Abstract
ST2 protein is a soluble splicing variant of ST2L protein, which is the receptor for interleukin-33 (IL-33). Previously, we reported that soluble ST2 suppressed the signal transduction of lipopolysaccharide (LPS) and cytokine production in monocytic cells. To investigate whether or not this inhibitory effect occurs in dendritic cells, which are the key players in innate and adaptive immunity, human monocyte-derived dendritic cells were pre-treated with soluble ST2 protein before LPS stimulation. Although soluble ST2 did not attenuate the LPS-induced maturation of dendritic cells, pre-treatment with soluble ST2 suppressed cytokine production and inhibited LPS signaling. Moreover, the proliferation of naive T cells was inhibited significantly by soluble ST2 pre-treatment. IL-33 had little effect on the cytokine production of immature monocyte-derived dendritic cells. Furthermore, soluble ST2 protein was internalized into dendritic cells, suggesting that soluble ST2 protein acts by a noncanonical mechanism other than the sequestration of IL-33.
Keywords: dendritic cells, LPS signal transduction, ST2, TLR4
Introduction
IL-33, a member of the IL-1 family, is considered to promote proinflammatory reactions and Th2-type immune responses.1 The specific receptor for IL-33 is ST2L protein, which forms a receptor complex with IL-1 receptor accessory protein. Soluble ST2 protein (sST2) is a variant of ST2L arising from alternative splicing. Because it binds with IL-33 to sequestrate the effect of IL-33, sST2 is considered an anti-inflammatory factor in cases such as asthma.2 In addition, we and other groups have reported that the serum level of sST2 rises in various diseases, including asthma, autoimmune diseases, cardiovascular diseases and sepsis, although the pathological relevance of these findings remains largely unknown.3,4,5,6,7,8,9
Before IL-33 was revealed to be a ligand of ST2L, sST2 was reported to inhibit the production of lipopolysaccharide (LPS)-induced proinflammatory cytokines in macrophages and to reduce the LPS-mediated mortality of mice.10 Recently, acute lung injury caused by LPS was again reported to be suppressed by sST2.11 We also reported that sST2 inhibited the effect of LPS in monocytic cells and suppressed IL-6 production remarkably by suppressing NF-κB activation.12 LPS is also known to stimulate the differentiation and maturation of monocytic cells to dendritic cells (DCs), which are the most potent antigen-presenting cells and critical regulators of immunity.13,14 Therefore, we extended these findings to the investigation of the immunoregulatory effects of sST2 on DCs mentioned herein.
Among the several types of DCs, monocyte-derived DCs (MDDC) can be induced as immature DCs from monocytes in peripheral blood by the stimulation of granulocyte macrophage-colony stimulating factor and interleukin (IL)-4, and this induction is important for the replenishment of DCs. Pathogen-associated molecular patterns such as LPS are recognized by pattern-recognition receptors as Toll-like receptors (TLRs) on DCs and induce further DC maturation and cytokine production. ST2 mRNA expression was recently reported in mouse bone marrow-derived DCs, and IL-33 was shown to activate DCs.15 Another report revealed that IL-33-activated DCs are crucial for the development of allergic airway inflammation of a model mouse,16 but the effects of IL-33 on DCs are still not fully understood. Moreover, the relevance of sST2 on DCs has not been reported yet.
In this study, we have verified the inhibitory effect of sST2 on the LPS-induced activation of human immature MDDCs (iMDDCs). sST2 attenuated the intracellular signal trunsduction of LPS, and subsequently the proinflammatory cytokine production, but it did not inhibit MDDC maturation. Furthermore, in this system, IL-33 did not activate iMDDCs and does not seem to be involved in the effect of sST2 on human iMDDCs, suggesting a noncanonical pathway independent of IL-33.
Materials and methods
Isolation of monocytes and differentiation to iMDDCs
Human peripheral blood mononuclear cells were isolated from the whole blood of healthy four donors by using Vacutainer CPT (BD, Franklin Lakes, NJ, USA). In compliance with the Office of Research Ethics at Jichi Medical University, informed consent was obtained from all individuals. Monocytes (Mo, purity >95%) were purified by positive selection with anti-CD14 magnetic beads (Miltenyi Biotech Co., Bergisch Gladbach, Germany) from peripheral blood mononuclear cells. iMDDCs were obtained by incubating 1×106 of Mo in 8 ml Mo-DC Differentiation Medium (Miltenyi Biotech) with 100 U/ml of penicillin and 100 U/ml of streptomycin in a 10-cm RepCell culture dish (Cell Seed Inc., Tokyo, Japan) for 7 days. On day 3, 8 ml fresh medium was added to feed the cells. After 7 days, iMDDCs were harvested by incubating the RepCell dish at 20 °C for 30 min.
iMDDCs were cultured in RPMI-1640 medium (Sigma, St Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (MultiSer; Thermo Electron, Scoresby, VIC, Australia), 100 U/ml penicillin and 100 U/ml streptomycin, at a density of 5×105 cells/ml for 3 days, in a humidified atmosphere with 5% CO2 at 37 °C.
To investigate the effect of sST2 on iMDDCs, iMDDCs left unstimulated overnight were pre-treated with sST2 for 60 min. Subsequently, pre-treated or nontreated iMDDCs were stimulated with LPS (Sigma; E. coli 055:B5), recombinant IL-33, flagellin, or green fluorescent protein (GFP) as controls for the indicated periods.
Mature MDDCs (mMDDCs) were obtained by incubating iMDDCs with LPS (500 ng/ml) for 2 days.
Enzyme-linked immunosorbent assay (ELISA)
Concentrations of IL-6 (Invitrogen, La Jolla, CA, USA), IL-8 (R&D Systems, Gaithersurg, MD, USA), IL-10 (GEN-PROBE, San Diego, CA, USA), IL-12p40 (United Biosource, Chevy Chase, MD, USA), IL-13 (Biosource), IL-33 (BioLegend, San Diego, CA, USA), sST2 (MBL, Aichi, Japan), and Interferon-α (PBL Interferonsource, Piscataway, NJ, USA) were determined by ELISA kits according to the manufacturers' instructions.
Flow cytometric analysis of MDDC surface markers
Stimulated MDDCs were preincubated with human Fc receptor blocking reagent (MBL, Nagoya, Japan) for 5 min at room temperature. To evaluate the differentiation and maturation of MDDCs, MDDCs were stained with Mo-DC Differentiation Inspector (Miltenyi Biotech) according to the manufacturer's instructions. The cells were washed and suspended in phosphate-buffered saline without calcium and magnesium (PBS(−)) containing 0.5% bovine serum albumin and 2 mM EDTA, then analyzed by an LSR Flow Cytometer (BD).
Adenosine 5′-triphosphate (ATP) measurement in iMDDCs
To estimate the cytotoxicity, intracellular ATP was measured by a luciferin-luciferase bioluminescence assay using the ‘Cell' ATP assay reagent (Toyo-Ink, Tokyo, Japan). The iMDDCs were seeded at 3×104/100 µl/well in a 96-well tissue culture plate and incubated in a CO2 incubator at 37 °C. The plate was incubated at room temperature for 30 min, and 100 µl ATP reagent was then added to each well. The plate was stirred for 1 min and then incubated in a luminometer (Lumat LB9507; Berthold Technologies, Bad Wildbad, Germany) for 10 min at 23 °C. The relative light intensity was then measured.
