Abstract
Hepatocellular carcinoma (HCC) is among the most lethal of human malignancies. During human multistep hepatocarcinogenesis, genomic gain represents an important mechanism in the activation of proto-oncogenes. In many circumstances, activated oncogenes hold clinical implications both as prognostic markers and targets for cancer therapeutics. Gain of chromosome 1q copy is one of the most frequently detected alterations in HCC and 1q21 is the most frequent minimal amplifying region (MAR). A better understanding of the physiological and pathophysiological roles of target genes within 1q21 amplicon will significantly improve our knowledge in HCC pathogenesis, and may lead to a much more effective management of HCC bearing amplification of 1q21. Such knowledge has long term implications for the development of new therapeutic strategies for HCC treatment. Our research group and others, focused on the identification and characterization of 1q21 target genes such as JTB, CKS1B, and CHD1L in HCC progression. In this review, we will summarize the current scientific knowledge of known target genes within 1q21 amplicon and the precise oncogenic mechanisms of CHD1L will be discussed in detail.
Keywords: amplification, 1q21, oncogene, CHD1L, hepatocellular carcinoma
Introduction
Primary liver cancer mainly refers to hepatocellular carcinoma (HCC), cholangiocarcinoma, and angiosarcoma. As the fifth most common neoplasm, HCC accounts for 85% to 95% of all primary liver cancers1 and ranks as the third-leading cause of cancer-related deaths worldwide2, 3, 4. The causes of HCC are perhaps better understood than those of any other major human cancer. The main risk factors include infections of hepatitis B virus (HBV) and hepatitis C virus (HCV), and intake of aflatoxin B1 (AFB1), which are responsible for about 80% HCC cases5. Other etiological factors, such as microcystin-contaminated drinking water, tobacco smoking, non-alcoholic steatohepatitis resulting from obesity and/or diabetes, and inherited metabolic diseases such as hereditary hemochromatosis6, 7, 8, have also been proposed to lead to HCC, although at a lower frequency. The incidence of HCC also increases progressively with age, suggesting that the accumulation of genetic alterations over time contributes to HCC development. HCC has a very poor prognosis, with a life expectancy of about 6 months from time of diagnosis, and a <3% 5-year survival rate for untreated cancer. Death often occurs due to liver failure associated with cirrhosis and/or rapid outgrowth of multilobular HCC. Although early HCC could be cured by surgical resection, the fact that many tumors are asymptomatic means that most patients at risk for HCC will not be diagnosed in time. These patients often present with inoperable HCC, usually with the large and/or multiple HCC nodules.
Detection of recurrent alterations in HCC
Genomic abnormalities play important roles in carcinogenesis. Over last several decades, the importance of chromosome aberrations in pathogenesis of cancer has been widely elucidated9. Like most cancers, HCC is a heterogeneous tumor with a complex variety of genetic changes10. Progressive genetic alteration causes a spectrum of events initiating with cell proliferation and progression from hyperplasia to dysplasia and ultimately to cancer11, 12. This model has become widely accepted and is applicable to many types of cancers, including HCC. The molecular analysis of HCC clarifies that the multistage process of hepatocarcinogenesis is complex and depends on a combination of genetic, viral, and environmental factors. In cancer cells, chromosomes are broken, truncated, deleted, amplified or translocated to other chromosomes. Some of aberrations are specific for tumorigenesis, whereas most of them happen randomly. Chromosomal abnormalities may lead to the inactivation of tumor suppressor genes (TSGs) or activation of oncogenes via amplification. As listed in Table 1, chromosomal losses are frequently detected in HCC patients at 1p (36%–37%), 4q (32%–70%), 6q (19%–37%), 8p (26%–77%), 13q (16%–55%), 16p (14%–70%), and 17p (10%–60%) while gains are often detected at 1q (46%–86%), 6p (20%–33%), 8q (31%–83%), 17q (29%–48%), and 20q (5%–37%)13, 14, 15, 16, 17, 18, 19, 20, 21, 22. In particular, gain of chromosome 1q21–23 and 8q22–24 has been associated with the early development of HCC16, 23, whereas gain of 3q has been linked to tumor recurrence and poor overall patient survival24. In addition, deletion of 8p has been correlated with HCC metastasis25.
Table 1. Summary of chromosomal gains and losses in hepatocellular carcinoma.
| Detected By Comparative Genomic Hybridization (CGH) |
By EIM |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chr | Marchio et al13 | Kusano et al14 | Sakakura et al15 | Wong et al16 | Guan et al17 | Chang et al18 | Niketeghad et al19 | Shiraishi et al20 | Balsara et al21 | Midorikara et al22 |
| Gains | ||||||||||
| 1q | 58 | 78 | 46 | 72 | 66 | 86 | 57 | 77 | 73 | 74 |
| 6p | 33 | 20 | ||||||||
| 8q | 60 | 66 | 31 | 48 | 48 | 77 | 52 | 52 | 83 | 48 |
| 17q | 33 | 43 | 30 | 29 | 48 | |||||
| 20q | 23 | 14 | 37 | 20 | 5 | 29 | 22 | |||
| Losses | ||||||||||
| 1p | 37 | 36 | ||||||||
| 4q | 70 | 32 | 48 | 43 | 40 | 59 | 33 | 35 | 40 | 48 |
| 6q | 37 | 23 | 19 | |||||||
| 8p | 65 | 29 | 28 | 37 | 32 | 77 | 26 | 44 | 35 | |
| 13q | 37 | 37 | 20 | 37 | 16 | 27 | 19 | 55 | 32 | |
| 16p | 54 | 46 | 33 | 30 | 70 | 50 | 14 | 52 | 63 | |
| 17p | 51 | 51 | 37 | 10 | 52 | 45 | 52 | 60 | 29 | |
Value means percentage of positive region. The number in square bracket corresponds with reference number.
