Abstract
The regulatory mechanism of Th2 bias at the maternal/fetal interface remains unclear. In this study, we characterized cytokine production in decidual stromal cells (DSCs), decidual immune cells (DICs) and embryo-derived trophoblast cells, and investigated the regulation of CXCL12/CXCR4 interaction on Th2 bias at the maternal/fetal interface in early human pregnancy. We found differential production of Th1-type and Th2-type cytokines by trophoblasts, DSCs and DICs. The secretion of these cytokines varied in different cell cocultures, conduced to Th2 bias. Flow cytometry showed that coculture of trophoblasts with DSCs and DICs significantly increased IL-4 and IL-10 production in trophoblasts, and IL-10 production in DSCs. However, the coculture of trophoblasts with DSCs and DICs significantly increased interferon (IFN)-γ expression in DSCs, and tumor-necrosis factor (TNF)-α expression in DICs. No change was seen in Th1-type cytokine production in trophoblasts, and in Th2-type cytokine production in DICs in all cocultures. Furthermore, pre-treatment with anti-CXCR4 neutralizing antibody upregulated the production of the Th1-type cytokines IFN-γ and TNF-α, and downregulated the production of the Th2-type cytokines IL-4 and IL-10, in trophoblasts, DSCs, DICs or their cocultures. Interestingly, rhCXCL12 inhibited production of the Th1-type cytokine TNF-α and enhanced the expression of the Th2-type cytokines such as IL-4 and IL-10 in DICs; this effect was abrogated by anti-CXCR4 antibody. Our present study has elucidated the individual contributions of component cells to the shaping of Th2 bias, and uncovered a complicated cross-talk via the CXCL12/CXCR4 signal at the maternal/fetal interface in early human pregnancy.
Keywords: CXCL12/CXCR4, maternal/fetal interface, Th1/Th2
Introduction
A unique cytokine network at the maternal/fetal interface plays a key role in the maintenance of pregnancy by modulating and integrating the immune and endocrine systems. It has been confirmed that Th2 bias is present at the maternal/fetal interface and in the periphery of successful pregnancy, and that a Th1 bias at the maternal/fetal interface is associated with pregnancy wastage.1,2,3,4,5 However, how the Th2 bias is developed and maintained at the maternal/fetal interface is still undefined.
It is beyond question that decidual lymphocytes play a major part in the local cytokine production and Th2 bias in normal pregnancy. In contrast to peripheral blood, a unique composition of immune cells can be found at the maternal/fetal interface with approximately 50%–70% natural killer cells, 15% macrophages and 10% CD3+ T lymphocytes. Decidual natural killer cells are CD56brightCD16−, and produce a variety of cytokines and growth factors that participate in spiral artery/tissue remodeling and placentation.6 Decidual macrophages are alternatively activated to exert anti-inflammatory actions. These cells produce few reactive oxygen derivatives, but do produce IL-10 and IL1-R antagonist.7 Placental γδT cells are activated to produce immunosuppressive IL-10 and transforming growth factor β2 which could inhibit the Th1 response.8 Decidual CD4+ and CD8+ T cells from miscarriages are defective in IL-4 and IL-10 production in comparison with decidual T cells from normal pregnancy.9 Consistent with this, the expression of a chemoattractant receptor homologous molecule on Th2 cells (a marker of IL-4-producing cells) in CD4+ and CD8+ T cells has been reported at the site of implantation in successful pregnancy.10
The commitment to Th1 or Th2 phenotypes can be brought about by multiple mechanisms, including the differential expression of distinct cytokine genes and transcriptional factors.11,12,13 Signal transducer and activator of transcription (STAT) 4 and STAT6 are key regulators in T-cell differentiation, and specifically mediate signals that emanate from the IL-12 and IL-4 receptors, respectively.14 Interferon (IFN)-γ, tumor-necrosis factor (TNF)-α and IL-12 promote the differentiation of naive Th0 cells into Th1 cells, whereas IL-4 is the most dominant factor for Th2 polarization. Various studies have categorized macrophages into M1 or M2 subsets based on cell surface phenotype, cytokine production and functional features, mirroring the Th1/Th2 paradigm.15 Both IL-4 and IL-10 can inhibit the development and function of Th1 cells and M1 macrophages, thus inhibiting allograft rejection.16 Decidual macrophages are characterized by an immunosuppressive phenotype (M2), and M2 polarization results in immune tolerance.17,18
DSCs are the major cellular component in decidua. In addition to secreting a series of cytokines, DSCs play a crucial role as non-professional antigen-presenting cells in the regulation of decidual CD4+ T-cell cytokine production which helps to maintain a balanced cytokine milieu at the maternal/fetal interface. DSCs can operate with trophoblasts in modulating trophoblast invasiveness and placentation.19,20 DSCs and trophoblasts can produce macrophage inhibitory cytokine 1 which modulates the dendritic cell phenotype with a tolerogenic subtype in decidua.21
As a key component of human placenta, trophoblasts are the only embryo-derived cells that interact directly with mother-derived cells. Human placental cytotrophoblasts produce the immunosuppressive cytokine IL-10 and the Th2-dominant cytokine IL-4 which promote trophoblast invasion and cause Th2 polarization and maternal–fetal tolerance. Placental trophoblasts decrease the production of Th1-type and Th17-type cytokines by peripheral T lymphocytes, inhibit the expression of transcription factors required for Th1 immunity and enhance the expression of transcription factors required for Th2 immunity.22
Our previous study has demonstrated that trophoblasts can confer to decidual dendritic cells the ability to induce differentiation of Th0 into Th2 cells via the secretion of thymic stromal lymphopoietin in early human pregnancy.23 Recently, we have confirmed that the first-trimester human trophoblast cells secrete chemokine (C-X-C motif) ligand 12 (CXCL12) which, in addition to inducing trophoblast proliferation and mediating crosstalk between trophoblasts and DSCs, can also recruit CD56brightCD16− natural killer cells into the decidua by its interaction with chemokine (C-X-C motif) receptor 4 (CXCR4).19,20,24 All these observations suggest that the chemokine CXCL12/CXCR4 signal might play an important role in the cross-talk between different functional cell types at the human maternal/fetal interface.
