Abstract
A number of studies have confirmed the potential for neuronal nicotinic acetylcholine receptor (NNR)-mediated neuroprotection and, more recently, its anti-inflammatory effects. The mechanistic overlap between these pathways and the ubiquitous effects observed following diverse insults suggest that NNRs modulate fundamental pathways involved in cell survival. These results have wide-reaching implications for the design of experimental therapeutics that regulate inflammatory and anti-apoptotic responses through NNRs and represent an initial step toward understanding the benefits of novel therapeutic strategies for the management of central nervous system disorders that target neuronal survival and associated inflammatory processes.
Keywords: neuroprotection, inflammation, nicotinic receptors, second messengers
Introduction
The role of neuroprotection may be central to the management of conditions such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, epilepsy, and ischemic optic neuropathy, as well as cerebrovascular disorders, traumatic brain injury, spinal cord injury, and retinal degeneration. Many of the underlying mechanisms responsible for damage to neural tissues are believed to be similar in a number of these conditions. Over 500 products have been investigated for neuroprotective effects, including those from the following categories: free radical scavengers, anti-excitotoxic agents, apoptosis inhibitors, anti-inflammatory agents, neurotrophic factors, and ion channel modulators. Extensive work assessing neuronal nicotinic acetylcholine receptor (NNR)-mediated neuroprotection supports a ubiquitous and broad role for NNRs in regulating the various pathways involved in cell survival, apoptosis, and death.
Inflammatory diseases affect more than 500 million patients in the major pharmaceutical markets, and the total prevalence is growing. Cytokine therapeutic agents play a major role in the treatment of these diseases. All existing or emerging therapies, including the T-cell modulators efalizumab and alefacept and the lymphocyte modulator abatacept, target specific cytokines, but none is directed toward several cytokines at once. Three recombinant anti-TNFα agents (etanercept, infliximab, and adalimumab) are currently used in the US for the treatment of inflammatory diseases such as rheumatoid arthritis (RA) and Crohn's disease (CD), and more than twenty other recombinant products that target 11 different cytokines are in clinical development for conditions such as RA, psoriasis, CD, asthma, multiple sclerosis, and osteoarthritis. In addition to inflammatory bowel disease, osteoarthritis, and sepsis, a number of clinical trials have also evaluated the role and contribution of anti-inflammatory agents in neurodegenerative diseases and other forms of dementia 1, 2, 3, 4. In addition, major public health conditions have been or are now being linked to insidious chronic inflammation. The “cholinergic anti-inflammatory pathway” and its role in immune responses and inflammatory cascades have attracted enormous interest due to the obvious relevance to a variety of debilitating human diseases, including atherosclerosis, diabetes, neurodegenerative diseases, osteoarthritis, sepsis, chronic obstructive pulmonary disease, and inflammatory bowel disease. Several clinical trials have also evaluated the role and contribution of anti-inflammatory agents in neurodegenerative diseases and other forms of dementia, potentially linking anti-inflammatory events with neuroprotective mechanisms4, 5.
NNR and neuroprotection
NNRs are heterogeneous in biological systems, partly as a consequence of the genetic diversity of subunit-encoding genes, the variable stoichiometry of the pentameric structure, the intrinsic biophysical properties of the resulting ligand-gated ion channel, and the cell-dependent coupling to secondary and tertiary messenger systems. Nine of the sixteen human genes that encode the subunits comprising the pentameric structures are expressed exclusively in the human brain, with predominant, but not exclusive, presynaptic localization of the pentameric receptor, which is implicated in the heterologous modulation of various neurotransmitters. Presynaptic nicotinic acetylcholine receptors (nAChRs) heterotypically modulate the release of non-cholinergic chemical messengers such as GABA, glutamate, serotonin, norepinephrine, dopamine (DA) growth factors, and various cytokines6.
The α4β2 and α7 NNR subtypes are the most abundant nicotinic receptor subtypes in the mammalian brain. Both appear to play a major role in cognitive processes such as learning and memory. Nicotinic receptors are found throughout the brain and have been shown to modulate multiple neuronal pathways involved in schizophrenia. Recent observations demonstrate that the gene products for the α4 and β2 subunits, which comprise the predominant mammalian brain NNR subtypes, can form heterogeneous targets7, 8. Human α4β2 NNRs expressed in transfected cell lines, as well as those expressed in vivo, are present as a mixture of two stoichiometries, (α4)2(β2)3 and (α4)3(β2)2. The former displays high sensitivity (HS), while the latter exhibits low sensitivity (LS) to agonist activation. The calcium permeability and affinity for nicotine of these two stoichiometries have also been shown to differ. The LS subtype has a lower affinity for nicotine and acetylcholine and displays high calcium permeability. Conversely, the HS subtype has a greater affinity for nicotine and acetylcholine and exhibits lower calcium permeability9. Although co-expression of the two isoforms in brain has been shown10 their specific roles in biological processes remain unknown. To date, studies evaluating neuroprotection have yet to provide clear evidence identifying the isoform that is present during biological insult and following NNR-mediated recovery. Either the α4, β2, or α5 subunit may represent the fifth subunit partner in the α4β2* protein target. Further references to α4β2*below reflect this uncertainty.
Convergence from many unrelated research areas has identified a primary role for one of the most abundant subtypes, the α7 nAChR, which is expressed in both the CNS and autonomic nervous system, in health and disease. Specifically, α7 NNRs are located in the hippocampus, thalamus, prefrontal cortex, subcortical basal ganglia, dopaminergic neurons in the ventral midbrain, and raphe serotonergic neurons. This nAChR subtype has been the subject of intense scrutiny in recent years, and it is becoming clear that it plays ubiquitous roles that range from cognitive processes to modulation of specific neurotransmitters and neuroprotection following various insults ranging from chemical toxicity to β-amyloid-induced cell death, normalization of sensory gating in schizophrenic patients and, more recently, as a central regulator of the inflammatory process. Similarly, a role for α4β2 in cognitive processes and neuroprotection has emerged, suggesting either redundant pathways within the same neurons or cell-specific expression of NNRs that regulate cell survival. Additional evidence, although limited, has suggested a potential role for α6β2* in protecting against nigrostriatal damage in mice. A number of excellent reviews have addressed various aspects of NNR-mediated neuroprotection 11, 12, 13, 14 and have provided an exhaustive review of the literature, including references to some of the early pioneering work. In the present review, I attempt to restrict references to the most recent work, with particular emphasis on the body of work dedicated to understanding the molecular and cellular pathways involved in α7 and α4β2* NNR-mediated neuroprotection and anti-inflammatory potential.
Preclinical evidence for neuroprotection
The potential for neuroprotection is supported by numerous in vitro studies demonstrating NNR-mediated protection of cells from a variety of toxic challenges, including brain injury15, oxygen-glucose deprivation16, 17, 18, oxidative stress16, β-amyloid toxicity (reviewed in14), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) kainite and glutamate excitotoxicity19, 20, 21, ethanol exposure22, and nerve growth factor (NGF) deprivation23, 24, 25, 26. Similarly, a number of studies using in vivo models have demonstrated neuroprotection by the reduction of β-amyloid expression in the APPsw mouse (a transgenic model of AD)27, surgically induced neuronal loss through nucleus basalis lesions28, chemically induced neurotoxicity mediated by MPTP toxicity and systemic kainic acid-induced excitotoxic effects (reviewed in19, 24), glutamate toxicity in the spastic Han-Wistar rat29, cerebral ischemia-reperfusion30, chronic ischemia models, and paraquat toxicity31. Interestingly, transient forebrain ischemia, which is ameliorated through NNR pathways, is associated with hyperphosphorylation of tau proteins in the hippocampus32. These broad neuroprotective effects following diverse insults can be recruited through several subtypes of the nicotinic receptor family of ligand-gated ion channels: the α4β2 nicotinic receptor17, 33, 34, 35 and the α7 nicotinic receptor13, 16, 18, 19, 36, 37, 38, 39, 40. Cytisine, a partial agonist of α4β2 and a full agonist of the α7 nicotinic receptor, is protective against beta amyloid toxicity in rat cortical neurons34.
