Abstract
Complex postsynaptic scaffolds determine the structure and signaling capabilities of glutamatergic synapses. Recent studies indicate that some of the same scaffold components contribute to the formation and function of nicotinic synapses on neurons. PDZ-containing proteins comprising the PSD-95 family co-localize with nicotinic acetylcholine receptors (nAChRs) and mediate downstream signaling in the neurons. The PDZ-proteins also promote functional nicotinic innervation of the neurons, as does the scaffold protein APC and transmembrane proteins such as neuroligin and the EphB2 receptor. In addition, specific chaperones have been shown to facilitate nAChR assembly and transport to the cell surface. This review summarizes recent results in these areas and raises questions for the future about the mechanism and synaptic role of nAChR trafficking.
Keywords: nicotinic receptor, postsynaptic, scaffold, synapse, PSD-95, trafficking, neuroligin
Introduction
Nicotinic acetylcholine receptors (nAChRs) are widely distributed throughout the central nervous system, and participate in numerous higher order functions1, 2, neurological disorders3, 4 and, of course, addiction5. The receptors comprise a family of subtypes in vertebrates, all of which are cation-selective ligand-gated ion channels6, 7, 8. At least 12 neuronal nAChR genes have been identified (α2-10, β2-4) which can form hetero- and homopentameric nAChRs7, 8, 9, 10. Activation of nAChRs is able to produce diverse effects because of receptor location and because of downstream signaling pathways engaged by the receptors in the postsynaptic cell. The signaling pathways often employ calcium because nAChR activation depolarizes the cell and can activate voltage-gated calcium channels11. In the case of homomeric α7-containing receptors (α7-nAChRs), significant calcium can enter directly through the receptor itself12, 13, 14. Increasing evidence indicates that nAChRs in general, and α7-nAChRs in particular, are concentrated both pre- and postsynaptically at a variety of glutamatergic and GABAergic synapses7, 15, 16, 17, 18. Critical determinants for nicotinic cholinergic transmission, therefore, are the mechanisms that target and anchor nAChRs at synaptic locations and couple the receptors to specific signal transduction machinery. Little is known about such mechanisms for neuronal nAChRs.
The best understood mechanisms determining receptor localization and function on neurons are those operative postsynaptically at glutamate spine synapses. In this case, a vast number of components have been identified, linked directly or indirectly to the postsynaptic AMPA and NMDA receptors responsible for excitatory neurotransmission (Figure 1). Central are the membrane associated guanylate kinases (MAGUKs) comprising the PSD-95 family, which contain PDZ domains that bind other proteins19. PSD-95 itself binds directly to the intracellular C-terminal of NMDA receptors, and together with other associated PSD-95 molecules, links numerous components in an elaborate postsynaptic scaffold. Included are AMPA receptors, bound via a TARP link, as well as components important for signal transduction such as calcium/calmodulin-dependent protein kinase II (CaMKII). SAP102 and PSD-93 are related members of the PSD-95 family and perform similar functions at glutamate synapses depending on the developmental stage and location of the synapse20, 21, 22. The fourth member of the family, SAP97, plays a different role, facilitating AMPA receptor trafficking to the surface membrane, for example23. Trafficking of AMPA receptors to the surface is a fundamental feature of synaptic plasticity24, 25. Trafficking within the surface membrane has also recently emerged as a critical determinant of synaptic responses26, 27, 28. Whether similar mechanisms might control the fate and function nAChRs has only recently emerged as a possibility.
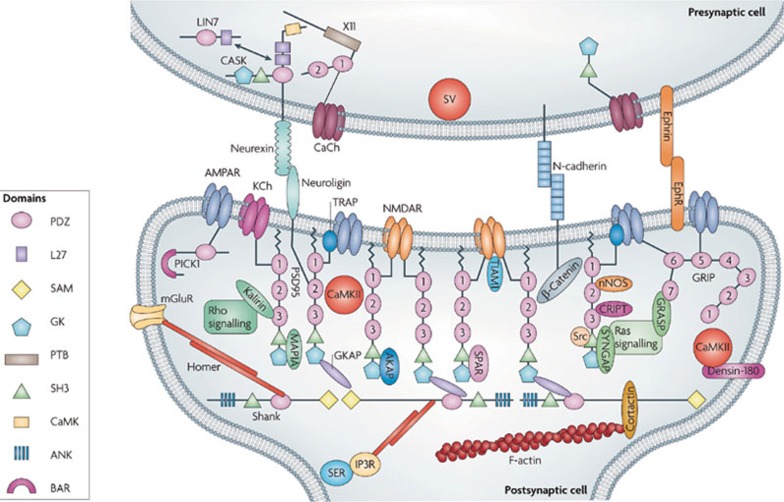
Figure 1.