Abbreviation: EIM, expression imbalance map.
Identification of minimal amplified region on chromosome 1q
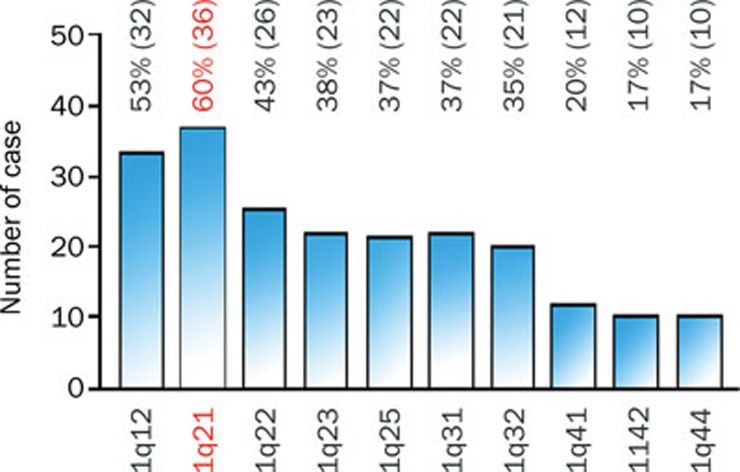
Using comparative genomic hybridization (CGH), we and other groups, have demonstrated that copy number gain of chromosome 1q is one of the most frequently detected alterations in HCC13, 14, 15, 16, 17, 18, 19, 20, 21, 22 and has been suggested as an early genomic alteration during HCC progression26. Several minimal amplified regions (MARs) on 1q were mapped including 1q12–q2217, 25, 1q23.3–q25.327 and 1q23.1–1q4328. Gains of 1q21–q25 have been linked to a more aggressive phenotype with metastatic potential in renal clear cell carcinoma and squamous cell carcinomas of lung29, 30. In ovarian cancer and neuroblastoma, 1q21–q22 amplification has been suggested to be correlated with a drug-resistant phenotype31, 32. In HCC, regional 1q21–q22 gains were found in 40% of advanced metastatic HCC cases26. In the past three years, our research group showed that 1q21 was the most frequently amplified region in chromosome 1q and its amplification has been detected in 36/60 (60%) of HCC specimens33 (Figure 1). This implies that 1q21 may harbor one or more oncogenes whose overexpression caused by amplification plays an important role in HCC pathogenesis.
Figure 1.
Amplification of different regions along chromosome 1q in 60 clinical HCC specimens. Amplification of 1q21 is most frequent chromosomal alteration in chromosome 1q in HCC.
Identification of cancer-related genes within 1q21 amplicon
In human solid tumors, the copy number alterations are believed to contribute to the tumorigenesis by affecting the functions of cancer-related genes in the altered chromosomal regions34. In HCC, isolation and identification of putative cancer-related oncogenes can improve our understanding of hepatocarcinogenesis. Inagaki et al reported a novel 1q21 amplicon in HCC-derived cell lines using high-density single nucleotide polymorphism (SNP) array35. Using fluorescence in situ hybridization (FISH) and real-time quantitative PCR, they identified three candidate targets CREB3L4, INTS3, and SNAPAP within 700-kb amplified 1q21 region and found these targets were all significantly overexpressed in tumors from HCC patients35 (Table 2). Wong et al found that the expression levels of JTB and SHC1 in 1q21 amplicon were significantly higher than the paired adjacent non-malignant liver tissues36 (Table 2). In addition, Midorikawa et al. demonstrated that a regional 1q21-23 gain (74%) was significantly associated with HCC by expression imbalance map (EIM) analysis which can detect mRNA expression imbalance linked to chromosomal regions, and they also identified two candidate genes SHC1 and CKS1B within 1q21 region22. Recently, our research group isolated a novel candidate oncogene CHD1L by using microdissected DNA from 1q21 and hybrid selection33. We found that amplification of CHD1L at genomic level and overexpression of CHD1L at protein level were detected in 50.6% (86/170) and 52.4% (163/311) of informative HCC tissues, respectively33 (Table 2).
Table 2. Candidate target genes in 1q21 amplicon in HCC.
| Gene | Location | Function | Overexpression in tumora | Reference |
|---|---|---|---|---|
| CREB3L4 | 1q21.3 | Androgen receptor signaling | 78% (14/18) | Inagaki et al35; Qi et al40 |
| INTS3 | 1q21.3 | Genome instability; DNA damage response | 78% (14/18) | Inagaki et al35; Zhang et al59; Huang et al60 |
| SNAPAP | 1q21.3 | Control exocytosis processes | 72% (13/18) | Inagaki et al35; Ruder et al58 |
| JTB | 1q21.3 | Apoptosis resistance | Pan et al41; Wong et al36; Kanome et al42 | |
| SHC1 | 1q21.3 | Cell proliferation; apoptosis; tyrosine phosphorylation; | Wong et al36; Huebner et al43; | |
| signaling | Kraut-Cohen et al44; Pinton et al45 | |||
| CKS1B | 1q21.2 | Cell cycle regulation | 63% (30/48) | Midorikawa et al22; Wang et al47; |
| Ganoth et al48; Shen et al50; Calvisi et al51 | ||||
| CHD1L | 1q21.1 | Cell proliferation; apoptosis; transcription regulation; | 52% (163/311) | Ma et al33; Ahel et al54; Chen et al56; |
| invasion and metastasis; DNA repair | Chen et al57 |
The percentage of HCC cases with higher expression of target gene than their adjacent nontumor tissues.