The α7 NNR
In cultured cells, nicotinic agonists demonstrate neuroprotection against β-amyloid toxicity37, 39, 41. In addition, chronic administration of nicotine to APPsw mice for 5.5 months dramatically reduced β-amyloid plaque expression27. In follow-up studies, Nordberg's group reported that nicotine treatment reduced insoluble forms of β-amyloid by 80%23, reduced GFAP-reactive astrocytes around plaques, and increased levels of the synaptic marker synaptophysin in as few as 10 days in the APPsw mice42. These data are relevant in that reduction of β-amyloid with anti-Aβ-antibodies leads to rapid recovery of associated neuritic dystrophy in living animals43. Furthermore, early cognitive deficits correlate with intracellular β-amyloid accumulation in 3×Tg-AD mice, and clearance of β-amyloid accumulation with immunotherapy reverses the early cognitive impairment44. Chronic administration of nicotine to 3×TG-AD mice (5 months via the drinking water) resulted in elevated levels of tau pathology45. No significant changes, however, have been observed in the tau pathology of humans chronically exposed to nicotine when compared to age-matched non-smokers46, 47, 48. In this respect, the 3×TG-AD mouse model may hold limited relevance to the effects of nicotine in humans. Shaw et al39 were the first to report that tyrosine phosphorylation of JAK2, which subsequently forms a complex with α7, was the initial transducing step prior to activation of PI3 kinase and Akt phosphorylation. The α7-JAK2 pathway was later confirmed in vivo49 and linked to the STAT3 pathway, suggesting an overlap between neuroprotective and anti-inflammatory pathways at the level of JAK2. β-amyloid binding to the α7 NNR subunit in hippocampal slices exposed to oxygen glucose deprivation has been shown to be necessary, as the neuroprotective effect of nicotine was lost in α7 knock-out mice16. Further studies in a mouse model of Alzheimer's disease have demonstrated clear inhibition of β-amyloid deposition and aggregation, and that these effects are mediated through a pro-survival cascade involving MAPK, Bcl-2, and NF-κB13. This cascade is activated by the α7 nicotinic receptor and is inhibited by β-amyloid39. The direct interaction of β-amyloid with the α7 nicotinic receptor is now well documented and has been demonstrated using electrophysiological and biochemical approaches. The interaction of nicotine with the α7 nAChR inhibits the interaction of Aβ(1–42) with the same receptor, and Aβ(1–42)-induced apoptosis is prevented by nicotine-induced activation of JAK237, 39. These effects can be shown by measuring markers of cytotoxicity, including cleavage of nuclear protein poly(ADP-ribose) polymerase (PARP), induction of caspase 3, or cell viability. In addition, TC-1698 [2-(3-pyridyl)-1-azabicyclo[3.2.2]nonane], a novel α7-selective agonist, exerts neuroprotective effects via activation of the JAK2/PI-3K cascade37. Cross-talk between β-amyloid toxicity and tau hyperphosphorylation has also been demonstrated. Aβ1–42 can potentiate hyperphosphorylation of tau proteins in cell lines, as well as in transgenic mice. Aβ1–42-induced tau phosphorylation and increases in GSK-3β phosphorylation were attenuated by selective α7 nicotinic ligands, and these effects were blocked by the antagonists methyllycaconitine and α-bungarotoxin50.
Neuroprotective effects against amyloid-β toxicity by an α7-nAChR “antagonist”51 also suggest involvement of mechanisms beyond the activation of channel opening. This indicates that the neuroprotective effects are associated with drug influences on messenger transduction and downstream signaling cascades. It is also possible that these effects are unrelated to channel function and changes in membrane potential.
The α4β2* NNR
A number of studies, however, have demonstrated that α4β2* neuronal nicotinic receptors function independently of α7 to induce neuroprotection and contribute to neuronal survival in vivo. Glutamate-induced neurotoxicity in primary cultures of rat cortical neurons21 and hippocampal slices52 was prevented in a time- and concentration-dependent manner by the α4β2* subtype. Evidence for this was shown using selective ligands and dihydro-beta-erythroidine, an α4β2* nAChR antagonist, whereas the α7 nAChR antagonist α-bungarotoxin had no effect. Furthermore, the neuroprotective effects required extracellular Ca2+. Similar neuroprotective effects are observed during glutamate-induced excitotoxicity in adult pig retinal ganglion cells20, suggesting potential opportunities for ophthalmic conditions. Transgenic mice have provided additional support for an independent role of α4β2* in mediating neuroprotection. Zanardi et al53 found that mice lacking the β2 subunit demonstrate increased susceptibility to hippocampal excitotoxic insult and diminished cognitive potential. Aged mice that lack the β2 neuronal nicotinic receptor subunit exhibit increased neuronal atrophy in the cortex and hippocampus54. In addition, compounds that are selective for α4β2* over α7 are neuroprotective in vitro. ABT-089, for example, is protective against glutamate toxicity in IMR-32 cells and rat cortical neurons55. Several Targacept compounds that are selective for α4β2* over α7, such as TC-1734, TC-2559, and TC-2403, are also protective against glutamate toxicity in cultured cells17, 56, and these effects are blocked by the an α4β2* antagonist DHβE34. In cultured nigral dopaminergic neurons, nicotine partially protects against MPP+ toxicity. This effect was blocked by the nicotinic antagonist d-tubocurarine, but not by the α7 antagonist α-bungarotoxin57.
There is considerable evidence for nicotinic neuroprotection in several in vivo models of PD. Nicotine is neuroprotective against 6-OHDA lesions of the nigrostriatal tract58, 59, 60, 61, 62 only at low doses60, 63. Nicotine also appears to be more effective against partial, but not complete dopaminergic lesions and when administered both before and after toxic insult24, 59. A-85380, SIB-1508Y (a β2-preferring agonists), TC-2403 (an α4β2 selective agonist), SIB-1553A (a β4-preferring agonist), and two selective α7 agonists were tested for neuroprotective effects in partial 6-OHDA lesion-induced rats62. Of the selective compounds, only A-85380 was effective alone, but it was not as effective as nicotine24, 62. At least one of the α7 compounds tested, however, was more potent at inducing desensitization than activation64. In addition, TC-2403 is known to undergo rapid first pass metabolism65, and so it is likely that sufficient quantities at doses of 0.2 and 0.4 mg/kg failed to enter the brain and were therefore unable to provide any neuroprotective effect. In α4 knock out mice, nicotine neuroprotection of DA neurons is lost in a methamphetamine model of toxicity60, supporting the relevance of the α4β2 subtype in neuroprotection.
In rodents with MPTP lesions of the nigrostriatal tract, the results of nicotine-induced neuroprotection are variable. This discrepancy in results can be attributed to the wide variety of protocols employed (reviewed in24, 66). For example chronic intermittent administration of nicotine is protective against MPTP toxicity, whereas chronic infusion of nicotine enhances MPTP toxicity24, 66. Nicotine, however, is always neuroprotective in primate models of MPTP toxicity. Chronic nicotine treatment administered via drinking water for several months before and during the toxic insult normalizes a number of parameters in the dopaminergic system. For example, nicotine administration attenuates the loss of tyrosine hydroxylase (TH; an enzyme involved in DA synthesis), DA, DAT (a marker of dopaminergic terminals), VMAT (another marker of dopaminergic terminals), and NNRs as a result of MPTP toxicity67. In addition, chronic nicotine administration in MPTP-treated primates normalizes nicotine-induced DA release, DA turnover, and synaptic plasticity68. These data imply that nicotine promotes an increase in DA neuronal processes and/or reduces nigrostriatal damage. Thus, it appears that nicotine administration reduces DA deficits resulting from nigrostriatal damage and supports the development of NNR ligands as promising therapeutics for PD.