Western blotting
The cells were lysed in NP-40 lysis buffer (10 mM HEPES/NaOH (pH 7.9), 10 mM KCl, 0.1 mM EDTA (pH 8.0), 0.1 mM EGTA (pH 8.0), 0.625% (w/v) NP-40, 20 mM NaF, 1 mM Na3OV4, 1 mM dithiothreitol (DTT) and 0.5 mM phenylmethylsulfonyl fluoride (PMSF)) supplemented with complete protease inhibitors (Roche, Basel, Switzerland). Proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Millipore, Bedfird, MA, USA). Membranes were probed using the designated antibodies. The prifmary antibodies were against IκBα (sc-371; Santa Cruz Biotechnolog, Santa Cruz, CA, USA), phospho-p38 MAPK (Thr180/Tyr182) (4631; Cell Signaling Technology, Beverly, MA, USA), p38 MAPK (9212; Cell Signaling Technology), phospho-JNK (Thr183/Tyr185) (4668; Cell Signaling Technology), JNK (9258; Cell Signaling Technology), phospho-p44/42 MAPK (Thy202/Tyr204) (4370; Cell Signaling Technology), p44/42 MAPK (4695; Cell Signaling Technology), or β-actin (sc-47778; Santa Cruz Biotechnology). Secondary antibodies were anti-mouse IgG-HRP (Bio Rad, Richmond, CA, USA) or anti-rabbit IgG-HRP (Jackson Laboratory, Bar Harbor, ME, USA). Samples were visualized using Image Quant LAS 4000 (GE Healthcare, Piscataway, NJ, USA) with ECL solution (GE Healthcare), and data were analyzed using the Image Quant TL v.7.0 program. The relative intensities of the bands were calculated by Image Quant LAS 4000 and normalized with each 0 time control.
Preparation of nuclear extract
Stimulated iMDDCs were lysed in NP-40 lysis buffer supplemented with protease inhibitors. As much supernatant as possible was removed by centrifugation. Pellets were suspended with an equal volume of the low buffer (20 mM KCl, 20 mM Tris HCl (pH 7.3), 0.2 mM EDTA (pH 8.0), 1.5 mM MgCl2, 25% (w/v) glycerol, 1 mM DTT and 0.5 mM PMSF) supplemented with protease inhibitors and then vortexed for 10 s. The suspensions were then mixed with the high buffer (600 mM KCl, 20 mM Tris-HCl (pH 7.3), 0.2 mM EDTA (pH 8.0), 1.5 mM MgCl2, 25% (w/v) glycerol, 1 mM DTT and 0.5 mM PMSF) supplemented with protease inhibitors. The volume of the high buffer was four times that of the low buffer, and the mixture was incubated for 30 min on ice with occasional vortexing. After centrifugation, the supernatant was harvested. Concentrations of NF-κBp65 in intranuclear homogenate were determined with the NF-κBp65 transcription factor assay kit according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI, USA).
Immunofluorescence
iMDDCs attached on cover glass were pre-treated with sST2 for 60 min at 37 °C and then fixed with 4% paraformaldehiyde for 20 min. Cells were permeabilized with 0.1% (w/v) Triton X-100 in PBS− for 30 min at room temperature. Subsequently, samples were blocked with PBS− containing 1% bovine serum albumin and 0.1% (w/v) Tween 20 for 30 min at room temperature. To detect NF-κBp65 protein, iMDDCs were incubated with anti-NF-κBp65 mouse monoclonal IgG1 (sc-8008, Santa Cruz Biotechnology) for 1 h at room temperature. An Alexa Fluor 488 F(ab′)2 fragment of goat anti-mouse IgG (H+L) was used as a secondary antibody. Samples were mounted using Prolong Gold anti-fade reagent with DAPI (Molecular Probes, Eugene, OR, USA) and then analyzed using a BZ-9000 microscope (Keyence, Osaka, Japan) and the BZ-2 analysis application.
Mixed lymphocyte reaction analysis
Naive CD4+ cells were purified by negative selection with the Naive CD4+ T-Cell Isolation Kit II (Miltenyi Biotech) from peripheral blood mononuclear cells of each four donors. sST2-pre-treated iMDDCs were incubated with LPS, recombinant IL-33, GFP, or PBS, and then cocultured autologously with naive CD4+ T cells prepared as described above. Briefly, the iMDDCs were seeded at 2×104/well in 96-well tissue culture plates and cocultured with the isolated naive CD4+ T cells at a 1∶10 ratio in RPMI-1640 medium alone, with 500 ng/ml LPS, with 100 ng/ml IL-33, with 500 ng/ml GFP, or with 10 µl of PBS for 5 days. Then, the cultured T cells were harvested and washed once with PBS, and resuspended in culture medium, and subjected to the proliferation assay with Cell Proliferation ELISA System (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions. Otherwise, naive CD4+ T cells without the coculture with iMDDCs, iMDDCs alone, or LPS-induced mMDDCs alone were stimulated as above, and assayed as control. Briefly, 100 µl/well of the cell culture was resuspended in duplicate in 96-well plates. A 10 µl/well BrdU labeling reagent was added. After overnight incubation, the cells were harvested by centrifugation at 300g for 10 min and were fixed with FixDenat solution, followed by incubation with peroxidase-labeled anti-BrdU for 120 min. After washing thrice, 100 µl/well of tetramethyl benzidine was added and incubated, and absorbance was measured by an ELISA plate reader.17
Real-time PCR
RNA was purified from monocytes, iMDDCs (CD209+CD83−), or mMDDCs (CD209+CD83+) using the RNeasy Mini kit (Qiagen, Hilden, Germany) and cDNA was synthesized using a High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA). Expression of genes was analyzed by real-time PCR using TaqMan Gene Expression Assays with StepOnePlus (Applied Biosystems). Levels of cDNA transcript were determined by real-time PCR with predesigned primers and normalized against the β-actin endogenous control (HuACTB; Applied Biosystems). The sequences for these probes were as follows: TLR4, Hs01060206_m1; IL-33, Hs00369211_m1; IL1RL1 (ST2), Hs00249389_m1. Each sample was assayed in triplicate with 40 cycles and the reactions were conducted in a 96-well plate format using StepOnePlus. The relative quantifications of these target genes were compared between the iMDDCs pre-treated with sST2 and the non-pre-treated ones.
Recombinant protein
The preparation of recombinant sST2 containing V5-His tags at the C terminus (sST2-V5-His) was described previously.12
The coding region of GFP105 (a gift from Dr T. Osumi)18 was inserted into a NotI/BstBI-digested pEF6/sST2-V5-His (pEF6/sST2-GFP105-V5-His). The expression and purification of recombinant sST2-GFP105-V5-His protein was performed in the same manner as the preparation of recombinant sST2-V5-His. Recombinant hIL-33 was purchased from Invitrogen (PHC9254).
Endocytosis analysis
LysoTracker probe (Red DND-99; Molecular Probes), a fluorescent acidotropic probe for labeling and tracking acidic organelles in live cells, was used to stain iMDDCs according to the manufacturer's instructions. iMDDCs were incubated with the macropinocytosis inhibitor, 5-(N-ethyl-N-isopropyl)amirolide (EIPA) (10, 20 and 30 µg/ml) or cytochalasin D (1, 5 and 10 µg/ml)19,20,21 at 37 °C for 30 min and washed twice with PBS−. The cells were then incubated with fresh medium containing sST2-GFP105-V5-His protein (100 ng/ml). After 1 h, the cells were washed with PBS−, fixed with 4% paraformaldehyde for 10 min at 4 °C, then analyzed under a BZ-9000 microscope.
Statistical analysis
The data are presented as means±s.e. The data were analyzed by the Tukey–Kramer test. A value of P<0.05 was considered significant.