In the present study, we investigated whether the CXCL12/CXCR4 axis was involved in the development of Th2 bias at the maternal/fetal interface. We first examined the extracellular and intracellular production of Th1/Th2-type cytokines in functional cells at the human maternal/fetal interface by using a Bioplex assay and flow cytometry (FCM). Thereafter, we investigated the interaction of different components and their regulatory roles in cytokine production through different cocultures. We found that the CXCL12/CXCR4 signal axis was actively involved in the development of Th2 bias at the maternal/fetal interface.
Materials and methods
Patient recruitment
The first-trimester human villous and decidual tissues were obtained from 30 women in healthy early pregnancy confirmed by ultrasound (age, 29.70±4.78 years; gestational age at sampling, 53.83±6.72 days, mean±s.d.), which were terminated for non-medical reasons. Each subject completed a signed, written consent form approved by the Human Investigation Committee in the Hospital of Obstetrics and Gynecology, Fudan University.
Isolation and primary culture of human first-trimester trophoblast cells
Trophoblast cells were isolated by trypsin-DNase I digestion and discontinuous Percoll gradient centrifugation, as described in our previous studies,19,23 and were cultured in Dulbecco's modified Eagle medium (DMEM)—high-glucose complete medium (2 mM glutamine, 25 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid, 100 IU/ml penicillin and 100 µg/ml streptomycin) supplemented with 20% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA, USA) in 5% CO2 at 37 °C.
Isolation and primary culture of DSCs and DICs
DSCs and DICs were isolated by trypsin-DNase I digestion and discontinuous Percoll gradient centrifugation, as described in our previous study.19 The densities of the recovered DSCs or decidual mononuclear cells were between 1.042 and 1.062 g/ml, and between 1.062 and 1.077 g/ml, respectively. The cells were cultured in DMEM/F12 or RPMI-1640 complete medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin in 5% CO2 at 37 °C.
Coculture of trophoblasts, DSCs and DICs
The freshly isolated trophoblasts, DSCs or trophoblasts with DSCs (1:1) were seeded at a density of 1×106 cell/ml per well in six-well plates overnight. The supernatants were aspirated completely, and the cells were washed with 1× phosphate-buffered saline. The same number of DICs were added in each well, and cocultured continuously for 48 h; the culture of DICs alone was also included. Some wells were treated with neutralizing antibodies against CXCR4 (20 µg/ml) or the isotype control (20 µg/ml). The supernatants were collected and centrifuged at 2000 g, then transferred to a new tube and stored at −80 °C for Bioplex assay. To increase cytokine production, phorbol myristate acetate (PMA, 25 ng/ml) and ionomycin (1 µg/ml) which are commonly used to stimulate intracellular cytokine production in vitro were added to the culture to activate cells for 4 h before the end of the 48 h culture, as optimized in our previous study.23 For intracellular cytokine analysis, a Golgi inhibitor brefeldin A (10 µg/ml) was used to block cytokine secretion into the media after the activation of cells by using PMA (25 ng/ml) and ionomycin (1 µg/ml) for 4 h before the end of the 48 h culture, and then the cells were harvested and analyzed by FCM to assess intracellular cytokine production.
Cytokine quantitation in supernatant by Bioplex assay
The supernatant from each indicated group was collected and centrifuged at 2000g, then transferred to a new tube and stored at −80 °C. The levels of cytokines, including IL-4, IL-10, TNF-α, IFN-γ and CXCL12, in the culture supernatants were quantified using the Bioplex assay following the manufacturer's instructions.
Monoclonal antibodies
FITC-conjugated anti-IFN-γ (mouse IgG1), anti-CD45, anti-cytokeratin (CK)-7, phycoerythrin-conjugated anti-TNF-α (mouse IgG1), vimentin, IL-4 (mouse IgG2b), phycoerythrin-Cy5.5-conjugated anti-IL-4 (mouse IgG1), allophycocyanin-conjugated anti-IFN-γ and anti-IL-10 (mouse IgG2b) monoclonal antibodies (mAbs), and the corresponding isotype controls, were purchased from Caltag Laboratories, Inc. (Burlingame, CA, USA).
FCM
To assess intracellular cytokine production, cells were treated with PMA (25 ng/ml), ionomycin (1 µg/ml) and brefeldin A (10 µg/ml) for 4 h in 24-well flat-bottom plates (Nunc, Roskilde, Denmark) before the end of the 48 h culture with or without neutralizing antibodies against CXCR4 (20 µg/ml) or rhCXCL12 (200 ng/ml). Thereafter, the floating cells (DICs) and attached cells (trophoblasts and DSCs) were harvested and resuspended in phosphate-buffered saline at a cell density of 3×106/ml. The cell suspension was placed in Falcon 2054 polystyrene round-bottom tubes (Becton Dickinson, Franklin Lakes, NJ, USA) in 100 µl aliquots for immunolabeling. The floating leukocytes were washed twice, immediately stained with CD45 mAb (a marker for leukocytes) using a standard immunofluorescence assay, and then were fixed, permeabilized and stained for TNF-α, IFN-γ, IL-4 and IL-10 mAbs for 30 min at 4 °C. The attached trophoblasts and DSCs were fixed, permeabilized and stained for CK-7 (a marker for trophoblasts), vimentin (a marker for DSCs), TNF-α, IFN-γ, IL-4 and IL-10 mAbs, and then washed twice and resuspended in phosphate-buffered saline for FCM analysis. In parallel, isotypic IgG was used as a control. Samples were analyzed in an FACSCalibur flow cytometer (Becton Dickinson) using CellQuest software (Becton Dickinson). Statistical analysis was conducted using the isotype-matched control as the reference. Typically, less than 1% positive cells were allowed beyond the statistical marker in the appropriate control.
