Abstract
Previous studies on lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P) using various approaches have shown that both the molecules can act as intercellular signaling molecules. The discovery of the Edg subfamily of G-protein-coupled receptors (GPCRs) (later renamed LPA1–3 and S1P1–5) for these molecules has opened up a new avenue for pathophysiological research on lysophospholipids. Genetic and molecular studies on lysophospholipid GPCRs have elucidated pathophysiological impacts and roles in cellular signaling pathways. Recently, lysophospholipid GPCR genes have been used to develop receptor subtype-selective agonists and antagonists. The discovery of FTY720, a novel immune modulator, along with other chemical tools, has provided a means of elucidating the functions of each lysophospholipid GPCR on an organ and the whole body level. This communication attempts to retrospectively review the development of agonists and antagonists for lysophospholipid GPCRs, provide integrated information on pharmacological tools for lysophospholipid GPCR signaling, and speculate on future drug development.
Keywords: lysophosphatidic acid, sphingosine 1-phosphate, agonist, antagonist, G-protein-coupled receptor, lysolipid
Discovery of GPCRs for LPA and S1P
Lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P) are two representative lysophospholipid mediators acting on G-protein-coupled receptors (GPCRs). LPA was first identified three decades ago as a factor affecting blood pressure, platelet aggregation, and smooth muscle contraction1, 2, 3, 4, 5 and rediscovered as a mitogenic serum lipid inducer of neurite retraction6, 7. The name 'lyso' originates from lysis of blood cells; thus, the non-specific detergent action of LPA has been doubted. In 1996, the high potency (nmol/L range) and GPCR implications of LPA were finally connected to the discovery of the first LPA receptor (LPA1, formerly known as Edg-2)8. Subsequently, LPA2 and LPA3 were identified as members of the endothelial differentiation gene (Edg) subfamily of GPCRs9, 10, 11. These three LPA receptors (Edg family) share a high homology with each other12.
Recently, the non-Edg family of LPA receptors, LPA4 (GPR23, p2y9), LPA5 (GPR92), and LPA6 (p2y5), were reported as members of the purinergic GPCR cluster13, 14, 15, 16, 17, 18, 19. Using the GPCR-Gα16 fusion expression system, Fujita's group reported GPR87 as another LPA receptor and P2Y10 as a dual receptor for both LPA and S1P20, 21. These results have not yet been confirmed by other research groups22. Instead, lysophosphatidylserine has been suggested to act as a ligand for P2Y10 without confirmation of LPA and S1P as ligands in NIH3T3 cells23.
S1P was initially reported 15 years ago to be a second messenger, mediating an increase in calcium levels due to PDGF and IgE signaling24, 25. The molecular target of S1P in the cytosol has not yet been identified. The initial finding of an S1P-induced calcium increase stimulated research in S1P biology and was linked to its recognition as an intercellular first messenger. The involvement of trimeric G proteins in S1P-induced actions as well as pertussis toxin sensitivity strongly suggested the presence of S1P GPCRs in the plasma membrane26, 27, 28, 29, 30. The discovery of S1P1 (formerly known as Edg-1) in 1998, along with four other receptors (S1P2–5) of the Edg subfamily GPCRs, became a milestone in S1P biology12, 31, 32, 33, 34, 35, 36.
Development of agonists and antagonists for LPA receptors
Prior to receptor cloning, various aspects of LPA receptor structure-activity relationships had been studied: 1) fatty acid chain length and the presence of double bonds, 2) acyl and alkyl linkage, 3) stereo-selectivity on the sn-2 position, and 4) modification of phosphate groups37. Oleoyl LPA (18:1) was considered the optimum ratio to increase Ca2+ levels in A431 cells38. Alkyl LPA showed a better effect than acyl LPA in platelet aggregation assays and an equal effect in other responses39, 40. Several groups reported a lack of stereo-selectivity on the sn-2 position of LPA. Early studies showed that modification of the phosphate group resulted in an absence of LPA responses38, 40.
The initial synthetic approach to LPA response was characterized using platelet aggregation assays. Replacement of the glycerol backbone with amino acids resulted in the production of N-palmitoyl serine phosphatidic acid (NPSPA) and N-palmitoyl tyrosine phosphatidic acid (NPTyrPA)40. These molecules were not active on platelet aggregation in humans, but did demonstrate activity as LPA receptor antagonists in Xenopus41. Later, NPSPA and NPTyrPA were reported as partial agonists of mammalian LPA receptors42, 43. Sugiura et al introduced the ethanolamine-based LPA mimetic, N-acyl aminoethanol phosphoric acid (NAEPA), as an equipotent agonist of platelet aggregation40. Later, research groups from the University of Virginia used NASPA and NAEPA as platforms to synthesize a series of VPC compounds37, 44. The phosphonate analogue of NAEPA lost its platelet aggregation properties40, but methylene phosphonate LPA was equipotent to LPA as an inhibitor of forskolin-driven cAMP accumulation in rat C6 glioma cells45. Jalink et al synthesized various phosphonate analogues along with fatty alcohol phosphates and the methyl ester of LPA (lysophosphatidylmethanol, LPM), but could not show a significant impact of these compounds on Ca2+ increase in A431 cells38. Ironically, these chemicals turned out to be selective or non-selective agonists of cloned LPA receptors (see details below). In the early era of LPA biology, suramin and lysophosphatidylglycerol were used to demonstrate GPCR involvement in LPA responses46 and as an antagonist of LPA-induced Ca2+ responses in Jurkat T cells47, respectively.
LPA GPCR agonists
Since the discovery of the three-Edg family of LPA receptors, the development of selective receptor-subtype agonists and antagonists has accelerated. The optimal chain length and the presence of double bonds have been found to vary depending on receptor subtype. For example, LPA3 showed a preference for unsaturated LPA similar to oleoyl LPA48, whereas LPA6 showed a preference for 2-acyl LPA19. Synthesis of LPA derivatives with phosphonate or thiophosphate groups instead of the phosphate group showed receptor-subtype selective activity similar to 1-oleoyl-2-O-methyl-rac-glycerophosphothionate (OMPT), 1-O-acyl-α-fluoromethylenephosphonate, and α-hydroxymethylenephosphonate LPA analogues as LPA3 receptor selective agonists49, 50, 51, 52. The phosphonate derivatives also provided a path toward the development of phosphatase-resistant long-lasting LPA derivatives53. A dialkyl phosphatidic acid (PA) 8:0 analog with a thiophosphate was reported as a potent and selective LPA3 agonist54, but later, agonistic activity on the LPA5 receptor and antagonistic activity on the LPA1,3 receptors was reported55. Based on computational modeling, dodecyl fatty alcohol phosphate was shown to be a specific LPA2 receptor agonist56, and later, oleoyl-thiophosphate was identified as a pan-agonist (LPA1–3)43. A methylene phosphonate LPA analogue was reported as a selective LPA2 agonist, but it recently was shown to exert agonistic and antagonistic activities on LPA5 and LPA3, respectively52, 57. T-15 (LPA1 agonist) and T-13 (LPA3 agonist) were both synthesized using a carbohydrate scaffold58. Darmstoff analogues were introduced as novel scaffolds for subtype-selective LPA receptor ligands59. Finally, octadecenyl phosphate was shown to be a selective agonist for LPA4 and LPA5 receptors55.