Model postsynaptic scaffold at a glutamate spine synapse. The postsynaptic density is comprised of membrane receptors and ion channels, scaffold and adaptor proteins, signaling proteins, cell-adhesion molecules and components of the cytoskeleton. Glutamate receptors, such as NMDARs (N-methyl-D-aspartate receptors) and AMPARs (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors), are located in the postsynaptic membrane, with the NMDARs at the center of the synapse and the AMPARs more peripheral. The PDZ-domain-containing scaffold proteins PSD95 (also known as DLG4) and the Src-homology domain 3 (SH3) and multiple ankyrin repeat domains (Shank) family form a two-layer protein network below the postsynaptic membrane, which is bridged by guanylate kinase-associated protein (GKAP). PSD95 forms membrane-perpendicular and roughly equally spaced filamentous structures, with its amino terminus attached to the membrane. Other signaling molecules occupy the spaces in the PSD95–GKAP–Shank protein web. Shank-family scaffolds are further linked to actin filaments. The domains of PSD95 and Shank [PDZ, SH3, guanylate kinase (GK), sterile-alpha motif (SAM) and ankyrin repeats (ANK) (see key)] are shown; other proteins are represented by simple shapes and are labeled. The presynaptic and postsynaptic membranes are connected by cell-adhesion molecules. Reprinted by permission from Macmillan Publishers Ltd: Nat Rev Neurosci 10(2), Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density, 87-99, copyright 2009.
Postsynaptic scaffolds at nicotinic synapses
Because nAChRs do not have intracellular N- or C-terminals, they were thought not likely to interact with PSD-95 family members. Surprisingly, the receptors do participate in PDZ-scaffolds in a variety of systems29, 30, 31. It is not clear whether the interactions between nAChRs and PSD-95 family members are direct or indirect in those cases, but it is clear that the scaffold proteins are essential for mediating nAChR function.
Best characterized are the roles of PSD-95 family members in regulating nAChR function on autonomic neurons. PSD-93 co-localizes with nAChRs in mouse superior cervical ganglion neurons and submandibular ganglion neurons, and apparently tethers guanylate kinase-associated protein (GKAP) and Shank at the sites31 as it does at glutamate synapses. Moreover, immunoprecipitation of solubilized components shows that PSD-93 forms a complex with ganglionic nAChRs. Most importantly, denervation studies demonstrate that PSD-93 promotes synaptic stability; synaptic clusters of nAChRs disperse much more rapidly in mice lacking PSD-9331.
All four PSD-95 family members are expressed by chick ciliary ganglion (CG) neurons29. Three of them – PSD-93, PSD-95, and SAP102 – co-assemble with heteromeric nAChRs, as judged by immunoprecipitation of complexes solubilized from heterologous expression systems. PDZ-containing puncta co-distribute both with α7-nAChRs and α3-containing heteromeric receptors (α3*-nAChRs) on CG neurons. Dispersing the puncta disrupts nicotinic downstream signaling pathways in the neurons; this was done by transfecting the cells with a construct encoding a 9 amino acid peptide from cysteine-rich PDZ-binding protein (CRIPT), that blocks PDZ-mediated protein-protein interactions. Receptor activation is no longer able to activate the transcription factor CREB and alter gene expression in the cells29. A more surprising result is that CRIPT dispersal of the PDZ-puncta also constrains functional innervation of the neurons. Neurons expressing CRIPT received fewer spontaneous excitatory postsynaptic potentials (EPSCs) than controls, though the mean EPSC amplitude and nAChR immunostaining on the somata did not appear to be substantially reduced (Figure 2). The results suggested that the postsynaptic PDZ-scaffold anchors components that exert trans synaptic effects, perhaps aligning presynaptic release sites over postsynaptic nAChR clusters or boosting presynaptic release capabilities.