Statistical analysis
One-way or two-way analysis of variance was used for the statistical comparisons of cytokine production. The post hoc Dunnett t test was used to compare the significance between the control and various treatments. All error bars in the figures indicate standard errors (s.e.). Statistical significance was set at P<0.05.
Results
Secretion of cytokines (Th1/Th2) in functional cells at the maternal/fetal interface in early human pregnancy
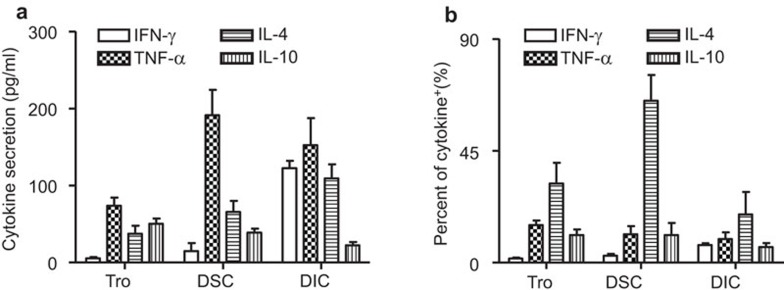
We first measured the cytokine secretion of primary functional cells at the maternal/fetal interface by the Bioplex assay. As shown in Figure 1a, the IFN-γ secretion from DICs was about 122.51±6.23 pg/ml; DSCs secreted very low levels of IFN-γ while trophoblasts secreted almost no IFN-γ. All three types of cells secreted TNF-α, with their respective secretion levels following the order: DSCs>DICs>trophoblasts. For Th2-type cytokines, IL-4 was secreted by all the cells, with their respective secretion levels following the order: DICs>DSCs>trophoblasts, while IL-10 was secreted by all the cells, with their respective secretion levels following the order: trophoblasts>DSCs>DICs. To determine intracellular cytokine production in each cell type, multiple staining and FCM were used for a panel of four cytokines (TNF-α, IFN-γ, IL-4 and IL-10). As shown in Figure 1b and Supplementary Figure 1, there was almost no expression of IFN-γ in trophoblasts and DSCs. The percentage of IFN-γ-positive DICs was about 7.06%±1.36%. About 15.11%±3.17%, 9.21%±5.7% and 9.51%±4.65% of human trophoblasts, DSCs, and DICs, respectively, were TNF-α-positive. However, IL-4-positive cells were found in 31.86%±16.50% of trophoblasts, 65.13%±17.95% of DSCs and 19.39%±15.69% of DICs. IL-10-positive cells were about 11.05%±4.67%, 11.03%±9.7% and 3.56%±3.07%, in trophoblasts, DSCs and DICs, respectively. Our current result indicates that all the examined functional cell types are involved in shaping of the Th2 bias milieu at the maternal/fetal interface of early pregnancy in humans.
Figure 1.
Expression of cytokines in the components of the maternal/fetal interface. (a) Secretion of cytokines by first-trimester trophoblasts, DSCs and DICs. Primary human trophoblasts, DSCs and DICs were cultured individually in six-well plates for 48 h. Before harvest, the cells were treated with PMA and ionomycin for 4 h. The supernatants were harvested, centrifuged and subjected to Bioplex assay. (b) Intracellular cytokine production in primary trophoblasts, DSCs and DICs. Isolated human trophoblasts, DSCs and DICs were treated with PMA, ionomycin and brefeldin A for 4 h, and then harvested and labeled for surface CD45, followed by intracellular labeling of CK-7, vimentin, TNF-α, IFN-γ, IL-4 and IL-10. The intracellular production of cytokines was analyzed by flow cytometry. The percentages of positive cells in the indicated cells summarize the findings of four independent experiments with 23 villi and four deciduas. Data represent the mean±s.e. of four experiments performed in triplicate wells with four different samples. CK, cytokeratin; DIC, decidual immune cell; DSC, decidual stromal cell; IFN, interferon; PMA, phorbol myristate acetate; s.e., standard error; TNF, tumor-necrosis factor.
