Abstract
Background
The addition of targeted agents to thoracic radiation has not improved outcomes in patients with locally advanced non-small cell lung cancer (NSCLC). In order to improve cure rates in locally advanced NSCLC, effective targeted therapies need to be identified that can be given safely with radiation therapy. Temsirolimus is an inhibitor of the mammalian target of rapamycin (mTOR) pathway and has single agent activity in lung cancer. Inhibition of the mTOR pathway has been shown to augment the cytotoxic effect of radiation in preclinical studies. There is scant clinical experience with mTOR inhibitors and radiation.
Methods
We performed a phase I study evaluating the combination of temsirolimus with thoracic radiation in patients with NSCLC.
Results
Ten patients were enrolled in the study. The dose limiting toxicities included sudden death, pneumonitis and pulmonary hemorrhage. The maximum tolerated dose of temsirolimus that could be administered safely with concurrent radiotherapy (35 Gy in 14 daily fractions) was 15 mg intravenously weekly. Of the 8 evaluable patients, 3 had a partial response and 2 had stable disease.
Conclusion
The combination of temsirolimus 15 mg weekly and thoracic radiation is well-tolerated and warrants further investigation, perhaps in a molecularly defined subset of patients.
Introduction
Approximately 26% of patients with non-small cell lung cancer (NSCLC) present with locally advanced disease which is not amenable to surgical resection.1 Concurrent administration of systemic chemotherapy along with thoracic radiation has been shown to improve survival over thoracic radiation alone in several randomized studies.2,3 However, even with the use of modern chemotherapy regimens and state of the art radiation techniques, the 3 year survival rate is at best only 30%.2,4 Moreover, concurrent chemoradiation is associated with significant toxicities including esophagitis and febrile neutropenia, and therefore considered only in the first line, potentially curative setting for patients with good performance status. While thoracic radiation alone is associated with fewer toxicities, 3 year survival is only 11%, largely due to distant relapse.5 Two large trials one exploring the substitution of pemetrexed for etoposide, and the other investigating the role of higher than conventional doses of thoracic radiation unfortunately have failed to improve overall survival in patients with locally advanced NSCLC.6,7 The addition of targeted agents to thoracic radiation thus far has not been successful.8,9 The only way to improve outcomes in patients with locally advanced NSCLC is to use targeted therapies in molecularly selected patients who receive chemoradiation.
Activation of the mammalian target of rapamycin (mTOR) pathway has been implicated in the development of several malignancies, including lung cancer.10,11 A member of the phosphatidylinositol 3-kinase (PI3K)-related family of kinases, mTOR is a 289-kDa protein serine/threonine kinase that was first identified as the cellular target of rapamycin and is involved in checkpoint regulation of the cell cycle regulation. Additionally, the mTOR pathway is responsible for upregulating downstream signaling of hypoxia inducible factor-1-α (HIF1-α) which promotes angiogenesis and cell proliferation.12 Temsirolimus is an inhibitor of the mTOR kinase and has demonstrated anti-proliferative and anti-angiogenic activity in multiple tumor types. Temsirolimus has been approved in the treatment of renal cell carcinoma, and is generally well-tolerated with observed grade 3 or 4 toxicities of temsirolimus including hyperglycemia (17%), hypophosphatemia (13%), anemia (9%), and hypertriglyceridemia (6%).13,14 In the phase II study reported by Ruengwetwattana and colleagues, 55 patients with untreated NSCLC were treated with temsirolimus 25 mg intravenously on a weekly basis.15 The clinical benefit rate was 35% with a partial response in 4 patients and stable disease for 8 weeks or more in 14 patients. Temsirolimus has appeal as an agent in combination with radiation for NSCLC because it has established anti-proliferative and anti-angiogenic activity in multiple epithelial tumors and has non-overlapping toxicities with radiation. Inhibition of the mTOR pathway and the downstream HIF1-α has been shown to augment the cytotoxic effect of radiation in vitro and in xenograft studies.16–18 However, there is scant clinical experience with temsirolimus in combination with radiation. The use of salvage temsirolimus along with involved field radiation in a single patient with refractory mantle cell lymphoma has been reported.19 A phase I study investigated the combination of temsirolimus combined with temozolamide and radiation in patients with glioblastoma multiforme, which was associated with grade 4/5 infections in 3 of 12 patients.20 The use of temsirolimus with thoracic radiotherapy for NSCLC has not been reported. We believe it is critical to test the safety and feasibility of single agent temsirolimus in combination with thoracic radiation before adding this agent in the setting of concurrent chemoradiation in patients with potentially curable locally advanced NSCLC. We therefore conducted a phase I study to establish the safety of temsirolimus in combination with thoracic radiation alone in patients who were not candidates for curative therapy with concurrent chemoradiation.
