Abstract
Acute-on-chronic liver failure (ACLF) is a severe life-threatening complication. Liver transplantation is the only available therapeutic option; however, several limitations have restricted its use in patients. The use of corticosteroids as an optional therapy for ACLF has received a great deal of interest. The rationale behind its use is the possible role of the immune system in initiating and perpetuating hepatic damage. In order to assess the relationship between myeloid dendritic cells (mDCs) and the efficacy of methylprednisolone (MP) treatment for hepatitis B virus (HBV)-associated ACLF patients, we recruited 30 HBV-associated ACLF patients who had received MP treatment at 10-day intervals; 26 patients received conservative medical (CM) management as a control. The functionality of DC subsets was lower in these ACLF patients compared with healthy subjects. In addition, compared with survivors, dead/transplanted patients had lower functional mDC in both groups. Furthermore, a decreased numbers of mDC at baseline was associated with high mortality of ACLF patients. Importantly, MP treatment resulted in a significant decrease in 28-day mortality, and all MP patients exhibited an initial rapid decrease in circulating mDC numbers within 10 days of MP treatment. Subsequently, MP survivors displayed a continuous increase in mDC numbers accompanied by a decrease in total bilirubin levels by more than 30%. However, MP dead/transplanted patients lacked these sequential responses compared with survivors. This evidence suggests strongly that the higher mDC numbers at baseline and the recovery of mDC number at the end of treatment may represent a prognostic marker for favorable response to corticosteroid treatment in ACLF patients.
Keywords: acute-on-chronic liver failure, methylprednisolone, myeloid dendritic cells, plasmacytoid dendritic cells
Introduction
Acute-on-chronic liver failure (ACLF) refers to acute hepatic insult characterized by jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in patients who had been diagnosed previously or undiagnosed with chronic liver disease.1 In some regions that have high hepatitis B virus (HBV) prevalence, the term HBV-associated acute-on-chronic liver failure has been introduced to describe acute aggravation of chronic hepatitis B liver failure,2 and this description constitutes about 70% of all the ACLF cases.1, 2 HBV-ACLF is associated with high mortality and is a severe life-threatening condition. Plasma exchange and hemodiafiltration have been used as artificial liver support systems for these patients, but these treatments are only partially effective. Liver transplantation is considered to be the standard treatment for these patients,3 but several limitations such as a limited numbers of donors, long waiting lists, high cost and multiple complications (e.g., rejection, problems associated with the long-term use of immunosuppressants, and perioperative morbidity and mortality) have restricted the use of liver transplantation in many ACLF patients.4, 5 Therefore, new strategies to delay or prevent disease progression of liver failure are required urgently.
There is paramount evidence that immune dysregulation occurs in HBV-ACLF patients.6, 7, 8, 9, 10, 11 These changes include exacerbated innate immunologic responses, such as the activation of monocytes/macrophages and lymphocytes and cytokine release from these inflammatory cells. Clinical findings and experimental rodent models suggest that corticosteroids can suppress the effects of these activated inflammatory cells.12, 13 However, only some of the HBV-ACLF patients treated with corticosteroids were cured without liver transplantation. As such, the identification of immune parameters as indicators for the efficacy of corticosteroid treatment is urgently needed.
Dendritic cells, as important professional antigen-presenting cells, participate actively in driving the progression of ACLF.7, 8, 14 Two distinct circulating dendritic cell (DC) subsets, myeloid and plasmacytoid DCs (mDCs and pDCs), have been identified in humans based on phenotypic and functional characterizations.15, 16 In general, human mDCs are well equipped to induce type 1 T helper cell polarization via the production of interleukin-12 (IL-12) or tumor-necrosis factor-α, which plays a crucial role in the development of liver damage. pDCs are characterized by their distinct capacity to produce large amounts of type-1 interferon (IFN), a key effector for inflammatory activation. Recent studies have demonstrated the functional impairment of mDCs and pDCs in patients with chronic HBV infection.17, 18, 19 Notably, these dendritic cells accumulate mostly in the liver of ACLF patients;7, 8, 14 however, any correlation between the DCs and corticosteroid efficacy is unknown.