Although alkyl LPA has been shown to exert equipotent activity on each Edg-family LPA receptor compared to acyl LPA60, alkyl LPA was found to be more potent than acyl LPA in platelet aggregation responses3. Therefore, the Edg-family LPA receptors were not able to account for the LPA response in platelets61. Recently, non-Edg (purinergic) LPA receptors have been identified. The LPA5/GPR92 receptor has demonstrated alkyl preference and a presence in platelets55. Farnesyl diphosphate, N-arachidonyl glycine, and carba-cyclic phosphatidic acid were shown to be selective agonists for LPA555, 62, 63. LPA6/p2y5 showed a preference for 2-acyl LPA over 1-acyl LPA19. Alkyl-OMPT, an LPA3 agonist, and 2-linoleoyl LPA showed better agonistic effects on the LPA6 receptor than 2-oleoyl LPA showed19, 64. In contrast, the methyl ester of LPA (LPM) was shown to be a pan-agonist for LPA1–5 although it was less potent than LPA65.
LPA GPCR antagonists
The development of LPA derivatives with a bulky group on the sn-2 position has demonstrated the stereo-selectivity of LPA receptors and has led to the further development of the LPA1/LPA3 selective antagonists VPC12249 and VPC3218366, 67. 2-Pyridyl-containing phosphonate was developed as a non-hydrolyzable LPA3 receptor antagonist68. Dioctylglyceropyrophosphate (DGPP) and Ki16425 were determined to be selective LPA1/LPA3 antagonists following screening of available lipid and non-lipid molecules69, 70. The thiophosphate version of PA 8:0 was shown to be the most potent LPA3 antagonist, whereas the dialkyl PA 8:0 analog with a thiophosphate was shown to be a potent and selective LPA3,5 agonist, as mentioned above54, 55. Tetradecyl-phosphonate was identified as a pan-antagonist (LPA1–3)43. Furthermore, farnesyl phosphate and farnesyl diphosphate potently and selectively blocked the LPA3-mediated Ca2+ increase71, although farnesyl diphosphate is a LPA5 selective agonist, as mentioned above55. T-14, which used a carbohydrate scaffold, was shown to be an LPA3 antagonist58.
Recently, virtual screening identified non-lipid LPA3 antagonist NSC161613, LPA2 antagonist H2L5186303, LPA4,5 antagonist 5987411, and LPA1,6 antagonist 576583455, 72. An LPA analogue of α-bromomethylene phosphonate was reported to act as a pan-antagonist to LPA1–4 and an autotoxin inhibitor52, 73.
Pharmacological tools for LPA GPCR signaling
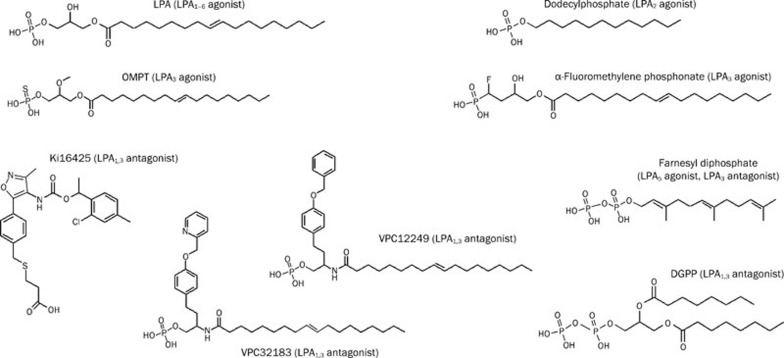
Commercially available chemicals for studying LPA receptor subtypes are currently in development, although the effects of previously developed chemicals on recently identified non-Edg LPA receptors have not been completely verified (Figure 1). For LPA1 or LPA3 receptor signaling, a combined application of LPA1,3 antagonists such as VPC12249, VPC32193, DGPP, and Ki16425 and LPA3 agonists such as OMPT and α-fluoromethylene phosphonate would be more favorable. For LPA2 receptor signaling, dodecyl phosphate is an adequate LPA2 selective agonist. For LPA5 receptor, farnesyl diphosphate could be used as a selective agonist. For LPA6 receptor, alkyl OMPT, an LPA3,6 agonist, could be used in combination with LPA1,3 antagonists (Table 1).
Figure 1.
Structures of commercially available agonists and antagonists for LPA GPCR signaling. Sources are Avanti polar lipid, Biomol international, Echelon bioscience, Enzo Life sciences, and Sigma-Aldrich.
Table 1. Agonistic and antagonistic characters of each compound on each LPA receptor. Numbers (nmol/L) mean EC50 or KD values for agonists and IC50 or KI values for antagonists.
| Name | LPA1 | LPA2 | LPA3 | LPA4 | LPA5 | LPA6 |
|---|---|---|---|---|---|---|
| Ki1642570 | Antagonist (250 nmol/L) | Antagonist (5600 nmol/L) | Antagonist (360 nmol/L) | |||
| DGPP69 | Antagonist (6600 nmol/L) | Antagonis (106 nmol/L) | ||||
| VPC3218367 | Antagonist (109 nmol/L) | Antagonist (175 nmol/L) | ||||
| VPC1224966 | Antagonist (137 nmol/L) | Antagonist (428 nmol/L) | ||||
| 2-Pyridyl phosphonate68 | Antagonist | |||||
| Thiophosphatidic acid 8:054 | Antagonist (360 nmol/L) | Antagonist (5 nmol/L) | ||||
| T1458 | Antagonist | |||||
| NSC16161372 | Antagonist (24 nmol/L) | |||||
| Compound 12120 | Antagonist (48 nmol/L) | Antagonist (230 nmol/L) | ||||
| H2L518630372 | Antagonist (7.2 nmol/L) | Antagonist (310 nmol/L) | ||||
| 598741155 | Antagonist (741 nmol/L) | Antagonist (1300 nmol/L) | ||||
| 576583455 | Antagonist (48 nmol/L) | Antagonist (292 nmol/L) | ||||
| α-bromomethylene phosphonate52, 55, 73, 117 | Antagonist (751 nmol/L) | Antagonist (304 nmol/L) | Antagonist (380 nmol/L) | Antagonist (167 nmol/L) | Weak agonist | |
| Tetradecyl-phosphonate43 | Antagonist (10000 nmol/L) | Antagonist (5500 nmol/L) | Antagonist (3100 nmol/L) | |||
| Farnesyl diphosphate55, 71 | Antagonist (2100 nmol/L) | Antagonist (155 nmol/L, 4600 nmol/L) | Antagonist (1980 nmol/L) | Agonist (40 nmol/L) | ||
| Carba cyclic PA55 | Agonist | |||||
| OMPT49 | Agonist (68 nmol/L) | |||||
| Alkyl OMPT19, 64 | Agonist (790 nmol/L) | Agonist (62 nmol/L) | Agonist | |||
| α-fluoromethylene phosphonate51 | Weak agonist | Weak agonist | Agonist (0.5 nmol/L) | |||
| α-hydroxymethylene- phosphonate52 | Agonist (393 nmol/L) | |||||
| Compound 8bo121 | Agonist (9.1 nmol/L) | Agonist (123 nmol/L) | ||||
| Dialkyl thiophosphatidic acid 8:054, 55 | Agonist (695 nmol/L) | Agonist (5720 nmol/L) | Agonist (3 nmol/L) | Agonist | ||
| Antagonist (382 nmol/L) | Antagonist (184 nmol/L) | |||||
| Dodecylphosphate56 | Agonist (700 nmol/L) | Antagonist (90 nmol/L) | ||||
| α-methylene phosphonate52, 55 | Agonist (>281 nmol/L) | Weak agonist (3900 nmol/L) | Agonist | |||
| Antagonist (1420 nmol/L) | ||||||
| T1558 | Agonist (5 nmol/L) | Agonist (50 nmol/L) | ||||
| T1358 | Week agonist (500 nmol/L) | Agonist (0.5 nmol/L) | ||||
| Octadecenyl phosphate55 | Agonist (608 nmol/L) | Agonist | ||||
| NPSPA11, 43, 122 | Weak agonist (1850 nmol/L) | Weak agonist | Weak agonist (1600 nmol/L) | |||
| NPTyrPA43, 55, 122 | Antagonist (3450 nmol/L) | Weak agonist (11000 nmol/L) | Antagonist (5570 nmol/L) | Agonist | ||
| NAEPA11 | Agonist | Agonist | Weak agonist | |||
| Oleoyl-thiophosphate43 | Agonist (193 nmol/L) | Agonist (244 nmol/L) | Agonist (546 nmol/L) | |||
| LPM65 | Agonist | Agonist | Agonist | Agonist | Agonist |
Development of agonist and antagonist for S1P receptors
In contrast to LPA, the structure-activity relationship of S1P has a very short history. Using cloned S1P receptors, sphinganine-1-phosphate (dihydro-S1P) and sphingosylphosphorylcholine (SPC) were shown to be equipotent and far less potent on each S1P receptor36, 74, 75, 76.
