receptors, increased release of calcium, ROS, endoperoxides and other prostanoids, which facilitates the greater occurrence of endothelium-dependent contraction in the hypertensive rat. The necessary increase in intracellular calcium can be triggered by receptor-dependent agonists, such as acetylcholine or ADP, or mimicked with calcium increasing agents, such as the calcium ionophore A23187. The abnormal increase in intracellular ROS can be mimicked by the exogenous addition of H2O2 or the generation of extracellular ROS by incubation of xanthine with xanthine oxidase. AA=arachidonic acid; ACh=acetycholine; ADP=adenosine diphosphate; H2O2=hydrogen peroxide;m=muscarinic receptors; P=purinergic receptors; PGD2=prostaglandin D2; PGE2=prostaglandin E2; PGF2α=prostaglandin F2α; PGI2=prostacyclin; PLA2=phospholipase A2; ROS=reactive oxygen species; TXA2= thromboxane A2; X+XO=xanthine plus xanthine oxidase. (Adapted from Tang and Vanhoutte, 2009, by permission).
Prostanoid receptors and Rho kinase
Thromboxane-prostanoid receptors (TP-receptors) are the most important prostanoid receptor subtype involved in endothelium-dependent contractions since TP receptor antagonists abolish these responses15, 57, 81. All prostanoids are able to bind with TP receptors, albeit with different affinities82. Thromboxane A2 is the most potent agonist at TP receptors. Endoperoxides and prostacyclin also activate TP receptors and both of them evoke transient contractions (probably due to their short half-life) which mimic acetylcholine-induced endothelium-dependent contractions56. Binding of EDCF to the TP receptors in turn activates the downstream Rho kinase pathway leading to the increased contractile activity of the vascular smooth muscle76.
In the SHR aorta, the gene expression levels and protein presence of TP receptors are not altered, but the responsiveness to endoperoxides is augmented compared to WKY preparations47, 48. This hyperresponsiveness contributes to the prominence of EDCF-mediated responses in the aorta of the SHR. Another crucial aspect in this prominence is that the vascular smooth muscle of aging WKY and of the SHR have lost the ability to respond with relaxation to prostacyclin, despite an unchanged expression of IP receptors and the large production of prostacyclin by endothelial cells exposed to acetycholine or A2318748, 56, 83, 84.
Interactions between NO, EDHF, and EDCF
In the SHR aorta, the concomitant release of NO inhibits endothelium-dependent contractions to acetylcholine85, 86, an observation that has lead to the systematic use of inhibitors of NO synthases when studying EDCF-mediated responses. In addition, previous exposure to endothelium-derived NO or exogenous NO-donors causes a long-term inhibition of EDCF-mediated responses87, 88. Likewise, in the renal artery of the rat, the absence of EDHF favours the occurrence of endothelium-dependent contractions9.
Alternatively, EDCF may also counteract the action of endothelium-derived relaxing factors. Thus, in WKY mesenteric artery, EDHF-mediated relaxations are attenuated by the release of EDCF89. This attenuation is explained best by the EDCF-induced activation of TP-receptors which depolarizes the vascular smooth muscle cells by inhibiting Kv and BKCa90, 91.
Physiological importance
In the early nineteenth century, Bayliss showed that an increase in the internal pressure in the carotid artery of the dog caused its constriction, a seminal observation leading to the concept of autoregulation92. In isolated basilar arteries of the same species, stretch induces a contraction which disappears after the removal of the endothelium, demonstrating an endothelium-dependent process14. This contraction is sensitive to both the COX inhibitor indomethacin and the calcium-influx blocker diltiazem, suggesting that the activity of COX (presumably in the endothelial cells) and the influx of extracellular calcium (presumably in the vascular smooth muscle cells) are required for the active response to stretch14. Likewise, in bovine coronary arteries, stretch elicits an endothelium-independent contraction which requires the activation of NAD(P)H oxidase93. Stretch also directly activates various cation channels on the smooth muscle cells of small arteries facilitating their contraction94, 95, 96. Oxygen-derived free radicals play a key role in endothelium-dependent contractions of the canine basilar artery66. Thus, it is tempting to speculate that the endothelium-dependent contraction evoked by stretch (resulting from activation of endothelial COX, the production of ROS and the hypersensitivity of the vascular smooth muscle) may initiate the autoregulatory response, at least in cerebral arteries.