In our present study, we examine the efficacy of methylprednisolone (MP) treatment in 30 HBV-associated ACLF patients compared with 26 matched conservative medical managed ACLF patients. We found that the higher mDC numbers at baseline or the recovery of mDC numbers at the end of treatment may represent a prognostic marker for favorable response to corticosteroid treatment in ACLF patients.
Materials and methods
Study subjects
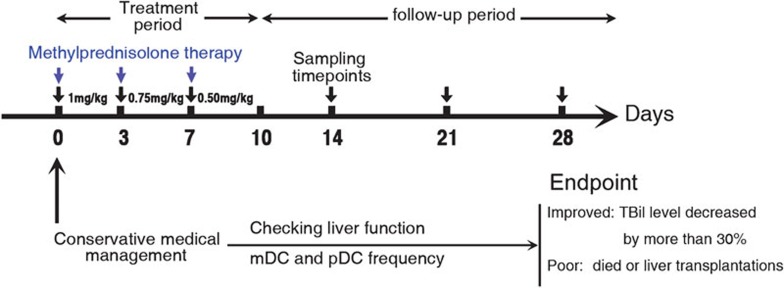
Thirty patients with HBV-related ACLF received MP treatment, 26 patients who received conservative medical (CM) management and 30 healthy controls (HCs) were enrolled in this study. The project was approved by the Ethics Committee of Beijing You An Hospital and Beijing 302 Hospital; all patients signed a written informed consent form in accordance with the Institutional Review Board guidelines for the protection of human subjects. All HBV-related ACLF patients had a history of chronic hepatitis or liver cirrhosis with serum HBsAg+ >6 months and had undergone the conventional treatment in this 4-week study. Briefly, conservative medical management includes nutritional support, hepatoprotective drugs, antiviral therapy, prevention and control complications, as well as liver support device usage. In the MP treatment group, patient's treatment further consisted of 1mg/kg/day (average: 80 mg/day) MP intravenously guttae for the first 3 days, thereafter 0.75 mg/kg/day (average: 60 mg/day) intravenously guttae continuously for the second 3 days and followed by 0.5 mg/kg/day(average: 40 mg/day) intravenously guttae until the end of the third 3-day period (Figure 1). The baseline clinical parameters were matched in the treatment and control groups (Table 1). Both groups of patients were divided subsequently into non-survivor (NS) patients (who died or received a liver graft) and survivors (who survived or whose total bilirubin level decreased by more than 30% at the end of study).
Figure 1.
Study protocols. mDC, myeloid dendritic cell; pDC, plasmacytoid dendritic cell.
Table 1. Clinical and biochemical features of two groups at baseline.
| Methylprednisolone-treated ACLF | Conservative managed ACLF | |
|---|---|---|
| Cases | 30 | 26 |
| Age (year) | 39.31±13.32 | 42.42±11.87 |
| Sex (male/female) | 23/7 | 20/6 |
| TBIL (µmol/l) | 393.12±133.27 | 395.42±174.38 |
| Creatinine (µmol/l) | 77.50±41.88 | 78.17±57.70 |
| PT (s) | 27.72±9.89 | 25.71±6.87 |
| PTA (%) | 31.18±9.94 | 33.27±6.46 |
| ALT (U/l) | 287.8 (26.2–3272.3) | 289.5 (15.9–313.1) |
| AST (U/l) | 233.9 (52.5–2521.0) | 284.1 (42.4–2859.3) |
| ALB (g/l) | 30.33±4.43 | 29.60±4.45 |
| MELD score | 24.36±6.49 | 23.03±5.93 |
| Encephalopathy (0/1/2/3/4) | 10/9/3/4/4 | 14/5/4/3/0 |
| Ascites (0/1/2/3) | 10/9/6/5 | 3/8/11/4 |
| Monocyte counts (/µl) | 819.81±624.69 | 888.43±450.26 |
| Lymphocyte counts (/µl) | 1205.36±618.48 | 1844.86±411.85 |
Abbreviations: ACLF, acute-on-chronic liver failure; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PT, prothrombin time; PTA, prothrombin activity; TBIL: total bilirubin.
Values are expressed as mean±standard deviation (s.d.) or median (average).