S1P GPCR agonists
Following the cloning of the S1P receptors, the development of S1P agonists and antagonists began. The importance of the D-erythro configuration of S1P was demonstrated using the cloned receptors77. The linkage of the immune modulator FTY720 to S1P receptors, however, boosted this area of research and opened a new direction for S1P biology78, 79, 80. Lymphopenia induction by inhibiting lymphocyte egress from lymphoid organs was shown to be mediated through the S1P1 receptor81. High-throughput screening (HTS) of an available chemical library showed that SEW2871 acted as an in vivo active heterocyclic S1P1 selective agonist81, 82 and compound 26 was synthesized as a potent 3,5-diphenyl-12,4-oxadiazole S1P1 agonist83. Later, using ultra-HTS, 3,5-diaryloxadiaxole (CYM5181) and dicyclohexylamide were found to be selective agonists for S1P1 and S1P3, respectively84. Using computational modeling, CYM-5442 was developed as an S1P1 selective agonist that was more potent than CYM518185. AUY954, an aminocarboxylate analogue of FTY720, was also introduced as an S1P1 selective agonist86. VPC01091, a cyclized analogue of FTY720, was shown to act as an orally active S1P1 agonist and an S1P3 antagonist87. KRP-203 is a pro-drug immune modulator similar to FTY720; the phosphorylated form of KRP-203 was shown to be a selective S1P1 agonist88, 89. Constrained azacyclic analogues of FTY720 showed selective agonist activities on S1P4 and S1P5 receptors90. Finally, phytosphingosine-1-phosphate was shown to act as a potent and selective agonist on the S1P4 receptor76.
S1P GPCR antagonists
Suramin was temporarily used as an S1P3 antagonist75, 91. Human S1P5 was also reported to be sensitive to suramin and its analogue NF02392.
Following screening of an available chemical library, JTE-013, a pyrazopyridine derivative, was identified as an S1P2 antagonist93, 94. Modification of the FTY720-phosphate structure led to the development of VPC23019 and VPC25239 as selective S1P1/S1P3 antagonists95. As mentioned above, VPC01091 is an orally active S1P1 agonist and S1P3 antagonist87. W146, hexyl phenyl amide phosphonate, was found to be a selective S1P1 antagonist96. VPC44116, an octyl analogue of W146 and γ-aminophosphonate analogue of VPC23019, antagonized lymphopenia and lung permeability via the S1P1 receptor97. SB64146 was reported to act as an inverse agonist on the S1P1 receptor98. Ascotricins A and B were isolated from a cultured broth of a fungus identified as Ascotricha chartarum and shown to inhibit the S1P1 receptor and S1P-mediated HUVEC migration99. Sankyo Co synthesized compound lead 2 (CL2), 2-(4-ethoxyphenoxy)-5-(3-octadecyl-5-oxo-4,5-dihydro-1H-pyrazol-1-yl) benzenesulfonate, which antagonized the S1P1>S1P3>S1P2 receptors100. Human S1P1 receptor-selective antagonist and agonist effects of a rat monoclonal antibody (4B5.2) in vivo were also reported101. Using a 3D database search, BML-241, 2-alkylthiazolidine-4-carboxylic acid, was found to act as an S1P3 antagonist, but its selectivity and potency were not recapitulated in CHO-K1 cells expressing the S1P3 receptor102, 103. A pharmacophore-based design of an S1P3 antagonist with a 3,4-dialkyoxybenzophenone scaffold was suggested104.
Pharmacological tools for S1P GPCR signaling
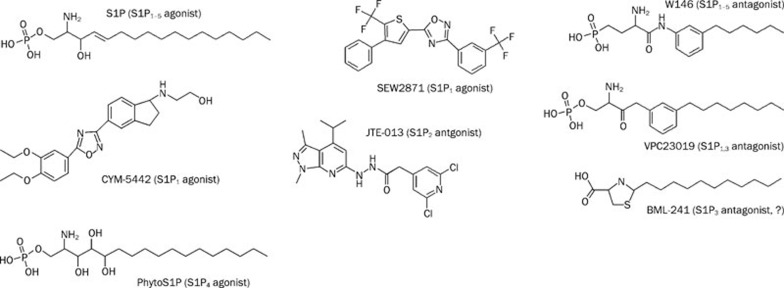
Commercially available tools for studying S1P receptor subtypes are highlighted in Figure 2. For S1P1 receptor signaling, CYM-5442 or SEW2871, both potent selective S1P1 agonists, and W146, a selective S1P1 antagonist, should be sufficient to elucidate S1P1 receptor involvement. S1P2 receptor signaling could be dissected using JTE-013, an S1P2 selective antagonist. For S1P3 GPCR signaling, a combined application of an S1P1,3 antagonist (VPC23019) and S1P1 antagonist (W146) or S1P1 agonist (CYM-5442) could be useful. Phytosphingosine 1-phosphate, an S1P4 selective agonist, could be used to study S1P4-mediated signaling. S1P1,3 antagonist (VPC23019)-insensitive, S1P2 antagonist (JTE-013)-insensitive, S1P4 agonist-non-responsive, and S1P- or FTY720-phosphate-sensitive signaling might be interpreted as S1P5 receptor or unidentified S1P receptor signaling (Table 2).