Neuroprotection conferred by nicotine in 6-OHDA and methamphetamine models of nigrostriatal damage is lost in α4 knockout mice60. Nigrostriatal protection through NNR has been consistent in studies using rat and non-human primates, but has demonstrated some discrepancies in mouse models of the disease69. The review by Quik et al reveals that the translational potential of cellular and in vivo models is of critical importance to understanding the clinical relevance of NNR-targeted therapies in various neurodegenerative conditions. For example, it is estimated that the α6* NNR represents approximately 40% of presynaptic nicotine-stimulated dopamine release in rodents, whereas, in non-human primates, the α6* component contributes as much as 70%67. Several NNR subtypes are emerging as likely candidates for modulating DA release and possibly nigrostriatal neuroprotection. These include α4β2*, α6-containing (α6*), and α7 NNRs, and studies with knockout mice indicate that a subpopulation of these receptors contains the α5 subunit (α4α5β2)70. The mRNA for the α7 subunit is found in DA cell bodies of the SN (reviewed in71); however, <3% of the nAChR binding sites in the striatum are attributable to α754. These sites are not reduced after 6-OHDA lesions, suggesting that α7 NNRs are not located at dopaminergic presynaptic terminals72. Much less is known on the molecular pathways of α4β2*-mediated neuroprotection than for alpha7 possibly in part because there are no known in vitro cell system that naturally express the α4β2* to allow molecular dissection of putative signaling cascade.
Acetylcholinesterase inhibitors
Donepezil pretreatment has been shown to prevent acute glutamate- and ionomycin-induced neurotoxicity, but not S-nitrosocysteine-induced neurotoxicity, suggesting that donepezil protects neurons against acute glutamate neurotoxicity via nAChRs before nitric oxide synthase activation. Neuroprotection was inhibited by NNR antagonists or phosphatidylinositol 3-kinase (PI3K) signaling inhibitors, and was associated with a decrease in the level of Akt phosphorylation. Neuroprotection was also inhibited by treatment with an inhibitor of mitogen-activated protein kinase (MAPK). These results suggest that donepezil protects neurons against moderate glutamate neurotoxicity via nAChR-PI3K-Akt and MAPK signaling pathways73. Kihara et al reported that nicotine and galantamine alone and in combination protected neurons against β-amyloid toxicity. Galantamine induced phosphorylation of Akt, an effector of PI3K, while PI3K inhibitors blocked the protective effect, as well as Akt phosphorylation. The FK1 antibody, which selectively blocks the allosterically potentiating ligand site on nAChRs, or suppression of α7 nAChR using an RNA interference technique significantly reduced galantamine-induced protection and Akt phosphorylation. These findings suggest that neuroprotection elicited by galantamine is mediated, at least in part, by the α7 nAChR-PI3K cascade. Galantamine has been shown to be neuroprotective in hippocampal slices subjected to oxygen glucose deprivation74 and in a transient global cerebral ischemia model in gerbils75. Even when applied 3 h post-ischemia, galantamine significantly increased the number of living pyramidal neurons after ischemia-reperfusion injury by reducing TUNEL, active caspase-3, and SOD-2 immunoreactivity. This effect was blocked with the nicotinic antagonist mecamylamine.
In addition to putative effects through the α7-PI3P-Akt pathway, galantamine was found to prevent β-amyloid(1–40) and thapsigargin-induced cell death in the human neuroblastoma cell line SH-SY5Y and in bovine chromaffin cells by upregulating the expression of the α7 receptor and the anti-apoptotic protein Bcl-276. The phosphatidylinositol 3-kinase-Akt pathway also mediates donepezil and galanthamine protection of cortical neurons against acute glutamate treatment77. Inhibition of acetylcholinesterase (AChE) results in an indiscriminate increase of ACh in cholinergic synapses. This is likely to result in indirect broad activation of muscarinic receptors as opposed to NNR ligands, which provide a more targeted approach. The pathways recruited through broad muscarinic activation may be additive or may potentially interfere with those recruited through selective NNR activation. A recent study found that activation of muscarinic receptors in astrocytoma cells modifies the expression of the p70S6K kinase involved in translational control78. Translational control is in part regulated by a cascade of phosphorylation-affecting proteins of the anti-apoptotic pathway controlled by mTOR (a mammalian target of rapamycin) and the pro-apoptotic pathway controlled by PKR. Muscarinic receptor activation with oxotremorine significantly increased the expression of phosphorylated p70S6K, eIF4E, and ERK without modifying mTOR activity in neuroblastoma cells or in the cerebral cortex and hippocampus of mice, suggesting stimulation of protein synthesis. Understanding the cross-talk between the mechanisms recruited through NNR and muscarinic activation may have clinical implications. Targeted selective activation of pathways such as α7 is preferable to broad non-selective recruitment of second and third messengers through indiscriminate cholinergic activation.
Dual effects of α4β2 and α7 NNR
Agonists of α4β2*, such as TC-1734, are known to induce ACh release in vivo. Since ACh is an α7 agonist, such compounds have the potential to act as indirect agonists of α7 neuronal nicotinic receptors, recruiting this subtype for further neuroprotection. A distinguishing feature of the activation of α4β2* in enhancing ACh release in select brain regions versus the broad impact of AChE inhibitors may rest on the intrinsic biophysical properties of NNRs, which exhibit strong rectifying properties that allow activation of hypofunctional pathways and inhibition of hyperactive pathways. This would result in true normalization of synaptic tone. Taken together, these data may indicate the necessity of targeting multiple subtypes in order to achieve optimal neuroprotection24, 62. Amyotrophic Lateral Sclerosis (ALS, sometimes called Maladie de Charcot or Lou Gehrig's Disease in the US) is a progressive, usually fatal, neurodegenerative disease associated with inflammation. In preclinical models of ALS, nicotine-induced neuroprotection was inhibited by either dihydro-beta-erythroidin or α-bungarotoxin, suggesting that it is mediated through both α4β2* and α7 NNRs, both of which have been identified on rat spinal motor neurons. These findings are consistent with the emerging role of these NNR subtypes in neuroprotective mechanims. Furthermore, based on the plethora of data implicating them in neuroprotection and anti-inflammatory pathways, these data suggest that targeting NNRs may be a useful strategy in ALS treatment79.
Mechanistic considerations
It has recently been shown that the correlation between AD pathology and cognitive decline represents a continuum from normal cognitive function to Mild Cognitive Impairment (MCI) in AD71, 80. In addition, progressive loss of cholinergic markers and severe depletion of cholinergic neurons (up to 85%) occur in AD patients81. Ideally, a therapy with neuroprotective capabilities should be administered at prodromic stages, such as upon diagnosis of Age-Associated Memory Impairment (AAMI) or MCI, to prevent further neurodegeneration. Autopsy analysis of the cortical tissue of smokers reveals lower β-amyloid plaque densities compared to non-smokers46, 47. The underlying mechanisms responsible for the decrease in β-amyloid plaques remain unclear, and it should be noted that nicotine is a very poor ligand for α7 receptors. Furthermore, nicotine lack of selectivity is limiting. We now have evidence that α7 interaction with JAK2 and subsequent activation leads to several downstream signaling pathways, including Ras-mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt), GSK-3β modulation of tau phosphorylation, and transcription factor signal transducers and activators of transcription-5. In addition, the α7 NNR agonist and GSK-3β inhibitor have no additive effect50. These observations suggest that α7 modulation can influence Aβ1–42-induced tau phosphorylation, possibly involving GSK-3β. The overlap between mechanisms underlying Aβ1–42 toxicity and tau phosphorylation could, if confirmed in vivo, provide novel strategies for targeting the hallmarks of AD pathobiology. Several ongoing clinical trials are assessing α7 compounds with various selectivity profiles, but the clinical end-points are focused on acute cognitive amelioration rather than long-term neuroprotection.
The newly discovered linkage of α7 NNR to the JAK2/PI3P/Akt/STAT3/NF-kB pathway is highly reminiscent of the mechanisms reported for Erythropoietin (EPO). Interestingly, both α4β2 and α7 have been implicated in the regulation of myelo- and erythropoiesis in bone marrow. Erythropoietin is a 165-amino acid (∼30 kDa) serum glycoprotein that is produced in fetal liver and adult kidney. It is responsible for the proliferation, survival, and differentiation of erythroid progenitor cells. The production and secretion of EPO is oxygen-dependent, and it stimulates the proliferation and maturation of erythroid cells. Upon binding of EPO to its receptor, it activates and causes dimerization of the receptor, as well as autophosphorylation of JAK2. EPO has been reported to have anti-inflammatory, anti-oxidative, anti-apoptotic, and neurotrophic properties that are relevant to cerebral ischemia. Its potential therapeutic role has been demonstrated in several animal models of cerebral ischemia, as well as in a clinical trial of ischemic stroke. The neuroprotective function of EPO is to target inflammation and improve cognitive and motor deficits manifested during traumatic brain injury82.