Patients and methods
Patient Selection
Patients with histologically or cytologically confirmed non-small cell lung cancer with an indication for palliative thoracic radiation were enrolled. Patients who were candidates for definitive chemoradiation with curative intent were excluded. Patients were required to have radiographically measurable disease, ECOG performance status of 0–2 and adequate hematologic, hepatic and renal function (leukocytes ≥3000; ANC ≥1500 cells/mm3; platelets ≥ 100,000/mm3; total bilirubin <1.5; AST/ALT less than 2.5 times the upper limit of normal levels, normal creatinine or CrCl>60 mL/min. Prior systemic therapies were allowed long as treatment was completed at least 4 weeks prior to study entry and all treatment related toxicities had resolved. Women of child-bearing potential and men were required to agree to the use of adequate contraception prior to study entry and for the duration of the study participation.
Patients were excluded from this study if they had received prior treatment with temsirolimus, or had received prior radiation therapy directed at the tumor volume to be treated with radiotherapy on this protocol. In addition, patients who were receiving any other investigational agents, hepatic enzyme-inducing anticonvulsants, or other CYP3A4 inducers were excluded. Patients with symptomatic brain metastasis were not allowed on the study. Other exclusion criteria included known hypersensitivity reactions to macrolide antibiotics, uncontrolled intercurrent illness including, not limited to symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, or psychiatric illness/social situation that would limit compliance with study requirements; known HIV-positivity on combination antiretroviral therapy. Written informed consent was obtained from all patients, and the trial was approved by the Washington University Human Research Protection Office.
Dosage and administration
Temsirolimus was administered as a weekly 30-minute intravenous infusion, with the starting dose of 20 mg on day 1. The other dose levels used on this study are described in table 1. Temsirolimus was administered on a weekly basis for a total of four weeks. Radiotherapy began on day 2 and consisted of daily fractions of 2.5Gy, 5 days per week to a total cumulative dose of 35 Gy for a total of 14 days. Radiotherapy treatments were designed at the discretion of the radiation oncologist per protocol such that > 95% of the dose encompassed the planning target volume (PTV) while restricting the volume of lung receiving in excess of 20 Gy to < 40%, the entire heart volume to < 25 Gy, and spinal cord to < 50 Gy. Routine supportive care was permitted per institutional guidelines.
Table 1.
Dose escalation schema
| Cohort | Temsirolimus weekly |
|---|---|
| −1 | 15 mg |
| 1 | 20 mg |
| 2 | 25 mg |
Study Design and Treatment Plan
The primary endpoint of the trial was maximum tolerated dose (MTD) of temsirolimus when given concurrently with thoracic radiation. Secondary endpoints included evaluation of safety of this regimen in patients with NSCLC, and determination of dose-limiting toxicities (DLT).
All toxicities were graded according to the NCI CTCAE v.3.0. DLT was defined as any grade 4 hematologic toxicity with the exception of anemia, any grade 4 non-hematologic toxicity related to study therapy, grade 3 or 4 pneumonitis or esophagitis, treatment delay of temsirolimus for more than 14 consecutive days due to study related toxicity, treatment delay of radiation therapy for more than 14 consecutive days because of study related toxicity, and death on study or within 30 days of receiving study therapy. The MTD was defined as the dose level which led to DLT in no more than one patient within the cohort.
