Abstract
Follicular helper T (TFH) cells represent a distinct subset of CD4+ helper T (TH) cells specialized in providing help to B cells. They are characterized by their unique transcriptional profile (Bcl6), surface marker expression (CXCR5, PD-1, ICOS and CD40L) and cytokine production pattern (IL-21 and IL-6). TFH cells provide help to B cells both to form germinal centers (GCs) and to differentiate into memory B cells and plasma cells for generation of humoral responses. However, there is emerging evidence that implicates TFH cells in the development of various human pathologies, such as autoimmune diseases, immunodeficiency and lymphoma. This review focuses on the current progress in this area including mouse and human studies. A clearer understanding of the mechanisms of TFH cell-mediated immunity and pathology may be exploited for rational development of therapeutic strategies.
Keywords: autoimmunity, B cells, immunodeficiency, lymphoma, TFH cells
Introduction
Antibody responses are a key component of the adaptive immune system. These responses are mostly dependent on help from antigen-specific CD4+ helper T (TH) cells. In recent years, follicular helper T (TFH) cells, a specialized subset of TH cells, have attracted much attention due to their ability to provide critical help to germinal center (GC) B cells. Although TFH and other TH cell subsets share many phenotypic and functional features, the recent identification of the master transcriptional regulator, B-cell lymphoma-6 (Bcl6), of TFH-cell differentiation has established TFH cells as a distinct subset of TH cells.1,2,3 TFH cells provide help to B cells both to generate and maintain the GCs as well as to differentiate into memory B cells and plasma cells. The plasma cells secrete antigen-specific antibodies for generation of humoral responses against a variety of pathogens, including viruses and bacteria.4,5 However, if TFH-cell function is not properly regulated, various pathologies ensue.6,7,8,9,10,11 Here we present a review of the current literature pertaining to the role of TFH cells in the development of pathologies, such as autoimmunity, immunodeficiency and lymphoma, and the implications of these studies for development of therapies.
Characteristics of TFH cells
Phenotypically, TFH cells can be characterized by the high expression of certain surface markers, such as chemokine receptor-5 (CXCR5), programmed death-1 (PD-1), inducible costimulator (ICOS) and CD40 ligand (CD40L).12 These markers contribute to the migration of TFH cells and the interaction of TFH cells with B cells to promote B-cell responses against pathogens. The binding of CXCR5 on TFH cells with its cognate ligand, CXCL-13, in GC facilitates the migration of TFH cells to B-cell follicles for their interaction with B cells.13,14 Upon TFH-cell activation, PD-1 induces an inhibitory signal to TFH cells, whereas ICOS functions as a costimulatory molecule, thus determining the outcome of the TFH cell-mediated B-cell response.15,16 Furthermore, CD40L, a member of the tumor necrosis factor family, is expressed on activated TFH cells, and its interaction with CD40 on B cells is crucial for immunoglobulin isotype switching.17 In addition, signaling lymphocytic activation molecule-associated protein (SAP) has been shown to play a critical role for TFH-cell function in GC formation and immune homoeostasis.18
Cytokines particularly IL-21 and IL-6 have been shown to play a key role in both TFH-cell differentiation and antigen-specific humoral responses. On the one hand, IL-21 has been considered as the master cytokine for the regulation of TFH-cell development that depends on the costimulation via CD40–CD40L and ICOS–ICOSL interactions. IL-21 induces production of IL-21 by TFH cells in an autocrine fashion, leading to the differentiation of TFH cells.19 Furthermore, IL-6 induces both IL-21 production as well as TFH-cell generation.20 A recent study by Eto et al.21 shows that TFH-cell differentiation requires both IL-6 and IL-21, and that these cytokines alone are not sufficient to drive TFH-cell differentiation, indicating the synergistic relationship between IL-21 and IL-6 that regulates the TFH-cell differentiation. On the other hand, IL-21 is critical for TFH cells to promote B-cell somatic hypermutation and immunoglobulin class switching.22 TFH cells can also produce other cytokines, similar to other TH cells such as TH1 and TH2 cells, which may be relevant to the development of various classes of antibodies.