The HMG-CoA reductase inhibitors (“statins”) have been shown in preclinical models to reduce neuronal injury and infarct size during acute ischemic stroke. There is additional experimental and clinical evidence that statins have beneficial effects on endothelial function, cerebral blood flow, and inflammation. These observations have motivated the initiation of clinical trials such as the Neuroprotection with Statin Therapy for Acute Recovery Trial (NeuSTART) to evaluate its potential83, 84.
Montaner et al 85, 86 conducted a double-blind, randomized, pilot clinical trial to study the safety and efficacy of simvastatin during the acute phase of ischemic stroke. They measured its efficacy on the evolution of several inflammatory markers (IL-6, IL-8, IL-10, monocyte chemoattractant protein-1, intercellular adhesion molecule-1, vascular cell adhesion molecule-1, C-reactive protein, sApo/Fas, tumor necrosis factor-alpha, E- and L-selectin), however no differences were found among the biomarkers studied regarding treatment allocation. These results suggest that larger trials are needed to identify any effects of statins on inflammatory biomarkers.
As stated above, the JAK2/PI3K/Akt/STAT3/NF-kB pathway is recruited through a number of endogenous and external stimuli. The recruitment of these signaling molecules in order to achieve the optimum balance for minimizing inflammatory cascades and achieving neuroprotection, however, may require targeting specific transducing molecules positioned at critical points in the signaling pathways. Extracellular signal-regulated kinase 1/2 (ERK1/2), one of the most characterized members of the MAPK family, mediates a range of activities from inflammation to cell death and survival. It has been argued that ERK1/2 activity generated by endogenous inflammatory factors may have detrimental effects, whereas ERK1/2 activity produced by exogenous signals (eg, growth factors) favors neuroprotection87.
Various additional strategies for neuroprotection have been used to attenuate brain inflammation via control of proliferation and production of proinflammatory cytokines, including derivatives of the insulin-like growth factor-188 and adenosine A2A analogues89, 90. Clinical proof of concept, however, remains to be established. Years of recreational human exposure to nicotine have provided some tantalizing observations of delayed onset in diseases such as Parkinson's disease where neurodegeneration and possibly inflammation are the primum movens of these conditions.
NNRs and inflammation
The “cholinergic anti-inflammatory pathway” and its role in immune responses and inflammatory cascades have attracted enormous interest due to their obvious relevance to a variety of debilitating human diseases. In this regard, evidence has emerged showing that the central nervous system (CNS) modulates the immune system through the reticuloendothelial system. In particular, this CNS modulation is mediated through the vagus nerve, utilizing the major vagal neurotransmitter ACh, which acts on α7 nAChRs on macrophages. Nicotinic receptors, such as the α7 subtype, are at the apex of key cellular pathways, both central and peripheral, and are involved in anti-inflammatory processes, as well as cell survival. This opens the door for treating a broad array of intractable diseases and conditions with inflammatory components, such as rheumatoid and osteoarthritis, inflammatory bowel diseases, and sepsis. This cholinergic vagal pathway appears to play an important role in modulating inflammatory responses, as evidenced by the fact that vagotomy increases LPS (lipopolysaccharide)-induced TNF-α serum levels and hepatic TNF-α responses. Conversely, electrical stimulation of the vagus nerve or treatment of vagotomized animals with ACh prevents the increase in TNF-α release. The role of α7 nAChRs in cholinergic modulation of TNF-α in LPS-stimulated macrophages has been confirmed using antisense oligonucleotides to the α7 nAChRs. Indeed, when the expression of this receptor is blocked, ACh does not have an effect on LPS-induced TNF-α release. This observation has been extended to in vivo models, which demonstrate that vagus nerve stimulation does not inhibit TNF-α release in α7 knockout mice. The key role played by α7 nAChRs in inflammatory processes is further supported by the observations that nicotine and α7 nAChR agonists, such as CAP55 and GTS-21, are effective in models of inflammation and protective in models of sepsis. Furthermore, they have been shown to inhibit local leukocyte recruitment and decrease endothelial cell activation. The α7 NNR ligands inhibit LPS-induced TNF-α release in murine-derived microglial cells, an effect that is attenuated by α-bungarotoxin. Furthermore, this inhibition appears to be mediated by a decrease in phosphorylation of p44/42 and p38 MAPK91.
The reduced incidence of ulcerative colitis among smokers suggests that nicotine may modulate the immune response that leads to this inflammatory bowel disease and that nicotinic receptors may be therapeutic targets for inflammatory diseases92, 93. The cellular and molecular mechanisms involved in the effects of nicotine, however, are not well understood, and attempts to treat ulcerative colitis with nicotine or other nicotinic ligands have provided conflicting results92, 93. Drug discovery efforts for these diseases have focused a great deal of energy on targeting TNF-α however, other pro-inflammatory cytokines contribute to disease and may better reflect disease progression. One such late mediator is high mobility group box chromosomal protein 1 (HMGB1, historically known as an abundant non-histone architectural chromosomal protein), whose release from macrophages is stimulated by LPS treatment94. Nicotine attenuates HMGB1 release from stimulated macrophages in vitro and reduces serum HMGB1 levels in experimental sepsis. The reduced HMGB1 levels in sepsis correlate with increased survival. Splenectomy also inactivates the cholinergic anti-inflammatory pathway, demonstrating the involvement of the RES95, 96. The LPS-stimulated increase in systemic TNF-α production is eliminated in splenectomized animals and, interestingly, nicotine decreases the survival of splenectomized animals with sepsis and bacterial clearance in septic peritonitis95, 96. As our understanding of the tissues involved in the cholinergic anti-inflammatory pathway from the CNS to the RES has unfolded, advances have also been made in understanding the molecular mechanisms involved. For example, the neuroprotective effects of nicotine and the α7-selective ligand TC-1698 can be traced to α7 activation and transduction of signals to PI3K and AKT (protein kinase B) via JAK239, all of which participate in a key cell survival pathway. Immunoprecipitation experiments indicate that the α7 receptor and JAK2 interact directly (also see Figure 1). Additional studies examined the effects of nicotine on LPS-treated and control peritoneal macrophages and found that nicotine treatment leads to phosphorylation of STAT349, another key component of the cellular anti-apoptotic cascade. This nicotine-mediated phosphorylation is inhibited by the α7-selective antagonists α-bungarotoxin and methyllycaconitine (MLA), as well as by AG490, a selective inhibitor of JAK2 phosphorylation. Immunoprecipitation studies support the previous findings showing that nicotine exposure recruits JAK2 and leads to an increased association between the kinase and α7 receptors. Studies examining LysM-Stat3fl/− mice, whose macrophages are deficient in STAT3, found that vagus nerve stimulation does not reduce peritoneal cytokine levels or intestinal inflammation, as it does in control animals. These data support the interaction of JAK2 and α7 and the critical role of STAT3 in the cholinergic anti-inflammatory pathway. LPS-stimulated release of TNF-α and neutrophil chemoattractant macrophage inflammatory proteins (MIP-1a and MIP-1b) are also inhibited by nicotine, and the mRNA levels of these inflammatory mediators are also modulated97. Therefore, cholinergic anti-inflammatory regulation occurs upstream of transcription. Nicotine also inhibits LPS-stimulated IκB phosphorylation, thereby preventing nuclear factor kappa B (NF-κB) activation, which is necessary for gene transcription of pro-inflammatory mediators97, 98.