Pathophysiological relevance
As mentioned already, endothelium-dependent contractions are exacerbated by aging, diabetes, hypertension and atherosclerosis41, 97, 98. Foe example, the blunted endothelium-dependent relaxations in response to acetylcholine in diabetic animals is partly due to the augmented production of EDCF, resulting from the over-expression and activation of COX and increased ROS production after the chronic exposure of the endothelial cells to high glucose levels99. In essential hypertensive patients, the blunted vasodilatation induced by acetylcholine can almost be normalized by the COX inhibitor indomethacin indicating that COX-derived vasoconstrictors are key players responsible for the abnormal endothelial response100. This indomethacin-sensitive impairment of the response to acetylcholine is accentuated by aging98. However, in secondary hypertension, inhibition of COX does not restore the acetylcholine-induced vasodilatation suggesting that EDCFs are not equally important in all cases of hypertension. It is likely that the prominence of endothelium-dependent contractions observed in arteries of aging and diseased (essential hypertension, diabetes) animals and human reflects the progressive inability of the endothelial cells to generate enough NO to curtail the production of EDCF40, 87, 88. Shifting from the normal release of NO (and EDHF) to that of EDCF likely plays an important role in the development of vascular disease40, 101.
Conclusion
Endothelial cells release COX-derived vasoconstrictor prostanoids and reactive oxygen species, which have been termed EDCF. In the SHR, prostacyclin becomes a prominent EDCF acting on TP-receptors, even more so that IP receptor signaling is impaired21, 56, 83. EDCF-mediated responses are amplified in aging normotensive animals (Koga et al, 1989; Wong et al, 2009), hypertensive12 and diabetic20, 50, 64, 102 animals. In humans, EDCF plays a role in the endothelial dysfunction that accompanies aging, atherosclerosis, myocardial infarction and essential hypertension98, 103, 104.
References
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial-cells in the relaxation of arterial smooth-muscle by acetylcholine. Nature. 1980;288:373–76. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–18. [PubMed] [Google Scholar]
- Luescher TF, Vanhoutte PM.The Endothelium: Modulator of Cardiovascular Function Boca RatonFL CRC Press; 1990
- Vanhoutte PM. The other endothelium-derived vasoactive factors. Circulation. 1993;87:V9–17. [Google Scholar]
- Gluais P, Edwards G, Weston AH, Vanhoutte PM, Feletou M. Hydrogen peroxide and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Eur J Pharmacol. 2005;513:219–24. doi: 10.1016/j.ejphar.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Gluais P, Edwards G, Weston AH, Falck JR, Vanhoutte PM, Feletou M. Role of SKCa and IKCa in endothelium-dependent hyperpolarizations of the guinea–pig isolated carotid artery. Br J Pharmacol. 2005;144:477–85. doi: 10.1038/sj.bjp.0706003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM.EDHF: the complete story CRC Press; 2006
- Shi Y, Ku DD, Man RY, Vanhoutte PM. Augmented endothelium-derived hyperpolarizing factor-mediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. J Pharmacol Exp Ther. 2006;318:276–81. doi: 10.1124/jpet.105.099739. [DOI] [PubMed] [Google Scholar]
- Michel FS, Man GS, Man RY, Vanhoutte PM. Hypertension and the absence of EDHF-mediated responses favour endothelium-dependent contractions in renal arteries of the rat. Br J Pharmacol. 2008;155:217–26. doi: 10.1038/bjp.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey JG, Vanhoutte PM. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982;51:439–47. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Purinergic endothelium-dependent and -independent contractions in rat aorta. Hypertension. 1993;22:577–83. doi: 10.1161/01.hyp.22.4.577. [DOI] [PubMed] [Google Scholar]
- Luescher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–8. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Shepherd JT, Vanhoutte PM. Endothelium-dependent contractions to calcium ionophore A23187, arachidonic acid, and acetylcholine in canine basilar arteries. Stroke. 1988;19:476–9. doi: 10.1161/01.str.19.4.476. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Shepherd JT, Vanhoutte PM. Endothelium-dependent contraction to stretch in canine basilar arteries. Am J Physiol. 1987;252:H671–3. doi: 10.1152/ajpheart.1987.252.3.H671. [DOI] [PubMed] [Google Scholar]