Multistep progression of hepatocarcinogenesis
The development and progression of HCC is typical of a multistage process. Malignant hepatocytes result from step-by-step changes that accumulate in mature hepatocytes or can be derived from stem cells37. The most widely accepted hypothesis describes a sequential process in which this stepwise transformation begins in the liver tissue that undergoing chronic hepatitis or cirrhosis caused by external stimuli (HBV or HCV infection, alcohol or metabolic diseases), progresses through a series of hyperplastic and dysplastic stages (low- and high-grade dysplastic) stages, and progresses further to become more malignant, making metastases possible38, 39. This malignant transformation of a normal cell into a cancer cell is thought to be caused by aberrant gene expression critical to cellular processes such as cell cycle control, cell growth, differentiation, apoptosis, cell adhesion and other functions at the cellular, molecular, and genetic levels. The tumor cells of early HCC are well differentiated and highly proliferative, and they go on to form less differentiated HCCs that frequently show a nodule-in-nodule appearance, a signature of advanced HCC. At this advanced stage, angiogenesis, tissue invasion, and metastases occur in up to 25% of cases37. Later on, the neoplastic cells become undifferentiated and are able to invade vessels and spread outside the liver, characteristics that define end-stage disease.
Roles of oncogenes in 1q21 during hepatocarcinogenesis
During human multistep hepatocarcinogenesis, DNA amplification represents an important mechanism in the activation of proto-oncogenes. In many circumstances, activated oncogenes hold clinical implications both as prognostic markers and targets for cancer therapeutics. Next, we will summarize the current findings of known target genes within 1q21 amplicon in HCC, and the role of a novel oncogene CHD1L identified by our laboratory during hepatocarcinogenesis will be described in detail.
CREB3L4, INT3, SNAPAP, and JTB
CREB3L4 (cyclic AMP responsive element binding protein3-like 4), belongs to the CREB/ATF family of transcriptional factor40. Immunostaining analysis showed that the expression of CREB3L4 at protein level were higher in prostate cancer than the adjacent noncancerous tissues40, whereas the functional role of CREB3L4 in HCC remains to be revealed. JTB (Jumping Translocation Breakpoint) is a transmembrane protein which suffers an unbalanced translocation in various types of cancers41. Wild-type JTB confers resistance to apoptosis induced by TGF-β142. The average level of JTB expression in HCC tumor cells was 1.9-fold higher than paired nontumor liver tissues36. To elucidate the role of JTB during hepatocarcinogenesis, Pan et al found that JTB could interact with HBs (Hepatitis B surface) and overexpression of JTB inhibited ultraviolet radiation-induced apoptosis which was compromised by co-overexpression of HBs41. However, little is known about the possible functional association of INTS3 and SNAPAP with HCC tumorigenesis.
SHC1
Huebner et al assigned the SHC1 gene to 1q2143. The SHC gene encodes 2 widely expressed overlapping proteins of 46 and 52 kilodalton (kDa) as well as a 66-kDa isoform, p66Shc. Shc is involved in signal transduction from receptor tyrosine kinases to downstream signal recipients such as Ras43, 44. The mitochondrial pro-apoptotic activity of p66Shc has been reported to be associated with its phosphorylation caused by protein kinase C β under oxidative conditions45. The expression level of p66Shc in tumor tissue has been reported to inversely correlate with an increased risk of disease recurrence in early-stage breast cancer46. Wong et al reported the expression of SHC1 gene at mRNA level was significantly higher than the adjacent nontumor liver tissues36. However, the oncogenic mechanism of SHC1 during HCC initiation and progression remains unclear.
CKS1B in 1q21.2
CKS1B (CDC28 protein kinase regulatory subunit 1B), also named Cks1 (cyclin kinase subunit 1), is a member of the highly conserved family of Cks/Suc 1 proteins47. Human Cks1 is required for SCFSkp2-mediated ubiquitination and degradation of p27kip1 that is indispensable for G1/S transition during cell cycle progression48. In this process, Cks1 interacts with Skp2, the substrate recognition component of SCF, thereby increasing its association with phosphorylated p27kip1, Skp2-bound p27kip1 is then degraded via ubiquitin-proteasome pathway49. So far, several groups have identified the potential role of Cks1 in HCC50, 51. Shen et al demonstrated that expression of Cks1 at mRNA and protein levels was significantly higher in HCC than those in the adjacent non-tumor liver tissue, and Cks1 expression was closely associated with poor differentiation and also negatively associated with p27kip1 in HCC50.
CHD1L in 1q21.1
CHD1L (Chromodomain Helicase/ATPase DNA Binding Protein 1-Like, also known as Amplified in Liver Cancer 1, ALC1) belongs to the SNF2-like subfamily of the Snf2 (sucrose non-fermenting 2) family. The full-length mRNA (2.98 kb) of CHD1L encodes an 897aa protein. The CHD1L protein contains highly conserved helicase motifs, which are also found in other family members such as Snf2, ISWI, and CHD152. The sequence homology of the helicase domain (107 aa) between CHD1L and CHD1 is 59%33. Unlike CHD1, however, CHD1L does not contain a chromo domain, which can recognize methylated histone tails. Instead, CHD1L contains a Macro domain, which is an ADP-ribose/PAR-binding element53. Ahel et al demonstrated that CHD1L, a chromatin remodeling enzyme, functions as a DNA damage-response protein via its association with known DNA factors and its rapid poly(ADP-ribose)-dependent recruitment to DNA damage sites54.