The identification of Bcl6 as a master regulator of TFH-cell differentiation has laid the basis for TFH cells as a distinct subset of TH cells. In contrast to the other TH cell subsets, such as TH1, TH2 and TH17 cells, which are controlled by T-bet, GATA3 and RORγt, respectively, TFH cells are dependent on Bcl6.1,2,3 Bcl6-deficient mice failed to develop TFH cells, suggesting that Bcl6 is critical for programming of TFH-cell differentiation.1,2,3 In contrast, Blimp-1, another transcription factor, acts as an antagonist of Bcl6 by specifically inhibiting TFH-cell differentiation.23 Therefore, Bcl6 and Blimp-1 are critical for TFH-cell differentiation, with promoting and regulatory role, respectively.
Although TFH cells act as a distinct subset of TH cells, they also show considerable plasticity. TFH cell-like features can also be exhibited by other subsets of T cells, including regulatory T (Treg) and invariant natural killer T (iNKT) cells.24,25,26,27 In particular, recent studies have described a population of follicular T regulatory (TFR) cells that share the phenotypic characteristics with TFH and Treg cells. Like classic TFH cells, the so-called TFR cells express high levels of CXCR5 and PD-1, and require Bcl6, SAP and CD28 for their development, but they express Foxp3 and can inhibit the GC reactions, similar to Treg cells. TFR cells are considered to be produced de novo from the progenitors of CXCR5−Foxp3 natural Treg cells.25,26,27 Interestingly, the expression of TFH cell-like phenotype is not just confined to conventional T cells. Chang et al.24 recently identified a subset of iNKT, iNKTFH, cells that share phenotypic, functional and ontological characteristics of TFH cells. These iNKTFH cells showed enhanced expression of CXCR5 and PD-1, and provided help to lipid antigen-reactive B cells via production of IL-21. Not surprisingly, the development of iNKTFH cells was found to be dependent on the transcriptional factor Bcl6 and CD28 signaling.24
TFH cells in autoimmunity
The hallmark of TFH-cell function is to help B cells to generate humoral immune responses, which is a complex physiological process that requires the formation of GC in secondary lymphoid tissues, such as spleen and lymph nodes. Specifically, TFH cells emit instructive signals to B cells to form and maintain GC. The GC provides a podium where TFH cells instruct B cells not only to differentiate into memory B cells, but also to class switch for antibodies through somatic hypermutation and isotype switching, resulting in the formation of antigen-specific high-affinity antibodies to combat infectious agents.28 However, unwanted antibody responses can come with the risk of autoimmune diseases (Figure 1). Many lupus-prone murine models show the spontaneous generation of GCs that is positively correlated with the production of autoantibodies.29,30 In human systemic lupus erythematosus (SLE) patients, autoreactive B cells actively participate in GC reactions, which eventually lead to the formation of pathogenic autoantibodies.31 A growing body of evidence further suggests that the aberrant function of TFH cells plays a critical role in generation of autoantibodies via autoreactive GC B cells, which inflict autoimmune pathologies in humans and mice (Table 1).
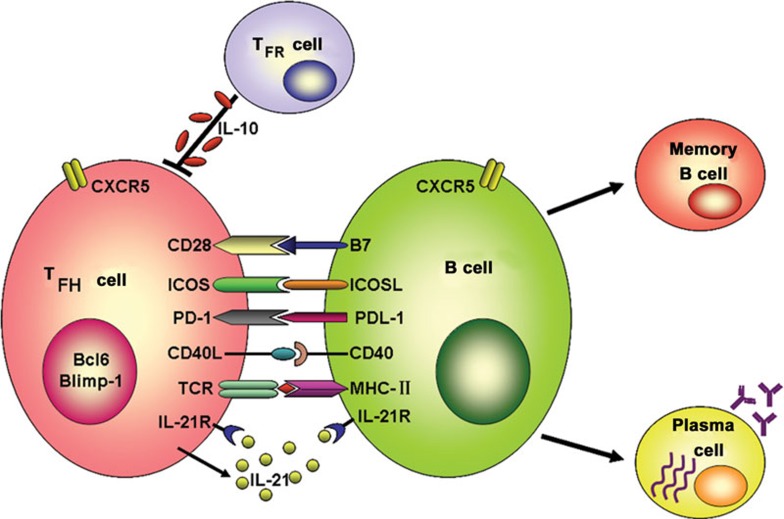
Figure 1.