Exclusion criteria were: history of variceal bleeding, recent infection, bleeding tendency and/or ascites ultrafiltration and/or dialysis during the last 2 months before enrolment; the presence of severe renal, respiratory or cardiac disease or any detectable tumor by ultrasonography, computed tomography or magnetic resonance imaging; evidence of extrahepatic biliary diseases (e.g., presence of primary sclerosing cholangitis or dilated common bile duct by ultrasonography); detection of hepatic, portal or splenic vein thromboses by Doppler ultrasonography; active substance abuse; lack of a supportive family; and unwillingness to sign the informed consent.
Flow cytometric analysis and cytokine release
The peripheral DC subset frequency was analyzed using protocols described previously by our team and with minor modifications.19, 20 In brief, freshly isolated peripheral blood mononuclear cells (PBMCs) were incubated with anti-lineage-1-FITC, anti-HLA-DR-PerCP and anti-CD11c-APC (BD Pharmingen, San Jose, CA, USA) or anti-CD123-APC (Miltenyi Biotec, Bergish Gladbach, Germany). The cells were fixed and analyzed by FACSCalibur and CellQuest software (BD Biosciences, San Jose, CA, USA). At least 200 000 cells per run were acquired. The absolute numbers of circulating DC subsets were calculated as per the formula: i.e., the counts of DCs (cells/µl)–(the DC frequency of PBMCs)×(lymphocyte cell count/µl+monocyte cell count/µl). Lymphocyte and monocyte counts were determined by an automated differential blood counter. DC subset function was evaluated by cytokine production. Freshly prepared PBMCs were stimulated in 24-well plates with either medium alone, 50 µg/ml poly(I:C) (Amersham Biosciences, Piscataway, NJ, USA) or 6 µg/ml CpG 2216 (Sangon, Shanghai, China) for 24 h at 37 °C. Then, supernatants were harvested and tested in duplicate for IFN-α or IL-12 by use of a commercial enzyme-linked immunosorbent assay kit according to the manufacturer's instructions (Biosource, Nivelles, Belgium). Sensitivity of the assays was 10 pg/ml.
Statistical analysis
All data were analyzed using SPSS 13.0 for Windows software (SPSS Inc., Chicago, IL, USA). Multiple comparisons were made among the different groups using the Kruskal–Wallis test for non-parametric data. Comparisons between various individuals were performed using the Mann–Whitney U test; comparisons between parameters in the same individual were performed using the Wilcoxon matched-pairs t-test. Follow-up time was calculated as the interval between the date of treatment and last follow-up or death. Survival rates were analyzed with the Kaplan–Meier method using the log-rank test to assess the difference between survival curves. For all tests, two-sided P values of <0.05 were considered to be statistically significant.
Results
Decreased numbers of functional DC subset cells in ACLF patients
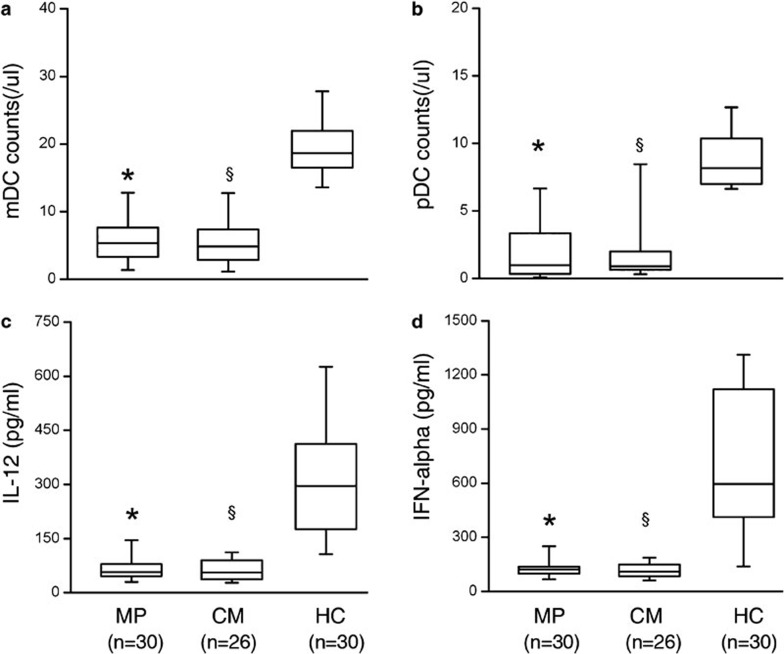
The frequency of DC subset cells in the blood between ACLF patients who received MP treatment or CM management was compared at baseline; two cell populations of mDCs defined as Lin-1−HLA-DR+CD11c+ and pDCs with the Lin-1−HLA-DR+CD123+ phenotype were monitored using flow cytometric analysis. In this study, mDCs identified by CD11c expression may, in part, include the CD11c+CD16+ DC subset. As shown in Figure 2a and b, the numbers of mDCs and pDCs were significantly lower (P<0.05) in the ACLF group compared with that in the HC group: i.e., mDCs: 5.98 cells/µl (range: 0.51–16.46 cells/µl) for the MP group; 5.66 cells/µl (range: 1.09–13.82 cells/µl) for the CM group vs. 19.49 cells/µl (range: 12.36–28.82 cells/µl) for HCs; and pDCs: 2.09 cells/µl (range: 0.02–7.92 cells/µl) for the MP group; 1.84 cells/µl (range: 0.19–9.50 cells/µl) for the CM group vs. 8.91 cells/µl (range: 6.36–17.49 cells/µl) for HCs. No significant difference in DC subset counts was found between the MP and CM groups.