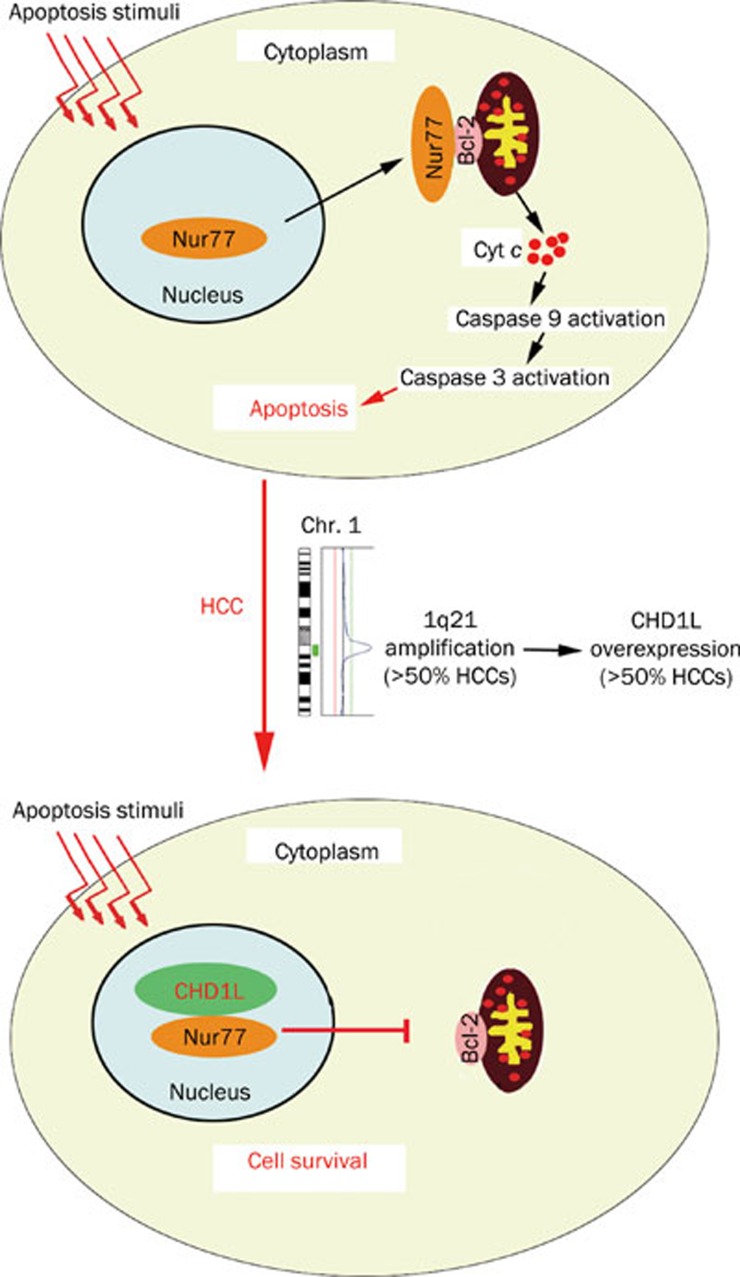
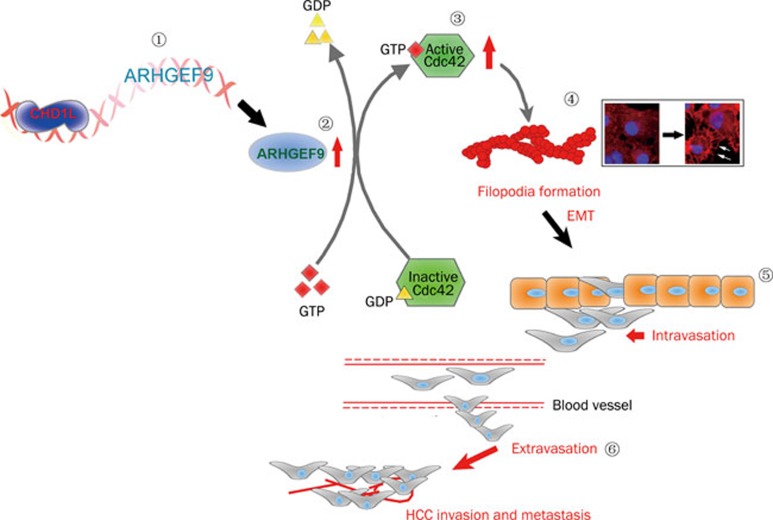
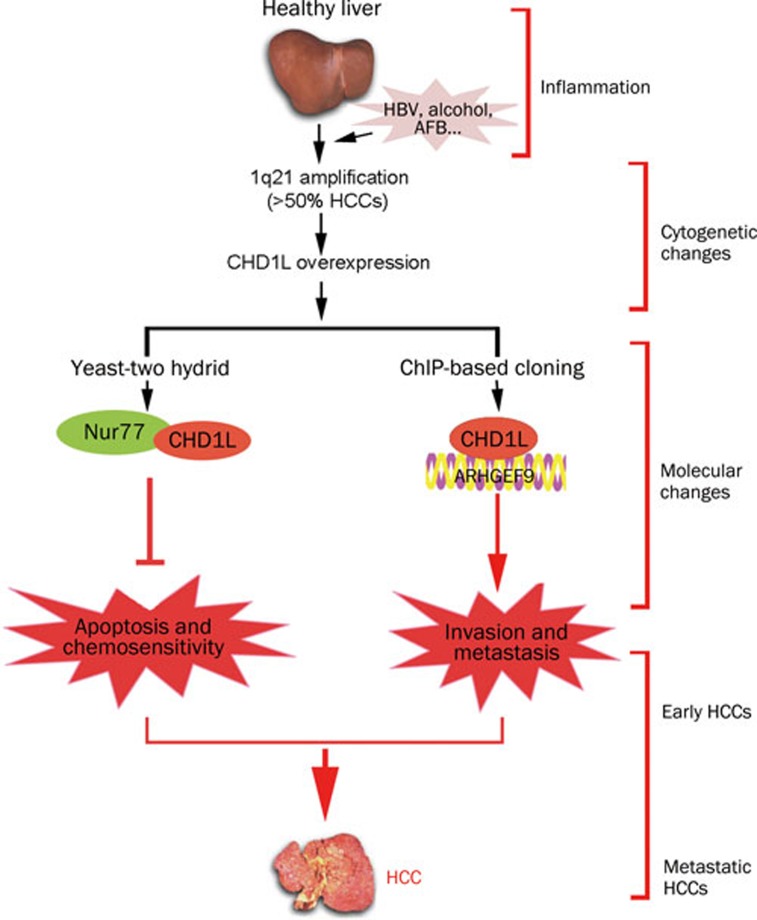
In 2008, the functional role of CHD1L in human tumorigenesis was first reported by our laboratory. We have demonstrated that 1) CHD1L-transfected cells are able to form more colonies in soft agar and caused tumor formation in nude mice; 2) the transformation ability of CHD1L is effectively inhibited by small interference RNA (siRNA) against CHD1L; 3) CHD1L can promote the G1/S transition and inhibit apoptosis in response to cellular stress33. Importantly, there is now a transgenic mouse model with ubiquitous CHD1L expression55. Spontaneous tumor formation was found in 10/41 (24.4%) of CHD1L-transgenic mice including 4 mice with HCCs, and no cases were seen in their 39 wild-type littermates55. Further, our mechanistic studies of CHD1L in the past two years have delineated CHD1L-involved hepatocarcinogenesis during HCC progression and metastasis. As a member of SNF2-like subfamily, CHD1L is a unique gene because most of its family members are likely to act as tumor suppressor genes (TSGs). Based on the identification of CHD1L target genes by means of yeast-two hybrid and chromatin immunoprecipitation (ChIP)-based cloning approaches, the underlying anti-apoptotic and metastatic mechanisms of CHD1L were well elucidated56, 57. Chen et al revealed that the anti-apoptotic ability of CHD1L was found to be linked to its interaction with Nur77, a critical member of a p53-independent apoptotic pathway56. As the first cellular protein identified to bind with Nur77, CHD1L was able to inhibit the nucleus-to-mitochondria translocation of Nur77, thereby causing the hindrance of cytochrome c (Cyt c) release into the cytoplasm, activation of caspase 9 and 3, as well as the initiation of apoptosis (Figure 2). Further, the C-terminal domain of CHD1L is proved to be responsible for the interaction with Nur77 and the inhibition of Nur77-mediated apoptosis. CHD1L was also found to be endowed with DNA binding activity57. A ChIP-based cloning strategy was conducted to isolate 35 CHD1L regulated genes, one of which was ARHGEF9, a guanine nucleotide exchange factor (GEF) for the Rho small GTPase Cdc42. ARHGEF9 was transcriptionally activated by CHD1L. Both in vitro and in vivo functional studies found that CHD1L could increase cell motility, induce filopodia formation and the epithelial-mesenchymal transition (EMT) via ARHGEF9-mediated Cdc42 activation, all contributing to tumor cell invasion and metastasis57 (Figure 3). Our further investigation of clinical HCC specimens exhibited significant association of CHD1L expression with not only clinicopathological features such as microsatellite tumor