Figure 2.
Structures of commercially available agonists and antagonists for S1P GPCR signaling. Sources are Avanti polar lipid, Biomol international, Cayman chemical, Echelon bioscience, Enzo Life sciences, Sigma-Aldrich, and Tocris.
Table 2. Agonistic and antagonistic characters of each compound on each S1P receptor. Numbers (nmol/L) mean EC50 or KD values for agonists and IC50 or KI values for antagonists.
| Name | SIP1 | SIP2 | SIP3 | SIP4 | SIP5 |
|---|---|---|---|---|---|
| AUY95486 | Agonist (1.2 nmol/L) | Agonist (1210 nmol/L) | Agonist (340 nmol/L) | ||
| CYM-544285 | Agonist (1.2 nmol/L) | ||||
| CYM-518184 | Agonist (3.4 nmol/L) | ||||
| SEW287181 | Agonist (13 nmol/L) | ||||
| Compound 2683 | Agonist (0.6 nmol/L) | Agonist (12000 nmol/L) | Agonist (70 nmol/L) | Agonist (1.0 nmol/L) | |
| Compound 1290 | Agonist (7.4 nmol/L) | Agonist (10.2 nmol/L) | |||
| Compound 1890 | Agonist (16.8 nmol/L) | Agonist (5.8 nmol/L) | |||
| VPC4411697 | Antagonist (30 nmol/L) | Antagonist (300 nmol/L) | Partial agonist (33 nmol/L) | ||
| W14696, 106 | Antagonist (36 nmol/L) | ||||
| SB64914698 | Antagonist (300 nmol/L) | ||||
| VPC2301995 | Antagonist (13.8 nmol/L) | Antagonist (1175 nmol/L) | Agonist (263 nmol/L) | Partial agonist (85.1 nmol/L) | |
| VPC2523995 | Antagonist (13.4 nmol/L) | Antagonist (97.7 nmol/L) | Agonist (166 nmol/L) | Partial agonist (11.5 nmol/L) | |
| CL2100 | Antagonist (4400 nmol/L) | Antagonist (37000 nmol/L) | Antagonist (6700 nmol/L) | ||
| VPC01091-P87 | Agonist (6.6 nmol/L) | Antagonist | Agonist | Partial agonist | |
| Ascotricins A and B99 | Antagonist (8200 nmol/L, 1800 nmol/L) | ||||
| JTE-01393 | Antagonist (17 nmol/L) | ||||
| BML-241102, 103 | Antagonist (?) | ||||
| TY-52156123 | Antagonist (110 nmol/L) | ||||
| DS-SG-44124 | Agonist | Agonist | Agonist | ||
| KRP-203-P88, 89 | Agonist (0.8 nmol/L) | Agonist (9.6 nmol/L) | |||
| PhytoS1P76 | Agonist (1.6 nmol/L) | ||||
| DihydroS1P36, 75, 76 | Agonist | Agonist | Agonist | Agonist (8.6 nmol/L) | Agonist |
| SPC34, 36, 75 | Partial agonist | Partial agonist | Partial agonist | Partial agonist | Partial agonist |
| FTY720-P78, 79 | Agonist (6.3 nmol/L), functional antagonist | Agonist (4.0 nmol/L) | Agonist (6.3 nmol/L) | Agonist (6.3 nmol/L) | |
| AFD-R79 | Agonist (2.5 nmol/L) | Agonist (4.0 nmol/L) | Agonist (4.0 nmol/L) | Agonist (1.3 nmol/L) |
Development of drugs acting on lysophospholipid GPCRs
Prior to the molecular cloning of GPCRs for LPA and S1P, a number of studies were conducted with the primary goal of determining the functions of lipid mediators at both the cellular and the organ level. These functions included platelet aggregation, smooth muscle contraction, and cell proliferation, among others1, 2, 3, 4, 5, 6, 105. The discovery of GPCRs allowed signal transduction studies to proceed in cells over-expressing the receptors and in receptor knock-down transgenic mouse models106, 107, 108, 109. The discovery of the pathophysiological significance of LPA and S1P, particularly on each receptor subtype, would contribute favorably to new drug development. In developing new medications acting on LPA or S1P receptors, subtype selectivity would be a major issue along with potency and efficacy to avoid side effects and ensure drug safety.
FTY720 was initially developed as an immune modulator for organ transplant patients110. At present, this compound is under clinical study for the treatment of multiple sclerosis111. Amira Pharmaceuticals reported LPA1 selective antagonist (4′-{4-[(R)-1-(2-chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}-biphnyl-4-yl)-acetic acid, AP2966, which showed good therapeutic potential in idiopathic pulmonary fibrosis and good pharmacokinetic profiles, including oral bioavailability112. Pfizer global research and development introduced the S1P1 selective agonists PF-A and PF-B. These agonists resulted in lymphopenia in rats and monkeys similar to FTY720 and reduced collagen-induced arthritis113. Using a different approach, Lpath Inc. recently introduced monoclonal antibodies against S1P and LPA. Humanized anti-S1P monoclonal antibody (mAb) sonepcizumab blocked the tumorigenic effect of S1P produced by cancer cells as well as the angiogenic effect induced during pathological angiogenesis114, 115. The LPA receptor pan-antagonist (LPA1–4) and autotoxin inhibitor, α-bromomethylene phosphonate LPA analogue, was shown to be an excellent anti-cancer agent116, 117.
Closing remarks
Following the initial discoveries of LPA activity 30 years ago and S1P activity 15 years ago, a dark dawn era in lysophospholipid biology occurred due to the lack of identifiable targets. The discovery of the Edg-family GPCRs for LPA and S1P shined light on this field. The first meeting that focused on lysophospholipid biology was a New York Academy of Science meeting conducted in 1999 at Rockefeller University118. Since 2001, FASEB summer research conferences on lysophospholipids have been held biannually. Lysophospholipid receptor nomenclature has been systematically assigned119, and more LPA receptors (purinergic or non-Edg LPA receptors) are being discovered each year. Every two years, we have had exciting findings including the linkage of FTY720 to the S1P receptor, discovery of an autotoxin as a LPA-producing lysoPLD, lysolipid-sensitive proton-sensing GPCRs (OGR1 subfamily), and finally, the development of new chemicals. This review integrates accumulated information regarding pharmacological tools for lysophospholipid GPCR signaling to compare their characteristics and provides valuable information such as available chemical sources. Using a combination of receptor expression patterns in each organ or cell, these pharmacological tools might prove useful in defining the pathophysiological impact and significance of lysophospholipids. As mentioned above, the control of lysophopholipid functions using specific agonists or antagonists will contribute toward novel drug development. At a 2009 FASEB meeting in Carefree (Arizona, USA), we began to see several of the chemicals described here, in addition to FTY720, being used in clinical applications112, 113, 114, 116. We now know more about many things than ever before. It is very likely that in the near future, the agonists/antagonists for LPA or S1P receptors will be on the market commercially and that there will be a section on lysophospholipid GPCRs in every basic pharmacology textbook.