Figure 2.
Decreased numbers of functional dendritic cell (DC) subsets in acute-on-chronic liver failure (ACLF) patients. (a, b) Distribution of circulating myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) in ACLF patients. (c, d) Cytokine-releasing capacity of total peripheral blood mononuclear cells induced by Toll-like receptor ligands. Boxes show the 10th, 25th, 75th and 90th percentiles and the median value (solid line) of each subset. *P<0.01 for MP patients compared with HC. §P<0.01 for CM patients compared with HC. P values are shown. CM, conservative medical managed (CM) ACLF patients; HC, healthy controls; MP, methylprednisolone (MP)-treated ACLF patients.
We examined the profiles of cytokine released by DC subsets in ACLF patients and in HCs. As shown in Figure 2c, a significant reduction of poly(I:C)-induced IL-12 production was found in the MP group and CM group compared with the HC group (313.9 pg/ml (range: 106.2–625.8 pg/ml)) (P<0.05), however, there was no significant difference in IL-12 production between the MP group (67.3 pg/ml (range: 27.8–230.4 pg/ml)) and the CM group (65.4 pg/ml (range: 22.9–148.3 pg/ml)).
A significant reduction in CpG-induced IFN-α production by pDCs was found in the MP group (130.9 pg/ml (range: 62.9–310.3 pg/ml)), and in the CM group (116.4 pg/ml (range: 60.9–197.0 pg/ml)), when compared with HCs (712.9 pg/ml (range: 138.5–1317.4 pg/ml)) (Figure 2d). However, there was no significant difference between the MP group and the CM group.
These data indicate that a significant reduction in the numbers of circulating DC subset cells occurs in ACLF patients.
ACLF outcome subgroup analysis
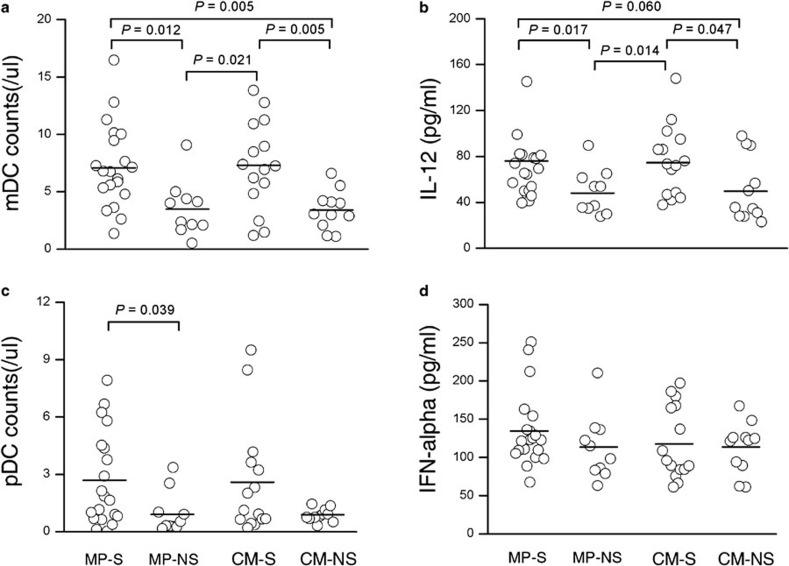
We further divided the ACLF patient group into either survivors who survived without receiving a liver graft or NS patients who underwent transplantation or died without transplantation. As shown in Figure 3a, the numbers of mDCs in NS patients at baseline were significantly lower than the numbers in survivors in both the MP group and the CM group (MP group: 7.21 cells/µl (range: 1.36–16.46 cells/µl) for survivor vs. 3.53 cells/µl (range: 0.51–9.06 cells/µl) for NS, P=0.012; CM