formation, venous infiltration and advanced tumor stage, but also the overall survival time and the disease-free survival rate (unpublished data). Furthermore, CHD1L was found to be significantly correlated with chemotherapy responsiveness and survival of recurrent HCC patients (unpublished data). Interestingly, the involvement of Nur77-mediated pathway in chemotherapeutic agent-induced apoptosis can dictate if CHD1L could confer resistance to chemotherapy (unpublished data). Taken together, we delineated two novel molecular mechanisms, CHD1L/Nur77 and CHD1L/ARHGEF9/Cdc42/EMT, during HCC progression and metastasis (Figure 4).
Figure 2.
The role of CHD1L in Nur77-mediated pathway during hepatocarcinogenesis. In normal condition, Nur77 localizes in nucleus. In response to apoptosis stimuli, Nur77 translocates from nucleus to mitochondria where it binds to Bcl-2 and induces its conformational change, thereby leading to cytochrome c (Cyt c) release into cytoplasm and the initiation of caspase activation and apoptosis. In HCC, CHD1L, which is overexpressed caused by 1q21 amplification, are able to inhibit the nucleus-to-mitochondria translocation of Nur77, resulting in the hindrance of cytochrome c (Cyt c) release, caspase activation and apoptosis.
Figure 3.
During HCC progression, CHD1L is overexpressed and activates ARHGEF9 transcription via a protein-DNA interaction ( ). CHD1L enhances the GTP loading onto Rho GTPase protein Cdc42 (the increased level of Cdc42-GTP) (
). CHD1L enhances the GTP loading onto Rho GTPase protein Cdc42 (the increased level of Cdc42-GTP) ( ) through the up-regulation of ARHGEF9 (
) through the up-regulation of ARHGEF9 ( ). CHD1L/ARHGEF9-induced Cdc42 activation leads to more filopodia formation as a characteristic of actin cytoskeletal rearrangement and the concomitant EMT phenotype (
). CHD1L/ARHGEF9-induced Cdc42 activation leads to more filopodia formation as a characteristic of actin cytoskeletal rearrangement and the concomitant EMT phenotype ( ). CHD1L-dependent EMT may result in a sequential process including the loss of cell-cell contact, the penetration of basement membrane (BM) and the entry of tumor cells into blood circulation (intravasation) (
). CHD1L-dependent EMT may result in a sequential process including the loss of cell-cell contact, the penetration of basement membrane (BM) and the entry of tumor cells into blood circulation (intravasation) ( ), finally the growth of tumor cells in the secondary sites in liver (intrahepatic metastases or microsatellite formation) or new organs (distant metastases) (
), finally the growth of tumor cells in the secondary sites in liver (intrahepatic metastases or microsatellite formation) or new organs (distant metastases) ( ).