Interaction between TFH and B cells to induce humoral responses. Activated TFH cells upregulate CXCR5 and migrate toward B-cell follicles to form GC. In GC, TFH cells interact with antigen-specific B cells through various molecules such as ICOS–ICOSL, PD-1–PDL-1, CD40–CD40L and IL-21R–IL-21, resulting in the production of memory B and plasma cells. The plasma cells secrete long-lived antibodies to combat infectious agents. However, aberrant TFH cell function leads to the production of autoantibodies that may result in autoimmune pathologies. TFR cells can inhibit the self-reactive B-cell responses such as autoantibody production via secretion of IL-10. CD40L, CD40 ligand; CXCR5, chemokine receptor-5; GC, germinal center; ICOS, inducible costimulator; PD-1, programmed death-1; TFH, follicular helper T; TFR, follicular T regulatory.
Table 1. TFH cells in autoimmunity.
| Autoimmune condition | Clinical manifestations | Phenotype of TFH cells | Mechanism of TFH cell dysfunction | Reference |
|---|---|---|---|---|
| SLE | Autoantibodies against dsDNA, hypergammaglobulinemia, thrombocytopenia, glomerulonephritis lymphadenopathy | CD4+CXCR5+PD-1hiICOShi | GC expansion TFHC cell overrepresentation | 51 |
| SS | Anti-SSA and SSB hypergammaglobulinemia, lymphadenopathy | CD4+CXCR5+PD-1hiICOShi | TFHC cell overrepresentation | 51 |
| Primary SS | Anti-SSA, SSB hypergamma-bulinemia salivary gland damage | CD4+CXCR5+CCR6+PD-1hiICOShi | TFHC cell overrepresentation | 55 |
| AITD | Autoantibodies, such as anti-TSH receptor, anti-thyroglobulin and anti-thyroperoxidase antibodies, thyroid dysfunction | CD4+CXCR5+PD-1hiICOShi | TFHC cell overrepresentation | 54 |
| RA | Circulating autoantibodies inflammation of joints | CD4+CXCR5+ICOShi | TFHC cell overrepresentation | 53 |
| JDM | Skin and muscle inflammation | CD4+CXCR5+ | Overrepresentation of TH2 and TH17 cell-like TFHC cells | 52 |
| Sanroque mouse | Hypergammaglobulinemia, lymphadenopathy, glomerulonephritis | CD4+CXCR5+PD-1hiICOShi | TFH cell overrepresentation, spontaneous GC formation, increased ICOS expression | 32 |
| BXSB-Yaa mouse | Hypergammaglobulinemia, lympgadenopathy, glomerulonephritis UV-induced dermatitis | CD4+CXCR5+PD-1hiICOShi | GC expansion, aberrant IL-21 secretion | 41 |
| Pristane-induced lupus in BALB/c mouse | Hypergammaglobulinemia, lympgadenopathy, glomerulonephritis | CD4+CXCR5+PD-1hiICOShi | GC expansion, overexpression of ICOS and IL-21 | 46 |
| NZB/W F1 mouse | Hypergammaglobulinemia, lympgadenopathy, glomerulonephritis | CD4+CXCR5+ICOShi | TFH cell overrepresentation, altered production of IL-21 and IL-17 | 45 |
Abbreviations: AITD, autoimmune thyroid diseases; CXCR5, chemokine receptor-5; GC, germinal center; ICOS, inducible costimulator; JDM, juvenile dermatomyositis; PD-1, programmed death-1; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, Sjogren's syndrome; TFH, follicular helper T; TFHC, circulating TFH; TSH, thyroid-stimulating hormone, UV, ultraviolet.