Results
Soluble ST2 protein inhibits the LPS-induced cytokine production of iMDDCs
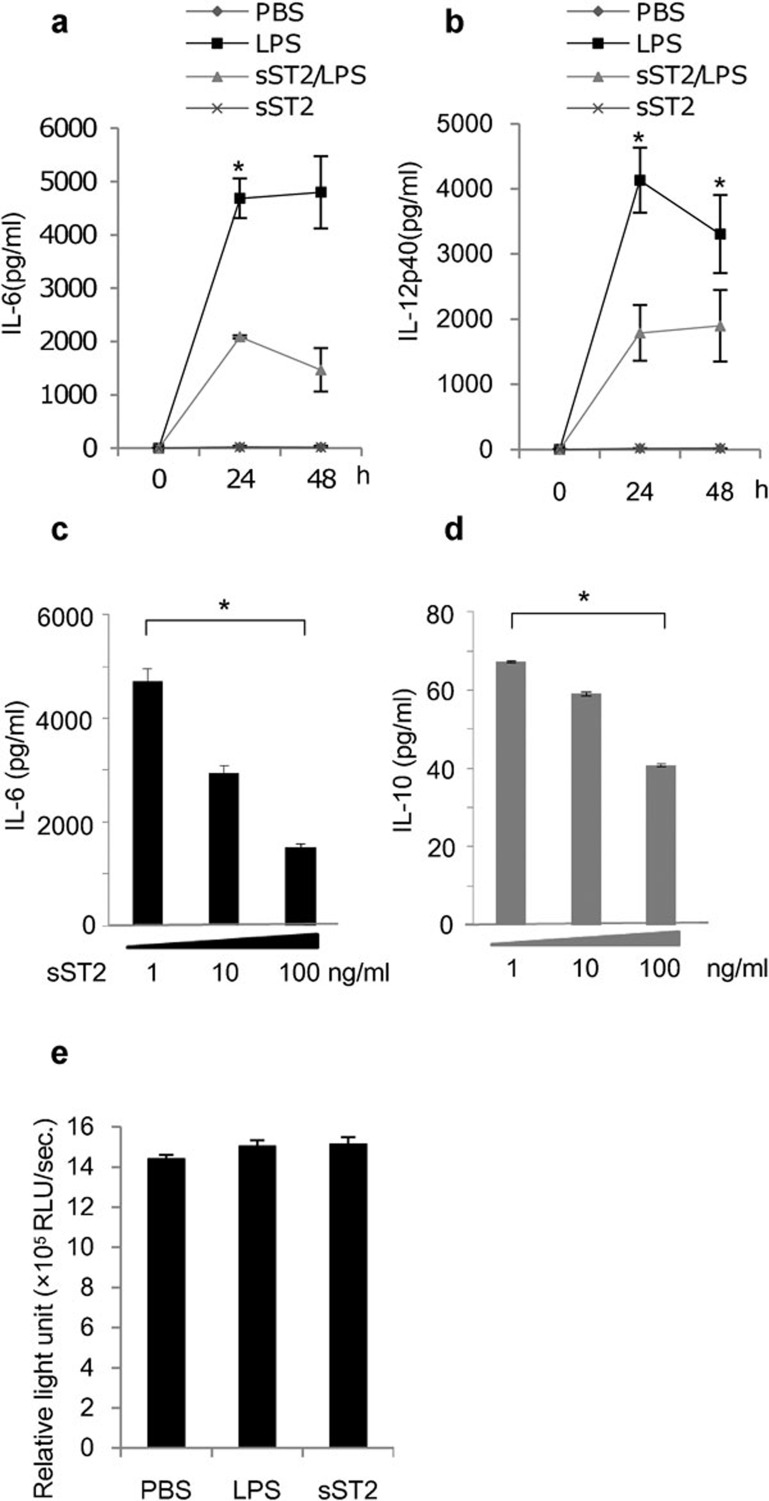
To investigate the effect of sST2 on iMDDCs activated by LPS, we first examined whether or not pre-treatment with sST2 could suppress the production of proinflammatory cytokines in human iMDDCs, as we previously reported that monocytic cells had done.12 Figure 1a and b shows that sST2 pre-treatment suppressed the secretion of IL-6 and IL-12p40 from iMDDCs stimulated by LPS, while also attenuating the productions of IL-8 (Figure 6b), IL-10 (Figure 1d) and tumor necrosis factor-α (data not shown) induced by LPS among the cytokines tested. The inhibition of cytokine production was dependent on the sST2 concentration (Figure 1c and d).
Figure 1.
sST2 inhibits the LPS-induced cytokine production of iMDDCs. (a) Time course of IL-6 secretion. iMDDCs (2.5×105/well) were pre-treated for 1 h with or without sST2 (100 ng/ml), and then the cells were stimulated with LPS (100 ng/ml) for 24 or 48 h. The amount of IL-6 was determined by ELISA. (b) Time course of IL-12p40 secretion was measured as described in (a). (c) Dose dependency of the inhibitory effect of sST2 was tested at various concentrations (1, 10 and 100 ng/ml) against IL-6 production for 24 h after LPS stimulation. (d) Dose dependency of sST2 to IL-10 was also tested as (c). (e) Possible cytotoxicity of sST2 was assayed by measuring the amount of ATP in iMDDCs (3×104/well) by the luciferase light-emitting method. The data are presented as the means of five experiments±s.d. Asterisks denote a statistically significant difference (*P<0.05). ATP, adenosine triphosphate; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; iMDDC, immature monocyte-derived dendritic cell; LPS, lipopolysaccharide; sST2, soluble ST2 protein.
We confirmed that sST2's inhibitory effect did not depend on the cytotoxicity according to the ATP assay (Figure 1e) or on apoptosis evaluated by annexin V with flow cytometry (data not shown). These data suggest that sST2 does not depend on cellular dysfunction or the induction of regulatory DCs for its suppressive effect on LPS stimulation.
Phenotypes of MDDCs analyzed by FACS
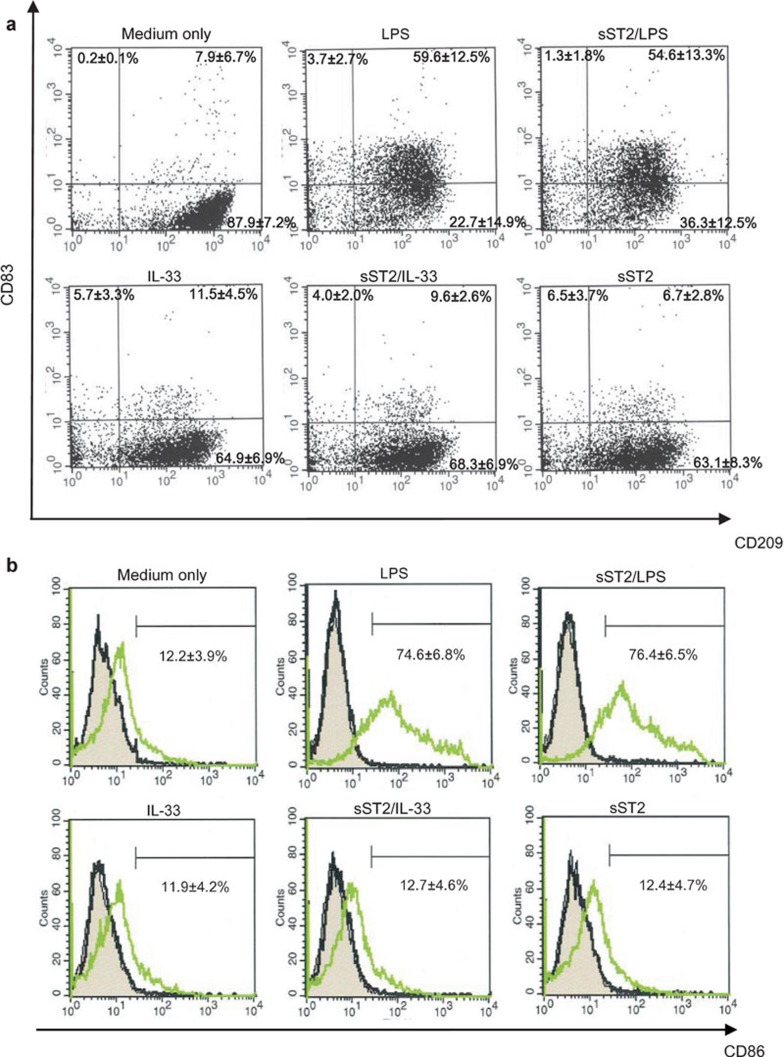
In our preliminary data, sST2 restrained the LPS-induced differentiation of THP-1 cells to DCs. Therefore, we used FACS analysis to examine whether or not pre-treatment with sST2 could suppress iMDDC differentiation to mMDDCs.