Th2 bias is regulated by interaction of the functional cells at the maternal/fetal interface
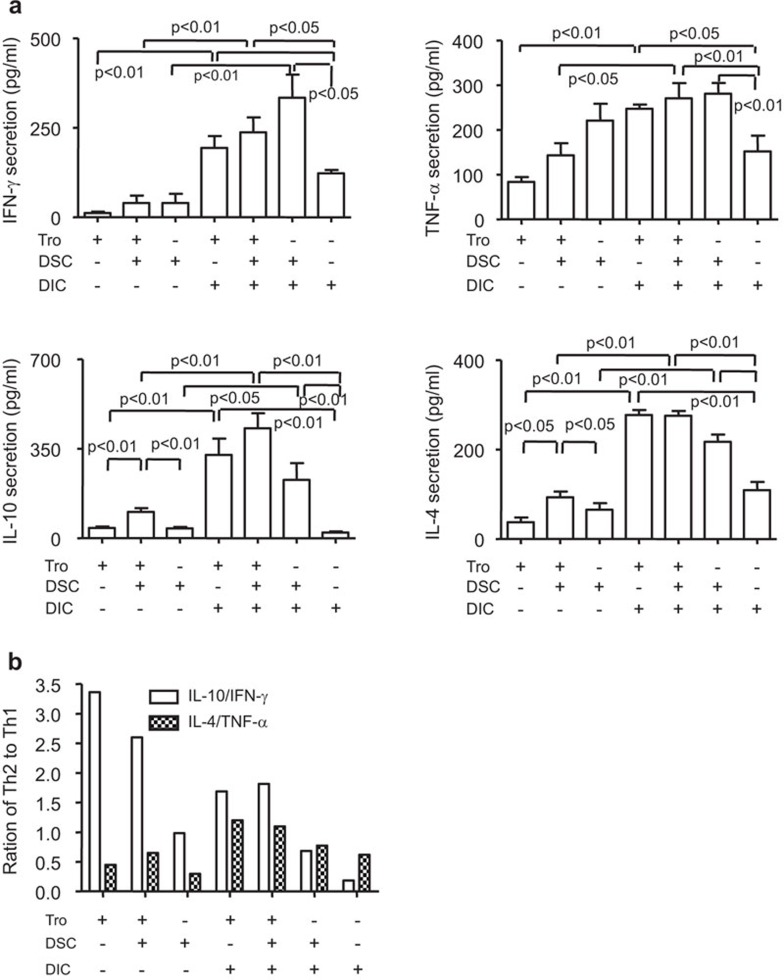
To investigate the interplay of the components involved in Th2 bias at the maternal/fetal interface, we first evaluated the secretion of cytokines in the supernatant from different cocultures. As shown in Figure 2a, the coculture of trophoblasts with DSCs increased Th2-type but not Th1-type cytokine secretion. Both Th1- and Th2-type cytokines were further increased in the coculture of trophoblasts, DSCs and DICs. The secretion of Th1-type cytokines was found to be highest in the DSC+DIC cocultures without or with trophoblast, followed by the trophoblast+DIC coculture, and lowest in the trophoblast+DSC coculture. In contrast, the secretion of Th2-type cytokines was highest in the trophoblast+DIC+DSC and trophoblast+DIC coculture, and lowest in the trophoblast+DSC coculture. We then investigated the ratios of IL-4/TNF-α and IL-10/IFN-γ, and found that the ratio of IL-4/TNF-α increased in the coculture, particularly in the coculture of all three cell types, compared to that of cell type alone (Figure 2b). However, the ratio of IL-10/IFN-γ was very high in trophoblasts alone, even with DSCs, which might be due to the limited secretion of IFN-γ by trophoblasts. Moreover, the ratio of IL-10/IFN-γ was much higher in the other coculture, especially in coculture of all three components compared to the corresponding single cell type. All these observations suggest that the interaction between trophoblasts, DSCs and DICs upregulates Th2 bias at the maternal/fetal interface.
Figure 2.
Th2 bias results from the interaction among trophoblasts, DSCs and DICs at the maternal/fetal interface. (a) Cytokine secretion in individual cultures or cocultures of primary trophoblasts, DSCs and DICs. Primary human trophoblasts, DSCs or DICs were cultured in six-well plates for 48 h. Before harvest, the cells were treated with PMA and ionomycin for 4 h. The supernatants were harvested, centrifuged and subjected to the Bioplex assay. Cytokine secretion from the indicated cells is shown, and the results summarize the findings of four independent experiments with 27 villi and four decidua. Data represent the mean±s.e. of four experiments performed in triplicate wells with four different samples. (b) The ratios of Th2/Th1-type cytokines in different coculture systems were obtained from the mean values in (a). DIC, decidual immune cell; DSC, decidual stromal cell; PMA, phorbol myristate acetate; s.e., standard error.
Reciprocal modulation of functional cells in cytokine production at the maternal/fetal interface
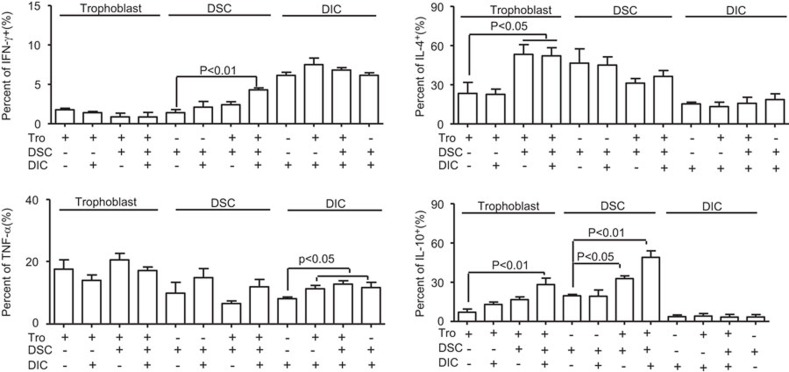
To define the individual contributions of functional cells to the Th2 bias at the maternal/fetal interface, intracellular cytokine production was analyzed by FCM. CK-7 and vimentin were used as markers of trophoblasts and DSCs, respectively, and CD45 was used as marker of decidual leukocytes. It was found in Figure 3 and Supplementary Figure 2 that the coculture of trophoblast cells with DICs significantly increased the production of IL-10 in trophoblast cells, and increased the production of the Th1-type cytokines TNF-α and IFN-γ in DICs. The production of the Th2-type cytokine IL-10 was increased in DSCs when cocultured with trophoblast cells. Although an upregulation of the Th2-type cytokines IL-4 and IL-10 in trophoblast cells was also observed, DSCs did not alter Th1-type cytokine production in trophoblast cells. Moreover, the addition of DSCs into coculture of trophoblast cells and DICs significantly increased IL-10 production in trophoblast cells. In contrast, the coculture of DSCs with DICs affected neither Th1 nor Th2 cytokine production in DSCs, but increased TNF-α production in DICs. Interestingly, the production of IL-10 and IFN-γ increased significantly in DSCs when cocultured with trophoblasts and DICs. Increased TNF-α expression in DICs was also observed in coculture containing all the three cell types.