TFH cells in murine models of autoimmune diseases
The contribution of TFH cells in autoimmune diseases has been mainly studied in murine models of SLE. Most data supporting the role of TFH cells in murine lupus come from studies using sanroque mouse model. Sanroque mice are having a single recessive mutation in the roquin gene that encodes a highly conserved protein, a member of the RING-type ubiquitin ligase protein family. These mice exhibit SLE-like pathologies, such as high-affinity anti-dsDNA antibodies, focal proliferative glomerulonephritis, necrotizing hepatitis, anemia and autoimmune thrombocytopenia. The sanroque mutation not only causes the formation of excessive TFH cells and GCs, but also disrupts a repressor of ICOS and results in aberrant production of IL-21.6 Using sanroque mice, Linterman et al.32 recently investigated the role of TFH cells in the development of murine lupus. They found that deletion of an allele of Bcl6 reduced the number of GC cells and ameliorated the SLE-like pathological reactions, suggesting that autoimmunity in sanroque mice is largely dependent on GC. Furthermore, they found that deficiency of SAP in sanroque mice resulted in a significant reduction in TFH cells and IL-21, and abrogated GC formation, autoantibody production and renal pathology. More convincingly, adoptive transfer of sanroque TFH cells into wild-type recipient mice led to the spontaneous GC formation that resulted in pathology, confirming the direct involvement of TFH cells in the pathogenesis of the lupus-like diseases.32 Overall, these data suggest that dysregulation of the GC response through excessive formation of TFH cells is responsible for autoimmunity in sanroque mice. However, some studies showed that roquin represses autoimmunity by limiting ICOS mRNA expression through a microRNA-independent post-translational repression.33,34 In addition, a recent study showed that the loss of roquin induced early death and immune dysregulation but not autoimmunity in sanroque mice.35 Therefore, future studies are required to completely elucidate the mechanisms behind pathogenesis of lupus in sanroque mice.
TH-cell differentiation is polarized toward TFH cells to generate B-cell responses against infections.36,37 These responses, if exaggerated by aberrant TFH-cell function, may also promote autoimmune diseases. A recent interesting study examined the role of TFH cells in the development of autoimmunity during chronic Salmonella infection in mice. The study showed that mice deficient in MyD88 when challenged with recombinant-attenuated Salmonella enterica serovar Typhimurium vaccine strain resulted in chronic infection, which was subsequently followed by the development of autoimmune pathologies, such as autoimmune hypergammaglobulinemia and deposition of immune complexes in the kidneys. In these mice, a population of TFH cell-like cells expressing the higher levels of PD-1, CXCR5, ICOS and IL-21 was found to be significantly expanding compared with healthy controls. In addition, blocking the function of these cells by anti-ICOS or anti-PD-1 antibodies ameliorated hyper-IgG in recombinant-attenuated Salmonella enterica serovar Typhimurium vaccine-infected MyD88-deficient mice. These observations suggest that the overrepresentation of TFH-like cells in chronic bacterial infection elicits autoimmune pathologies in a PD-1- and ICOS-dependent fashion.38
Aberrant production of TFH cell-associated cytokines, particularly IL-21 has been shown to be critical for the development of autoimmunity in lupus-prone mice. IL-21 is mainly produced by TFH cells and appears to be crucial for TFH-cell differentiation and GC reactions.18,39,40 Several studies using lupus-prone mouse models have provided strong evidence for the involvement of IL-21 in autoimmunity. Ozaki et al.41 showed that BXSB-Yaa mice, which reveal features of lupus, had overexpansion of TFH cells and excessive production of IL-21. Additionally, blocking IL-21 in the lupus-prone MRLlpr mice led to reduced lupus pathologies, such as pathogenic autoantibodies, lymphadenopathy and deposition of immune complexes in the kidney.42 Consistently, when MLRlpr mice were made deficient in IL-21R, they showed reduced splenomegaly, lymphadenopathy and autoantibody production, and lacked spontaneous GC formation and plasma-cell accumulation.43 Moreover, the role of IL-21 is also shown in another model of murine lupus, NZB/W F1 mice, which exhibit clinical features that resemble those of human SLE, including autoantibody production and immune complex-mediated nephritis.44,45 Upon intranasal administration of anti-CD3 antibodies, NZB/W F1 mice exhibited suppressed lupus development characterized by reduced levels of autoantibodies and diminished glomerulonephritis.45 It was found that the treatment with anti-CD3 antibody affected the function of CD4+CXCR5+ICOS+ TFH cells, characterized by decreased IL-21 and IL-17 production in these mice which was associated with the autoimmune pathologies.45 Moreover, a recent in vivo study found IL-27 to be crucial for promoting autoimmune pathologies through inducing the production of IL-21 by TFH cells in a murine model of pristane-induced lupus.46 Using IL-27α-receptor-deficient (IL-27Rα−/−) mice, Batten et al.46 showed that IL-27 is important for IL-21 production and TFH-cell survival. They found that mice deficient in IL-27Rα, when treated with pristane, exhibited diminished autoantibody production and mild renal lesions compared with pristane-treated wild-type mice.