Acknowledgments
This work was supported by the National Research Foundation, 2010 Korea–Japan Joint Research Grant (2010-616-E00015).
References
- Gerrard JM, Kindom SE, Peterson DA, Peller J, Krantz KE, White JG. Lysophosphatidic acids. Influence on platelet aggregation and intracellular calcium flux. Am J Pathol. 1979;96:423–38. [PMC free article] [PubMed] [Google Scholar]
- Tokumura A, Fukuzawa K, Yamada S, Tsukatani H. Stimulatory effect of lysophosphatidic acids on uterine smooth muscles of non-pregant rats. Arch Int Pharmacodyn Ther. 1980;245:74–83. [PubMed] [Google Scholar]
- Simon MF, Chap H, Douste-Blazy L. Human platelet aggregation induced by 1-alkyl-lysophosphatidic acid and its analogs: a new group of phospholipid mediators. Biochem Biophys Res Commun. 1982;108:1743–50. doi: 10.1016/s0006-291x(82)80113-7. [DOI] [PubMed] [Google Scholar]
- Tsukatani H, Yamada S, Tokumura A, Miyamoto T, Takauchi K. Isolation of an acute hypotensive substance from bovine brain lipid fraction. Chem Pharm Bull (Tokyo) 1976;24:2294–300. doi: 10.1248/cpb.24.2294. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH. Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem. 1995;270:12949–52. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- Tigyi G, Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992;267:21360–7. [PubMed] [Google Scholar]
- Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–83. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273:7906–10. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274:27776–85. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- Im DS, Heise CE, Harding MA, George SR, O'Dowd BF, Theodorescu D, et al. Molecular cloning and characterization of a lysophosphatidic acid receptor, Edg-7, expressed in prostate. Mol Pharmacol. 2000;57:753–9. [PubMed] [Google Scholar]
- Lynch KR, Im DS. Life on the edg. Trends Pharmacol Sci. 1999;20:473–5. doi: 10.1016/s0165-6147(99)01401-7. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for Lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–6. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Dubin AE, Chun J. LPA4/GPR23 is an LPA receptor utilizing Gs, Gq/Gi-mediated calcium signaling and G12/13-mediated Rho activation. J Biol Chem. 2007;282:4310–7. doi: 10.1074/jbc.M610826200. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a New G12/13- and Gq-coupled Lysophosphatidic Acid Receptor That Increases cAMP, LPA5. J Biol Chem. 2006;281:23589–97. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, et al. Lysophosphatidic Acid Binds to and Activates Gpr92, a G Protein-Coupled Receptor Highly Expressed in Gastro-Intestinal Lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Ishii S, Hamano F, Noguchi K, Shimizu T. LPA4/p2y9/GPR23 mediates Rho-dependent morphological changes in a rat neuronal cell line. J Biol Chem. 2007;282:5814–24. doi: 10.1074/jbc.M610767200. [DOI] [PubMed] [Google Scholar]
- Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, et al. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329–34. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, et al. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem. 2009;284:17731–41. doi: 10.1074/jbc.M808506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861–6. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- Murakami M, Shiraishi A, Tabata K, Fujita N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2008;371:707–12. doi: 10.1016/j.bbrc.2008.04.145. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Herr D, Mutoh T, Chun J. Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol. 2009;9:15–23. doi: 10.1016/j.coph.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Okutani M, Arima N, Inoue A, Makide K, Aoki J.Identification of a novel receptor for lysophosphatidylserine FASEB Summer Research Conference 2009. Poster #24.
- Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–60. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature. 1996;380:634–6. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Goodemote KA, Mattie ME, Berger A, Spiegel S. Involvement of a pertussis toxin-sensitive G protein in the mitogenic signaling pathways of sphingosine 1-phosphate. J Biol Chem. 1995;270:10272–7. doi: 10.1074/jbc.270.17.10272. [DOI] [PubMed] [Google Scholar]
- van Koppen C, Meyer zu Heringdorf M, Laser KT, Zhang C, Jakobs KH, Bunemann M, et al. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J Biol Chem. 1996;271:2082–7. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- Bunemann M, Brandts B, zu Heringdorf DM, van Koppen CJ, Jakobs KH, Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by sphingosine-1-phosphate. J Physiol. 1995;489:701–7. doi: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F, Tomura H, Sho K, Kimura T, Sato K, Im DS, et al. Sphingosine 1-phosphate stimulates hydrogen peroxide generation through activation of phospholipase C-Ca2+ system in FRTL-5 thyroid cells: possible involvement of guanosine triphosphate-binding proteins in the lipid signaling. Endocrinology. 1997;138:220–9. doi: 10.1210/endo.138.1.4883. [DOI] [PubMed] [Google Scholar]
- Im DS, Fujioka T, Katada T, Kondo Y, Ui M, Okajima F. Characterization of sphingosine 1-phosphate-induced actions and its signaling pathways in rat hepatocytes. Am J Physiol. 1997;272:G1091–9. doi: 10.1152/ajpgi.1997.272.5.G1091. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–5. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- An S, Bleu T, Huang W, Hallmark OG, Coughlin SR, Goetzl EJ. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–82. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takuwa N, Yatomi Y, Gonda K, Shigematsu H, Takuwa Y. EDG3 is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signaling characteristics distinct from EDG1 and AGR16. Biochem Biophys Res Commun. 1999;260:203–8. doi: 10.1006/bbrc.1999.0886. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Graler MH, Bernhardt G, Hobson JP, Lipp M, Spiegel S. Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood. 2000;95:2624–9. [PubMed] [Google Scholar]
- Yamazaki Y, Kon J, Sato K, Tomura H, Sato M, Yoneya T, et al. Edg-6 as a putative sphingosine 1-phosphate receptor coupling to Ca2+ signaling pathway. Biochem Biophys Res Commun. 2000;268:583–9. doi: 10.1006/bbrc.2000.2162. [DOI] [PubMed] [Google Scholar]
- Im DS, Heise CE, Ancellin N, O'Dowd BF, Shei GJ, Heavens RP, et al. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J Biol Chem. 2000;275:14281–6. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- Lynch KR, Macdonald TL. Structure-activity relationships of lysophosphatidic acid analogs. Biochim Biophys Acta. 2002;1582:289–94. doi: 10.1016/s1388-1981(02)00183-x. [DOI] [PubMed] [Google Scholar]