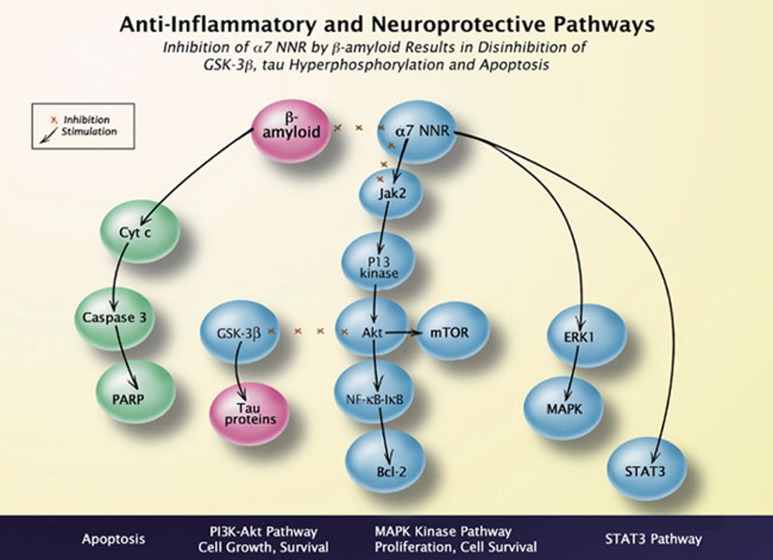
Figure 1.
Relationship of 7 nAChRs to anti-apoptotic and anti-inflammatory pathways. Schematic showing α7 nAChR-mediated activation of JAK2 and cross-talk mechanisms between α7 nAChRs and β-amyloid-activated pathways. α7-mediated neuroprotection via the JAK2 pathway intersects with the anti-inflammatory pathway mediated through STAT3/NF-κB. Abbreviations: Akt (protein kinase B); Bcl-2 (B cell lymphoma 2 protein); IκB (Inhibitor kappa B); JAK2 (Janus kinase 2); NF-κB (nuclear factor kappa B and transcription factor complex); mTOR: mammalian target of rapamycin (kinase); STAT3 (Signal Transducer and Activator of Transcription3). PARP: Poly (ADP-ribose) polymerase.
Secreted lymphocyte antigen-6/urokinase-type plasminogen activator receptor-related protein-1 (SLURP-1) is a 9 kDa secreted protein encoded by the ARS B gene that shows structural similarity to the snake venom toxin α-bungarotoxin99. Acetylcholine elicits current responses in control and SLURP-1-treated Xenopus oocytes expressing recombinant human α7 nAChRs. Furthermore, SLURP-1 significantly increases both ACh potency and efficacy (more than a 10-fold increase) via the α7 nAChR. It is localized to human skin, exocervix, gums, stomach, and esophagus and has been implicated in maintaining the physiological and structural integrity of the keratinocyte layers of the skin. Mutations in the SLURP-1 gene result in Mal de Meleda (MDM), a rare autosomal recessive genetic disease that is characterized by inflammatory palmoplantar keratoderma. SLURP-1 expression is regulated by retinoic acid, epidermal growth factor, and interferon-gamma100, 101, 102"/>. The SLURP-1 protein has been identified in several biological fluids such as sweat, saliva, tears, and urine from normal volunteers101. In palmoplantar sections from MDM patients, as well as in their sweat, mutant SLURP-1, including the new variant R71H-SLURP-1, was either absent or barely detectable. Thus, SLURP-1 acts as a positive allosteric modulator of α7 NNRs100 and most MDM mutations in SLURP-1 affect either the expression, integrity, or stability of the protein. This results in inflammatory manifestations, raising the intriguing possibility that, in addition to acetylcholine, endogenous peptides may actively modulate the α7 cholinergic cascade.
The role of α4β2 in inflammatory processes is also emerging. (E)-metanicotine (an α4β2-selective ligand103) inhibits IL-8 and TNF-α production in human macrophages and in cells of the inflamed muc osa104. Conversely, pro-inflammatory cytokines shift the neuronal nicotinic receptor assembly to the α4β2 configuration over α4β2 or α4β2β4, suggesting bi-directional regulation of cytokines and nicotinic receptor expression105.
In addition, recent studies have implicated distinct participation of α4β2* and α7 in the regulation of B-lymphocyte development and activation and have suggested that the CD40 pathway contributes to these effects. These studies support a role for both α4β2 and α7 in the regulation of inflammatory/immune processes106. Lymphocytic cholinergic activity in the regulation of immune function is supported by studies in mutant mice and the emerging of cholinergic role in immune function may provide the basis for targeted immunotherapy107.
Summary
A number of studies have confirmed the potential for nicotinic acetylcholine receptor-mediated neuroprotection and, more recently, its anti-inflammatory effects. The mechanistic overlap between these pathways and the ubiquitous effects observed following diverse insults have suggested that NNRs modulate fundamental pathways involved in cell survival (Figure 1). Neuroprotection mediated by α7 after a variety of cellular insults are initiated via activation of JAK2, triggering downstream cellular signaling events that include activation of phosphoinositide-3-kinase/Akt, GSK-3β, and the transcription factors STAT3 and NF-kB. This leads to inhibition of neuronal cell apoptosis or macrophage activation via the cholinergic anti-inflammatory pathway. Additional mechanisms through the MAPK/ERK pathways participate in the regulation of cell survival and apoptosis. These results have wide-reaching implications for the design of experimental therapeutics that regulate inflammatory and anti-apoptotic responses through the NNR. They also represent an initial step toward understanding the benefits of novel therapeutic strategies targeting neuronal survival and management of associated inflammatory processes for the management of CNS disorders. In addition, selective nicotinic ligands that affect the pro-inflammatory pathway from the transcriptional level upward provide a new therapeutic class possessing a potentially better mechanism of action for the treatment of inflammatory and autoimmune disorders. These ligands achieve this by modulating a broad array of cytokines and cellular pathways that are involved in cell homeostasis, an effect that would not be possible by targeting individual proteins. The mechanistic overlap between the signaling pathways involved in neuroprotective mechanisms and in controlling inflammation may provide the tools needed to control and break the vicious cycle of cell death and inflammation. The aptly named Janus kinase, the deity of gateways, beginnings, and endings in roman mythology, resides at the crossroad of these bimodal signaling cascades and may provide a convenient target via NNR modulation for novel therapies designed to manage the disruption of regulatory proteins that are central to cellular homeostasis. The present therapeutic armamentarium is lacking drugs directed toward some of the fundamental pathways involved in cell survival and chronic inflammation that have been increasingly implicated in some of the most devastating diseases, including atherosclerosis, diabetes, neurodegenerative diseases, chronic obstructive pulmonary diseases, inflammatory bowel diseases, and other untractable diseases. The potential to target neuronal survival and chronic inflammation could be a turning point in our ability to manage some of the most costly public health issues of our time. Although there is mounting evidence for the potential of NNRs to target the hallmark of diseases that may constitute the biggest public health challenges, the regulatory path for such therapies remains to be established and only global pharmaceutical companies with a strategic interest in these areas have the means to undertake the task of extended clinical trials and to influence the regulatory bodies to pave the way for such new therapies.