- Yang D, Feletou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension. 2003;41:143–8. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- Yang D, Feletou M, Boulanger CM, Wu HF, Levens N, Zhang JN, et al. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–10. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM, Vanhoutte PM. Endothelium-dependent contractions to arachidonic acid are mediated by products of cyclooxygenase. Am J Physiol. 1985;248:H432–7. doi: 10.1152/ajpheart.1985.248.4.H432. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension. 1989;13:859–64. doi: 10.1161/01.hyp.13.6.859. [DOI] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Prostanoids and reactive oxygen species: team players in endothelium-dependent contractions. Pharmacol Ther. 2009;122:140–9. doi: 10.1016/j.pharmthera.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vanhoutte PM. Reactive oxygen-derived free radicals are key to the endothelial dysfunction of diabetes. J Diabetes. 2009;1:151–62. doi: 10.1111/j.1753-0407.2009.00030.x. [DOI] [PubMed] [Google Scholar]
- Feletou M, Verbeuren TJ, Vanhoutte PM. Endothelium-dependent contractions in SHR: a tale of prostanoid TP and IP receptors. Br J Pharmacol. 2009;156:563–74. doi: 10.1111/j.1476-5381.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CM, Morrison KJ, Vanhoutte PM. Mediation by M3–muscarinic receptors of both endothelium–dependent contraction and relaxation to acetylcholine in the aorta of the spontaneously hypertensive rat. Br J Pharmacol. 1994;112:519–24. doi: 10.1111/j.1476-5381.1994.tb13104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension. 1989;14:542–8. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, et al. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci USA. 2009;106:11418–23. doi: 10.1073/pnas.0813307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon EB, Golbabaie A, van Breemen C. In the presence of L-NAME SERCA blockade induces endothelium-dependent contraction of mouse aorta through activation of smooth muscle prostaglandin H2/thromboxane A2 receptors. Br J Pharmacol. 2002;137:545–53. doi: 10.1038/sj.bjp.0704884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MS, Delansorne R, Man RY, Vanhoutte PM. Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2008;295:H289–96. doi: 10.1152/ajpheart.00116.2008. [DOI] [PubMed] [Google Scholar]
- Tang EH, Leung FP, Huang Y, Feletou M, So KF, Man RY, et al. Calcium and reactive oxygen species increase in endothelial cells in response to releasers of endothelium-derived contracting factor. Br J Pharmacol. 2007;151:15–23. doi: 10.1038/sj.bjp.0707190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Feletou M, Ku DD, Man RY, Vanhoutte PM. The calcium ionophore A23187 induces endothelium-dependent contractions in femoral arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007;150:624–32. doi: 10.1038/sj.bjp.0706999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Paysant J, Badier-Commander C, Verbeuren T, Vanhoutte PM, Feletou M. In SHR aorta, calcium ionophore A-23187 releases prostacyclin and thromboxane A2 as endothelium-derived contracting factors. Am J Physiol Heart Circ Physiol. 2006;291:H2255–64. doi: 10.1152/ajpheart.01115.2005. [DOI] [PubMed] [Google Scholar]
- Randriamampita C, Tsien RY. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–14. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Wolf MJ, Wang J, Turk J, Gross RW. Depletion of intracellular calcium stores activates smooth muscle cell calcium-independent phospholipase A2. A novel mechanism underlying arachidonic acid mobilization. J Biol Chem. 1997;272:1522–6. doi: 10.1074/jbc.272.3.1522. [DOI] [PubMed] [Google Scholar]
- Wolf MJ, Gross RW. The calcium-dependent association and functional coupling of calmodulin with myocardial phospholipase A2. Implications for cardiac cycle-dependent alterations in phospholipolysis. J Biol Chem. 1996;271:20989–92. doi: 10.1074/jbc.271.35.20989. [DOI] [PubMed] [Google Scholar]