).
Figure 4.
Healthy liver that undergoes chronic hepatitis or cirrhosis, progresses through hyperplastic and dysplastic stages. During these stages, cytogenetic alterations often occur including amplication of 1q21 which has been detected in over 50% of HCC specimens. Therefore, CHD1L is amplified and overexpression in HCC cases. By using molecular biological techniques, we delineated two molecular pathways (CHD1L)-(Nur77)-(Cyt c) and (CHD1L)-(ARHGEF9)-(Cdc42)-(EMT), which led to evasion of apoptosis, the decreased sensitivity to chemotherapy and the promotion of tissue invasion and metastasis.
Conclusions and future directions
During HCC progression, regional chromosomal gain represents a major mechanism in the activation of proto-oncogenes. Gain of 1q is one of the most frequently detected alterations in HCC and has been identified as an genomic event associated with early development of HCC25. Due to the highest frequency of copy number gain at 1q21, much effort has been devoted to identifying target genes which are responsible for 1q21 amplicon. However, the extensive and complicated nature of chromosome aberrations makes difficult for identifying the biologically relevant alterations and their functional significance. In this decade, some research groups focused on the identification and characterization of 1q21 target genes such as CHD1L, CKS1B, and SHC1 in HCC progression. Based on our recent work, CHD1L is proved to be amplified and overexpression in HCC cases. By using molecular biological techniques, we demonstrated two molecular pathways (CHD1L)-(Nur77)-(Cyt c) and (CHD1L)-(ARHGEF9)-(Cdc42)-(EMT), which led to evasion of apoptosis, the decreased sensitivity to chemotherapy and the promotion of tissue invasion and metastasis. Although more and more candidate target genes within 1q21 amplicon have been identified, for majority of target genes, the precise mechanisms underlying HCC initiation and progression however, remain unclear. It is also possible that coactivation of these target genes leads to HCC development and progression. To better elucidate the relationship between genomic aberrations and hepatocarcinogenesis, functional and mechanistic studies are needed to clarify the roles of these target genes.
References
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–87. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–8. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Beaugrand M. Hepatocellular carcinoma: present status and future prospects. J Hepatol. 2003;38:S136–49. doi: 10.1016/s0168-8278(02)00432-4. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211, v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Adams LA, Angulo P. Recent concepts in non-alcoholic fatty liver disease. Diabet Med. 2005;22:1129–33. doi: 10.1111/j.1464-5491.2005.01748.x. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–8. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127:S79–86. doi: 10.1016/j.gastro.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Debernardi S, Lillington D, Young BD. Understanding cancer at the chromosome level: 40 years of progress. Eur J Cancer. 2004;40:1960–7. doi: 10.1016/j.ejca.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Unsal H, Yakicier C, Marcais C, Kew M, Volkmann M, Zentgraf H, et al. Genetic heterogeneity of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1994;91:822–6. doi: 10.1073/pnas.91.2.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Marchio A, Meddeb M, Pineau P, Danglot G, Tiollais P, Bernheim A, et al. Recurrent chromosomal abnormalities in hepatocellular carcinoma detected by comparative genomic hybridization. Genes Chromosomes Cancer. 1997;18:59–65. [PubMed] [Google Scholar]
- Kusano N, Shiraishi K, Kubo K, Oga A, Okita K, Sasaki K. Genetic aberrations detected by comparative genomic hybridization in hepatocellular carcinomas: their relationship to clinicopathological features. Hepatology. 1999;29:1858–62. doi: 10.1002/hep.510290636. [DOI] [PubMed] [Google Scholar]
- Sakakura C, Hagiwara A, Taniguchi H, Yamaguchi T, Yamagishi H, Takahashi T, et al. Chromosomal aberrations in human hepatocellular carcinomas associated with hepatitis C virus infection detected by comparative genomic hybridization. Br J Cancer. 1999;80:2034–9. doi: 10.1038/sj.bjc.6690638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N, Lai P, Lee SW, Fan S, Pang E, Liew CT, et al. Assessment of genetic changes in hepatocellular carcinoma by comparative genomic hybridization analysis: relationship to disease stage, tumor size, and cirrhosis. Am J Pathol. 1999;154:37–43. doi: 10.1016/S0002-9440(10)65248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XY, Fang Y, Sham JS, Kwong DL, Zhang Y, Liang Q, et al. Recurrent chromosome alterations in hepatocellular carcinoma detected by comparative genomic hybridization. Genes Chromosomes Cancer. 2000;29:110–6. [PubMed] [Google Scholar]
- Chang J, Kim NG, Piao Z, Park C, Park KS, Paik YK, et al. Assessment of chromosomal losses and gains in hepatocellular carcinoma. Cancer Lett. 2002;182:193–202. doi: 10.1016/s0304-3835(02)00083-6. [DOI] [PubMed] [Google Scholar]