References
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–47. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- McGeer EG, Klegeris A, McGeer PL. Inflammation, the complement system and the diseases of aging. Neurobiol Aging. 2005;26 Suppl 1:94–7. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Bencherif M. Convergence of alpha 7 nicotinic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: central role for JAK2 activation of STAT3 and NF-kappaB. Brain Res. 2009;1256:1–7. doi: 10.1016/j.brainres.2008.11.053. [DOI] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr Alzheimer Res. 2005;2:355–65. doi: 10.2174/1567205054367883. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann N Y Acad Sci. 2004;1035:104–16. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–24. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Liu R, Morley BJ, Lindstrom JM. Structurally different neuronal nicotinic acetylcholine receptor subtypes purified and characterized using monoclonal antibodies. J Neurosci. 1987;7:4005–16. doi: 10.1523/JNEUROSCI.07-12-04005.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is up-regulating by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–7. [PubMed] [Google Scholar]
- Moroni M, Bermudez I. Stoichiometry and pharmacology of two human alpha4beta2 nicotinic receptor types. J Mol Neurosci. 2006;30:95–6. doi: 10.1385/JMN:30:1:95. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR.et al. Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the beta2 subunit J Pharmacol Exp Ther 19992891090–103. [PubMed] [Google Scholar]
- Quik M, O'Neill M, Perez XA. Nicotine neuroprotection against nigrostriatal damage: importance of the animal model. Trends Pharmacol Sci. 2007;28:229–35. doi: 10.1016/j.tips.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang J, Zhu H, Qin C, Chen Q, Zhao B. Dissecting the signaling pathway of nicotine-mediated neuroprotection in a mouse Alzheimer disease model. FASEB J. 2007;21:61–73. doi: 10.1096/fj.06-5841com. [DOI] [PubMed] [Google Scholar]
- Dineley KT. Beta-amyloid peptide — nicotinic acetylcholine receptor interaction: the two faces of health and disease. Front Biosci. 2007;12:5030–8. doi: 10.2741/2445. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Charriez CM, Guseva MV, Scheff SW. Nicotinic receptor modulation for neuroprotection and enhancement of functional recovery following brain injury or disease. Ann N Y Acad Sci. 2004;1035:316–34. doi: 10.1196/annals.1332.019. [DOI] [PubMed] [Google Scholar]
- Egea J, Rosa AO, Sobrado M, Gandía L, López MG, García AG. Neuroprotection afforded by nicotine against oxygen and glucose deprivation in hippocampal slices is lost in alpha7 nicotinic receptor knockout mice. Neuroscience. 2007;145:866–72. doi: 10.1016/j.neuroscience.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Bohme GA, Caldwell WS, Letchworth SR, Traina VM, Obinu MC.et al. TC-1734: an orally active neuronal nicotinic acetylcholine receptor modulator with antidepressant, neuroprotective and long-lasting cognitive effects CNS Drug Rev 200410147–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AO, Egea J, Gandía L, López MG, García AG. Neuroprotection by nicotine in hippocampal slices subjected to oxygen-glucose deprivation: involvement of the alpha7 nAChR subtype. J Mol Neurosci. 2006;30:61–2. doi: 10.1385/JMN:30:1:61. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Chae JS, Jung ME, Bing G, Ko KH, Kim WK.et al. Repeated intracerebroventricular infusion of nicotine prevents kainate-induced neurotoxicity by activating the alpha7 nicotinic acetylcholine receptor Epilepsy Res 200773292–8. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Smith O, Linn DM, Linn CL. Acetylcholine neuroprotection against glutamate-induced excitotoxicity in adult pig retinal ganglion cells is partially mediated through alpha4 nAChRs. Exp Eye Res. 2006;83:1135–45. doi: 10.1016/j.exer.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Ueda M, Iida Y, Kitamura Y, Kawashima H, Ogawa M, Magata Y.et al. 5-Iodo-A-85380, a specific ligand for alpha 4 beta 2 nicotinic acetylcholine receptors, prevents glutamate neurotoxicity in rat cortical cultured neurons Brain Res 2008119946–52. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Al-Namaeh M, Manaye KF, Taylor RE. Protective effects of nicotine on ethanol-induced toxicity in cultured cerebellar granule cells. Neurotox Res. 2003;5:315–21. doi: 10.1007/BF03033151. [DOI] [PubMed] [Google Scholar]
- Hellström-Lindahl E, Court J, Keverne J, Svedberg M, Lee M, Marutle A.et al. Nicotine reduces A beta in the brain and cerebral vessels of APPsw mice Eur J Neurosci 2004192703–10. [DOI] [PubMed] [Google Scholar]
- O'Neill MJ, Murray TK, Lakics V, Visanji NP, Duty S. The role of neuronal nicotinic acetylcholine receptors in acute and chronic neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2002;1:399–411. doi: 10.2174/1568007023339166. [DOI] [PubMed] [Google Scholar]
- Parain K, Hapdey C, Rousselet E, Marchand V, Dumery B, Hirsch EC. Cigarette smoke and nicotine protect dopaminergic neurons against the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine Parkinsonian toxin. Brain Res. 2003;984:224–32. doi: 10.1016/s0006-8993(03)03195-0. [DOI] [PubMed] [Google Scholar]
- Takada Y, Yonezawa A, Kume T, Katsuki H, Kaneko S, Sugimoto H.et al. Nicotinic acetylcholine receptor-mediated neuroprotection by donepezil against glutamate neurotoxicity in rat cortical neurons J Pharmacol Exp Ther 2003306772–7. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Hellström-Lindahl E, Lee M, Johnson M, Mousavi M, Hall R.et al. Chronic nicotine treatment reduces beta-amyloidosis in the brain of a mouse model of Alzheimer's disease (APPsw) J Neurochem 200281655–8. [DOI] [PubMed] [Google Scholar]
- Nanri M, Yamamoto J, Miyake H, Watanabe H. Protective effect of GTS-21, a novel nicotinic receptor agonist, on delayed neuronal death induced by ischemia in gerbils. Jpn J Pharmacol. 1998;76:23–9. doi: 10.1254/jjp.76.23. [DOI] [PubMed] [Google Scholar]
- Hildebrandt IJ, Termeer JL, Toledo D, Cohen RW. Characterization of chronic nicotine exposure on the survival of the spastic Han-Wistar rat. Nicotine Tob Res. 2003;5:827–36. doi: 10.1080/14622200310001614593. [DOI] [PubMed] [Google Scholar]
- Nanri M, Miyake H, Murakami Y, Matsumoto K, Watanabe H. GTS-21, a nicotinic agonist, attenuates multiple infarctions and cognitive deficit caused by permanent occlusion of bilateral common carotid arteries in rats. Jpn J Pharmacol. 1998;78:463–9. doi: 10.1254/jjp.78.463. [DOI] [PubMed] [Google Scholar]
- Khwaja M, McCormack A, McIntosh JM, Di Monte DA, Quik M. Nicotine partially protects against paraquat-induced nigrostriatal damage in mice; link to alpha6beta2* nAChRs. J Neurochem. 2007;100:180–90. doi: 10.1111/j.1471-4159.2006.04177.x. [DOI] [PubMed] [Google Scholar]
- Morioka M, Kawano T, Yano S, Kai Y, Tsuiki H, Yoshinaga Y.et al. Hyperphosphorylation at serine 199/202 of tau factor in the gerbil hippocampus after transient forebrain ischemia Biochem Biophys Res Commun 2006347273–8. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Maeda T, Kume T, Kochiyama H, Akaike A, Shimohama S.et al. Nicotine protects cultured cortical neurons against glutamate-induced cytotoxicity via alpha7-neuronal receptors and neuronal CNS receptors Brain Res 1997765135–40. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Urushitani M, Sawada H, Kimura J, Kume T.et al. Stimulation of alpha4beta2 nicotinic acetylcholine receptors inhibits beta-amyloid toxicity Brain Res 1998792331–4. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Kihara T. Nicotinic receptor-mediated protection against beta-amyloid neurotoxicity. Biol Psychiatry. 2001;49:233–9. doi: 10.1016/s0006-3223(00)01100-8. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H.et al. alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity J Biol Chem 200127613541–6. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Papke RL, Bhatti BS, Shaw S, Bencherif M. The neuroprotective effect of 2-(3-pyridyl)-1-azabicyclo[3.2.2]nonane (TC-1698), a novel alpha7 ligand, is prevented through angiotensin II activation of a tyrosine phosphatase. J Pharmacol Exp Ther. 2004;309:16–27. doi: 10.1124/jpet.103.061655. [DOI] [PubMed] [Google Scholar]