As shown in Figure 2a, iMDDCs upregulated CD83 expression by LPS irrespective of sST2 pre-treatment, and CD83 expression was not enhanced with the incubation of IL-33 or sST2 alone. sST2 also had little effect on CD86 expression (Figure 2b). The result of FACS shows that pre-treatment with sST2 did not suppress the maturation of iMDDCs by LPS.
Figure 2.
Characterization of MDDC phenotypes after various stimuli. iMDDCs were pre-treated for 1 h with or without sST2 (100 ng/ml), and the cells were stimulated with LPS (100 ng/ml) or IL-33 for 48 h at 37 °C. (a) The expression levels of CD83(PE) and CD209(APC) were detected by flow cytometry using the Mo-DC Differentiation Inspector. (b) The expression levels of CD86(PE) were detected using the anti-human CD86 antibody. A gray area is presenting MDDCs without staining after various stimuli. A black line shows MDDCs stained by mouse Ig-G1 as isotype control. A green line is MDDCs stained by anti-human CD86 antibody. The percentage of the each positive quadrant area is indicated as the means of three experiments±s.d. Mo-DC, monocyte-dendritic cell; IL, interleukin; iMDDCs, immature monocyte-derived dendritic cell; LPS, lipopolysaccharide; MDDC, monocyte-derived dendritic cell; sST2, soluble ST2 protein.
LPS signaling pathway was attenuated by soluble ST2 protein
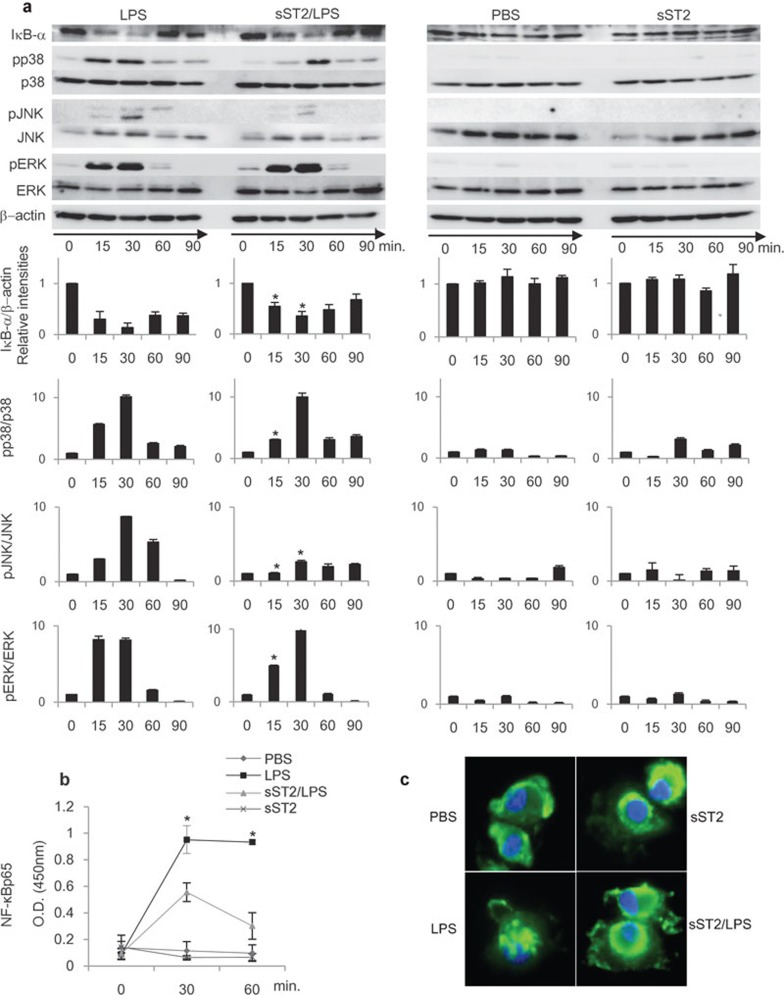
To further elucidate the mechanism underlying sST2's inhibitory effect on LPS-induced cytokine production in iMDDCs, LPS signaling pathways were examined in detail through the activation of three types of MAP kinase (p38, JNK and ERK), the nuclear localization of NF-κB, and the degradation of IκBα.
The phosphorylation of MAP kinases and the degradation of IκBα were analyzed by immunoblotting. As shown in Figure 3a, sST2 inhibited the degradation of IκBα by LPS stimulation. Furthermore, LPS significantly induced the phosphorylation of these three MAP kinases in MDDCs, while sST2 pre-treatment suppressed the LPS-induced phosphorylation of p38 and JNK, and to a lesser extent with ERK (Figure 3a). We compared the relative intensity of the each band using Image Quant LAS 4000 for quantification, and the result showed that sST2 pre-treatment most suppressed the LPS signaling after 15 min of stimulation. We then examined whether or not sST2 pre-treatment inhibit the nuclear localization of NF-κB. The amount of NF-κBp65 in nuclear extracts was analyzed, and sST2 pre-treatment significantly decreased the amount of NF-κB protein in the nucleus compared to the iMDDCs stimulated only by LPS (Figure 3b).
Figure 3.
LPS signaling pathway was attenuated by sST2. (a) sST2 inhibited IκBα degradation and phosphorylation of MAPKs. iMDDCs with or without sST2 pre-treatment were stimulated by LPS (500 ng/ml) for 15, 30, 60, or 90 min, and the cell lysates were resolved by SDS–PAGE and transferred to PVDF membranes. The amounts of phosphorylated, total MAPKs, or IκBα were detected using specific antibodies. The relative intensities of the bands were calculated by LAS4000 and normalized with the values at 0 min. β-actin, p38, JNK, or ERK levels were used as control for equal loading. The representative result of three experiments is presented. Data indicated mean values±s.d. An asterisk denotes a statistically significant difference (*P<0.05). (b) Nuclear extracts were separated from iMDDCs with the same stimulus and time course for 15, 30, or 60 min as in (a), and then the amounts of NF-κBp65 in nuclear extracts were analyzed with NF-κBp65 transcription factor assay kit. The data are presented as means of three experiments±s.d. An asterisk denotes a statistically significant difference (*P<0.05). (c) sST2 inhibited nuclear translocation of NF-κBp65. iMDDCs were treated as indicated for 60 min, then incubated with anti-NF-κB p65 antibody for 60 min at room temperature. Representative data of three experiments are presented. iMDDCs, immature monocyte-derived dendritic cell; LPS, lipopolysaccharide; MAPK, MAP kinase; PVDF, polyvinylidene fluoride; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; sST2, soluble ST2 protein.
By fluorescence microscopy, the localization of NF-κB protein was further analyzed with anti-NF-κBp65 antibody. While NF-κB protein showed a nuclear shift when stimulated by LPS, pre-treatment with sST2 markedly suppressed the nuclear localization of NF-κB (Figure 3c).
These results reveal that sST2 suppresses LPS signal transduction in iMDDCs, eventually causing the inhibition of cytokine production.
Soluble ST2 attenuates the interaction of naive T-lymph cells and MDDCs
CD83-positive MDDCs are functionally matured MDDCs with strong antigen presentation ability in vitro, and we showed above that sST2 does not directly influence CD83 expression on LPS-stimulated iMDDCs (Figure 2).