- Niketeghad F, Decker HJ, Caselmann WH, Lund P, Geissler F, Dienes HP, et al. Frequent genomic imbalances suggest commonly altered tumour genes in human hepatocarcinogenesis. Br J Cancer. 2001;85:697–704. doi: 10.1054/bjoc.2001.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi K, Okita K, Kusano N, Harada T, Kondoh S, Okita S, et al. A comparison of DNA copy number changes detected by comparative genomic hybridization in malignancies of the liver, biliary tract and pancreas. Oncology. 2001;60:151–61. doi: 10.1159/000055313. [DOI] [PubMed] [Google Scholar]
- Balsara BR, Pei J, De Rienzo A, Simon D, Tosolini A, Lu YY, et al. Human hepatocellular carcinoma is characterized by a highly consistent pattern of genomic imbalances, including frequent loss of 16q23.1–24.1. Genes Chromosomes Cancer. 2001;30:245–53. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1083>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Midorikawa Y, Tsutsumi S, Nishimura K, Kamimura N, Kano M, Sakamoto H, et al. Distinct chromosomal bias of gene expression signatures in the progression of hepatocellular carcinoma. Cancer Res. 2004;64:7263–70. doi: 10.1158/0008-5472.CAN-04-1275. [DOI] [PubMed] [Google Scholar]
- Lau SH, Guan XY. Cytogenetic and molecular genetic alterations in hepatocellular carcinoma. Acta Pharmacol Sin. 2005;26:659–65. doi: 10.1111/j.1745-7254.2005.00126.x. [DOI] [PubMed] [Google Scholar]
- Poon TC, Wong N, Lai PB, Rattray M, Johnson PJ, Sung JJ. A tumor progression model for hepatocellular carcinoma: bioinformatic analysis of genomic data. Gastroenterology. 2006;131:1262–70. doi: 10.1053/j.gastro.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Qin LX, Tang ZY, Sham JS, Ma ZC, Ye SL, Zhou XD, et al. The association of chromosome 8p deletion and tumor metastasis in human hepatocellular carcinoma. Cancer Res. 1999;59:5662–5. [PubMed] [Google Scholar]
- Wang Y, Wu MC, Sham JS, Zhang W, Wu WQ, Guan XY. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer. 2002;95:2346–52. doi: 10.1002/cncr.10963. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Nishida N, Itoh T, Komeda T, Fukuda Y, Ikai I, et al. Discrete breakpoint mapping and shortest region of overlap of chromosome arm 1q gain and 1p loss in human hepatocellular carcinoma detected by semiquantitative microsatellite analysis. Genes Chromosomes Cancer. 2005;42:34–43. doi: 10.1002/gcc.20117. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Nishida N, Itoh T, Kuno M, Minata M, Komeda T, et al. Comprehensive allelotyping of well-differentiated human hepatocellular carcinoma with semiquantitative determination of chromosomal gain or loss. Genes Chromosomes Cancer. 2002;35:329–39. doi: 10.1002/gcc.10126. [DOI] [PubMed] [Google Scholar]
- Gronwald J, Storkel S, Holtgreve-Grez H, Hadaczek P, Brinkschmidt C, Jauch A, et al. Comparison of DNA gains and losses in primary renal clear cell carcinomas and metastatic sites: importance of 1q and 3p copy number changes in metastatic events. Cancer Res. 1997;57:481–7. [PubMed] [Google Scholar]
- Petersen S, Aninat-Meyer M, Schluns K, Gellert K, Dietel M, Petersen I. Chromosomal alterations in the clonal evolution to the metastatic stage of squamous cell carcinomas of the lung. Br J Cancer. 2000;82:65–73. doi: 10.1054/bjoc.1999.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh K, Takano M, Koshikawa T, Hirai M, Yoshida S, Mano Y, et al. Gains of 1q21–q22 and 13q12–q14 are potential indicators for resistance to cisplatin-based chemotherapy in ovarian cancer patients. Clin Cancer Res. 1999;5:2526–31. [PubMed] [Google Scholar]
- Hirai M, Yoshida S, Kashiwagi H, Kawamura T, Ishikawa T, Kaneko M, et al. 1q23 gain is associated with progressive neuroblastoma resistant to aggressive treatment. Genes Chromosomes Cancer. 1999;25:261–9. doi: 10.1002/(sici)1098-2264(199907)25:3<261::aid-gcc8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Ma NF, Hu L, Fung JM, Xie D, Zheng BJ, Chen L, et al. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology. 2008;47:503–10. doi: 10.1002/hep.22072. [DOI] [PubMed] [Google Scholar]
- Vissers LE, Veltman JA, van Kessel AG, Brunner HG. Identification of disease genes by whole genome CGH arrays. Hum Mol Genet. 2005;14:R215–23. doi: 10.1093/hmg/ddi268. [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Yasui K, Endo M, Nakajima T, Zen K, Tsuji K, et al. CREB3L4, INTS3, and SNAPAP are targets for the 1q21 amplicon frequently detected in hepatocellular carcinoma. Cancer Genet Cytogenet. 2008;180:30–6. doi: 10.1016/j.cancergencyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Wong N, Chan A, Lee SW, Lam E, To KF, Lai PB, et al. Positional mapping for amplified DNA sequences on 1q21–q22 in hepatocellular carcinoma indicates candidate genes over-expression. J Hepatol. 2003;38:298–306. doi: 10.1016/s0168-8278(02)00412-9. [DOI] [PubMed] [Google Scholar]
- Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–9. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Caramella D, Bartolozzi C, Di Coscio G. Long-term follow-up study of adenomatous hyperplasia in liver cirrhosis. Ital J Gastroenterol. 1994;26:163–8. [PubMed] [Google Scholar]
- Theise ND, Park YN, Kojiro M. Dysplastic nodules and hepatocarcinogenesis. Clin Liver Dis. 2002;6:497–512. doi: 10.1016/s1089-3261(02)00006-5. [DOI] [PubMed] [Google Scholar]