- Trepakova ES, Csutora P, Hunton DL, Marchase RB, Cohen RA, Bolotina VM. Calcium influx factor directly activates store-operated cation channels in vascular smooth muscle cells. J Biol Chem. 2000;275:26158–63. doi: 10.1074/jbc.M004666200. [DOI] [PubMed] [Google Scholar]
- Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–20. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- Wong MS, Man RY, Vanhoutte PM. Calcium-independent phospholipase A2 plays a key role in the endothelium-dependent contractions to acetylcholine in the aorta of SHR. Am J Physiol Heart Circ Physiol. 2010;298:H1260–6. doi: 10.1152/ajpheart.01068.2009. [DOI] [PubMed] [Google Scholar]
- Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, Cohen RA, Bolotina VM. Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J Biol Chem. 2001;276:7782–90. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- Garavito RM, DeWitt DL. The cyclooxygenase isoforms: structural insights into the conversion of arachidonic acid to prostaglandins. Biochim Biophys Acta. 1999;1441:278–87. doi: 10.1016/s1388-1981(99)00147-x. [DOI] [PubMed] [Google Scholar]
- Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, et al. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res. 2009;104:228–35. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. COX-1 and vascular disease. Clin Pharmacol Ther. 2009;86:212–5. doi: 10.1038/clpt.2009.108. [DOI] [PubMed] [Google Scholar]
- Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension. 1995;25:517–23. doi: 10.1161/01.hyp.25.4.517. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Davidge ST. Prostaglandin H synthase and vascular function. Circ Res. 2001;89:650–60. doi: 10.1161/hh2001.098351. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Endothelium-dependent contractions to acetylcholine, ATP and the calcium ionophore A 23187 in aortas from spontaneously hypertensive and normotensive rats. Fundam Clin Pharmacol. 2004;18:321–6. doi: 10.1111/j.1472-8206.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Ospina JA, Duckles SP, Krause DN. 17beta-estradiol decreases vascular tone in cerebral arteries by shifting COX-dependent vasoconstriction to vasodilation. Am J Physiol Heart Circ Physiol. 2003;285:H241–50. doi: 10.1152/ajpheart.00018.2003. [DOI] [PubMed] [Google Scholar]
- Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM, Boulanger CM. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res. 1995;76:1003–10. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–18. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- Tang EH, Ku DD, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2−/− knockout but not COX1−/− knockout mice. J Cardiovasc Pharmacol. 2005;46:761–5. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- Shi Y, Man RY, Vanhoutte PM. Two isoforms of cyclooxygenase contribute to augmented endothelium-dependent contractions in femoral arteries of 1-year-old rats. Acta Pharmacol Sin. 2008;29:185–92. doi: 10.1111/j.1745-7254.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Ishida K, Kobayashi T, Kamata K. Pyrrolidine dithiocarbamate reduces vascular prostanoid-induced responses in aged type 2 diabetic rat model. J Pharmacol Sci. 2009;110:326–33. doi: 10.1254/jphs.09116fp. [DOI] [PubMed] [Google Scholar]
- Camacho M, Lopez-Belmonte J, Vila L. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and prostaglandin I synthase activity. Circ Res. 1998;83:353–65. doi: 10.1161/01.res.83.4.353. [DOI] [PubMed] [Google Scholar]
- Blanco-Rivero J, Cachofeiro V, Lahera V, Aras-Lopez R, Marquez-Rodas I, Salaices M, et al. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension. 2005;46:107–12. doi: 10.1161/01.HYP.0000171479.36880.17. [DOI] [PubMed] [Google Scholar]
- Asano H, Shimizu K, Muramatsu M, Iwama Y, Toki Y, Miyazaki Y, et al. Prostaglandin H2 as an endothelium-derived contracting factor modulates endothelin-1-induced contraction. J Hypertens. 1994;12:383–90. [PubMed] [Google Scholar]
- Gluais P, Vanhoutte PM, Feletou M. Mechanisms underlying ATP-induced endothelium-dependent contractions in the SHR aorta. Eur J Pharmacol. 2007;556:107–14. doi: 10.1016/j.ejphar.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005;146:834–45. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- Ito T, Kato T, Iwama Y, Muramatsu M, Shimizu K, Asano H, et al. Prostaglandin H2 as an endothelium-derived contracting factor and its interaction with endothelium-derived nitric oxide. J Hypertens. 1991;9:729–36. doi: 10.1097/00004872-199108000-00006. [DOI] [PubMed] [Google Scholar]
- Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor–mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]
- Moncada S, Gryglewski RJ, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–65. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Nakayama N, Ishida K, Kobayashi T, Kamata K. Eicosapentaenoic acid improves imbalance between vasodilator and vasoconstrictor actions of endothelium-derived factors in mesenteric arteries from rats at chronic stage of type 2 diabetes. J Pharmacol Exp Ther. 2009;329:324–34. doi: 10.1124/jpet.108.148718. [DOI] [PubMed] [Google Scholar]
- Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–6. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Zou MH, Leist M, Ullrich V. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am J Pathol. 1999;154:1359–65. doi: 10.1016/S0002-9440(10)65390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, So KF, Man RY, Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions in femoral arteries of rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007;152:1033–41. doi: 10.1038/sj.bjp.0707439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–74. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol. 1989;257:H33–7. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Schugel J, Cosentino F, Vanhoutte PM. Endothelium-dependent contractions to oxygen-derived free radicals in the canine basilar artery. Am J Physiol. 1993;264:H859–64. doi: 10.1152/ajpheart.1993.264.3.H859. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–7. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Paolocci N, Biondi R, Bettini M, Lee CI, Berlowitz CO, Rossi R, et al. Oxygen radical-mediated reduction in basal and agonist-evoked NO release in isolated rat heart. J Mol Cell Cardiol. 2001;33:671–9. doi: 10.1006/jmcc.2000.1334. [DOI] [PubMed] [Google Scholar]
- Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18:195–9. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Hibino M, Okumura K, Iwama Y, Mokuno S, Osanai H, Matsui H, et al. Oxygen-derived free radical-induced vasoconstriction by thromboxane A2 in aorta of the spontaneously hypertensive rat. J Cardiovasc Pharmacol. 1999;33:605–10. doi: 10.1097/00005344-199904000-00013. [DOI] [PubMed] [Google Scholar]
- Yang D, Levens N, Zhang JN, Vanhoutte PM, Feletou M. Specific potentiation of endothelium-dependent contractions in SHR by tetrahydrobiopterin. Hypertension. 2003;41:136–42. doi: 10.1161/01.hyp.0000047669.93078.a7. [DOI] [PubMed] [Google Scholar]
- Cohen RA. Does EDCF contribute to diabetic endothelial cell dysfunction. Dialog Cardiovasc Med. 2002;7:225–31. [Google Scholar]
- Jin N, Packer CS, Rhoades RA. Reactive oxygen-mediated contraction in pulmonary arterial smooth muscle: cellular mechanisms. Can J Physiol Pharmacol. 1991;69:383–8. doi: 10.1139/y91-058. [DOI] [PubMed] [Google Scholar]
- Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- Chan CK, Mak JC, Man RY, Vanhoutte PM. Rho kinase inhibitors prevent endothelium-dependent contractions in the rat aorta. J Pharmacol Exp Ther. 2009;329:820–6. doi: 10.1124/jpet.108.148247. [DOI] [PubMed] [Google Scholar]
- Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol. 2004;11:171–8. doi: 10.1038/nsmb725. [DOI] [PubMed] [Google Scholar]
- Li H, Gutterman DD, Rusch NJ, Bubolz A, Liu Y. Nitration and functional loss of voltage-gated K+ channels in rat coronary microvessels exposed to high glucose. Diabetes. 2004;53:2436–42. doi: 10.2337/diabetes.53.9.2436. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Azma T, Nakahata K, Iranami H, Kimoto Y, Dojo M, et al. Inhibitory effect of high concentration of glucose on relaxations to activation of ATP-sensitive K+ channels in human omental artery. Arterioscler Thromb Vasc Biol. 2004;24:2290–5. doi: 10.1161/01.ATV.0000148006.78179.c7. [DOI] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gap junction inhibitors reduce endothelium-dependent contractions in the aorta of spontaneously hypertensive rats. J Pharmacol Exp Ther. 2008;327:148–53. doi: 10.1124/jpet.108.140046. [DOI] [PubMed] [Google Scholar]