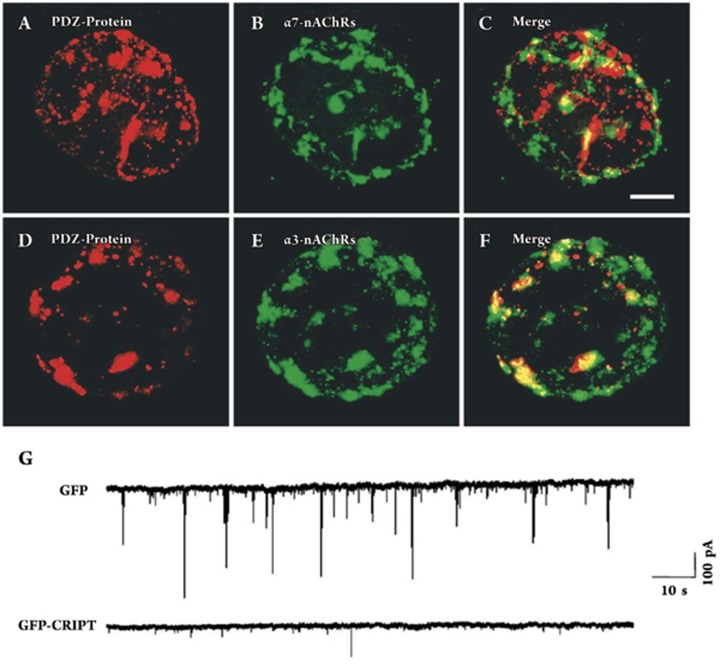
Figure 2.
PDZ proteins codistribute with CG synaptic nAChRs; disrupting PDZ interactions in postsynaptic cells diminishes synaptic transmission. (A) Freshly dissociated E15 CG neurons were immunostained with a monoclonal antibody (mAb) that recognizes the PSD-95 family of PDZ proteins and costained with either goat polyclonal antisera for α7-nAChRs (B) or with mAb 35 for α3β4*-nAChRs (E), and the paired images merged (C, F). Both α7-nAChR and α3β4*-nAChR clusters colocalize with PDZ. Scale bar: 10 μm. (G) CG neurons in culture for 7 days develop α3β4*-nAChR clusters that colocalize with PDZ proteins. CG neurons were transfected on day 1 in culture with GFP-CRIPT, which disrupts all PSD-95 family PDZ interactions. Whole-cell patch-clamp recording revealed many spontaneous EPSCs in control cells transfected with GFP (upper trace) but only relatively few EPSCs in cells transfected with GFP-CRIPT (bottom trace). Reprinted from Neuron, 38(5), Conroy WG, Liu Z, Nai Q, Coggan JS, Berg DK, PDZ-containing proteins provide a functional postsynaptic scaffold for nicotinic receptors in neurons, 759-71, 2003, with permission from Elsevier.
Postsnaptic PDZ-scaffolds at nicotinic synapses are not likely to be confined to autonomic neurons. In hippocampal neurons, α7-nAChRs appear to co-localize with PSD-9530. What the PDZ-protein is doing there and how it might be associated with α7-nAChRs remain interesting questions. The same report showed that a Wnt-7a signaling pathway promoted accumulation of presynaptic α7-nAChRs co-localized with the scaffold protein adenomatous polyposis coli (APC). Jacob and co-workers demonstrated that postsynaptically APC is localized with α3*-nAChRs on chick CG neurons, rather than with α7-nAChRs32. PSD-93 forms part of the APC complex with α3*-nAChRs on CG neurons. In addition, the complex contains End binding protein 1 (EB1), macrophin, IQ motif containing GTPase activating protein 1 (IQGAP1), and 14-3-3 which together link α3*-nAChRs to the cytoskeleton and stabilize the postsynaptic complex33.