group: 7.30 cells/µl (range: 1.19–13.82 cells/µl) for survivor vs. 3.42 cells/µl (range: 1.09–6.60 cells/µl) for NS, P=0.005). In addition, as shown in Figure 3b, a significant reduction in poly(I:C)-induced IL-12 production was found in the NS group compared with survivors (MP group: 76.6 pg/ml (range: 39.5–230.4 pg/ml)) for survivors vs. 48.7 pg/ml (range: 27.8–89.4 pg/ml), P=0.017; CM group: 75.8 pg/ml (range: 37.7–148.0 pg/ml)) for survivors vs. 51.3 pg/ml (range: 22.9–97.7 pg/ml), P=0.047). Interestingly, the decreased pDC numbers were found only in NS patients in the MP group (2.67 cells/µl (range: 0.02–7.92 cells/µl) for survivors vs. 0.96 cells/µl (range: 0.15–3.36 cells/µl) for NS, P=0.039, Figure 3c). Furthermore, there was no significant difference in CpG-induced IFN-α production by pDCs between survivors and NS patients (Figure 3d).
Figure 3.
Decreased numbers of functional myeloid dendritic cells (mDCs) in survived acute-on-chronic liver failure (ACLF) patients at baseline. (a, c) Distribution of circulating mDCs and plasmacytoid DCs (pDC) in survived and non-survived ACLF patients. (b, d) Cytokine-releasing capacity of total peripheral blood mononuclear cells (PBMCs) induced by Toll-like receptor ligands in survived and non-survived ACLF patients. Each circle represents an individual. Horizontal bars represent the median values of indicated index. P values are shown. NS, non-survived ACLF patients; S, survived ACLF patients.
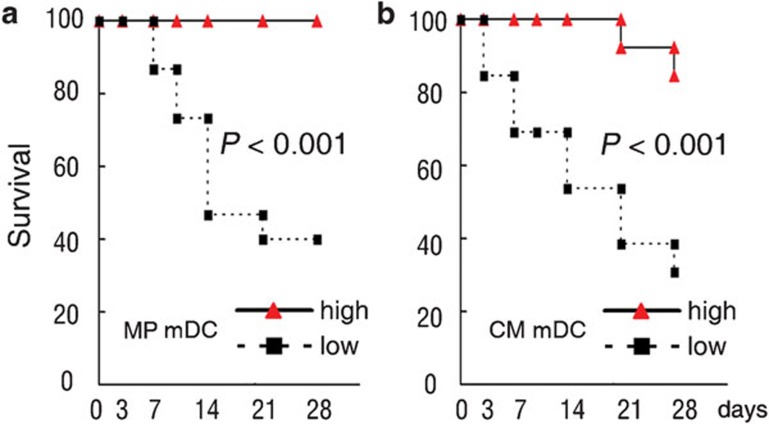
Decreased circulating mDCs at baseline predicts poor survival of ACLF patients
To investigate the association of mDCs with the survival of ACLF patients, ACLF patients in MP group and CM group were divided into two groups (high mDCs and low mDCs group) by the median value of circulating mDC frequency, respectively. The results showed that the low mDC group had significantly poorer survival rates compared with the high mDC group (P<0.001) (Figure 4a for MP group and Figure 4b for CM group).
Figure 4.
Kaplan–Meier curves showing the 28-day survival rate with respect to the myeloid dendritic cell (mDC) numbers: (a) methylprednisolone (MP); (b) conservative medical management (CM).