A conventional 3+3 design was used to define MTD. However, a maximum of six patients were planned to be treated at the initial temsirolimus dose level 1 (20 mg) to ensure tolerability before dose escalation. If a DLT occurred in 0 or 1 patients, then the plan was to enter additional patients in the next higher dose cohort (dose level 2). However, if DLTs were observed in 2 or more patients enrolled in dose level 1, additional six patients would be enrolled in the next lower dose cohort (dose level-1). There was no intra-patient dose escalation. The patients were evaluated for a minimum of 90 days after initiation of temsirolimus, or recovery to grade 1 toxicities, whichever occurred later (excluding alopecia).
Follow-up studies
Toxicity assessments were performed weekly during the first five weeks and then every four weeks thereafter to evaluate for DLT. Physical exams were performed every two weeks during the first four weeks and then every four weeks thereafter. Radiologic evaluation was performed at baseline and at eight weeks after initiation of treatment. Response was defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 criteria.
Results
Patient characteristics
Between March 2009 and February 2011, 10 patients were enrolled in this study (Figure 1 and Table 2), of which 5 were men and 5 were women. The median age of these patients was 62.5 years (range 46–83). Patients were treated on two dose levels: 4 patients received treatment on dose level 1 (temsirolimus 20 mg) and 6 received treatment on dose level -1 (temsirolimus 15 mg). Patients received a median of 3.5 doses of temsirolimus. Five patients received all 4 doses of temsirolimus. Eight patients completed their course of radiation therapy.
Figure 1.
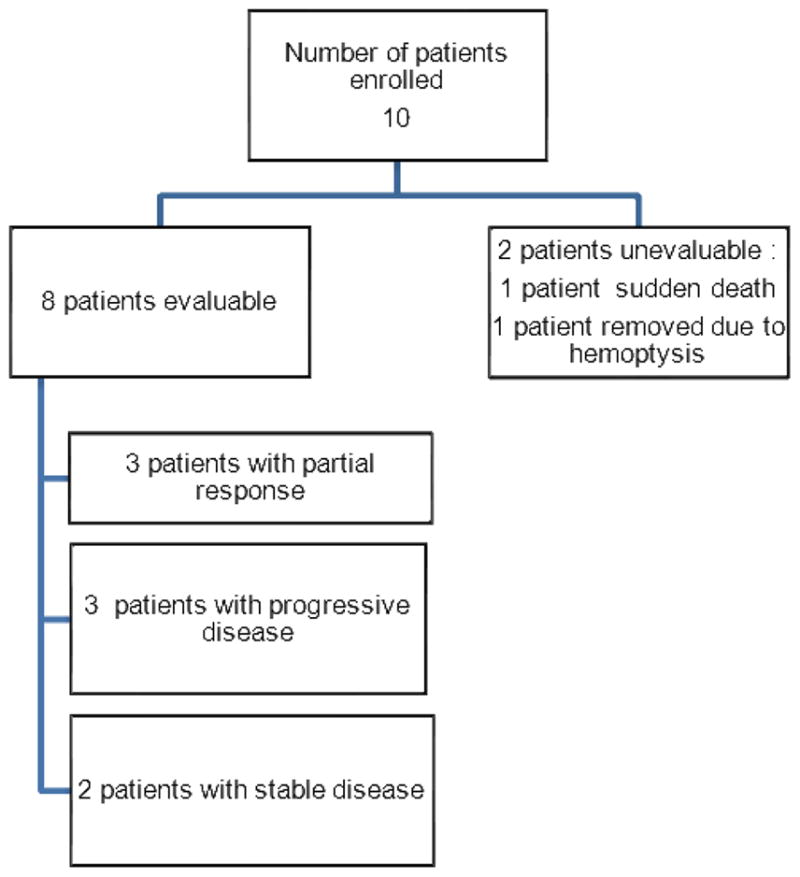
Flow diagram of patients enrolled on study
Table 2.