- Jalink K, Hengeveld T, Mulder S, Postma FR, Simon MF, Chap H, et al. Lysophosphatidic acid-induced Ca2+ mobilization in human A431 cells: structure-activity analysis. Biochem J. 1995;307:609–16. doi: 10.1042/bj3070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguen G, Gaige B, Grevy JM, Rogalle P, Bellan J, Wilson M, et al. Structure-activity analysis of the effects of lysophosphatidic acid on platelet aggregation. Biochemistry. 1999;38:8440–50. doi: 10.1021/bi9816756. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Tokumura A, Gregory L, Nouchi T, Weintraub ST, Hanahan DJ. Biochemical characterization of the interaction of lipid phosphoric acids with human platelets: comparison with platelet activating factor. Arch Biochem Biophys. 1994;311:358–68. doi: 10.1006/abbi.1994.1249. [DOI] [PubMed] [Google Scholar]
- Liliom K, Bittman R, Swords B, Tigyi G. N-palmitoyl-serine and N-palmitoyl-tyrosine phosphoric acids are selective competitive antagonists of the lysophosphatidic acid receptors. Mol Pharmacol. 1996;50:616–23. [PubMed] [Google Scholar]
- Hooks SB, Ragan SP, Hopper DW, Honemann CW, Durieux ME, Macdonald TL, et al. Characterization of a receptor subtype-selective lysophosphatidic acid mimetic. Mol Pharmacol. 1998;53:188–94. doi: 10.1124/mol.53.2.188. [DOI] [PubMed] [Google Scholar]
- Durgam GG, Virag T, Walker MD, Tsukahara R, Yasuda S, Liliom K, et al. Synthesis, structure-activity relationships, and biological evaluation of fatty alcohol phosphates as lysophosphatidic acid receptor ligands, activators of PPARgamma, and inhibitors of autotaxin. J Med Chem. 2005;48:4919–30. doi: 10.1021/jm049609r. [DOI] [PubMed] [Google Scholar]
- Lynch KR, Hopper DW, Carlisle SJ, Catalano JG, Zhang M, MacDonald TL. Structure/activity relationships in lysophosphatidic acid: the 2-hydroxyl moiety. Mol Pharmacol. 1997;52:75–81. doi: 10.1124/mol.52.1.75. [DOI] [PubMed] [Google Scholar]
- Proll MA, Clark RB. Potent Gi-mediated inhibition of adenylyl cyclase by a phosphonate analog of monooleylphosphatidate. Mol Pharmacol. 1991;39:740–4. [PubMed] [Google Scholar]
- van der Bend RL, Brunner J, Jalink K, van Corven EJ, Moolenaar WH, van Blitterswijk WJ. Identification of a putative membrane receptor for the bioactive phospholipid, lysophosphatidic acid. EMBO J. 1992;11:2495–501. doi: 10.1002/j.1460-2075.1992.tb05314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Casey G, Mills GB. Effect of lysophospholipids on signaling in the human Jurkat T cell line. J Cell Physiol. 1995;163:441–50. doi: 10.1002/jcp.1041630303. [DOI] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett. 2000;478:159–65. doi: 10.1016/s0014-5793(00)01827-5. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Erickson JR, Goddard GJ, Yu S, Liu S, Cheng KW, et al. Identification of a phosphothionate analogue of lysophosphatidic acid (LPA) as a selective agonist of the LPA3 receptor. J Biol Chem. 2003;278:11962–9. doi: 10.1074/jbc.M209168200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Qian L, Prestwich GD. Synthesis of monofluorinated analogues of lysophosphatidic acid. J Org Chem. 2003;68:5320–30. doi: 10.1021/jo020729l. [DOI] [PubMed] [Google Scholar]
- Xu Y, Aoki J, Shimizu K, Umezu-Goto M, Hama K, Takanezawa Y, et al. Structure-activity relationships of fluorinated lysophosphatidic acid analogues. J Med Chem. 2005;48:3319–27. doi: 10.1021/jm049186t. [DOI] [PubMed] [Google Scholar]
- Jiang G, Xu Y, Fujiwara Y, Tsukahara T, Tsukahara R, Gajewiak J, et al. Alpha-substituted phosphonate analogues of lysophosphatidic acid (LPA) selectively inhibit production and action of LPA. Chem Med Chem. 2007;2:679–90. doi: 10.1002/cmdc.200600280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos WL, Heasley BH, Jarosz R, Carter KM, Lynch KR, Macdonald TL. Synthesis and biological evaluation of phosphonic and thiophosphoric acid derivatives of lysophosphatidic acid. Bioorg Med Chem Lett. 2004;14:3473–6. doi: 10.1016/j.bmcl.2004.04.061. [DOI] [PubMed] [Google Scholar]
- Durgam GG, Tsukahara R, Makarova N, Walker MD, Fujiwara Y, Pigg KR, et al. Synthesis and pharmacological evaluation of second-generation phosphatidic acid derivatives as lysophosphatidic acid receptor ligands. Bioorg Med Chem Lett. 2006;16:633–40. doi: 10.1016/j.bmcl.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W, et al. Unique Ligand Selectivity of the GPR92/LPA5 Lysophosphatidate Receptor Indicates Role in Human Platelet Activation. J Biol Chem. 2009;284:17304–19. doi: 10.1074/jbc.M109.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag T, Elrod DB, Liliom K, Sardar VM, Parrill AL, Yokoyama K, et al. Fatty alcohol phosphates are subtype-selective agonists and antagonists of lysophosphatidic acid receptors. Mol Pharmacol. 2003;63:1032–42. doi: 10.1124/mol.63.5.1032. [DOI] [PubMed] [Google Scholar]
- Gajewiak J, Tsukahara R, Tsukahara T, Fujiwara Y, Yu S, Lu Y, et al. Alkoxymethylenephosphonate analogues of (Lyso) phosphatidic acid stimulate signaling networks coupled to the LPA2 receptor. Chem Med Chem. 2007;2:1789–98. doi: 10.1002/cmdc.200700111. [DOI] [PubMed] [Google Scholar]
- Tamaruya Y, Suzuki M, Kamura G, Kanai M, Hama K, Shimizu K, et al. Identifying specific conformations by using a carbohydrate scaffold: discovery of subtype-selective LPA-receptor agonists and an antagonist. Angew Chem Int Ed Engl. 2004;43:2834–7. doi: 10.1002/anie.200454065. [DOI] [PubMed] [Google Scholar]
- Gududuru V, Zeng K, Tsukahara R, Makarova N, Fujiwara Y, Pigg KR, et al. Identification of Darmstoff analogs as selective agonists and antagonists of lysophosphatidic acid receptors. Bioorg Med Chem Lett. 2006;16:451–6. doi: 10.1016/j.bmcl.2005.08.096. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tanaka M, Arai H, Aoki J, Prestwich GD. Alkyl lysophosphatidic acid and fluoromethylene phosphonate analogs as metabolically-stabilized agonists for LPA receptors. Bioorg Med Chem Lett. 2004;14:5323–8. doi: 10.1016/j.bmcl.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Hooks SB, Santos WL, Im DS, Heise CE, Macdonald TL, Lynch KR. Lysophosphatidic acid-induced mitogenesis is regulated by lipid phosphate phosphatases and is Edg-receptor independent. J Biol Chem. 2001;276:4611–21. doi: 10.1074/jbc.M007782200. [DOI] [PubMed] [Google Scholar]
- Oh DY, Yoon JM, Moon MJ, Hwang JI, Choe H, Lee JY, et al. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem. 2008;283:21054–64. doi: 10.1074/jbc.M708908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, et al. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem. 2006;281:22786–93. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Xu Y, Simper T, Jiang G, Aoki J, Umezu-Goto M, et al. Phosphorothioate analogues of alkyl lysophosphatidic acid as LPA3 receptor-selective agonists. ChemMedChem. 2006;1:376–83. doi: 10.1002/cmdc.200500042. [DOI] [PubMed] [Google Scholar]