- Ren K, Puig V, Papke RL, Itoh Y, Hughes JA, Meyer EM. Multiple calcium channels and kinases mediate alpha7 nicotinic receptor neuroprotection in PC12 cells. J Neurochem. 2005;94:926–33. doi: 10.1111/j.1471-4159.2005.03223.x. [DOI] [PubMed] [Google Scholar]
- Shaw S, Bencherif M, Marrero MB. Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against Abeta-(1-42) amyloid. J Biol Chem. 2002;277:44920–4. doi: 10.1074/jbc.M204610200. [DOI] [PubMed] [Google Scholar]
- Thatcher GR, Bennett BM, Reynolds JN. NO chimeras as therapeutic agents in Alzheimer's disease. Curr Alzheimer Res. 2006;3:237–45. doi: 10.2174/156720506777632925. [DOI] [PubMed] [Google Scholar]
- Seo J, Kim S, Kim H, Park CH, Jeong S, Lee J.et al. Effects of nicotine on APP secretion and Abeta- or CT(105)-induced toxicity Biol Psychiatry 200149240–7. [DOI] [PubMed] [Google Scholar]
- Unger C, Svedberg MM, Yu WF, Hedberg MM, Nordberg A. Effect of subchronic treatment of memantine, galantamine, and nicotine in the brain of Tg2576 (APPswe) transgenic mice. J Pharmacol Exp Ther. 2006;317:30–6. doi: 10.1124/jpet.105.098566. [DOI] [PubMed] [Google Scholar]
- Brendza RP, Bacskai BJ, Cirrito JR, Simmons KA, Skoch JM, Klunk WE.et al. Anti-Abeta antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice J Clin Invest 2005115428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–88. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Green KN, Liang K, Tran L, Chen Y.et al. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer's disease Proc Natl Acad Sci U S A 20051023046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G, Roder H, Nunomura A, Takeda A, Friedlich AL, Zhu X.et al. Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation Neuroreport 1999102411–5. [DOI] [PubMed] [Google Scholar]
- Ulrich J, Johannson-Locher G, Seiler WO, Stähelin HB. Does smoking protect from Alzheimer's disease? Alzheimer-type changes in 301 unselected brains from patients with known smoking history. Acta Neuropathol. 1997;94:450–4. doi: 10.1007/s004010050732. [DOI] [PubMed] [Google Scholar]
- Perry E, Martin-Ruiz C, Lee M, Griffiths M, Johnson M, Piggott M.et al. Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases Eur J Pharmacol 2000393215–22. [DOI] [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ.et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway Nat Immunol 20056844–51. [DOI] [PubMed] [Google Scholar]
- Hu M, Waring JF, Gopalakrishnan M, Li J. Role of GSK-3beta activation and alpha7 nAChRs in Abeta(1-42)-induced tau phosphorylation in PC12 cells. J Neurochem. 2008;106:1371–7. doi: 10.1111/j.1471-4159.2008.05483.x. [DOI] [PubMed] [Google Scholar]
- Martin SE, de Fiebre NE, de Fiebre CM. The alpha7 nicotinic acetylcholine receptor-selective antagonist, methyllycaconitine, partially protects against beta-amyloid1-42 toxicity in primary neuron-enriched cultures. Brain Res. 2004;1022:254–6. doi: 10.1016/j.brainres.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Hao J, Perez D, Penzo M, Maldonado HM, Gonzalez MT.et al. Tobacco cembranoids protect the function of acute hippocampal slices against NMDA by a mechanism mediated by alpha4beta2 nicotinic receptors J Neurosci Res 200582631–41. [DOI] [PubMed] [Google Scholar]
- Zanardi A, Ferrari R, Leo G, Maskos U, Changeux JP, Zoli M. Loss of high-affinity nicotinic receptors increases the vulnerability to excitotoxic lesion and decreases the positive effects of an enriched environment. FASEB J. 2007;21:4028–37. doi: 10.1096/fj.07-8260com. [DOI] [PubMed] [Google Scholar]
- Zoli M, Picciotto MR, Ferrari R, Cocchi D, Changeux JP. Increased neurodegeneration during ageing in mice lacking high-affinity nicotine receptors. EMBO J. 1999;18:1235–44. doi: 10.1093/emboj/18.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JP, Donnelly-Roberts D, Briggs CA, Anderson DJ, Gopalakrishnan M, Xue IC.et al. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine]: I. A potent and selective cholinergic channel modulator with neuroprotective properties J Pharmacol Exp Ther 1997283235–46. [PubMed] [Google Scholar]
- Bencherif M, Bane AJ, Miller CH, Dull GM, Gatto GJ. TC-2559: a novel orally active ligand selective at neuronal acetylcholine receptors. Eur J Pharmacol. 2000;409:45–55. doi: 10.1016/s0014-2999(00)00807-4. [DOI] [PubMed] [Google Scholar]
- Jeyarasasingam G, Tompkins L, Quik M. Stimulation of non-alpha7 nicotinic receptors partially protects dopaminergic neurons from 1-methyl-4-phenylpyridinium-induced toxicity in culture. Neuroscience. 2002;109:275–85. doi: 10.1016/s0306-4522(01)00488-2. [DOI] [PubMed] [Google Scholar]
- Abin-Carriquiry JA, McGregor-Armas R, Costa G, Urbanavicius J, Dajas F. Presynaptic involvement in the nicotine prevention of the dopamine loss provoked by 6-OHDA administration in the substantia nigra. Neurotox Res. 2002;4:133–9. doi: 10.1080/10298420290015863. [DOI] [PubMed] [Google Scholar]
- Costa G, Abin-Carriquiry JA, Dajas F. Nicotine prevents striatal dopamine loss produced by 6-hydroxydopamine lesion in the substantia nigra. Brain Res. 2001;888:336–42. doi: 10.1016/s0006-8993(00)03087-0. [DOI] [PubMed] [Google Scholar]
- Ryan RE, Ross SA, Drago J, Loiacono RE. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol. 2001;132:1650–6. doi: 10.1038/sj.bjp.0703989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Otero R, Méndez-Alvarez E, Hermida-Ameijeiras A, López-Real AM, Labandeira-García JL. Effects of (-)-nicotine and (-)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson's disease. Biochem Pharmacol. 2002;64:125–35. doi: 10.1016/s0006-2952(02)01070-5. [DOI] [PubMed] [Google Scholar]
- Visanji NP, O'Neill MJ, Duty S. Nicotine, but neither the alpha4beta2 ligand RJR2403 nor an alpha7 nAChR subtype selective agonist, protects against a partial 6-hydroxydopamine lesion of the rat median forebrain bundle. Neuropharmacology. 2006;51:506–16. doi: 10.1016/j.neuropharm.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Blum M, Wu G, Mudó G, Belluardo N, Andersson K, Agnati LF.et al. Chronic continuous infusion of (-)nicotine reduces basic fibroblast growth factor messenger RNA levels in the ventral midbrain of the intact but not of the 6-hydroxydopamine-lesioned rat Neuroscience 199670169–77. [DOI] [PubMed] [Google Scholar]
- Zwart R, De Filippi G, Broad LM, McPhie GI, Pearson KH, Baldwinson T.et al. 5-Hydroxyindole potentiates human alpha 7 nicotinic receptor-mediated responses and enhances acetylcholine-induced glutamate release in cerebellar slices Neuropharmacology 200243374–84. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Schmitt JD, Bhatti BS, Crooks P, Caldwell WS, Lovette ME.et al. The heterocyclic substituted pyridine derivative (+/-)-2-(-3-pyridinyl)-1-azabicyclo[2.2.2]octane (RJR-2429): a selective ligand at nicotinic acetylcholine receptors J Pharmacol Exp Ther 1998284886–94. [PubMed] [Google Scholar]
- Quik M. Smoking, nicotine and Parkinson's disease. Trends Neurosci. 2004;27:561–8. doi: 10.1016/j.tins.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Quik M, Parameswaran N, McCallum SE, Bordia T, Bao S, McCormack A.et al. Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates J Neurochem 2006981866–75. [DOI] [PubMed] [Google Scholar]
- Quik M, Chen L, Parameswaran N, Xie X, Langston JW, McCallum SE.et al. Chronic oral nicotine normalizes dopaminergic function and synaptic plasticity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned primates J Neurosci 2006264681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Polonskaya Y, McIntosh JM, Kulak JM. Differential nicotinic receptor expression in monkey basal ganglia: effects of nigrostriatal damage. Neuroscience. 2002;112:619–30. doi: 10.1016/s0306-4522(02)00106-9. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC.et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice Mol Pharmacol 2004651526–35. [DOI] [PubMed] [Google Scholar]