The identification of TFR cells has further deepened our understanding of how overexpansion of GC is prevented from autoantibody production.25,26,27,47 Lim et al.47 first reported that TFR cells migrate to follicles in human tonsils and elicit suppressive effect on the TFH cell-mediated B-cell responses, such as antibody production, B-cell survival and expression of activation-induced cytosine deaminase. In vivo studies in mice have further provided direct evidence to show a critical role for TFR cells in inhibiting the GC responses.25,26,27
TFH cells in human autoimmune diseases
Although less clearer than that in murine models, the implications of TFH cells in the development of human autoimmune diseases have started to be appreciated.6,32 Earlier studies have provided indirect evidence to indicate the role of TFH cells in promoting human autoimmunity, e.g., production of pathogenic autoantibodies in SLE patients caused by dysregulated GC reactions,31,48 presence of the aggregates of T and B cells containing plasmablasts in the kidneys of patients with lupus nephritis49 and reduced GC reactions upon blocking CD40L–CD40 interactions in patients with active SLE.50 Recent studies have further provided clearer evidence on the role of TFH cells in promotion of both systemic and organ-specific autoimmune diseases in humans.51,52,53,54,55 Simpson et al.51 performed an elegant study investigating the role of TFH cells in patients with SLE and Sjogren's syndrome. They found an overrepresented population of CD4+CXCR5+ TFH cell-like cells in the blood, referred to as circulating TFH (TFHC) cells, of a subset of the SLE patients (14 out of 46), which expressed ICOS and PD-1 compared with healthy controls. Furthermore, the expansion of TFHC cells was found to be positively correlated with severity of the SLE as evidenced by high autoantibody titer, glomerulonephritis and thrombocytopenia that resulted in end-organ damages. The alteration of TFHC cells has been reported in patients with various autoimmune diseases, such as rheumatoid arthritis, primary Sjogren's syndrome, juvenile dermatomyositis and autoimmune thyroid diseases, where TFHC cells are present at a higher frequency and show positive correlation with serum autoantibody titer in the patients.52,53,54,55 Overall, these observations suggest an important role for TFHC cells in human autoimmunity. However, it should be noted that although TFHC cells resemble TFH cells in terms of ICOS and PD-1 expression, TFHC cells in SLE patients do not express Bcl6 and IL-21 that are hallmarks of classic TFH cells.51 This discrepancy poses a pertinent question as to whether TFHC and TFH cells are indeed related. It may be that TFHC cells are a subset of TFH cells residing in the lymphoid organs that may downregulate Bcl6 while migrating to the systemic circulation as TFHC cells. This is in accordance with the fact that TFH cells may downregulate Bcl6 over weeks after antigenic challenge.56 Recently, Morita et al.52 shed light on the relationship between TFHC (CD4+CXCR5+) and TFH cells and convincingly demonstrated that TFHC cells share functional, and to some extent phenotypic, properties of TFH cells present in the lymphoid organs of humans and mice, and constitute a subset of circulating pool of memory TFH cells. They found TFHC cells to be potent at providing help for B-cell responses, such as production of plasmablasts and promotion of class switching through IL-21. Furthermore, TFHC cells showed expression of ICOS and PD-1 but not Bcl6, similar to TFHC cells in lupus patients.52 Evidence from other recent studies also points toward a relationship between TFHC and TFH cells. The patients deficient in ICOS and CD40L lack TFHC cells, whereas TFHC cells expressing ICOS are overrepresented in SLE and rheumatoid arthritis patients.10,51,53
TFH cells and immunodeficiency