Improved survival ratios correlate with mDC restoration in MP-treated ACLF patients
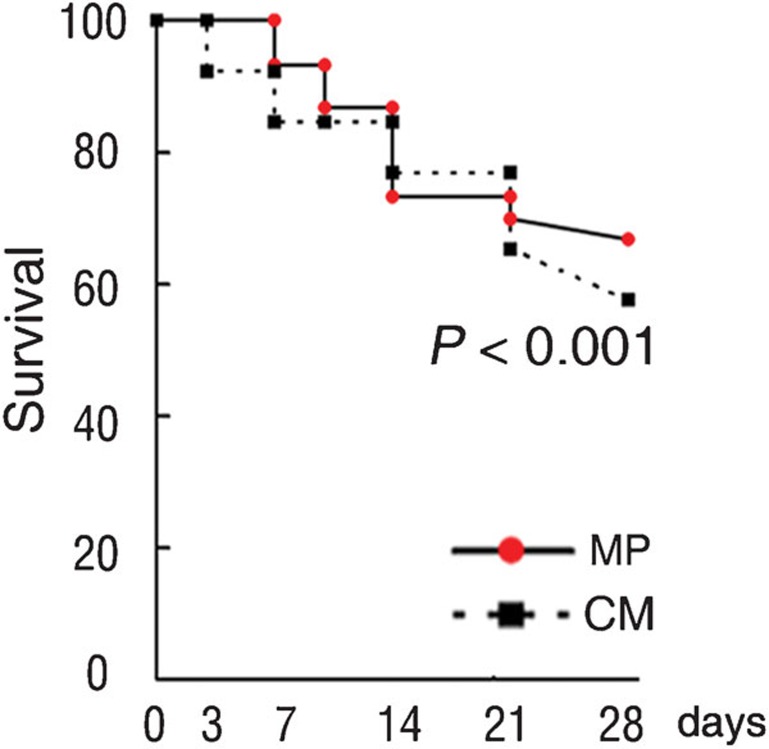
We monitored further the effect of MP treatment on the survival ratios of enrolled patients in this study. It was found that the survival ratios were increased significantly in the MP group compared with that in CM group at the end of 28-day follow-up period (log-rank test with P<0.001; Figure 5). These data suggest that MP therapy can improve the survival ratios in these ACLF patients.
Figure 5.
Kaplan–Meier curves showing the 28-day survival rate with respect to methylprednisolone (MP) and conservative medical management (CM) therapy.
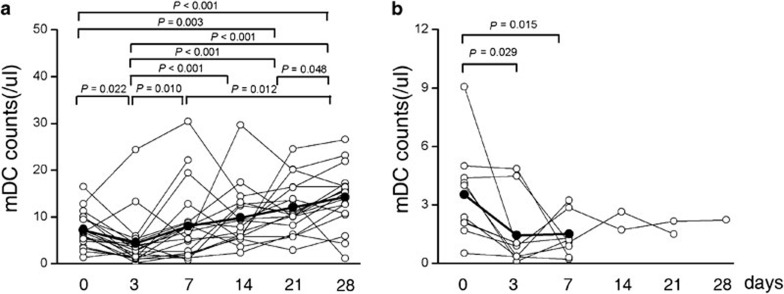
We further found that MP patients exhibited an initially rapid decrease in circulating mDC numbers within 10 days of MP treatment. Subsequently, MP survivors displayed a continuous increase in mDC numbers accompanied by a total bilirubin level decrease of more than 30% (Figure 6a). However, MP dead/transplanted patients lacked these sequential responses compared with survivors. mDCs numbers remained persistently low in this cohort of patients (Figure 6b). There was no microbiological evidence of sepsis during the study period. These data suggest that the recovery of mDC numbers at the end of treatment may represent a prognostic marker for favorable response to corticosteroid treatment in ACLF patients.
Figure 6.
The numbers of myeloid dendritic cells (mDCs) in methylprednisolone-treated patients during the 28-day fellow-up periods. Distribution of circulating mDCs in surviving (a) and non-surviving (b) acute-on-chronic liver failure patients. The P values between different time points are shown. Each symbol represents one individual and each line represents the changes in an individual patient's mDC numbers. The solid line depicts the mean numbers of mDCs.