Patient characteristics
| Characteristics | No. of patients |
|---|---|
| Age | |
| Median | 62.5 |
| Range | 46–83 |
| Sex | |
| Male | 5 |
| Female | 5 |
| ECOG Performance status | |
| 0 | 1 |
| 1 | 9 |
| Stage | |
| IV | 9 |
| Recurrent | 1 |
| Histology | |
| Adenocarcinoma | 5 |
| Squamous cell carcinoma | 3 |
| Poorly differentiated NSCLC | 2 |
| Prior radiotherapy | 2 |
| Head and neck radiation | 1 |
| Gamma knife for brain metastasis | 1 |
| Prior chemotherapy | 6 |
| Platinum doublet or triplet | 6 |
| Docetaxel | 1 |
| Pemetrexed | 1 |
| rlotinib | 1 |
Toxicities
Four patients were enrolled at dose level 1. One patient registered to the protocol received two doses of temsirolimus and three doses of radiation and expired at home. This event was considered a DLT per protocol, even though the event was attributed to the patient’s lung cancer and not related to protocol therapy. Another patient treated at dose level 1 experienced grade 3 pneumonitis, grade 4 fatigue and grade 4 lymphopenia related to protocol therapy meeting the definition of DLT (table 3). This patient developed pneumonitis on day 10 of radiation, after receiving 2 doses of temsirolimus. This patient was able to complete radiation therapy to a total dose of 35 Gy, but did not receive any additional doses of temsirolimus. The V20 was 22% and the mean lung dose was 9.8 Gy. Since two patients had DLT, we de-escalated the dose of temsirolimus to dose level -1. Of the first three patients treated at dose level-1, one patient developed grade 3 pulmonary hemorrhage from a fungating right mainstem bronchus mass after receiving only 2 radiation fractions to a total dose of 5 Gy, and underwent bronchial artery embolization No other DLTs occurred at this dose level when three additional patients were enrolled. The MTD was determined to be dose level -1, which comprised of temsirolimus 15 mg intravenously weekly with concurrent radiotherapy.
Table 3.
Toxicity profile
| Toxicity | Cohort (1) (N=4) | Cohort (−1) (N=6) | ||||
|---|---|---|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | |
| Cardiovascular | 3 | 1 | ||||
| Constitutional | 3 | 1*^ | 3 | |||
| Gastrointestinal | 2 | 1 | 5 | |||
| Lymphopenia | 1 | 1*^ | 2 | 2 | ||
| Neutropenia | 2 | |||||
| Decreased platelet count | 1 | 2 | ||||
| Other hematological | 1 | 2 | 1 | |||
| Infection | 2 | 1 | ||||
| Metabolic/Laboratory | 3 | 3 | 2 | |||
| Pain | 2 | 4 | ||||
| Pulmonary | 2 | 1*^ | 2 | 1* | ||
| Neurologic | 2 | 4 | ||||
| Dermatologic | 1 | 3 | ||||
| Hemorrhage/ Thromboembolic | 1 | 1 | 2 | |||
| Sudden Death | 1* | |||||
Dose limiting toxicities
This patient developed all of the following: grade 3 pneumonitis, grade 4 fatigue and grade 4 lymphopenia.
Antitumor activity
Two patients were not evaluable for response. One patient was removed from study due to sudden death at home. She had received 1 dose of temsirolimus (dose level 1) and 3 days of radiation and her death was thought to be unrelated to the study treatment. A second patient was removed from study following 1 dose of temsirolimus and 2 days radiation due to hemoptysis. Eight patients were evaluable for anti-tumor activity. Two of the 8 evaluable patients had stable disease, 3 patients had a partial response, and 3 patients had progressive disease with new lesions outside the radiation treatment field.