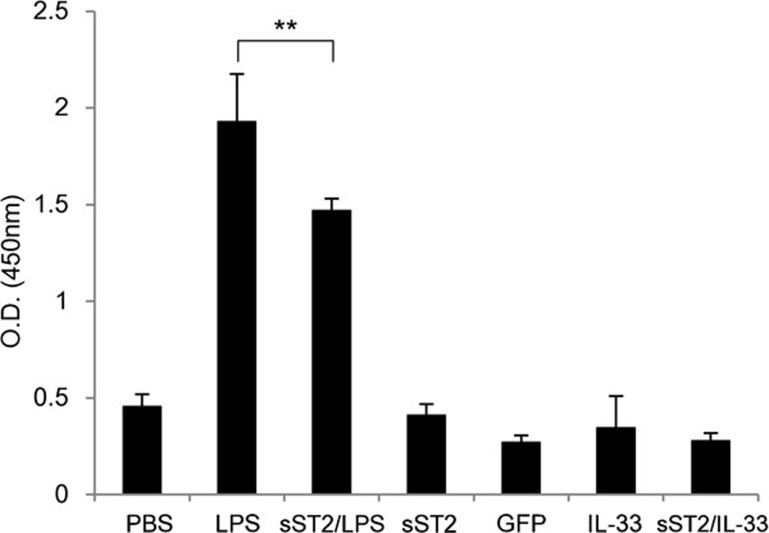
To determine whether or not the suppression of LPS signaling in iMDDCs by sST2 actually plays a role in the function of iMDDCs, we next examined the ability of LPS-stimulated iMDDCs to stimulate naive CD4+ T-cell proliferation. iMDDCs were cultured with naive CD4+ T cells in the presence or absence of LPS, sST2, IL-33, GFP, or PBS for 5 days, and the proliferation of naive CD4+ T cells was assayed by BrdU incorporation.17 The addition of IL-33 or sST2 into the culture had little effect on lymphocyte proliferation compared to the control, GFP protein, or PBS (Figure 4), and otherwise, iMDDCs without T cells incorporate little BrdU (data not shown). In contrast, LPS markedly induced BrdU incorporation, and the proliferation of naive CD4+ T cells was inhibited significantly when iMDDCs were pre-treated with sST2 (Figure 4).
Figure 4.
sST2 attenuates the proliferation of naive T-lymph cells by stimulated MDDCs autologously. iMDDCs were seeded at 2×104/well in 96-well tissue culture plates and cocultured with naive CD4+ T cells at a 1:10 ratio with or without 500 ng/ml LPS, 100 ng/ml IL-33, or 500 ng/ml GFP for 5 days. LPS-induced mMDDCs without T cells and PBS-treated iMDDCs were employed as controls. BrdU incorporation was measured to assay the proliferation of naive CD4+ T cells. The data are presented as the means of three experiments±s.d. A double asterisk denotes a statistically significant difference (**P<0.01). GFP, green fluorescent protein; IL, interleukin; iMDDCs, immature monocyte-derived dendritic cell; LPS, lipopolysaccharide; MDDC, monocyte-derived dendritic cell; mMDDC, mature monocyte-derived dendritic cell; PBS, phosphate-buffered saline; sST2, soluble ST2 protein.
IL-33 is not involved in the suppressive effect of sST2 on iMDDCs
It is known widely that sST2 functions as a decoy receptor of IL-33.2 Therefore, it should be examined whether or not the IL-33/ST2L pathway is involved in the sST2-induced suppression of cytokine production in iMDDCs.
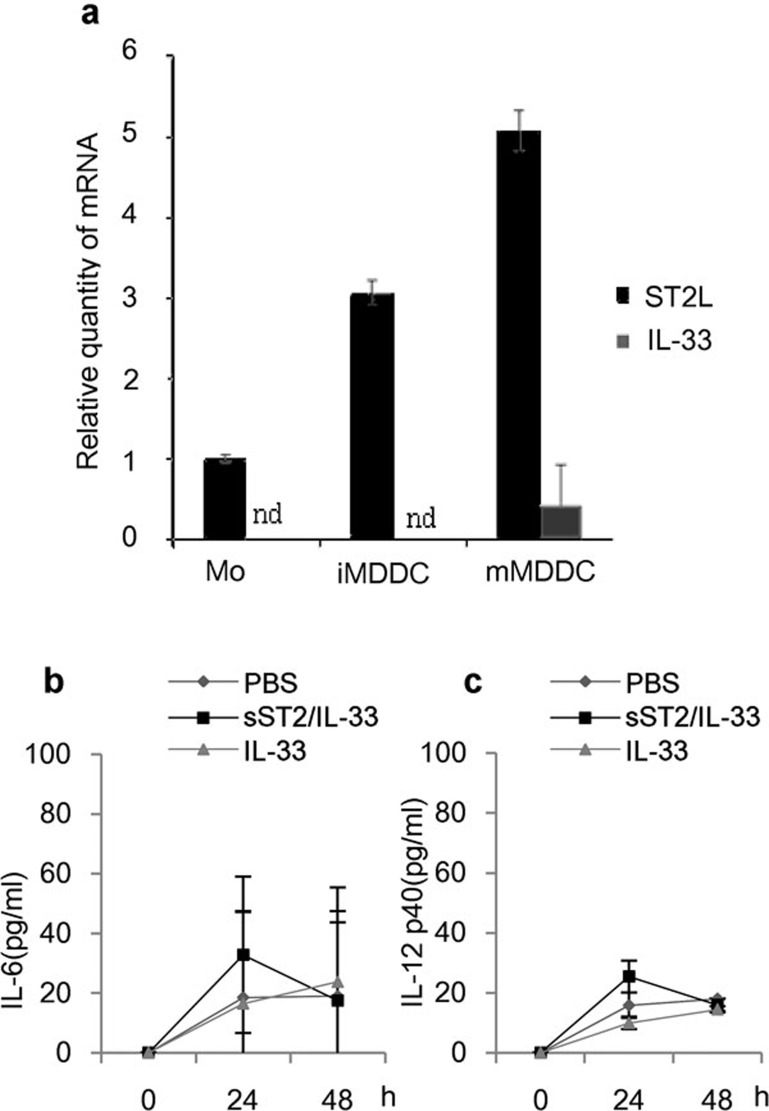
First, the ST2L messenger level in monocytes, iMDDCs and mMDDCs was examined using real-time PCR, and ST2L expression was detected and upregulated as cells maturated from monocytes to mMDDCs, whereas the expression of IL-33 mRNA was detected only in mMDDCs (Figure 5a). Additionally, IL-33 protein was not detected in the conditioned medium of sST2-treated, LPS-stimulated, or sST2/LPS-treated iMDDCs by ELISA (data not shown). Recombinant IL-33 was then added exogenously to the culture medium of iMDDCs, but neither IL-6 nor IL-12p40 was produced (Figure 5b and c). This suggested that sST2 inhibits cytokine production noncanonically and not by acting as a decoy receptor of IL-33.
Figure 5.
The involvement of IL-33/ST2L on cytokine production in iMDDCs. (a) IL-33 and ST2L mRNA levels were analyzed by real-time PCR in various maturation states of MDDCs. (b) iMDDCs were pre-treated for 1 h with or without sST2 (100 ng/ml), and then the cells were stimulated with IL-33 (50 ng/ml) for 24 or 48 h. The amount of IL-6 was determined by ELISA. (c) IL-12p40 production by IL-33 stimulation was assayed as described in (b). The data are presented as the means of three experiments±s.d. ELISA, enzyme-linked immunosorbent assay; IL, interleukin; iMDDCs, immature monocyte-derived dendritic cell; MDDC, monocyte-derived dendritic cell; sST2, soluble ST2 protein.
sST2 did not suppress the TLR5 pathway
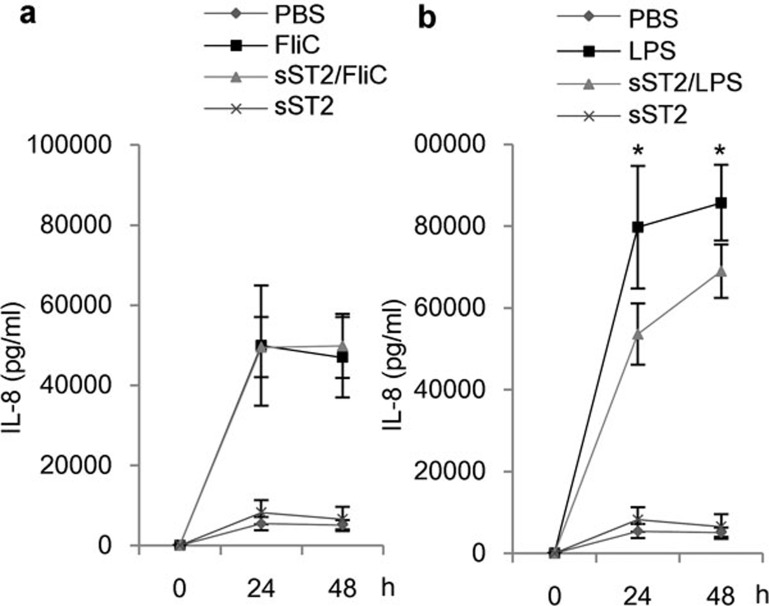
We showed that sST2 suppressed the LPS stimulus in iMDDCs. In order to verify whether or not this phenomenon is specific to the LPS/TLR4 pathway, we used flagellin as a ligand of TLR5, which was reported to activate iMDDCs.22 We tried to measure the cytokine production from flagellin-stimulated MDDCs, and IL-6 and IL-12p40 were not detectable by ELISA, but IL-8 was detectable and induced by flagellin.