- Endo T, Kano K, Motoki R, Hama K, Okudaira S, Ishida M, et al. Lysophosphatidylmethanol is a pan lysophosphatidic acid receptor agonist and is produced by autotaxin in blood. J Biochem. 2009;146:283–93. doi: 10.1093/jb/mvp068. [DOI] [PubMed] [Google Scholar]
- Heise CE, Santos WL, Schreihofer AM, Heasley BH, Mukhin YV, Macdonald TL, et al. Activity of 2-substituted lysophosphatidic acid (LPA) analogs at LPA receptors: discovery of a LPA1/LPA3 receptor antagonist. Mol Pharmacol. 2001;60:1173–80. doi: 10.1124/mol.60.6.1173. [DOI] [PubMed] [Google Scholar]
- Heasley BH, Jarosz R, Lynch KR, Macdonald TL. Initial structure-activity relationships of lysophosphatidic acid receptor antagonists: discovery of a high-affinity LPA1/LPA3 receptor antagonist. Bioorg Med Chem Lett. 2004;14:2735–40. doi: 10.1016/j.bmcl.2004.03.076. [DOI] [PubMed] [Google Scholar]
- Heasley BH, Jarosz R, Carter KM, Van SJ, Lynch KR, Macdonald TL. A novel series of 2-pyridyl-containing compounds as lysophosphatidic acid receptor antagonists: development of a nonhydrolyzable LPA3 receptor-selective antagonist. Bioorg Med Chem Lett. 2004;14:4069–74. doi: 10.1016/j.bmcl.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Fischer DJ, Nusser N, Virag T, Yokoyama K, Wang D, Baker DL, et al. Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol Pharmacol. 2001;60:776–84. [PubMed] [Google Scholar]
- Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- Liliom K, Tsukahara T, Tsukahara R, Zelman-Femiak M, Swiezewska E, Tigyi G. Farnesyl phosphates are endogenous ligands of lysophosphatidic acid receptors: inhibition of LPA GPCR and activation of PPARs. Biochim Biophys Acta. 2006;1761:1506–14. doi: 10.1016/j.bbalip.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fells JI, Tsukahara R, Fujiwara Y, Liu J, Perygin DH, Osborne DA, et al. Identification of non-lipid LPA3 antagonists by virtual screening. Bioorg Med Chem. 2008;16:6207–17. doi: 10.1016/j.bmc.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich GD, Gajewiak J, Zhang H, Xu X, Yang G, Serban M. Phosphatase-resistant analogues of lysophosphatidic acid: agonists promote healing, antagonists and autotaxin inhibitors treat cancer. Biochim Biophys Acta. 2008;1781:588–94. doi: 10.1016/j.bbalip.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS, Clemens J, Macdonald TL, Lynch KR. Characterization of the human and mouse sphingosine 1-phosphate receptor, S1P5 (Edg-8): structure-activity relationship of sphingosine1-phosphate receptors. Biochemistry. 2001;40:14053–60. doi: 10.1021/bi011606i. [DOI] [PubMed] [Google Scholar]
- Ancellin N, Hla T. Differential pharmacological properties and signal transduction of the sphingosine 1-phosphate receptors EDG-1, EDG-3, and EDG-5. J Biol Chem. 1999;274:18997–9002. doi: 10.1074/jbc.274.27.18997. [DOI] [PubMed] [Google Scholar]
- Candelore MR, Wright MJ, Tota LM, Milligan J, Shei GJ, Bergstrom JD, et al. Phytosphingosine 1-phosphate: a high affinity ligand for the S1P4/Edg-6 receptor. Biochem Biophys Res Commun. 2002;297:600–6. doi: 10.1016/s0006-291x(02)02237-4. [DOI] [PubMed] [Google Scholar]
- Lim HS, Park JJ, Ko K, Lee MH, Chung SK. Syntheses of sphingosine-1-phosphate analogues and their interaction with EDG/S1P receptors. Bioorg Med Chem Lett. 2004;14:2499–503. doi: 10.1016/j.bmcl.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–9. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Im DS. Linking Chinese medicine and G-protein-coupled receptors. Trends Pharmacol Sci. 2003;24:2–4. doi: 10.1016/s0165-6147(02)00012-3. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–48. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, et al. S1P1-selective in vivo active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–15. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Li Z, Chen W, Hale JJ, Lynch CL, Mills SG, Hajdu R, et al. Discovery of potent 3,5-diphenyl-1,2,4-oxadiazole sphingosine-1-phosphate-1 (S1P1) receptor agonists with exceptional selectivity against S1P2 and S1P3. J Med Chem. 2005;48:6169–73. doi: 10.1021/jm0503244. [DOI] [PubMed] [Google Scholar]
- Schurer SC, Brown SJ, Gonzalez-Cabrera PJ, Schaeffer MT, Chapman J, Jo E, et al. Ligand-binding pocket shape differences between sphingosine 1-phosphate (S1P) receptors S1P1 and S1P3 determine efficiency of chemical probe identification by ultrahigh-throughput screening. ACS Chem Biol. 2008;3:486–98. doi: 10.1021/cb800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Jo E, Sanna MG, Brown S, Leaf N, Marsolais D, et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol. 2008;74:1308–18. doi: 10.1124/mol.108.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Mi Y, Pally C, Beerli C, Chen A, Guerini D, et al. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem Biol. 2006;13:1227–34. doi: 10.1016/j.chembiol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Zhu R, Snyder AH, Kharel Y, Schaffter L, Sun Q, Kennedy PC, et al. Asymmetric synthesis of conformationally constrained fingolimod analogues discovery of an orally active sphingosine 1-phosphate receptor type-1 agonist and receptor type-3 antagonist. J Med Chem. 2007;50:6428–35. doi: 10.1021/jm7010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro J, Kudou S, Iwai S, Takahashi M, Hakamata Y, Kinoshita M, et al. Use of sphingosine-1-phosphate 1 receptor agonist, KRP-203, in combination with a subtherapeutic dose of cyclosporine A for rat renal transplantation. Transplantation. 2006;82:804–12. doi: 10.1097/01.tp.0000232687.78242.cd. [DOI] [PubMed] [Google Scholar]
- Song J, Matsuda C, Kai Y, Nishida T, Nakajima K, Mizushima T, et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther. 2008;324:276–83. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- Hanessian S, Charron G, Billich A, Guerini D. Constrained azacyclic analogues of the immunomodulatory agent FTY720 as molecular probes for sphingosine 1-phosphate receptors. Bioorg Med Chem Lett. 2007;17:491–4. doi: 10.1016/j.bmcl.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Himmel HM, Meyer Zu Heringdorf D, Graf E, Dobrev D, Kortner A, Schuler S, et al. Evidence for Edg-3 receptor-mediated activation of I(K.ACh) by sphingosine-1-phosphate in human atrial cardiomyocytes. Mol Pharmacol. 2000;58:449–54. doi: 10.1124/mol.58.2.449. [DOI] [PubMed] [Google Scholar]