Discussion
Non-small cell lung cancer is a molecularly diverse disease, and a driver mutation has been identified in 54% of patients21 Despite the striking benefit seen with the use of agents such as erlotinib and crizotinib in molecularly selected patients with advanced stage NSCLC, empiric use of targeted agents has not improved the outcomes in patients with locally advanced NSCLC. The use of the vascular endothelial growth factor inhibitor, bevacizumab, with radiation has been associated with development of trachea-esophageal fistula.9 Maintenance therapy with gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, following completion of chemoradiation in molecularly unselected NSCLC was associated with worse overall survival.8 Induction chemotherapy followed by gefitinib and concurrent thoracic radiation in patients with unresectable adenocarcinoma, selected by light/never smoking status only, did not meet pre-defined criteria for feasibility.22 In order to improve cure rates in locally advanced NSCLC, rationally selected, effective targeted therapies need to be identified that can be given safely with radiation therapy. Future studies of targeted agents with radiation need to be directed towards molecularly selected patient populations.
This is the first report to the best of our knowledge, of the use of temsirolimus along with thoracic radiation in patients with lung cancer. The outcomes for patients with locally advanced NSCLC treated with definitive chemoradiation have reached a plateau.23 The successful incorporation of targeted agents, such as inhibitors of mTOR in this setting requires better understanding molecular markers of response that can be used to select patients. Somatic mutations in tuberous sclerosis complex 1 (TSC1) gene and inactivation of neurofibromatosis type 2 gene (NF2) were recently identified as potential markers of response to mTOR therapy, through sequencing of the tumor genome of a single patient with metastatic bladder cancer, who achieved complete response of greater than 2 years duration to everolimus.24 Inactivating somatic mutations of STK11 (LKB1) reported in 34% of lung adenocarcinomas and 19% of squamous cell carcinomas could potentially identify patients likely to respond to temsirolimus given the sensitivity of a cell line with loss of STK11 to rapamycin.25–27 Mutations in PIK3CA have been shown to sensitize cancer cells to everolimus, but a concurrent mutation in KRAS or BRAF is predictive for resistance to mTOR inhibition.28 Future studies of mTOR inhibitors in conjunction with thoracic radiation or chemoradiation should select patients based on these biomarkers. This strategy has already been employed in an ongoing phase II study of sunitinib or temsirolimus in patients with advanced rare tumors, in which patient selection is based on suspected or known germline mutations in PTEN, TS1/2, LKB1 and NF1 or 2 (NCT01396408).
Conclusions
The combination of temsirolimus 15mg weekly and thoracic radiation is well-tolerated and warrants further investigation, perhaps in a molecularly defined subset of patients.
Acknowledgments
We would like to thank patients who participated in this study and physicians, nurses and research coordinators at Washington University Oncology Clinic for their care of these patients. The authors also wish to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842. This study was funded by Pfizer pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morgensztern D, Goodgame B, Baggstrom MQ, Gao F, Govindan R. The effect of FDG-PET on the stage distribution of non-small cell lung cancer. J Thorac Oncol. 2008;3:135–9. doi: 10.1097/JTO.0b013e3181622c2c. [DOI] [PubMed] [Google Scholar]
- 2.Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21:2004–10. doi: 10.1200/JCO.2003.04.197. [DOI] [PubMed] [Google Scholar]
- 3.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology J Clin Oncol. 2008;26:5755–60. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 4.Jalal SI, Riggs HD, Melnyk A, et al. Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann Oncol. 2012;23:1730–8. doi: 10.1093/annonc/mdr565. [DOI] [PubMed] [Google Scholar]
- 5.Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990;323:940–5. doi: 10.1056/NEJM199010043231403. [DOI] [PubMed] [Google Scholar]
- 6.Bradley JD, Paulus R, Komaki R, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: Results on radiation dose in RTOG 0617. ASCO Meeting Abstracts. 2013;31:7501. [Google Scholar]