When stimulated by LPS, IL-8 production from iMDDCs with sST2 pre-treatment decreased by about 20% (Figure 6b); however, sST2 had little effect on IL-8 production when iMDDCs were stimulated by flagellin (Figure 6a). Therefore, it is revealed that sST2 suppresses the LPS/TLR4 pathway, but not flagellin/TLR5 pathway.
Figure 6.
Flagellin stimulation was not inhibited by sST2. (a) iMDDCs were stimulated by flagellin (FliC, 100 ng/ml) for 24 or 48 h, and secreted IL-8 was determined by ELISA. The data are presented as the means of three experiments±s.d. (b) iMDDCs were stimulated by LPS (100 ng/ml), and the amount of IL-8 was determined as (a). Pre-treatment with sST2 decreased the amount of secreted IL-8 slightly. ELISA, enzyme-linked immunosorbent assay; IL, interleukin; iMDDCs, immature monocyte-derived dendritic cell; LPS, lipopolysaccharide; sST2, soluble ST2 protein.
Soluble ST2 protein suppressed TLR4 expression in iMDDCs
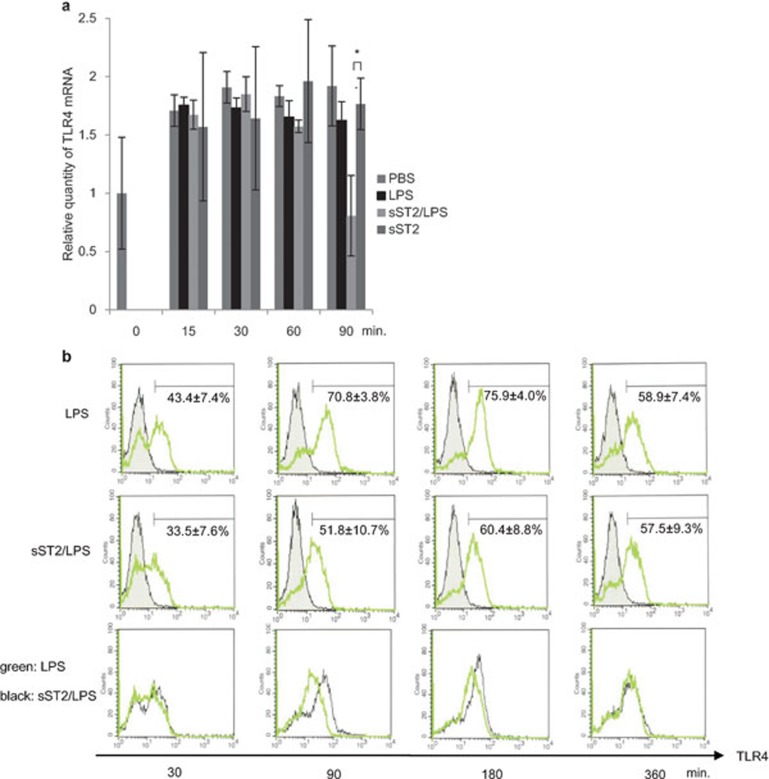
We examined whether or not the suppressive effect of sST2 on LPS-induced cytokine production was correlated with TLR4 expression. TLR4 mRNA levels were examined by real-time PCR in iMDDCs after incubation with LPS, sST2, or sST2/LPS.
The results showed that, with sST2 pre-treatment, TLR4 mRNA expression was downregulated 90 min after LPS stimulation (Figure 7a). Without LPS stimulation, sST2 pre-treatment did not change TLR4 expression, suggesting that the effect of sST2 on TLR4 expression is dependent on LPS stimulation.
Figure 7.
TLR4 expression is suppressed by sST2. (a) Relative quantity of TLR4 mRNA was analyzed by real-time PCR after various stimuli (sST2, LPS, sST2/LPS) and normalized against endogenous β-actin. The data are presented as the means of three experiments±s.d. An asterisk denotes a statistically significant difference (*P<0.05). (b) iMDDCs were pre-treated with or without sST2 (100 ng/ml) and treated with LPS (500 ng/ml) for 30 to 360 min. TLR4 protein on the cell surface was analyzed by FACS with anti-TLR4-PE. The figures of the bottom column were showing the expression of TLR4 of LPS-stimulated iMDDCs (the upper column, red) superimposed on the middle column (sST2/LPS; green). Representative data of three experiments are presented. FACS, fluorescence-activated cell sorting; iMDDCs, immature monocyte-derived dendritic cell; LPS, lipopolysaccharide; sST2, soluble ST2 protein; TLR4, Toll-like receptor 4.
The downregulation of TLR4 was also confirmed by FACS. TLR4 protein expression on the cell surface decreased 90 to 180 min after LPS stimulation with sST2 pre-treatment, and this downregulation recovered after 6 h (Figure 7b). The downregulation of TLR4 expression may play a partial role in sST2's inhibition of cytokine production.
Soluble ST2 protein translocates into MDDCs
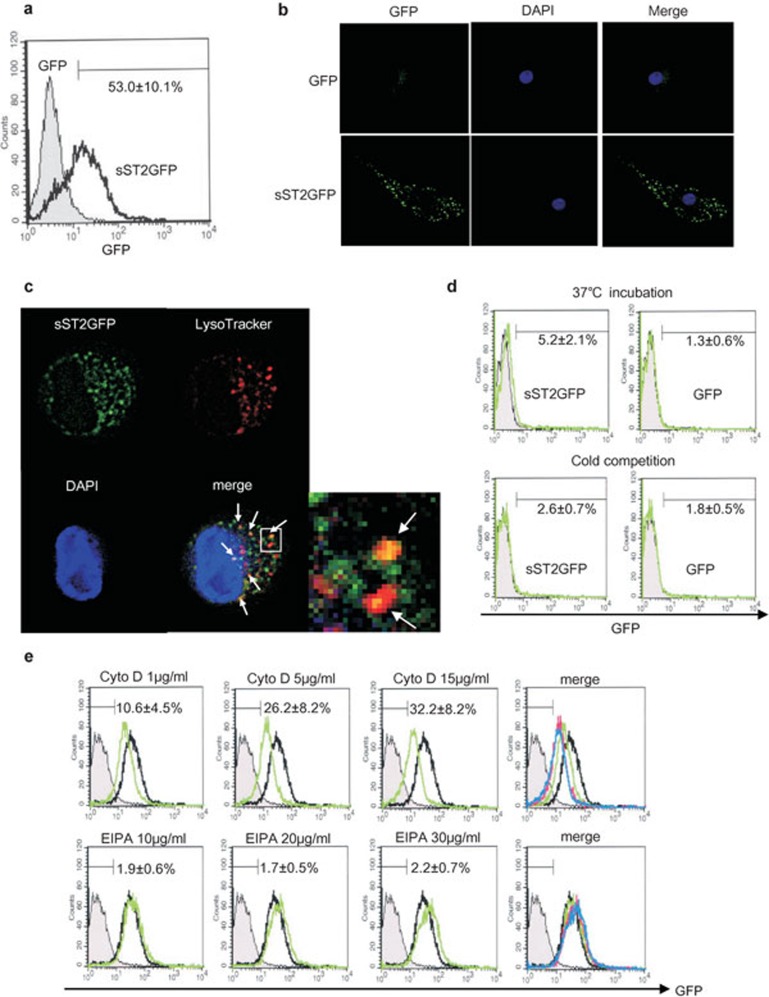
To elucidate the noncanonical mechanism by which sST2 inhibits cytokine production in iMDDCs, sST2-GFP fusion protein was incubated with iMDDCs without any detergent to investigate the binding property of sST2 to iMDDCs. When sST2-GFP protein was incubated with iMDDCs for 1 h, slight binding was observed by FACS (Figure 8d). But when the incubation was prolonged to 16 h, the intensity of GFP with iMDDCs increased markedly (Figure 8a). Subsequently, we investigated the localization of sST2-GFP in iMDDCs using fluorescence microscopy, and sST2-GFP was observed to be attached on the surface of iMDDCs or internalized into iMDDCs with speckled perinuclear pattern (Figure 8b). Furthermore, we confirmed that at least a part of sST2-GFP was internalized to the iMDDCs and colocalized with lysosome stained by LysoTracker (Red DND-99) (Figure 8c). Little binding was detected in the case of cold incubation for 1 h, suggesting that sST2 binding to iMDDCs is followed by engulfment of sST2 with energy consumption.