Figure 3.
Th1- and Th2-type cytokine production in coculture of maternal/fetal interface components. Isolated human trophoblasts, DSCs and DICs were seeded in six-well plates for 48 h. Before harvest, the cells were treated with PMA, ionomycin and brefeldin A for 4 h. Cells were labeled for surface expression of CD45 and for intracellular CK-7, vimentin, IFN-γ, TNF-α, IL-4 and IL-10. Intracellular cytokine production was analyzed by flow cytometry. The percentages of positive cells in the indicated cells are shown, and the results summarize the findings of four independent experiments with 29 villi and four decidua. Data represent the mean±s.e. of four experiments performed in triplicate wells with four different samples. CK, cytokeratin; DIC, decidual immune cell; DSC, decidual stromal cell; IFN, interferon; PMA, phorbol myristate acetate; s.e., standard error; TNF, tumor-necrosis factor.
CXCL12/CXCR4 axis is involved in Th2 bias at the maternal/fetal interface
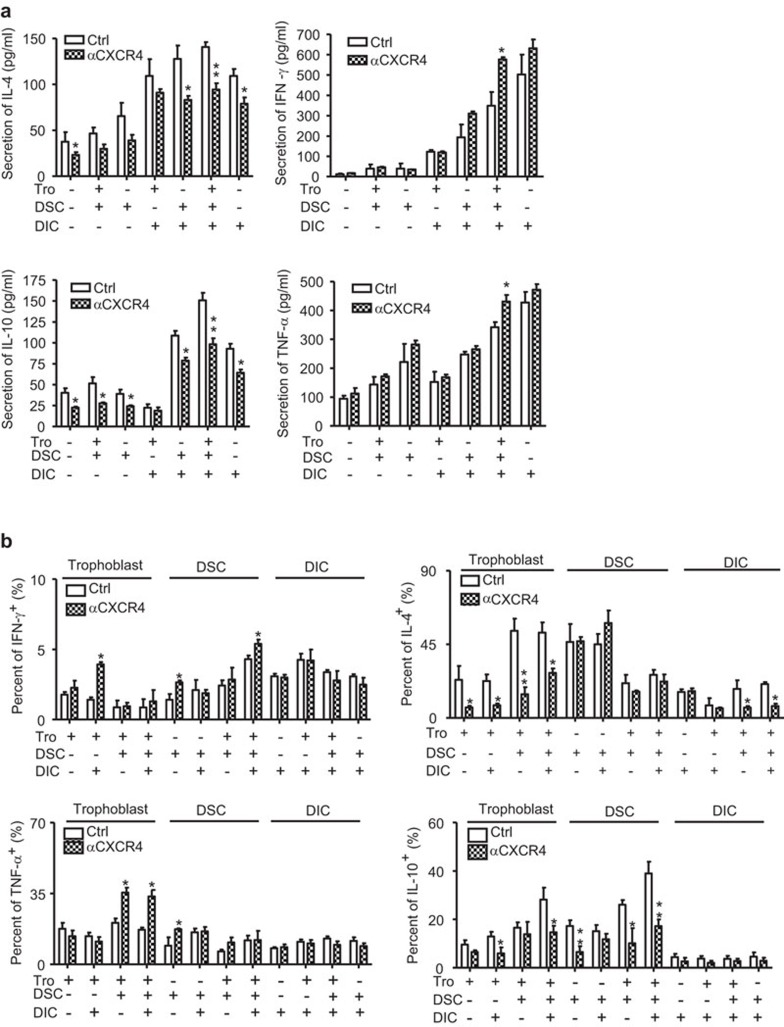
Our previous study has demonstrated that the CXCL12/CXCR4 axis plays an important role in the cross-talk between trophoblasts, DSCs and DICs.23,24,25,26 In the present study, we investigated how this axis was involved in the development of the Th2 bias at the maternal/fetal interface. As shown in Figure 4a, treatment with anti-CXCR4 antibody significantly inhibited IL-4 and IL-10 secretion in all cultures, especially in coculture of two or three cell types. Furthermore, the treatment significantly increased the secretion of Th1-type cytokines, such as IFN-γ and TNF-α, in the coculture of all the three types of cells, but not in the culture of a single cell type, or in the coculture of two cell types. FCM analysis of intracellular cytokine production (Figure 4b) demonstrated a decrease of IL-4 expression in trophoblasts and DICs after treatment with anti-CXCR4 antibody. A decrease of IL-10 expression in trophoblasts and DSCs in different cultures was also observed after this treatment. In contrast, treatment with anti-CXCR4 antibody significantly increased IFN-γ and TNF-α production in trophoblasts and DSCs, but not in DICs.
Figure 4.
The CXCL12/CXCR4 signal is involved in the development of Th2 bias at the maternal/fetal interface. Primary human trophoblasts, DSCs and DICs were seeded in six-well plates for 48 h. In the coculture system, the ratio of trophoblasts/DSCs/DICs was 1:1:1. A neutralizing antibody against CXCR4, or the isotype control antibody (20 µg/ml) were added in some wells. Before harvest, the cells were treated with PMA, ionomycin or brefeldin A for 4 h. The secretion (a) and intracellular expression (b) of cytokines were determined by Bioplex and flow cytometry, respectively. (a) The effect of CXCL12/CXCR4 on the secretion of Th1- and Th2-type cytokines at the maternal/fetal interface. (b) The effect of CXCL12/CXCR4 on the intracellular expression of Th1- and Th2-type cytokines in three types of cells at the maternal/fetal interface. The findings of four independent experiments are summarized with 35 villi and four deciduas. Data represent the mean±s.e. of four experiments performed in triplicate wells with four different samples. *P<0.05, compared to the isotype antibody-treated corresponding cells. αCXCR4 indicates pre-treatment with 20 µg/ml of neutralizing antibody against CXCR4. DIC, decidual immune cell; DSC, decidual stromal cell; PMA, phorbol myristate acetate; s.e., standard error; Tro, primary human trophoblasts.