- Kato T, Iwama Y, Okumura K, Hashimoto H, Ito T, Satake T. Prostaglandin H2 may be the endothelium-derived contracting factor released by acetylcholine in the aorta of the rat. Hypertension. 1990;15:475–81. doi: 10.1161/01.hyp.15.5.475. [DOI] [PubMed] [Google Scholar]
- Dickinson JS, Murphy RC. Mass spectrometric analysis of leukotriene A4 and other chemically reactive metabolites of arachidonic acid. J Am Soc Mass Spectrom. 2002;13:1227–34. doi: 10.1016/S1044-0305(02)00456-7. [DOI] [PubMed] [Google Scholar]
- Rapoport RM, Williams SP. Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 1996;28:64–75. doi: 10.1161/01.hyp.28.1.64. [DOI] [PubMed] [Google Scholar]
- Levy JV. Prostacyclin-induced contraction of isolated aortic strips from normal and spontaneously hypertensive rats (SHR) Prostaglandins. 1980;19:517–25. doi: 10.1016/s0090-6980(80)80002-5. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Nitric oxide and inactivation of the endothelium-dependent contracting factor released by acetylcholine in spontaneously hypertensive rat. J Cardiovasc Pharmacol. 2004;43:815–20. doi: 10.1097/00005344-200406000-00011. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Nitric oxide inactivates endothelium-derived contracting factor in the rat aorta. Hypertension. 1992;19:442–5. doi: 10.1161/01.hyp.19.5.442. [DOI] [PubMed] [Google Scholar]
- Tang EH, Feletou M, Huang Y, Man RY, Vanhoutte PM. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am J Physiol Heart Circ Physiol. 2005;289:H2434–40. doi: 10.1152/ajpheart.00568.2005. [DOI] [PubMed] [Google Scholar]
- Feletou M, Tang EH, Vanhoutte PM. Nitric oxide the gatekeeper of endothelial vasomotor control. Front Biosci. 2008;13:4198–217. doi: 10.2741/3000. [DOI] [PubMed] [Google Scholar]
- Sekiguchi F, Nakahira T, Kawata K, Sunano S. Responses to endothelium-derived factors and their interaction in mesenteric arteries from Wistar-Kyoto and stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2002;29:1066–74. doi: 10.1046/j.1440-1681.2002.03778.x. [DOI] [PubMed] [Google Scholar]
- Scornik FS, Toro L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am J Physiol. 1992;262:C708–13. doi: 10.1152/ajpcell.1992.262.3.C708. [DOI] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F. Thromboxane A2–induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Czeta. Circ Res. 2003;93:656–63. doi: 10.1161/01.RES.0000095245.97945.FE. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–31. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res. 2003;92:23–31. doi: 10.1161/01.res.0000051860.84509.ce. [DOI] [PubMed] [Google Scholar]
- Wu X, Davis MJ. Characterization of stretch-activated cation current in coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H1751–61. doi: 10.1152/ajpheart.2001.280.4.H1751. [DOI] [PubMed] [Google Scholar]
- Setoguchi M, Ohya Y, Abe I, Fujishima M. Stretch-activated whole-cell currents in smooth muscle cells from mesenteric resistance artery of guinea-pig. J Physiol. 1997;501:343–53. doi: 10.1111/j.1469-7793.1997.343bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol. 2001;91:973–83. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Tang EH. Endothelium-dependent contractions: when a good guy turns bad. J Physiol. 2008;586:5295–304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Versari D, Salvetti A. Endothelium, aging, and hypertension. Curr Hypertens Rep. 2006;8:84–9. doi: 10.1007/s11906-006-0045-4. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–74. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–9. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelial dysfunction and atherosclerosis. Eur Heart J. 1997;18 Suppl E:E19–29. doi: 10.1016/s0195-668x(97)90005-1. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Jakubowski JA, Cohen RA. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol. 1989;257:H1327–33. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk. Circulation. 2002;106:640–2. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- Boulanger CM. Secondary endothelial dysfunction: hypertension and heart failure. J Mol Cell Cardiol. 1999;31:39–49. doi: 10.1006/jmcc.1998.0842. [DOI] [PubMed] [Google Scholar]