Discussion
This study characterized circulating DC subsets and revealed their correlation with the efficacy in ACLF patient treatment with MP. We found that decreased mDC numbers at baseline were associated with a high mortality in ACLF patients. Furthermore, the survivors on MP therapy exhibited an initially rapid decrease and then recovery of circulating mDC numbers, while NS lacked this alteration. In addition to an enhanced liver function, improved survival ratios were also observed in these MP-treated ACLF patients during the clinical follow-up.
The development of ACLF involves dysregulation of both the innate and adaptive immune systems. Many different cell types, including T lymphocytes, monocytes and DCs, are primed in ACLF, and these are believed to play a pivotal role in the pathogenesis of this disease.6, 7, 8, 9, 10, 11 Corticosteroids have been used for the treatment of severe acute hepatic failure by either oral or intravenous administration. Mechanically, corticosteroids can suppress the effects of activated macrophages, DC subsets and other inflammatory cells,10, 11 which indicates that there is a reciprocal effect on the efficacy of therapy. However, this therapy has not been shown to have a satisfactory clinical effect and has not been established definitively.12, 13 As such, we speculate that finding immune markers as a surrogate for therapeutic efficacy would define the beneficial effects of this therapy. The decrease in DC numbers in peripheral blood may be an important phenomenon of ACLF pathogenesis. Previous data from our laboratory have indicated that both mDCs and pDCs infiltrate extensively the liver of ACLF patients and have expressed mature phenotypes.8, 14 Therefore, this DC migration from the blood to the liver may lead to the decreased DC numbers found in the blood, while infiltrated DCs in liver may be involved in the pathogenesis of ACLF. This study found that the decreased numbers of mDC at baseline was associated with a high mortality of ACLF patients and further indicated that the mDC numbers of the host may determine the outcome of ACLF patients. Importantly, within 10 days of MP therapy, circulating mDC numbers initially rapidly decreased, possibly indicating the apoptosis of circulating mDCs. These data are in agreement with previous studies that showed that glucocorticoid can induce the apoptosis of DCs.21 The sequential responses were uniquely found in survivors but not in NS patients because mDC recovery occurred drastically and almost simultaneously with improved liver function. Although our data suggest that a strong mDC recovery may be an important factor for favorable responses to MP treatment, it was rational to speculate that the rebound of circulating mDC numbers may not simply be a direction result of the redistribution of mDCs from the liver, where they function as effectors of ACLF pathogenesis, to the blood. The altered hepatic microenvironment or improved liver function or the recruitment of mDC or mDCs generation at bone marrow may be one of the factors which account for the rebound of mDCs.
Notably, the present study was conducted on HBV-associated ACLF patients relative to acute hepatic failure.12, 13, 22 In addition, the control and treatment groups were homogeneous in the etiology of liver disease (all patients had chronic HBV infection related liver disease) and were comparable in terms of their baseline clinical characteristics (Table 1). Therefore, this design, at least to some extent, provides more convincing data related to the efficacy of MP in the treatment of ACLF patients. However, there are still several limitations existing in this study. Firstly, we did not randomize the patients. Secondly, we did not document the mDC alterations in livers of the studied patients because liver biopsy carries a high risk in ACLF patients. However, the present study also highlights mDC numbers that should be considered as an indicator for ACLF patients who are capable of responding to MP treatment.
In conclusion, we evaluated the efficacy of MP-treated ACLF patient with altered numbers of mDCs. We found that there was a potential role for mDC numbers in the prediction of prognosis in these patients. Therefore, these findings will help to provide patients with better therapeutic options.
Acknowledgments
This study is supported by the National Natural Science Foundation of China (Nos. 30801039 and 81072424) and the major projects of viral hepatitis from Beijing Municipal Science and Technology Commission (No. H020920020890). We thank the volunteers who generously participated in this study.
The authors have no financial conflict of interest.