Figure 8.
sST2-GFP is internalized into iMDDCs. (a) iMDDCs were incubated with sST2-GFP (100 ng/ml) for 16 h at 37 °C. The fluorescence intensity of GFP was analyzed by FACS. One of five similar experiments is presented. The percentage of positive cells are indicated as the means of three experiments±s.d. (b) sST2-GFP is attached to iMDDCs which were incubated with sST2-GFP or GFP as described in (a) and observed with a fluorescence microscope (green, sST2-GFP; blue, DAPI). (c) sST2-GFP and lysosomes were co-localized in iMDDCs. The lysosomes in iMDDCs treated with sST2-GFP were stained with LysoTracker probes (Red DND-99) and observed with a fluorescence microscope (green, sST2-GFP; red, lysosome; yellow, sST2-GFP and the lysosome merged). The representative data of three similar experiments are presented. (d) Cold competition of sST2-GFP binding to iMDDCs. iMDDCs were incubated with sST2-GFP on ice or at 37 °C for 1 h. The fluorescence intensity of GFP was analyzed by FACS (upper panel green, 37 °C; lower panel green, on ice; gray area, unstained control). The percentage of positive cells are indicated as the means of five experiments±s.d. (e) Inhibition of macropinocytosis. iMDDCs were pre-treated with cytochalasin D (1, 5 and 15 µg/ml) or EIPA (10, 20 and 30 µg/ml) for 30 min, then sST2-GFP (100 ng/ml) was added for 16-h culture. After incubation, the cells were washed twice with PBS−, and then fluorescence was analyzed by FACS. The gray area shows the control without staining, and the black line shows iMDDCs incubated with sST2-GFP only. The merged panel presented cytochalasin D (green; 1 µg/ml, pink; 5 µg/ml, blue; 15 µg/ml) or EIPA (green; 10 µg/ml, pink; 20 µg/ml, blue; 30 µg/ml) lines. The percentage of positive cells are indicated as the means of five experiments±s.d. EIPA, 5-(N-ethyl-N-isopropyl)amirolide; DAPI, 4,6-diamidino-2-phenylindole; FACS, fluorescence-activated cell sorting; GFP, green fluorescent protein; iMDDCs, immature monocyte-derived dendritic cell; LPS, lipopolysaccharide; sST2, soluble ST2 protein.
Besides, the absorption of sST2-GFP into the iMDDC was not suppressed by cyotochalasin D or EIPA, each of which inhibits micropinocytosis (Figure 8e). This suggested that sST2 was translocated into iMDDCs by endocytosis.
Discussion
In this study, we confirmed that sST2 inhibits LPS stimulation in human MDDCs. We previously reported that sST2 inhibited LPS stimulation in monocytic cells.12 Monocytes are known to differentiate to DCs by LPS along with granulocyte macrophage-colony stimulating factor; and, in our preliminary investigation, the differentiation was also inhibited by sST2 (Takezako N et al., 2006, unpubl. data). As mentioned in this paper, iMDDCs pre-treated with sST2 were inhibited on LPS-induced cytokine production (Figure 1a and b), but, unlike monocyte differentiation, sST2 did not suppress the maturation of iMDDC by LPS (Figure 2). The discrepancy between monocytes and iMDDCs in terms of differentiation and maturation by LPS could be explained by the difference in susceptibility to sST2.
sST2 is thought to be a decoy receptor for IL-33, and its biological function is considered to be dependent on the sequestration of IL-33, which is the generally accepted mechanism by which sST2 functions in several pathologic states, such as airway inflammation,2 cardiovascular diseases,8,23 and severe sepsis.24 However, it is intriguing that IL-33 did not induce cytokine production in human iMDDCs (Figure 5b and c) and had little effect on the maturation of iMDDC (Figure 2), whereas IL-33 reportedly activated and maturated mouse bone marrow-derived DCs.15,16 This disagreement could also be attributable to the difference between human iMDDCs and mouse bone marrow-derived DCs; moreover, the limited expression of IL-33 in human iMDDC (Figure 5a) and absence of IL-33 in the conditioned medium of cultured iMDDCs make it difficult to consider that endogenous IL-33 plays a role in sST2's inhibitory effect on human iMDDCs.
These findings suggest that the mechanism of sST2 suppressing human iMDDCs is noncanonical and independent on the sequestration of IL-33. To elucidate the noncanonical mechanism of sST2 function, we further investigate, in detail, the way in which sST2 inhibits the LPS signaling pathway. Consistent with our previous observation on monocytic cells,12 the activation of NF-κB and MAP kinases by LPS was markedly inhibited by sST2 pre-treatment (Figure 3). This suppressive effect is thought to be a key to the inhibition of cytokine production. On the other hand, sST2 did not inhibit the flagellin-activated TLR5 pathway (Figure 6), suggesting that sST2's inhibitory effect is not general to TLR-dependent signal. So far, 13 different TLRs have been identified and TLRs 1–9 are conserved in humans and mice. The expression of TLRs varies with species, DC subtypes and maturation stages. Human MDDCs express the extracellular TLRs (TLRs 1, 2, 4, 5, 6) and the endosomal TLRs (TLRs 3, 8), but do not express TLR9.25,26 We tried to investigate the effect of sST2 on TLR9 using CpG, but iMDDCs were not stimulated by it. The effect of sST2 on other TLR pathways (TLR1/2, TLR2/6 and TLR7/8) remain be solved.
TLR4 expression was reported to be downregulated by sST2,10 and that was also the case in iMDDCs. However, the downregulation of TLR4 mRNA by sST2 was observed after 90 min of LPS stimulation (Figure 7a), and the TLR4 protein level was also decreased, though slightly (Figure 7b). LPS signal transduction is thought to occur in minutes or, in any case, much less than 1 h, so it seems that the downregulation of TLR4 may play a partial, rather than a crucial, role in the inhibitory effect of sST2.
Upstream from NF-κB and MAP kinases in the TLR4 signaling pathway, the molecule affected directly by sST2 is one of our major interests. The direct interaction between sST2 and LPS was also tested by the BIAcore system, but binding was not observed (data not shown). We are now planning to examine the direct binding of sST2 and TLR4, employing HEK293T cells overexpressing TLR4.