Trans synaptic regulation
The ability of CRIPT to reduce nicotinic innervation of CG neurons suggested that the postsynaptic PDZ-scaffold organized components required for trans synaptic control of synapse formation. Neuroligin (NL) fulfills some of the criteria for such a component. CG neurons express several forms of NL and their binding partners α- and β-neurexins34, 35. Overexpression of tagged NL demonstrates that it co-localizes with nAChRs and can transcellularly induce accumulation of presynaptic components in adjacent neurites overlying the nAChR clusters34. Electrophysiological analysis of synaptic events indicates that NL increases the frequency of spontaneous miniature EPSCs (mEPSCs) recorded in the neurons without increasing mEPSC amplitude. This is most readily consistent with a presynaptic effect, though other possibilities remain. A dissection of the NL subdomains revealed that both the extracellular and intracellular domains were required for maximal mEPSC frequency, and further that the intracellular domain by itself functioned as a dominant negative (Figure 3). Unexpectedly, overexpression of NL boosted mEPSC frequency even if the construct lacked the PDZ-binding domain. The results suggested that high levels of NL can function as their own synapse-nucleating event and need not tether directly to a PDZ-scaffold.
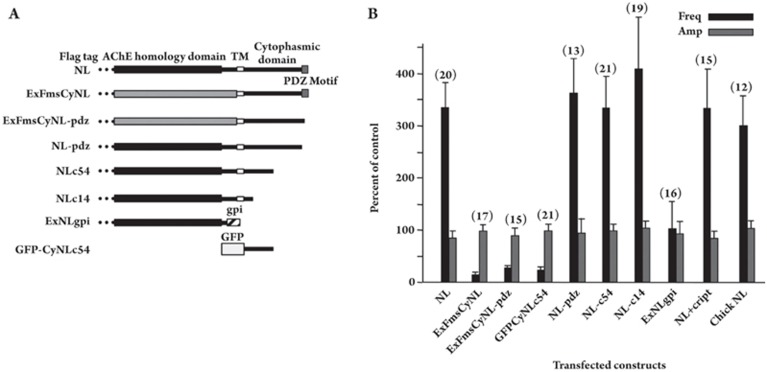
Figure 3.
Required NL domains for induction of nicotinic synapses. (A) NL constructs were prepared for transfection of E8 CG neurons in culture. NL, rat NL-1 full length with an 8-amino acid FLAG epitope fused on the N-terminus; ExFmsCyNL, NL-1 construct in which the extracellular domain was replaced by the extracellular domain of Fms; ExFmsCyNL-pdz, ExFmsCyNL lacking the C-terminus PDZ-binding domain of NL-1; NL-pdz, NL-1 lacking the 5-amino acid C-terminus representing a PDZ-binding motif; NLc54 and NLc14, NL-1 truncated after the first 54 and 14 amino acids, respectively, of the cytoplasmic domain; ExNLgpi, extracellular AChE-like domain of NL-1 with a gpi linkage site at the C-terminus; GFPCyNLc54, GFP fused to the first 54 amino acids of the cytoplasmic domain of NL-1. Flag tag, 8 amino acids attached at the N-terminus; AChE homology domain, extracellular domain of NL-1 homologous to the equivalent region of AChE; TM, transmembrane domain; Cytoplasmic domain, cytoplasmic portion of NL-1; PDZ motif, PDZ-binding motif; GFP, green fluorescent protein sequence. (B) Changes in mEPSC frequency caused by individual NL-1 constructs. The frequency and amplitude of mEPSCs recorded (in TTX) from neurons 5–8 days after transfection with the indicated NL-1 constructs were expressed as a percent of the values obtained from control neurons in the same cultures and then averaged across experiments to obtain mean±SEM for the normalized values (n=# of neurons). CRIPT refers to a GFP-CRIPT construct that disperses PDZ-scaffold proteins. Chick NL, chick full-length NL-1. All of the normalized values for frequency, except for ExNLgpi, were significantly different (P<0.05) from control (100%) where control represents cells transfected with GFP and untransfected cells; none of the normalized values for amplitude were significantly different from control (100%). The results indicate that extracellular and proximal cytoplasmic sequences of NL are necessary to enhance mEPSC frequency, while dominant-negative effects are observed for constructs having only the NL cytoplasmic sequence or the cytoplasmic sequence attached to an inappropriate extracellular sequence. PDZ interactions are not required for the effects. Reprinted from Dev Biol 307 (1), Conroy WG, Nai Q, Ross B, Naughton G, Berg DK, Postsynaptic neuroligin enhances presynaptic inputs at neuronal nicotinic synapses, 79–91, 2007, with permission from Elsevier.