- 7.Vokes E, Wang L, Vansteenkiste J, et al. Preliminary safety and treatment delivery data during concurrent phase of chemoradiation therapy of the PROCLAIM trial: a phase 3 trial of pemetrexed, cisplatin, and radiotherapy followed by consolidation pemetrexed versus etoposide, cisplatin, and radiotherapy followed by consolidation cytotoxic chemotherapy of choice in patients with stage III nonsquamous cell lung cancer. J Thorac Oncol : official publication of the International Association for the Study of Lung Cancer. 2013;8:S551–S2. [Google Scholar]
- 8.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–6. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 9.Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28:43–8. doi: 10.1200/JCO.2009.24.7353. [DOI] [PubMed] [Google Scholar]
- 10.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–62. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 11.Wislez M, Spencer ML, Izzo JG, et al. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer Res. 2005;65:3226–35. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- 12.Hudson CC, Liu M, Chiang GG, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–14. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 14.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–18. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 15.Reungwetwattana T, Molina JR, Mandrekar SJ, et al. Brief report: a phase II “window-of-opportunity” frontline study of the MTOR inhibitor, temsirolimus given as a single agent in patients with advanced NSCLC, an NCCTG study. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:919–22. doi: 10.1097/JTO.0b013e31824de0d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Kon T, Wang H, et al. Enhancement of hypoxia-induced tumor cell death in vitro and radiation therapy in vivo by use of small interfering RNA targeted to hypoxia-inducible factor-1alpha. Cancer Res. 2004;64:8139–42. doi: 10.1158/0008-5472.CAN-03-2301. [DOI] [PubMed] [Google Scholar]
- 17.Arvold ND, Guha N, Wang D, et al. Hypoxia-induced radioresistance is independent of hypoxia-inducible factor-1A in vitro. Int J Radiat Oncol Biol Phys. 2005;62:207–12. doi: 10.1016/j.ijrobp.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Williams KJ, Telfer BA, Xenaki D, et al. Enhanced response to radiotherapy in tumours deficient in the function of hypoxia-inducible factor-1. Radiother Oncol. 2005;75:89–98. doi: 10.1016/j.radonc.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Kirova YM, Chargari C, Amessis M, Vernant JP, Dhedin N. Concurrent involved field radiation therapy and temsirolimus in refractory mantle cell lymphoma (MCL) Am J Hematol. 2010;85:892. doi: 10.1002/ajh.21803. [DOI] [PubMed] [Google Scholar]
- 20.Sarkaria JN, Galanis E, Wu W, et al. Combination of temsirolimus (CCI-779) with chemoradiation in newly diagnosed glioblastoma multiforme (GBM) (NCCTG trial N027D) is associated with increased infectious risks. Clin Cancer Res. 2010;16:5573–80. doi: 10.1158/1078-0432.CCR-10-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kris MG, Johnson BE, Kwiatkowski DJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI’s Lung Cancer Mutation Consortium (LCMC) ASCO Meeting Abstracts. 2011;29:CRA7506. [Google Scholar]
- 22.Niho S, Ohe Y, Ishikura S, et al. Induction chemotherapy followed by gefitinib and concurrent thoracic radiotherapy for unresectable locally advanced adenocarcinoma of the lung: a multicenter feasibility study (JCOG 0402) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:2253–8. doi: 10.1093/annonc/mds012. [DOI] [PubMed] [Google Scholar]
- 23.Govindan R, Bogart J, Vokes EE. Locally advanced non-small cell lung cancer: the past, present, and future. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3:917–28. doi: 10.1097/JTO.0b013e318180270b. [DOI] [PubMed] [Google Scholar]
- 24.Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makowski L, Hayes DN. Role of LKB1 in lung cancer development. Br J Cancer. 2008;99:683–8. doi: 10.1038/sj.bjc.6604515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 27.Contreras CM, Akbay EA, Gallardo TD, et al. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis Model Mech. 2010;3:181–93. doi: 10.1242/dmm.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohseni M, Park BH. PIK3CA and KRAS mutations predict for response to everolimus therapy: now that’s RAD001. J Clin Invest. 2010;120:2655–8. doi: 10.1172/JCI44026. [DOI] [PMC free article] [PubMed] [Google Scholar]