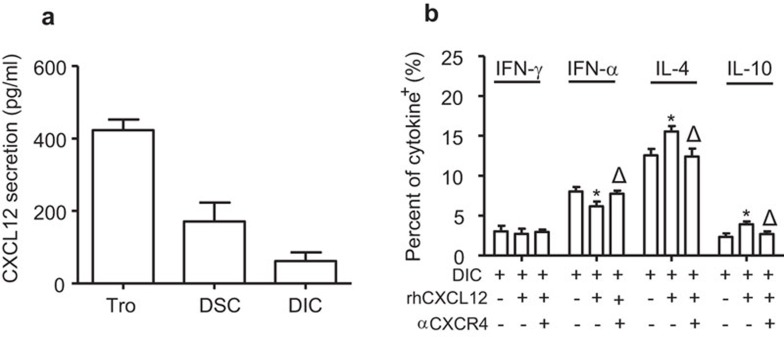
CXCL12 promotes Th2 bias at the maternal/fetal interface
Because CXCL12 is secreted by trophoblasts, secreted at lower levels by DSCs and minimally secreted by DICs (Figure 5a), we further evaluated the effect of exogenous CXCL12 on Th1- and Th2-type cytokine production in DICs. As shown in Figure 5b, human recombinant CXCL12 alone increased Th2-type IL-4 and IL-10 production while decreasing Th1-type TNF-α expression in DIC cells. Pre-treatment with anti-CXCR4 antibody eliminated the effect of CXCL12 on the cytokine production in DICs. Together, our data suggest that CXCL12/CXCR4 axis is involved in the development of Th2 bias at the maternal/fetal interface.
Figure 5.
CXCL12 promotes Th2-type and inhibits Th1-type cytokine production in DICs in early human pregnancy. (a) Primary human trophoblasts, DSCs and DICs were cultured in six-well plates for 48 h. Before harvest, the cells were treated with PMA and ionomycin for 4 h. The supernatants were harvested, centrifuged and then subjected to Bioplex assay. The findings of four independent experiments are summarized with 12 villi and four deciduas. Data represent the mean±s.e. of four experiments performed in triplicate wells with four different samples. (b) Primary human DICs were seeded in six-well plates for 48 h. rhCXCL12 (200 ng/ml) and/or neutralizing antibody against CXCR4 (20 µg/ml) were added in some wells. Before harvest, the cells were treated with PMA and ionomycin for 4 h. The secretion of cytokines was determined by the Bioplex assay. The findings of four independent experiments are summarized with 20 villi and four deciduas. Data represent the mean±s.e. of four experiments performed in triplicate wells with four different samples. *P<0.05, compared to the corresponding isotype antibody-treated cells. ΔP<0.05, compared to rhCXCL12-treated DICs. rhCXCL12: treatment with 200 ng/ml of recombinant human CXCL12. αCXCR4 indicates pretreatment with 20 µg/ml of neutralizing antibody against CXCR4. DIC, decidual immune cell; DSC, decidual stromal cell; PMA, phorbol myristate acetate; s.e., standard error.
Discussion
In the present study, we have shown that coculture of two or three of these cell types results in increased secretion of both pro-inflammatory Th1-type and anti-inflammatory Th2-type cytokines. Interestingly, increased Th1 cytokine production was mainly from the maternally originated DICs and DSCs, whereas Th2 cytokine production was from trophoblasts and DSCs. The net increase in the coculture brings about Th2 bias, especially in the coculture of all three cell types. Unexpectedly, the highest levels of Th2-type cytokines IL-4 and IL-10 were seen whenever DICs were present in the culture, suggesting that DICs are required for the production of these Th2-type cytokines in trophoblasts and DSCs. Our data also indicate that maternal immune cells attempt to attack allogeneic fetal placental trophoblasts by producing the Th1-type cytokine TNF-α, while trophoblasts intend to defend themselves by producing the Th2-type cytokines IL-4 and IL-10, and that DSCs play an intermediary role in reconciling the relationship between trophoblasts and DICs and creating a harmonious Th2-type cytokine microenvironment. This conclusion differs slightly from that of other reports in which trophoblasts were believed to promote Th2 polarization.22,25 The explanation for this discrepancy might be that others treated peripheral T cells rather than decidual T cells with trophoblast-cultured supernatant, rather than direct coculture with the trophoblast. Our unpublished data show similar effects of trophoblasts and DSCs on peripheral immune cells.
During pregnancy, the uterine mucosa is characterized by a large number of maternal immune cells. These immune cells are found in close contact with embryo-derived trophoblast cells. From an immunological perspective, a successful pregnancy is paradoxical, because interactions between maternal immune cells and fetal trophoblasts which express paternally inherited alloantigens, should provoke maternal immune responses that attack fetal antigens. Interestingly, depletion of immune cells, instead of helping the pregnancy, results in termination of pregnancy.1 Therefore, there appears to be a complex but compatible and harmonious dialogue among the multiple cell types at the maternal/fetal interface modulated by some regulatory factors to support a successful pregnancy.
Cytokines are an array of regulatory factors that are secreted by a large number of immune and non-immune cells, and are involved extensively in intercellular communication. It is widely recognized that cytokines play a fundamental role at the maternal/fetal interface in early human pregnancy, and that cytokine dysregulation must be linked to pregnancy wastage.2,3,4,5,26 For many years, it has been believed that the predominance of Th2-type cytokines, such as IL-4 and IL-10, over Th1-type cytokines, such as IFN-γ and TNF-α, at the maternal/fetal interface was fundamental for a successful pregnancy.2,3,4 However, the potential mechanism of development of Th2 bias at the maternal/fetal interface remains unclear.