Other transmembrane “synaptogenic” molecules are also expressed by CG neurons and can augment nicotinic innervation. These include the cell adhesion molecules L1 and SynCAM, both of which can act in CG neurons in culture to increase the number of synaptic contacts the cells receive36. This implies a trans synaptic effect. Electroporation studies confirmed that both endogenous NL and L1 act to provide this function in vivo. They are required for CG neurons to receive the expected number of presynaptic boutons overlying postsynaptic nAChR complexes (Figure 4). SynCAM, in contrast, is not critical for synapse formation in vivo but may nonetheless contribute to synaptic maturation36.
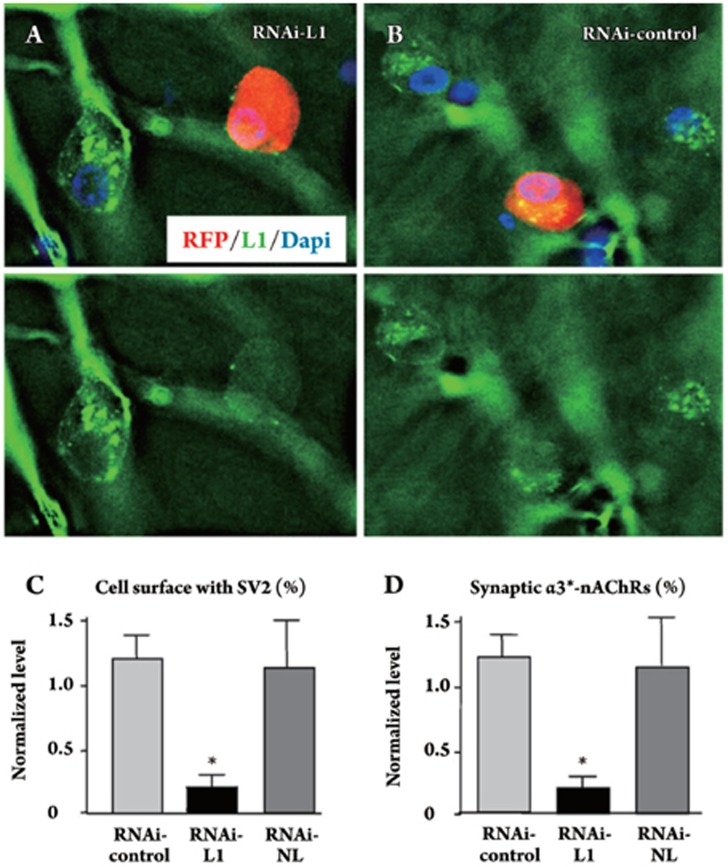
Figure 4.
L1 RNAi show reduces innervation of α3*-nAChR clusters in ovo. (A) CG neurons transfected in culture with a construct encoding an RNAi against chicken L1 and RFP as a marker (red) showed lower levels of endogenous L1 as seen by immunostaining (green) than did nearby untransfected cells. This is most evident when viewing the images with the RFP fluorescence deleted to reveal the L1 stain (bottom). (B) An RNAi with a scrambled sequence (RNAi-control) had no effect on L1 levels. (C) Electroporation of CG neurons in ovo revealed significant reductions in SV2 levels abutting RNAi-L1 transfected cells compared to RNAi-control transfected cells, similar to the pattern seen with the L1Cyt-GFP construct. In contrast transfection with an RNAi construct that targets NL (RNAi-NL) had no affect on SV2 levels. (D) Quantifying the proportion of α3*-nAChR clusters with SV2 clusters apposed, to assay for potential synapses, revealed a similar pattern. Cells transfected with RNAi-L1, but not with RNAi-NL, had a significantly reduced fraction of α3*-nAChR clusters receiving SV2 puncta. *P<0.05 compared to RNAi-control by ANOVA with Bonferroni post-tests. Scale bars: 10 μm. Reprinted from Mol Cell Neurosci 39 (1), Triana-Baltzer GB, Liu Z, Gounko NV, Berg DK, Multiple cell adhesion molecules shaping a complex nicotinic synapse on neurons, 74-82, 2008, with permission from Elsevier.