- Niedernberg A, Scherer CR, Busch AE, Kostenis E. Comparative analysis of human and rat S1P5 (edg8): differential expression profiles and sensitivities to antagonists. Biochem Pharmacol. 2002;64:1243–50. doi: 10.1016/s0006-2952(02)01289-3. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Satoh H, Yanase M, Inoue Y, Tomiya T, Arai M, et al. Antiproliferative property of sphingosine 1-phosphate in rat hepatocytes involves activation of Rho via Edg-5. Gastroenterology. 2003;124:459–69. doi: 10.1053/gast.2003.50049. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, et al. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res. 2003;58:170–7. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–41. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–41. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- Foss FW Jr, Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, et al. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem. 2007;15:663–77. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CM, Long J, Gorshkova I, Fujiwara Y, Connell M, Belmonte KE, et al. Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the sphingosine 1-phosphate receptor-1. FASEB J. 2006;20:509–11. doi: 10.1096/fj.05-4810fje. [DOI] [PubMed] [Google Scholar]
- Yonesu K, Ohnuki T, Ono Y, Takatsu T, Nara F. Ascotricins A and B, novel antagonists of sphingosine-1-phosphate receptor 1 from Ascotricha chartarum Berk. SANK 14186. J Antibiot (Tokyo) 2009;62:359–64. doi: 10.1038/ja.2009.40. [DOI] [PubMed] [Google Scholar]
- Yonesu K, Kawase Y, Inoue T, Takagi N, Tsuchida J, Takuwa Y, et al. Involvement of sphingosine-1-phosphate and S1P1 in angiogenesis: analyses using a new S1P1 antagonist of non-sphingosine-1-phosphate analog. Biochem Pharmacol. 2009;77:1011–20. doi: 10.1016/j.bcp.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Dembrow D, Van Brocklyn JR, Graler M, Huang MC. An IgM-kappa rat monoclonal antibody specific for the type 1 sphingosine 1-phosphate G protein-coupled receptor with antagonist and agonist activities. Immunol Lett. 2004;93:63–9. doi: 10.1016/j.imlet.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Koide Y, Hasegawa T, Takahashi A, Endo A, Mochizuki N, Nakagawa M, et al. Development of novel EDG3 antagonists using a 3D database search and their structure-activity relationships. J Med Chem. 2002;45:4629–38. doi: 10.1021/jm020080c. [DOI] [PubMed] [Google Scholar]
- Jongsma M, Hendriks-Balk MC, Michel MC, Peters SL, Alewijnse AE. BML-241 fails to display selective antagonism at the sphingosine-1-phosphate receptor, S1P3. Br J Pharmacol. 2006;149:277–82. doi: 10.1038/sj.bjp.0706872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide Y, Uemoto K, Hasegawa T, Sada T, Murakami A, Takasugi H, et al. Pharmacophore-based design of sphingosine 1-phosphate-3 receptor antagonists that include a 3,4-dialkoxybenzophenone scaffold. J Med Chem. 2007;50:442–54. doi: 10.1021/jm060834d. [DOI] [PubMed] [Google Scholar]
- Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–67. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem. 2009;78:743–68. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol. 2008;160:25–46. doi: 10.1007/112_0507. [DOI] [PubMed] [Google Scholar]
- Skoura A, Hla T. Lysophospholipid receptors in vertebrate development, physiology, and pathology. J Lipid Res. 2009;50:S293–8. doi: 10.1194/jlr.R800047-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–54. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Lynch KR. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr Opin Immunol. 2002;14:569–75. doi: 10.1016/s0952-7915(02)00374-6. [DOI] [PubMed] [Google Scholar]
- Brown BA, Kantesaria PP, McDevitt LM. Fingolimod: a novel immunosuppressant for multiple sclerosis. Ann Pharmacother. 2007;41:1660–8. doi: 10.1345/aph.1G424. [DOI] [PubMed] [Google Scholar]
- Swaney JS, Chapman C, Correa LD, Bain G, Bundey RA, Prodanovich PP, et al. A novel, small molecule, LPA1 receptor-selective antagonist, inhibits bleomycin-induced inflammation and fibrosis in the mouse. FASEB Summer Research Conference. 2009.
- Nagiec M, Funckes-Shippy C, Hiebsch R, Radi Z, Walker J, Gierse J, et al. Targeting S1P signaling for inflammation. FASEB Summer Research Conference. 2009.
- Sabbadini R, Cavalli A, Wocjiak J, Visentin B, Matteo R, Campbell M, et al. Antibodies against bioactive lipids. FASEB Summer Research Conference. 2009.
- Caballero S, Swaney J, Moreno K, Afzal A, Kielczewski J, Stoller G, et al. Anti-sphingosine-1-phosphate monoclonal antibodies inhibit angiogenesis and sub-retinal fibrosis in a murine model of laser-induced choroidal neovascularization. Exp Eye Res. 2009;88:367–77. doi: 10.1016/j.exer.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich GD. Dual activity LPA receptor pan-antagonist/antotaxin inhibitors as anti-cancer agents: evaluation in vivo using engineered human tumors. FASEB Summer Research Conference. 2009. [DOI] [PMC free article] [PubMed]
- Zhang H, Xu X, Gajewiak J, Tsukahara R, Fujiwara Y, Liu J, et al. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 2009;69:5441–9. doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Lynch KR. Preface: the omnific lysophospholipid growth factors. Ann N Y Acad Sci. 2000;905:xi–xiv. doi: 10.1111/j.1749-6632.2000.tb06531.x. [DOI] [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, et al. International Union of Pharmacology. XXXIV. Lysophospholipid Receptor Nomenclature. Pharmacol Rev. 2002;54:265–9. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Fells JI, Tsukahara R, Liu J, Tigyi G, Parrill AL. Structure-based drug design identifies novel LPA3 antagonists. Bioorg Med Chem. 2009;17:7457–64. doi: 10.1016/j.bmc.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli MH, Abele S, Binkert C, Bravo R, Buchmann S, Bur D, et al. 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem. 2010;53:4198–211. doi: 10.1021/jm100181s. [DOI] [PubMed] [Google Scholar]
- An S, Bleu T, Zheng Y, Goetzl EJ. Recombinant human G protein-coupled lysophosphatidic acid receptors mediate intracellular calcium mobilization. Mol Pharmacol. 1998;54:881–8. doi: 10.1124/mol.54.5.881. [DOI] [PubMed] [Google Scholar]
- Murakami A, Takasugi H, Ohnuma S, Koide Y, Sakurai A, Takeda S, et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: investigation based on a new S1P3 receptor antagonist. Mol Pharmacol. 2010;77:704–13. doi: 10.1124/mol.109.061481. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim YL, Sacket SJ, Kim HL, Han M, Park DS, et al. Sphingosine 1-phosphate (S1P) induces shape change in rat C6 glioma cells through the S1P2 receptor: development of an agonist for S1P receptors. J Pharm Pharmacol. 2007;59:1035–41. doi: 10.1211/jpp.59.7.0017. [DOI] [PubMed] [Google Scholar]