DSC is another important cell type at the maternal/fetal interface, which originates from the proliferation and differentiation of fibroblast-like stromal cell precursors in the endometrium and is the major cellular component of human decidua.27 DSCs are involved in a series of immune regulatory processes, such as the production of cytokines as well as antigen phagocytosis and presentation,28,29,30 indicating that DSCs might be an important regulator on Th2 bias at the maternal/fetal interface.
As the critical component in human placenta, embryo-derived trophoblasts may also play an important role in shaping the Th2 bias at the maternal/fetal interface.31,32 Pregnancy wastage is accompanied by downregulation of both pro- and anti-inflammatory cytokines in trophoblasts.33 Trophoblasts can produce cytokines such as IL-10 and TNF-α,32 and cytokine gene polymorphism in the promoter regions of TNF-α and IL-10 is associated with recurrent pregnancy loss.34
Chemokines play well-established roles as attractants of naive and effector T cells. Different chemokine receptors are preferentially expressed on Th1 cells or Th2 cells, which is dependent on the genetic programs activated by STAT4 and STAT6.35 Our current study has demonstrated that rhCXCL12 can promote the production of Th2 cytokines while inhibiting Th1 cytokine production from DICs, and that an anti-CXCR4 antibody can reverse the effect of CXCL12 on cytokine production in DICs. Furthermore, Th1-type cytokines can be upregulated, while Th2-type cytokines can be downregulated by the anti-CXCR4 antibody in immune and non-immune cells at the maternal/fetal interface. These results suggest that the CXCL12/CXCR4 axis is involved in the regulation of Th1/2-type cytokine production and the development of the Th2 bias at the maternal/fetal interface. Our data also show that the effect of anti-CXCR4 or rhCXCL12 is not dramatic which suggest that they may not explain the whole phenotype and that other mediators play important roles in the dialogue of different types of cells and Th2 bias at the maternal/fetal interface. How the chemokine CXCL12/CXCR4 signal regulates the cross-talk among trophoblasts, DSCs and DICs, and the production of their cytokines will be determined in future studies.
In summary, we have demonstrated that fetal trophoblast cells and maternal DSCs and DICs produce various cytokines. In vitro coculture of these cell types results in the production of both Th1-type and Th2-type cytokines in the supernatant, but Th2-type cytokines predominate over Th1-type cytokines. FCM analysis uncovered the respective contributions of functional cells to the shaping of the Th2 bias at the maternal/fetal interface. Blockade of the CXCL12/CXCR4 signal switches the Th2 to a Th1 bias in the coculture while rhCXCL12 enhances Th2 and reduces Th1 cytokine expression in DICs. Our study sheds light on the complicated interaction among functional cells through the CXCL12/CXCR4 signal, which mediates a Th2 bias at the maternal/fetal interface. Combined with the observation that decreased Th2 cytokine production and CXCL12/CXCR4 expression are observed in the decidua and villi from miscarriage (unpublished data), the current study deepens our understanding of the mechanisms of Th2 bias at the maternal/fetal interface. It can also be speculated that dysregulation of CXCL12/CXCR4 expression during pregnancy might result in detrimental effects on the outcome of pregnancy.
It is worth noticing that FACS (fluorescence activated cell sorting) staining of cells has a lot of intrinsic problems to be correlated with Bioplex or ELISA results. For example, percentage of positive cells may not correlate with final production of the cytokines because a few positive cells may account of the production of most of the cytokines in the supernatant. In addition, the definition of positive or negative in FACS results is very arbitrary. The quantification and comparison between different treatments are relative. In deed, results in Figure 1a showed significant difference with those in Figure 1b. Thinking of these reasons, we will sort each cell population and use ELISA to accurately measure the cytokine production in the cell extract in a future study.
Acknowledgments
This work was supported by the Key Project and Major International Joint Research Project of NSFC 30730087, 30910103909, the Program for Outstanding Medical Academic Leader (all to DJ Li), NSFC 81070537, NSFC 31171437, the Shanghai PuJiang Talent Program 10PJ1401600 (all to MR Du) and the Natural Science Research Program for colleges and universities of Jiangsu Province 10KJB320021 (to R Zhu).