Yet another transmembrane component interacting with nAChRs on CG neurons is the EphB2 receptor (EphB2R). Transsynaptic interactions between EphB2Rs on postsynaptic cells and the transmembrane protein ephrinB-1 on presynaptic neurons can influence the clustering and function of NMDA receptors37, 38, 39. On CG neurons, EphB2Rs co-localize with α7-nAChRs on somatic spines embedded within lipid rafts40. Activation of the EphB2Rs with an ephrinB-1 fragment had two effects: it physically constrained α7-nAChRs from dispersal following spine collapse or lipid raft dispersal, and it augmented nicotinic activation of the transcription factor CREB40. How it does this and what the physical basis is for EphB2R/α7-nAChR interactions remain open questions.
Trafficking and chaperones
Current expectations about nAChR trafficking have been shaped in part by recent results showing that glutamate receptor trafficking both to and within the plasma membrane determines synaptic function and plasticity41, 42, 43. Early studies identified an “up-regulation” of functional nAChRs on the cell surface in response to chronic nicotine exposure44, 45, 46. It has now become clear that the up-regulation results from a variety of post-translational mechanisms including protein assembly and both trafficking to and stabilization within the surface membrane47, 48, 49, 50, 51,. Moreover, both the mechanism and the extent of up-regulation appear to be cell-type specific52.
Trafficking of nAChRs to the cell surface depends on chaperones. This may be most pronounced for α7-nAChRs which cannot be expressed by many cell types 53. Ric-3 has been identified as a chaperone that helps assemble and traffic α7-nAChRs to the cell surface54, 55, 56. Yeast-2-hybrid analysis has identified other chaperones mediating assembly and transport of neuronal nAChRs. One is 14-3-3η which interacts with α4 nAChR subunits and increases the steady-state levels of α4β2-nAChRs on the cell surface57. A second is VILIP-1 which also regulates α4β2-nAChR surface expression58.
Receptor internalization is also likely to depend on specific scaffold components and contribute importantly to the regulation of nicotinic signaling. An interesting example is provided by the SNARE-dependent activity-induced internalization of α7-nAChRs; replacement from an internal pool is required to maintain downstream signaling59. Yet other scaffold proteins control α7-nAChR clustering as demonstrated by the report on PICK160. The use of proteomics to identify proteins that interact specifically with individual nAChR subtypes will almost certainly divulge new and interesting players controlling nAChR trafficking and stabilization at synaptic sites61.
Rapid trafficking of nAChRs in the surface membrane is only beginning to be examined. Early studies on muscle nAChRs demonstrated that receptors can be mobile in the plasma membrane and traffic to sites of nerve-muscle contact62, 63, 64. Relatively rapid and reversible diffusion of the receptors can also occur in muscle membrane65. Recently it has been shown that nAChRs on autonomic neurons are capable of rapid lateral diffusion into and out of synapses depending on innervation and cellular conditions66. How this trafficking is regulated and what role it might play in nicotinic signaling in the CNS will be interesting issues to resolve.
Future directions
Although it is clear that the PSD-95 family of MAGUKs is critical for the maintenance of normal nicotinic synapse function, the roles of individual members have not yet been defined. Studies in glutamatergic systems have shown that PSD-95 family members exert both common and unique effects on synaptic function42. A similar level of complexity may exist at nicotinic synapses. Determining how and which PSD-95 family members specify nAChR expression, localization, and activity will be an important part of understanding the nature of nicotinic synapses.