- Qi H, Fillion C, Labrie Y, Grenier J, Fournier A, Berger L, et al. AIbZIP, a novel bZIP gene located on chromosome 1q21.3 that is highly expressed in prostate tumors and of which the expression is up-regulated by androgens in LNCaP human prostate cancer cells. Cancer Res. 2002;62:721–33. [PubMed] [Google Scholar]
- Pan JS, Cai JY, Xie CX, Zhou F, Zhang ZP, Dong J, et al. Interacting with HBsAg compromises resistance of jumping translocation breakpoint protein to ultraviolet radiation-induced apoptosis in 293FT cells. Cancer Lett. 2009;285:151–6. doi: 10.1016/j.canlet.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Kanome T, Itoh N, Ishikawa F, Mori K, Kim-Kaneyama JR, Nose K, et al. Characterization of Jumping translocation breakpoint (JTB) gene product isolated as a TGF-beta1-inducible clone involved in regulation of mitochondrial function, cell growth and cell death. Oncogene. 2007;26:5991–6001. doi: 10.1038/sj.onc.1210423. [DOI] [PubMed] [Google Scholar]
- Huebner K, Kastury K, Druck T, Salcini AE, Lanfrancone L, Pelicci G, et al. Chromosome locations of genes encoding human signal transduction adapter proteins, Nck (NCK), Shc (SHC1), and Grb2 (GRB2) Genomics. 1994;22:281–7. doi: 10.1006/geno.1994.1385. [DOI] [PubMed] [Google Scholar]
- Kraut-Cohen J, Muller WJ, Elson A. Protein-tyrosine phosphatase epsilon regulates Shc signaling in a kinase-specific manner: increasing coherence in tyrosine phosphatase signaling. J Biol Chem. 2008;283:4612–21. doi: 10.1074/jbc.M708822200. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, et al. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–63. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- Davol PA, Bagdasaryan R, Elfenbein GJ, Maizel AL, Frackelton AR Jr. Shc proteins are strong, independent prognostic markers for both node-negative and node-positive primary breast cancer. Cancer Res. 2003;63:6772–83. [PubMed] [Google Scholar]
- Wang XC, Tian J, Tian LL, Wu HL, Meng AM, Ma TH, et al. Role of Cks1 amplification and overexpression in breast cancer. Biochem Biophys Res Commun. 2009;379:1107–13. doi: 10.1016/j.bbrc.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, et al. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321–4. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- Rother K, Li YY, Tschop K, Kirschner R, Muller GA, Mossner J, et al. Expression of cyclin-dependent kinase subunit 1 (Cks1) is regulated during the cell cycle by a CDE/CHR tandem element and is downregulated by p53 but not by p63 or p73. Cell Cycle. 2007;6:853–62. doi: 10.4161/cc.6.7.4017. [DOI] [PubMed] [Google Scholar]
- Shen DY, Fang ZX, You P, Liu PG, Wang F, Huang CL, et al. Clinical significance and expression of cyclin kinase subunits 1 and 2 in hepatocellular carcinoma. Liver Int. 2009;30:119–25. doi: 10.1111/j.1478-3231.2009.02106.x. [DOI] [PubMed] [Google Scholar]
- Calvisi DF, Ladu S, Pinna F, Frau M, Tomasi ML, Sini M, et al. SKP2 and CKS1 promote degradation of cell cycle regulators and are associated with hepatocellular carcinoma prognosis. Gastroenterology. 2009;137:1816–26. doi: 10.1053/j.gastro.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Flaus A, Martin DM, Barton GJ, Owen-Hughes T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;34:2887–905. doi: 10.1093/nar/gkl295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karras GI, Kustatscher G, Buhecha HR, Allen MD, Pugieux C, Sait F, et al. The macro domain is an ADP-ribose binding module. EMBO J. 2005;24:1911–20. doi: 10.1038/sj.emboj.7600664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahel D, Horejsi Z, Wiechens N, Polo SE, Garcia-Wilson E, Ahel I, et al. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science. 2009;325:1240–3. doi: 10.1126/science.1177321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Huang JD, Hu L, Zheng BJ, Chen L, Tsang SL, et al. Transgenic CHD1L expression in mouse induces spontaneous tumors. PLoS One. 2009;4:e6727. doi: 10.1371/journal.pone.0006727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hu L, Chan TH, Tsao GS, Xie D, Huo KK, et al. Chromodomain helicase/adenosine triphosphatase DNA binding protein 1-like (CHD1l) gene suppresses the nucleus-to-mitochondria translocation of nur77 to sustain hepatocellular carcinoma cell survival. Hepatology. 2009;50:122–129. doi: 10.1002/hep.22933. [DOI] [PubMed] [Google Scholar]
- Chen L, Chan TH, Yuan YF, Hu L, Huang J, Ma S, et al. CHD1L promotes hepatocellular carcinoma progression and metastasis in mice and is associated with these processes in human patients. J Clin Invest. 2010;120:1178–91. doi: 10.1172/JCI40665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruder C, Reimer T, Delgado-Martinez I, Hermosilla R, Engelsberg A, Nehring R, et al. EBAG9 adds a new layer of control on large dense-core vesicle exocytosis via interaction with Snapin. Mol Biol Cell. 2005;16:1245–57. doi: 10.1091/mbc.E04-09-0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wu J, Yu X. Integrator3, a partner of single-stranded DNA-binding protein 1, participates in the DNA damage response. J Biol Chem. 2009;284:30408–15. doi: 10.1074/jbc.M109.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Gong Z, Ghosal G, Chen J. SOSS complexes participate in the maintenance of genomic stability. Mol Cell. 2009;35:384–93. doi: 10.1016/j.molcel.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]