Immunodeficiency is a pathological condition where the immune response is compromised or absent. Defects in humoural immune response lead to the humoral immunodeficiencies, such as common variable immunodeficiency (CVID), X-linked hyper IgM syndrome (HIGM) and X-linked lymphoproliferative disease (XLP). The clinicopathological presentation of these diseases includes severely impaired humoral immune responses characterized by the absence of GC and altered production of antigen-specific memory B cells and antibodies.57 The underlying etiology and molecular mechanisms of these diseases remain unclear. Recent studies have, however, implicated various genes such as ICOS, CD40L and SAP to be involved in pathogenesis of the diseases. Deficiency of ICOS or CD19 can cause CVID, whereas SAP deficiency results in XLP. HIGM develops as a result of the absence of CD40L. Mice that are deficient in ICOS, CD40L or SAP reflect clinical features similar to those of patients of the immunodeficiencies.58
Since ICOS, CD40L and SAP are highly expressed by TH cells, especially TFH cells, and that mutations in them can lead to altered development and/or function of TH/TFH cells, it is highly likely that TFH cells play a critical role in humoral immunodeficiencies. Bossaller et al. recently studied the frequencies of CXCR5+ TFH cells in CVID patients and ICOS-deficient mice and found that CXCR5+ TFH cells were severely reduced in the peripheral blood of CVID patients compared with healthy individuals. Consistently, ICOS-deficient mice were significantly depleted of CXCR5+ TFH cells in the blood and lymphoid tissues, and that the lack of GC was associated with reduced numbers of CXCR5+ TFH cells in B-cell follicles.10 Furthermore, deletions of CD19 can also cause CVID, which is characterized by defective B-cell responses and lack of TFH cells.59 Similar to these findings, individuals suffering from XLP and SAP-deficient mice also exhibit severely impaired B-cell responses with altered development and/or function of TFH cells.60 Ma et al. showed that XLP patients had reduced numbers of CD4+ T cells compared with healthy individuals. The CD4+ T cells produced lower quantities of IL-10 and failed to provide help for B-cell responses.61 Similarly, mice deficient in SAP show severe functional defects in CD4+ T cells as evidenced by their inability to confer long-lived humoral responses.62 Studies further demonstrate that CD4+ T-cell function in HIGM patients, which lack CD40L, is strictly compromised.63
TFH cells and lymphoma
Emerging evidence supports the relationship between TFH cells and lymphoma, particularly peripheral T-cell lymphoma (PTCL). PTCLs are a rare family of lymphomas with unfavorable prognosis. They may be classified into three types, anaplastic large-cell lymphoma, angioimmunoblastic T-cell lymphoma (AITL) and unspecified PTCL. AITL is the most widely studied type of PTCL, and thus considered as the prototype of T-cell lymphoma. Recent studies have suggested that TFH cells and neoplastic AITL cells share the expression of many phenotypic markers, and that the AITL cells may be derived from TFH cells.64,65,66,67,68 Examples of these markers expressed by both TFH cells and neoplastic AITL cells include Bcl6, CXCR5, PD-1, CD40L, OX40 and CXCL13. However, neoplastic AITL cells, in contrast to TFH cells, express CD10, which is a cell surface zinc metalloendopeptidase expressed in a variety of normal and neoplastic tissues. Thus, CD10 may be used as a phenotypic marker to distinguish neoplastic AITL cells from TFH cells. More recently, the expression of TFH cell markers (Bcl6, PD-1 and CXCL13) and CD10 has also been demonstrated in a subset of non-AITL PTCL, analogous to the neoplastic AITL cells.11 In a recent study, de Leval et al.69 using gene-expression profiling demonstrated that the AITL cells are characterized by the overexpression of several genes of normal TFH cells, including CXCL13, Bcl6 and CD40L. Rodríguez Pinilla et al.70 showed an atypical subset of primary cutaneous small/medium-sized pleomorphic CD4+ T-cell lymphomas, which not only expressed TFH cell markers, such as PD-1, CXCL13 and Bcl6, but also localized in vicinity of B cells, leading to the formation of clusters. Although these data support the derivation of the AITL cells from TFH cells, the underlying molecular mechanism for neoplastic transformation of TFH cells into AITL and primary cutaneous small/medium-sized pleomorphic CD4+ T-cell lymphomas remains obscure.