The synaptic capabilities of nAChRs are likely to be determined in part by their precise spatio-temporal regulation on neurons. New technologies have recently been developed to characterize the dynamics of endogenous synaptic receptors with high temporal and spatial resolution. Prominent among these is single-particle-tracking studies using semiconductor quantum dots (QDs). Recently QD analysis has demonstrated that lateral diffusion of glutamate receptors is a critical mechanism shaping synaptic responses at glutamate synapses26. These new tools will be useful to resolve unanswered questions about the dynamics of nAChRs in central excitatory synapses. It will be exciting to apply these tools for the first time to the analysis of nAChRs on neurons.
Acknowledgments
Grant support was provided by the NIH (No NS012601 and NS035469) and the Tobacco-Related Disease Research Program (No 16RT-0167). David GOMEZ-VARELA is a FICYT Fellow(Spain); Catarina C FERNANDES is a Fundação Calouste Gulbenkian Fellow (Portugal).
References
- Bannon AW, Decker MW, Holladay MW, Curzon P, Donnelly-Roberts D, Puttfarcken PS, et al. Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science. 1998;279:77–81. doi: 10.1126/science.279.5347.77. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–7. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Levin ED. Nicotinic system involvement in Alzheimer's and Parkinson's diseases. Implications for therapeutics. Drugs Aging. 1997;11:206–28. doi: 10.2165/00002512-199711030-00005. [DOI] [PubMed] [Google Scholar]
- Raggenbass M, Bertrand D. Nicotinic receptors in circuit excitability and epilepsy. J Neurobiol. 2002;53:580–9. doi: 10.1002/neu.10152. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–7. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol. 1997;15:193–222. doi: 10.1007/BF02740634. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–6. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–43. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Graham A, Court JA, Martin-Ruiz CM, Jaros E, Perry R, Volsen SG, et al. Immunohistochemical localisation of nicotinic acetylcholine receptor subunits in human cerebellum. Neuroscience. 2002;113:493–507. doi: 10.1016/s0306-4522(02)00223-3. [DOI] [PubMed] [Google Scholar]
- Papke RL. The kinetic properties of neuronal nicotinic receptors: genetic basis of functional diversity. Prog Neurobiol. 1993;41:509–31. doi: 10.1016/0301-0082(93)90028-q. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–24. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiery A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc Natl Acad Sci USA. 1993;90:6971–5. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Klein RC, Fayuk D, Yakel JL. Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci. 2003;23:9024–31. doi: 10.1523/JNEUROSCI.23-27-09024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, et al. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Dani JA. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci. 2005;25:6084–91. doi: 10.1523/JNEUROSCI.0542-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–41. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature “silent” connections in the developing hippocampus. Proc Natl Acad Sci USA. 2003;100:2059–64. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci USA. 2008;105:20953–8. doi: 10.1073/pnas.0811025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–20. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–6. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, et al. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–67. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–18. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, et al. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–5. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–97. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A, Choquet D. New concepts in synaptic biology derived from single-molecule imaging. Neuron. 2008;59:359–74. doi: 10.1016/j.neuron.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Conroy WG, Liu Z, Nai Q, Coggan JS, Berg DK. PDZ-containing proteins provide a functional postsynaptic scaffold for nicotinic receptors in neurons. Neuron. 2003;38:759–71. doi: 10.1016/s0896-6273(03)00324-6. [DOI] [PubMed] [Google Scholar]