References
- Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Fan ST, Lo CM, Wei WI, Yong BH, Lai CL, et al. Live-donor liver transplantation for acute-on-chronic hepatitis B liver failure. Transplantation. 2003;76:1174–1179. doi: 10.1097/01.TP.0000087341.88471.E5. [DOI] [PubMed] [Google Scholar]
- O'Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764–1776. doi: 10.1053/j.gastro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Chan AC, Fan ST, Lo CM, Liu CL, Chan SC, Ng KK, et al. Liver transplantation for acute-on-chronic liver failure. Hepatol Int. 2009;3:571–581. doi: 10.1007/s12072-009-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Fan ST, Lo CM, Wei WI, Yong BH, Lai CL, et al. Live-donor liver transplantation for acute-on-chronic hepatitis B liver failure. Transplantation. 2003;76:1174–1179. doi: 10.1097/01.TP.0000087341.88471.E5. [DOI] [PubMed] [Google Scholar]
- Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, et al. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- Zou Z, Li B, Xu D, Zhang Z, Zhao JM, Zhou G, et al. Imbalanced intrahepatic cytokine expression of interferon-gamma, tumor necrosis factor-alpha, and interleukin-10 in patients with acute-on-chronic liver failure associated with hepatitis B virus infection. J Clin Gastroenterol. 2009;43:182–190. doi: 10.1097/MCG.0b013e3181624464. [DOI] [PubMed] [Google Scholar]
- Zou Z, Xu D, Li B, Xin S, Zhang Z, Huang L, et al. Compartmentalization and its implication for peripheral immunologically-competent cells to the liver in patients with HBV-related acute-on-chronic liver failure. Hepatol Res. 2009;39:1198–1207. doi: 10.1111/j.1872-034X.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, et al. Interleukin-17-producing CD4+ T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81–91. doi: 10.1002/hep.23273. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Zou ZS, Huang A, Zhang Z, Fu JL, Xu XS, et al. Hyper-activated pro-inflammatory CD16 monocytes correlate with the severity of liver injury and fibrosis in patients with chronic hepatitis B. PLoS One. 2011;6:e17484. doi: 10.1371/journal.pone.0017484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Zhang JY, Zeng Z, Tien P, Wang FS. Skewed ratios between CD3+ T cells and monocytes are associated with poor prognosis in patients with HBV-related acute-on-chronic liver failure. Biochem Biophys Res Commun. 2010;402:30–36. doi: 10.1016/j.bbrc.2010.09.096. [DOI] [PubMed] [Google Scholar]
- Balow JE, Rosenthal AS. Glucocorticoid suppression of macrophage migration inhibitory factor. J Exp Med. 1973;137:1031–1041. doi: 10.1084/jem.137.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Amraoui Z, Gueydan C, Bruyns C, Le Moine O, Vandenabeele P, et al. Methylprednisolone differentially regulates IL-10 and tumour necrosis factor (TNF) production during murine endotoxaemia. Clin Exp Immunol. 1996;106:91–96. doi: 10.1046/j.1365-2249.1996.d01-799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zou ZS, Fu JL, Cai L, Jin L, Liu YJ, et al. Severe dendritic cell perturbation is actively involved in the pathogenesis of acute-on-chronic hepatitis B liver failure. J Hepatol. 2008;49:396–406. doi: 10.1016/j.jhep.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738–746. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- van der Molen RG, Sprengers D, Biesta PJ, Kusters JG, Janssen HL. Favorable effect of adefovir on the number and functionality of myeloid dendritic cells of patients with chronic HBV. Hepatology. 2006;44:907–914. doi: 10.1002/hep.21340. [DOI] [PubMed] [Google Scholar]
- Duan XZ, Zhuang H, Wang M, Li HW, Liu JC, Wang FS. Decreased numbers and impaired function of circulating dendritic cell subsets in patients with chronic hepatitis B infection (R2) J Gastroenterol Hepatol. 2005;20:234–242. doi: 10.1111/j.1440-1746.2004.03529.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang H, Chen D, Yao J, Fu J, Jin L, et al. Response to interferon-alpha treatment correlates with recovery of blood plasmacytoid dendritic cells in children with chronic hepatitis B. J Hepatol. 2007;47:751–759. doi: 10.1016/j.jhep.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Brokaw JJ, White GW, Baluk P, Anderson GP, Umemoto EY, McDonald DM. Glucocorticoid-induced apoptosis of dendritic cells in the rat tracheal mucosa. Am J Respir Cell Mol Biol. 1998;19:598–605. doi: 10.1165/ajrcmb.19.4.2870. [DOI] [PubMed] [Google Scholar]
- Viruet EJ, Torres EA. Steroid therapy in fulminant hepatic failure secondary to autoimmune hepatitis. P R Health Sci J. 1998;17:297–300. [PubMed] [Google Scholar]