Since TFH cells are critical constituents of the micromilieu of B-cell lymphoma, and can influence the development of neoplastic B cells, they are likely to have an important role in development of B-cell lymphoma. This concept is supported by a recent study which described a population of TFH cell-like cells in the vicinity of follicular lymphoma that develops from GC B cells. These cells shared surface markers of TFH cells, such as CD4, CXCR5 and ICOS, and could be divided into two functionally distinct subpopulations, CD4+CXCR5hiICOShiCD25+ and CD4+CXCR5hiICOShiCD25− T cells. In contrary to TFH cells, the gene profiling of these cells showed that they expressed many genes, tumor necrosis factor, LTA, IL-4 and CD40LG, which are involved in the process of lymphoma development.71
Concluding remarks
In recent years, significant advances have been made in understanding the immunobiology of TFH cells and relevance of these cells with various diseases. TFH cells have surfaced as a distinct subset of TH cells that provide help to B cells, and play a critical role in generation and maintenance of humoroul immune responses. Emerging evidence also illustrates that the aberrations in TFH function may lead to development of a variety of pathologies, such as autoimmune diseases, immunodeficiencies and lymphomas. A better understanding of the role of TFH cells both in protective immunity and pathology is crucial for designing specific therapies for pathologies like autoimmune diseases and immunodeficiencies. For example, TFH cell-associated molecules, such as PD-1, ICOS and CD40L may be interesting targets for treating autoimmunity and immunodeficiency. Suppressing the expression of these molecules may contain autoimmune responses, whereas promoting their expression may ameliorate immunodeficiency. Specifically, further studies are required to answer important mechanistic questions behind the TFH cell-mediated development of pathologies and to address the potential to target TFH cells:
What is the basis for the aberrations in TFH-cell function and the subsequent development of pathologies in autoimmunity and immunodeficiency in the mouse models and patients?
What is the role of TFR cells in controlling autoimmune diseases and what approaches can be taken to promote the role of these cells?
How to translate the knowledge on TFH cells gained from mouse model studies into human autoimmune diseases and is it possible for circulating TFHC cells to be a biomarker in disease diagnosis and treatment?
Acknowledgments
This work was supported by grants from Canadian Institutes of Health Research and Manitoba Health Research Council (to XY). SS was a recipient of Dr Allan R. Ronald Studentship and Manitoba Health Research Council PhD Studentship. XY is Canada Research Chair in Infection and Immunity. The authors would like to thank Ms Ying Peng for her assistance in figure drawing.
References
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Good-Jacobson KL. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Gomez-Martin D, Diaz-Zamudio M, Jorge Romo-Tena, Ibarra-Sanchez MJ, Alcocer-Varela J. Follicular helper T cells poise immune responses to the development of autoimmune pathology. Autoimmun Rev. 2011;10:325–330. doi: 10.1016/j.autrev.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Dong W, Zhu P, Wang Y, Wang Z. Follicular helper T cells in systemic lupus erythematosus: a potential therapeutic target. Autoimmun Rev. 2011;10:299–304. doi: 10.1016/j.autrev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmstrom P, Wahren-Herlenius M, et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organs of patients with Sjogren's syndrome. Arthritis Rheum. 2003;48:3187–3201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;117:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Pinilla SM, Atienza L, Murillo C, Pérez-Rodríguez A, Montes-Moreno S, Roncador G, et al. Peripheral T-cell lymphoma with follicular T-cell markers. Am J Surg Pathol. 2008;32:1787–1799. doi: 10.1097/PAS.0b013e31817f123e. [DOI] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 2005;106:1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1 high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE. 2011;6:17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2004;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2011;13:35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg I, Agua-Doce A, Hernández A, Almeida C, Oliveira VG, Faro J, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- Luzina IG, Atamas SP, Storrer CE, daSilva LC, Kelsoe G, Papadimitriou JC, et al. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70:578–584. [PubMed] [Google Scholar]
- Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Tan AH, Hu X, Athanasopoulos V, Simpson N, Silva DG, et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450:299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- Glasmacher E, Hoefig KP, Vogel KU, Rath N, Du L, Wolf C, et al. Roquin binds inducible costimulator mRNA and effectors of mRNA decay to induce microRNA-independent post-transcriptional repression. Nat Immunol. 2010;11:725–733. doi: 10.1038/ni.1902. [DOI] [PubMed] [Google Scholar]