- Quik M, Kulak JM. Nicotine and nicotinic receptors; relevance to Parkinson's disease. Neurotoxicology. 2002;23:581–94. doi: 10.1016/s0161-813x(02)00036-0. [DOI] [PubMed] [Google Scholar]
- Quik M, Bordia T, Forno L, McIntosh JM. Loss of alpha-conotoxin MII- and A85380-sensitive nicotinic receptors in Parkinson's disease striatum. J Neurochem. 2004;88:668–79. doi: 10.1111/j.1471-4159.2004.02177.x. [DOI] [PubMed] [Google Scholar]
- Takada-Takatori Y, Kume T, Ohgi Y, Izumi Y, Niidome T, Fujii T.et al. Mechanism of neuroprotection by donepezil pretreatment in rat cortical neurons chronically treated with donepezil J Neurosci Res 2008863575–83. [DOI] [PubMed] [Google Scholar]
- Sobrado M, Roda JM, López MG, Egea J, García AG. Galantamine and memantine produce different degrees of neuroprotection in rat hippocampal slices subjected to oxygen-glucose deprivation. Neurosci Lett. 2004;365:132–6. doi: 10.1016/j.neulet.2004.04.067. [DOI] [PubMed] [Google Scholar]
- Lorrio S, Sobrado M, Arias E, Roda JM, García AG, López MG. Galantamine postischemia provides neuroprotection and memory recovery against transient global cerebral ischemia in gerbils. J Pharmacol Exp Ther. 2007;322:591–9. doi: 10.1124/jpet.107.122747. [DOI] [PubMed] [Google Scholar]
- Arias E, Alés E, Gabilan NH, Cano-Abad MF, Villarroya M, García AG.et al. Galantamine prevents apoptosis induced by beta-amyloid and thapsigargin: involvement of nicotinic acetylcholine receptors Neuropharmacology 200446103–14. [DOI] [PubMed] [Google Scholar]
- Takatori Y. Mechanisms of neuroprotective effects of therapeutic acetylcholinesterase inhibitors used in treatment of Alzheimer's disease. Yakugaku Zasshi. 2006;126:607–16. doi: 10.1248/yakushi.126.607. [DOI] [PubMed] [Google Scholar]
- Deguil J, Perault-Pochat MC, Chavant F, Lafay-Chebassier C, Fauconneau B, Pain S. Activation of the protein p7OS6K via ERK phosphorylation by cholinergic muscarinic receptors stimulation in human neuroblastoma cells and in mice brain. Toxicol Lett. 2008;182:91–6. doi: 10.1016/j.toxlet.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Nakamizo T, Kawamata J, Yamashita H, Kanki R, Kihara T, Sawada H.et al. Stimulation of nicotinic acetylcholine receptors protects motor neurons Biochem Biophys Res Commun 20053301285–9. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–41. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Giacobini E. Cholinergic function and Alzheimer's disease 1. Int J Geriatr Psychiatry. 2003;18:S1–S5. doi: 10.1002/gps.935. [DOI] [PubMed] [Google Scholar]
- Cotena S, Piazza O, Tufano R. The use of erythtropoietin in cerebral diseases. Panminerva Med. 2008;50:185–92. [PubMed] [Google Scholar]
- Elkind MS, Sacco RL, MacArthur RB, Fink DJ, Peerschke E, Andrews H.et al. The Neuroprotection with Statin Therapy for Acute Recovery Trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke Int J Stroke 20083210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr JD. Statins in the spectrum of neurologic disease. Curr Atheroscler Rep. 2008;10:11–8. doi: 10.1007/s11883-008-0003-5. [DOI] [PubMed] [Google Scholar]
- Montaner J, Chacón P, Krupinski J, Rubio F, Millán M, Molina CA.et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial Eur J Neurol 20081582–90. [DOI] [PubMed] [Google Scholar]
- Montaner J. Treatment with statins in the acute phase of ischemic stroke. Expert Rev Neurother. 2005;5:211–21. doi: 10.1586/14737175.5.2.211. [DOI] [PubMed] [Google Scholar]
- Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–69. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- Guan J. Insulin-like growth factor-1 and its derivatives: potential pharmaceutical application for ischemic brain injury. Recent Patents CNS Drug Discov. 2008;3:112–27. doi: 10.2174/157488908784534630. [DOI] [PubMed] [Google Scholar]
- Chen JF, Pedata F. Modulation of ischemic brain injury and neuroinflammation by adenosine A2A receptors. Curr Pharm Des. 2008;14:1490–9. doi: 10.2174/138161208784480126. [DOI] [PubMed] [Google Scholar]
- Jennings JS, Gerber AM, Vallano ML. Pharmacological strategies for neuroprotection in traumatic brain injury. Mini Rev Med Chem. 2008;8:689–701. doi: 10.2174/138955708784567377. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J.et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors J Neurochem 200489337–43. [DOI] [PubMed] [Google Scholar]
- McGilligan VE, Wallace JM, Heavey PM, Ridley DL, Rowland IR. Hypothesis about mechanisms through which nicotine might exert its effect on the interdependence of inflammation and gut barrier function in ulcerative colitis. Inflamm Bowel Dis. 2007;13:108–15. doi: 10.1002/ibd.20020. [DOI] [PubMed] [Google Scholar]
- Scott DA, Martin M. Exploitation of the nicotinic anti-inflammatory pathway for the treatment of epithelial inflammatory diseases. World J Gastroenterol. 2006;12:7451–9. doi: 10.3748/wjg.v12.i46.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Hackett JT, Cox ME, Van Hoek M, Lindstrom JM, Parsons SJ. Regulation of the neuronal nicotinic acetylcholine receptor by SRC family tyrosine kinases. J Biol Chem. 2004;279:8779–86. doi: 10.1074/jbc.M309652200. [DOI] [PubMed] [Google Scholar]
- Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA.et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis J Exp Med 20062031623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF.et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis J Infect Dis 20051912138–48. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E.et al. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7 Clin Exp Immunol 2006146116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J.et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation J Exp Med 20052011113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki Y, Yoshikawa K, Fukuda H, Fujii YX, Misawa H, Kawashima K. Immune system expression of SLURP-1 and SLURP-2, two endogenous nicotinic acetylcholine receptor ligands. Life Sci. 2007;80:2365–8. doi: 10.1016/j.lfs.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J.et al. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda Hum Mol Genet 2003123017–24. [DOI] [PubMed] [Google Scholar]
- Favre B, Plantard L, Aeschbach L, Brakch N, Christen-Zaech S, de Viragh PA.et al. SLURP1 is a late marker of epidermal differentiation and is absent in Mal de Meleda J Invest Dermatol 2007127301–8. [DOI] [PubMed] [Google Scholar]
- Mastrangeli R, Donini S, Kelton CA, He C, Bressan A, Milazzo F.et al. ARS component B: structural characterization, tissue expression and regulation of the gene and protein (SLURP-1) associated with Mal de Meleda Eur J Dermatol 200313560–70. [PubMed] [Google Scholar]
- Bencherif M, Lovette ME, Fowler KW, Arrington S, Reeves L, Caldwell WS.et al. RJR-2403: a nicotinic agonist with CNS selectivity I In vitro characterization J Pharmacol Exp Ther 19962791413–21. [PubMed] [Google Scholar]
- Spoettl T, Paetzel C, Herfarth H, Bencherif M, Schoelmerich J, Greinwald R.et al. (E)-metanicotine hemigalactarate (TC-2403-12) inhibits IL-8 production in cells of the inflamed mucosa Int J Colorectal Dis 200722303–12. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Days EL, Kaasch T, González de Mendoza M, Owen L, Persiyanov K.et al. Pro-inflammatory cytokines modify neuronal nicotinic acetylcholine receptor assembly J Neuroimmunol 200516688–101. [DOI] [PubMed] [Google Scholar]
- Skok MV, Grailhe R, Agenes F, Changeux JP. The role of nicotinic receptors in B-lymphocyte development and activation. Life Sci. 2007;80:2334–6. doi: 10.1016/j.lfs.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Fujii T, Takada-Takatori Y, Kawashima K. Basic and clinical aspects of non-neuronal acetylcholine: expression of an independent, non-neuronal cholinergic system in lymphocytes and its clinical significance in immunotherapy. J Pharmacol Sci. 2008;106:186–92. doi: 10.1254/jphs.fm0070109. [DOI] [PubMed] [Google Scholar]