Supplementary Information
References
- Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bi-directional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon. Immunol Today. 1993;7:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Raghupathy R. Pregnancy: success and failure within the Th1/Th2/Th3 paradigm. Semin Immunol. 2001;13:219–227. doi: 10.1006/smim.2001.0316. [DOI] [PubMed] [Google Scholar]
- Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–482. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- Raghupathy R, Kalinka J. Cytokine imbalance in pregnancy complications and its modulation. Front Biosci. 2008;13:985–994. doi: 10.2741/2737. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- Fan DX, Duan J, Li MQ, Xu B, Li DJ, Jin LP. The decidual gamma-delta T cells up-regulate the biological functions of trophoblasts via IL-10 secretion in early human pregnancy. Clin Imunol. 2011;141:284–292. doi: 10.1016/j.clim.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli GF, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- Michimata T, Sakai M, Miyazaki S, Saito S. Decrease of T-helper 2 and T-cytotoxic 2 cells at implantation sites occurs in unexplained recurrent spontaneous abortion with normal chromosomal content. Hum Reprod. 2003;18:1523–1528. doi: 10.1093/humrep/deg280. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Ouyang W, Szabo SJ, Jacobson NG, Guler ML, Gorham JD, et al. T helper differentiation proceeds through Stat1-dependent, Stat4-dependent and Stat4-independent phases. Curr Top Microbiol Immunol. 1999;238:13–26. doi: 10.1007/978-3-662-09709-0_2. [DOI] [PubMed] [Google Scholar]
- Rincón M, Flavell RA. Transcriptional control in the Th1/Th2 decision. Curr Biol. 1997;7:729–732. doi: 10.1016/s0960-9822(06)00368-x. [DOI] [PubMed] [Google Scholar]
- Agarwal1 S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- Wurster AL, Tanaka T, Grusby MJ. The biology of Stat4 and Stat6. Oncogene. 2000;19:2577–2584. doi: 10.1038/sj.onc.1203485. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Bashyam H. Th1/Th2 cross-regulation and the discovery of IL-10. J Exp Med. 2007;204:237. doi: 10.1084/jem.2042fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63:460–471. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Nagamatsu T, Schust DJ. The immunomodulatory roles of macrophages at the maternal–fetal interface. Reprod Sci. 2010;17:209–218. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]
- Wu X, Li DJ, Yuan MM, Zhu Y, Wang MY. The expression of CXCR4/CXCL12 in first-trimester human trophoblast cells. Biol Reprod. 2004;70:1877–1885. doi: 10.1095/biolreprod.103.024729. [DOI] [PubMed] [Google Scholar]
- Zhou WH, Du MR, Dong L, Yu J, Li DJ. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal cells in human first-trimester pregnancy. Hum Reprod. 2008;23:2669–2679. doi: 10.1093/humrep/den308. [DOI] [PubMed] [Google Scholar]
- Segerer SE, Rieger L, Kapp M, Dombrowski Y, Muller N, Dietl J, et al. MIC-1 (a multifunctional modulator of dendritic cell phenotype and function) is produced by decidual stromal cells and trophoblasts. Hum Reprod. 2012;27:200–209. doi: 10.1093/humrep/der358. [DOI] [PubMed] [Google Scholar]
- Liu F, Guo J, Tian T, Wang H, Dong F, Huang H, et al. Placental trophoblasts shifted Th1/Th2 balance toward Th2 and inhibited Th17 immunity at fetomaternal interface. APMIS. 2011;119:597–604. doi: 10.1111/j.1600-0463.2011.02774.x. [DOI] [PubMed] [Google Scholar]
- Guo PF, Du MR, Wu HX, Lin Y, Jin LP, Li DJ. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood. 2010;116:2061–2069. doi: 10.1182/blood-2009-11-252940. [DOI] [PubMed] [Google Scholar]
- Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16− NK cells into decidua by way of expressing and secreting of CXCL12/SDF-1. J Immunol. 2005;175:61–68. doi: 10.4049/jimmunol.175.1.61. [DOI] [PubMed] [Google Scholar]
- Kanai T, Fujii T, Kozuma S, Yamashita T, Miki A, Kikuchi A, et al. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol Hum Reprod. 2001;7:195–200. doi: 10.1093/molehr/7.2.195. [DOI] [PubMed] [Google Scholar]
- Clark DA, Croitoru K. TH1/TH2, 3 imbalance due to cytokine-producing NK, gammadelta T and NK-gammadelta T cells in murine pregnancy decidua in success or failure of pregnancy. Am J Reprod Immunol. 2001;45:257–265. doi: 10.1111/j.8755-8920.2001.450501.x. [DOI] [PubMed] [Google Scholar]
- Richards RG, Brar AK, Frank GR, Hartman SM, Jikihara H. Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of prolactin and insulin-like growth factor binding protein-1 expression. Biol Reprod. 1995;52:609–615. doi: 10.1095/biolreprod52.3.609. [DOI] [PubMed] [Google Scholar]
- Olivares EG, Montes MJ, Oliver C, Galindo JA, Ruiz C. Cultured human decidual stromal cells express B7-1 (CD80) and B7-2 (CD86) and stimulate allogeneic T cells. Biol Reprod. 1997;57:609–615. doi: 10.1095/biolreprod57.3.609. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H. IL-15 expression at human endometrium and decidua. Biol Reprod. 2000;63:683–687. doi: 10.1095/biolreprod63.3.683. [DOI] [PubMed] [Google Scholar]
- Ruiz C, Montes MJ, Abadia-Molina AC, Olivares EG. Phagocytosis by fresh and cultured human decidual stromal cells: opposite effects of interleukin-1 alpha and progesterone. J Reprod Immunol. 1997;33:15–26. doi: 10.1016/s0165-0378(96)01009-1. [DOI] [PubMed] [Google Scholar]
- Royle C, Lim S, Xu B, Tooher J, Ogle R, Hennessy A. Effect of hypoxia and exogenous IL-10 on the pro-inflammatory cytokine TNF-alpha and the anti-angiogenic molecule soluble Flt-1 in placental villous explants. Cytokine. 2009;47:56–60. doi: 10.1016/j.cyto.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Torricelli M, Voltolini C, Bloise E, Biliotti G, Giovannelli A, de Bonis M, et al. Urocortin increases IL-4 and IL-10 secretion and reverses LPS-induced TNF-alpha release from human trophoblast primary cells. Am J Reprod Immunol. 2009;62:224–231. doi: 10.1111/j.1600-0897.2009.00729.x. [DOI] [PubMed] [Google Scholar]
- Scott VL, Shack LA, Eells JB, Ryan PL, Donaldson JR, Coats KS. Immunomodulator expression in trophoblasts from the feline immunodeficiency virus (FIV)-infected cat. Virol J. 2011;8:336. doi: 10.1186/1743-422X-8-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Kaur A. Recurrent pregnancy loss: TNF-α and IL-10 polymorphisms. J Hum Reprod Sci. 2011;4:91–94. doi: 10.4103/0974-1208.86090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Gunst KV, Sarvetnick N. STAT4/6-dependent differential regulation of chemokine receptors. Clin Immunol. 2006;118:250–257. doi: 10.1016/j.clim.2003.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.