It is possible that sST2 has a specific receptor on the cell surface of MDDC, and TLR4 or CD14 would be the one of the candidates. To investigate the binding and fate of sST2 incubated with iMDDCs, we undertook the FACS analysis with sST2-GFP fusion protein without using any detergent. As shown in Figure 8, the specific binding of sST2-GFP fusion protein to iMDDC is not strong after the 1-h incubation; however, a marked shift in flow cytometry was observed after 16 h (Figure 8a). With the analysis by a fluorescence microscopy, at least a part of sST2-GFP was shown to be translocated into the cells and colocalized with lysosome (Figure 8c). Whole these procedures were done without any detergent. This translocation was not dependent on macropinocytosis (Figure 8e), and was specific to sST2-GFP compared with GFP protein. Recent study indicated that TLR4 first induces TIRAP-MyD88 signaling and then is endocytosed and activates TRAM-TRIF signaling.27 LPS was endocytosed by a receptor-mediated mechanism dependent on dyamin and clathrin and colocalized with TLR4 on early endosome28 and TLR4 endocytosis after LPS stimulation required phospholipase Cγ-2-inositol 1,4,5-trisphosphate-subsequent calcium signaling cascade.29 Endocytosis is a process of ligand uptake mediated by receptors such as the mannose receptor and Fc receptor, whereby ligands and immune complexes are internalized. In addition, endocytosis pathways could be subdivided into four categories: clathrin-mediated endocytosis, caveolae-mediated endocytosis, macropinocytosis and phagocytosis. Based on the results obtained, we suppose that a specific binding protein for sST2 on the cell surface, followed by internalization by endocytosis, could be involved in this process.
In several disorders, serum concentrations of sST2 were elevated,3,4,5,6,7,8,9 and the binding ability of sST2 towards IL-33 is generally thought to play a key role in the pathogenetic relevance of sST2.2,8,23,24 IL-33 is considered to be involved in a broad spectrum of diseases: not only in Th2-related diseases such as asthma and atopic dermatitis, but also in other inflammatory diseases such as inflammatory bowel disease, type 2 diabetes, and cardiovascular diseases, in which IL-33 is thought to protect the cardiovascular system.30 Recently, a high level of serum sST2 is considered to be a predictor of a high likelihood of mortality in patients with acute and chronic heart failure,7,8 pulmonary diseases.31
sST2 levels were also reported to increase in patients with sepsis and the sST2 levels correlate with severity and mortality of sepsis,24 and IL-33 was shown to attenuate sepsis.32 Sepsis is a systemic inflammatory condition following bacterial infection, associated with mortality rates of 20–50%. Sepsis moves through different phases with periods of excessive immunity called cytokine storm alternating with periods of immune suppression. Recent evidence has also identified that immunosuppression occurring after severe systemic inflammation is a result of depletion in DC numbers and following dysfunction in DC activity.33 The pathogenetic role of elevated sST2 in sepsis is still unclear, but suppressive effect of sST2 on DCs could play a role in septic patients other than the sequestration of IL-33.
We tested the overall effect of sST2 on T-cell activation via DCs, and found that sST2 pre-treatment of iMDDCs inhibited T-cell proliferation in a mixed lymphocyte reaction (Figure 4). This inhibition could result from the reduced production of cytokines from iMDDCs, especially in IL-12, which is an activator of T-cell proliferation. These results suggest that sST2 could inhibit DC function and attenuate immune reaction without sequestrating IL-33. This hypothesis would add to our understanding of the pathophysiological roles of sST2 in broad range of diseases.
Acknowledgments
We thank Dr Morisada Hayakawa, Dr Nobuhiko Kamoshita and Dr Masaki Kashiwada, Jichi Medical University, for the helpful discussions. The authors are grateful to Ms Reiko Izawa and Ms Chihiro Aoki for their excellent technical support and clerical assistance.
References
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- Lloyd CM. IL-33 family members and asthma - bridging innate and adaptive immune responses. Curr Opin Immunol. 2010;22:800–806. doi: 10.1016/j.coi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa K, Arai T, Okazaki H, Minota S, Tominaga S. Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem Biophys Res Commu. 2001;284:1104–1108. doi: 10.1006/bbrc.2001.5090. [DOI] [PubMed] [Google Scholar]
- Talabot-Ayer D, McKee T, Gindre P, Bas S, Baeten DL, Gabay C, et al. Distinct serum and synovial fluid interleukin (IL)-33 levels in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Joint Bone Spine. 2012;79:32–37. doi: 10.1016/j.jbspin.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- Daniels LB, Clopton P, Iqbal N, Tran K, Maisel AS. Association of ST2 levels with cardiac structure and function and mortality in outpatients. Am Heart J. 2010;160:721–728. doi: 10.1016/j.ahj.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Tajima S, Oshikawa K, Tominaga S, Sugiyama Y. The increase in serum soluble ST2 protein upon acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2003;124:1206–1214. doi: 10.1378/chest.124.4.1206. [DOI] [PubMed] [Google Scholar]
- Sweet MJ, Leung BP, Kang D, Sogaard M, Schulz K, Trajkovic V, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol. 2001;166:6633–6639. doi: 10.4049/jimmunol.166.11.6633. [DOI] [PubMed] [Google Scholar]
- Yin H, Li XY, Yuan BH, Zhang BB, Hu SL, Gu HB, et al. Adenovirus-mediated overexpression of soluble ST2 provides a protective effect on lipopolysaccharide-induced acute lung injury in mice. Clin Exp Immunol. 2011;164:248–255. doi: 10.1111/j.1365-2249.2011.04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezako N, Hayakawa M, Hayakawa H, Aoki S, Yanagisawa K, Endo H, et al. ST2 suppresses IL-6 production via the inhibition of IkB degradation induced by the LPS signal in THP-1 cells. Biochem Biophys Res Commun. 2006;341:425–432. doi: 10.1016/j.bbrc.2005.12.206. [DOI] [PubMed] [Google Scholar]
- Verhasselt V, Buelens C, Willems F, de Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123:1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41:1675–1686. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- Nguyen XD, Eichler H, Dugrillon A, Piechaczek C, Braun M, Klüter H. Flow cytometric analysis of T cell proliferation in a mixed lymphocyte reaction with dendritic cells. J Immunol Methods. 2003;275:57–68. doi: 10.1016/s0022-1759(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Hashiguchi N, Tsukamoto T, Osumi T. Variant forms of green and blue fluorescent proteins adapted for the use in mammalian cells. Bioimages. 1998;6:1–7. [Google Scholar]
- Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- Meier O, Boucke K, Hammer SV, Keller S, Stidwill RP, Hemmi S, et al. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J Cell Biol. 2002;158:1119–1131. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MD, Kozel TR. Macrophage uptake, intracellular localization, and degradation of poly-g-D-glutamic acid, the capsular antigen of Bacillus anthracis. . Infect Immun. 2009;77:532–538. doi: 10.1128/IAI.01009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The toll-like receptor 5 stimulus bacterial fragellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, Andrew N. J. McKenzie ANJ et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerwerf JJ, Tanck MW, van Zoelen MA, Wittebole X, Laterre PF, van der Poll T. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. 2010;36:630–637. doi: 10.1007/s00134-010-1773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ, et al. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother. 2010;10:1573–1582. doi: 10.1007/s00262-010-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Granucci F. Regulation of antigen uptake, migration, and lifespan of dendritic cell by Toll-like receptors. J Mol Med. 2010;9:873–880. doi: 10.1007/s00109-010-0638-x. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husebye H, Halaas Ø, Stenmark H, Tunheim G, Sandanger Ø, Bogen B, et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CY, Veckman V, Limmer K, David M. Phospholipase Cγ-2 and intracellular calcium are required for lipopolysaccharide-induced Toll-like receptor 4 (TLR4) endocytosis and interferon regulatory factor 3 (IRF3) Activation. J Biol Chem. 2012;287:3704–3709. doi: 10.1074/jbc.C111.328559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rumayor A, Camargo CA, Green SM, Baggish AL, O'Donoghue M, Januzzi JL. Soluble ST2 plasma concentrations predict 1-year mortality in acutely dyspneic emergency department patients with pulmonary disease. Am J Clin Pathol. 2008;130:578–584. doi: 10.1309/WMG2BFRC97MKKQKP. [DOI] [PubMed] [Google Scholar]
- Alves-Filho JC, Sônego F, Souto FO, Freitas A, Verri WA, Jr, Auxiliadora-Martins M, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708–712. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res. 2001;24:273–287. doi: 10.1385/IR:24:3:273. [DOI] [PubMed] [Google Scholar]