- Bertossi A, Aichinger M, Sansonetti P, Lech M, Neff F, Pal M, et al. Loss of Roquin induces early death and immune deregulation but not autoimmunity. J Exp Med. 2011;208:1749–1756. doi: 10.1084/jem.20110578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Lu L, Hua C, Qin L, Zhao P, Wang J, et al. High frequency of CD4+CXCR5+ TFH cells in patients with immune-active chronic hepatitis B. PLoS ONE. 2011;6:e21698. doi: 10.1371/journal.pone.0021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HJ, Yang H, Yang JY, Seo SU, Chang SY, Seong JK, et al. Expansion of Tfh-like cells during chronic Salmonella exposure mediates the generation of autoimmune hypergammaglobulinemia in MyD88-deficient mice. Eur J Immunol. 2012;42:618–628. doi: 10.1002/eji.201141748. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- Rankin AL, Guay H, Herber D, Bertino SA, Duzanski TA, Carrier Y, et al. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Fas(lpr/lpr)/J mice. J Immunol. 2012;188:1656–1667. doi: 10.4049/jimmunol.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+CD25−LAP+ regulatory T cell and is associated with down-regulation of IL-17+CD4+ICOS+CXCR5+ follicular helper T cells. J Immunol. 2008;181:6038–6050. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, et al. IL-27 supports germinal center formation by enhancing IL-21 production and the function of T follicular helper cells. J Exp Med. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci USA. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, et al. In situ B cell-mediated immune responses and tubuleinterstitial inflammation in human lupus nephritis. J Immunol. 2011;186:1849–1860. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer AC, Slota R, Fischer R, Gur H, Girschick H, Yarboro C, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154−CD40 interactions. J Clin Invest. 2003;112:1506–1520. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao C, et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin Dev Immunol. 2012;2012:827480. doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Ma J, Liu Y, Tong J, Tian J, Chen J, et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 2012;97:943–950. doi: 10.1210/jc.2011-2003. [DOI] [PubMed] [Google Scholar]
- Li XY, Wu ZB, Ding J, Zheng ZH, Li XY, Chen LN, et al. Role of the frequency of blood CD4+CXCR5+CCR6+ T cells in autoimmunity in patients with sjogren's syndrome. Biochem Biophys Res Commun. 2012;422:238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, Conley ME, et al. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124:1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG, Deenick EK, Palendira U, Ma CS. T cell–B cell interactions in primary immunodeficiencies. Ann N Y Acad Sci. 2012;1250:1–13. doi: 10.1111/j.1749-6632.2011.06361.x. [DOI] [PubMed] [Google Scholar]
- van Zelm MC, Reisli I, van der Burg M, Castaño D, van Noesel CJ, van Tol MJ, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354:1901–1912. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- Nichols KE, Ma CS, Cannons JL, Schwartzberg PL, Tangye SG. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005;203:180–199. doi: 10.1111/j.0105-2896.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Ma CS, Hare NJ, Nichols KE, Dupré L, Andolfi G, Roncarolo MG, et al. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115:1049–1059. doi: 10.1172/JCI23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;412:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- Korthäuer U, Graf D, Mages HW, Brière F, Padayachee M, Malcolm S, et al. Defective expression of T-cell CD40 ligand causes X-lined immunodeficiency with hyper IgM. Nature. 1993;361:539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30:802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, Boye K, Martin N, Copie-Bergman C, Plonquet A, Fabiani B, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am J Surg Pathol. 2006;30:490–494. doi: 10.1097/00000478-200604000-00009. [DOI] [PubMed] [Google Scholar]
- Grogg KL, Attygalle AD, Macon WR, Remstein ED, Kurtin PJ, Dogan A. Angioimmunoblastic T-cell lymphoma: a neoplasm of germinal-center T-helper cells. Blood. 2005;106:1501–1502. doi: 10.1182/blood-2005-03-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogg KL, Jung S, Erickson LA, McClure RF, Dogan A. Primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma: a clonal T-cell lymphoproliferative disorder with indolent behavior. Mod Pathol. 2008;21:708–715. doi: 10.1038/modpathol.2008.40. [DOI] [PubMed] [Google Scholar]
- Yu H, Shahsafaei A, Dorfman DM. Germinal-center T-helper-cell markers PD-1 and CXCL13 are both expressed by neoplastic cells in angioimmunoblastic T-cell lymphoma. Am J Clin Pathol. 2009;131:33–41. doi: 10.1309/AJCP62WRKERPXDRT. [DOI] [PubMed] [Google Scholar]
- de Leval L, Rickman DS, Thielen C, Reynies A, Huang YL, Delsol G, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–4963. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- Rodríguez Pinilla SM, Roncador G, Rodríguez-Peralto JL, Mollejo M, García JF, Montes-Moreno S, et al. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. Am J Surg Pathol. 2009;33:81–90. doi: 10.1097/PAS.0b013e31818e52fe. [DOI] [PubMed] [Google Scholar]
- Amé-Thomas P, le Priol J, Yssel H, Caron G, Pangault C, Jean R, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia. 2012;26:1053–1063. doi: 10.1038/leu.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]