- Farias GG, Valles AS, Colombres M, Godoy JA, Toledo EM, Lukas RJ, et al. Wnt-7a induces presynaptic colocalization of alpha 7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. J Neurosci. 2007;27:5313–25. doi: 10.1523/JNEUROSCI.3934-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MJ, Zhao S, Bredt DS, Sanes JR, Feng G. PSD93 regulates synaptic stability at neuronal cholinergic synapses. J Neurosci. 2004;24:378–88. doi: 10.1523/JNEUROSCI.3865-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temburni MK, Rosenberg MM, Pathak N, McConnell R, Jacob MH. Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein. J Neurosci. 2004;24:6776–84. doi: 10.1523/JNEUROSCI.1826-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MM, Yang F, Giovanni M, Mohn JL, Temburni MK, Jacob MH. Adenomatous polyposis coli plays a key role, in vivo, in coordinating assembly of the neuronal nicotinic postsynaptic complex. Mol Cell Neurosci. 2008;38:138–52. doi: 10.1016/j.mcn.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy WG, Nai Q, Ross B, Naughton G, Berg DK. Postsynaptic neuroligin enhances presynaptic inputs at neuronal nicotinic synapses. Dev Biol. 2007;307:79–91. doi: 10.1016/j.ydbio.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Ross BS, Conroy WG. Capabilities of neurexins in the chick ciliary ganglion. Dev Neurobiol. 2008;68:409–19. doi: 10.1002/dneu.20598. [DOI] [PubMed] [Google Scholar]
- Triana-Baltzer GB, Liu Z, Gounko NV, Berg DK. Multiple cell adhesion molecules shaping a complex nicotinic synapse on neurons. Mol Cell Neurosci. 2008;39:74–82. doi: 10.1016/j.mcn.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–56. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–64. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–5. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- Liu Z, Conroy WG, Stawicki TM, Nai Q, Neff RA, Berg DK. EphB receptors co-distribute with a nicotinic receptor subtype and regulate nicotinic downstream signaling in neurons. Mol Cell Neurosci. 2008;38:236–44. doi: 10.1016/j.mcn.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–27. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–52. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Mauceri D, Gardoni F, Marcello E, Di Luca M. Dual role of CaMKII-dependent SAP97 phosphorylation in mediating trafficking and insertion of NMDA receptor subunit NR2A. J Neurochem. 2007;100:1032–46. doi: 10.1111/j.1471-4159.2006.04267.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–84. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235:619–28. [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem. 1985;45:427–33. doi: 10.1111/j.1471-4159.1985.tb04005.x. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol. 2005;68:1839–51. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, et al. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–72. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Nelson ME, Kuryatov A, Olale F, Cooper J, Keyser K, et al. Chronic nicotine treatment up-regulates human alpha3 beta2 but not alpha3 beta4 acetylcholine receptors stably transfected in human embryonic kidney cells. J Biol Chem. 1998;273:28721–32. doi: 10.1074/jbc.273.44.28721. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, et al. Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–18. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ST, Millar NS. Host cell-specific folding and assembly of the neuronal nicotnic acetylcholine receptor alpha7 subunit. J Neurochem. 1997. pp. 2140–51. [DOI] [PubMed]
- Castillo M, Mulet J, Gutierrez LM, Ortiz JA, Castelan F, Gerber S, et al. Dual role of the RIC-3 protein in trafficking of serotonin and nicotinic acetylcholine receptors. J Biol Chem. 2005;280:27062–8. doi: 10.1074/jbc.M503746200. [DOI] [PubMed] [Google Scholar]
- Millar NS. RIC-3: a nicotinic acetylcholine receptor chaperone. Br J Pharmacol. 2008;153 Suppl 1:S177–83. doi: 10.1038/sj.bjp.0707661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treinin M. RIC-3 and nicotinic acetylcholine receptors: biogenesis, properties, and diversity. Biotechnol J. 2008;3:1539–47. doi: 10.1002/biot.200800179. [DOI] [PubMed] [Google Scholar]
- Jeanclos EM, Lin L, Treuil MW, Rao J, DeCoster MA, Anand R. The chaperone protein 14-3-3eta interacts with the nicotinic acetylcholine receptor alpha 4 subunit. Evidence for a dynamic role in subunit stabilization. J Biol Chem. 2001;276:28281–90. doi: 10.1074/jbc.M011549200. [DOI] [PubMed] [Google Scholar]
- Lin L, Jeanclos EM, Treuil M, Braunewell KH, Gundelfinger ED, Anand R. The calcium sensor protein visinin-like protein-1 modulates the surface expression and agonist sensitivity of the alpha 4beta 2 nicotinic acetylcholine receptor. J Biol Chem. 2002;277:41872–8. doi: 10.1074/jbc.M206857200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tearle AW, Nai Q, Berg DK. Rapid activity-driven SNARE-dependent trafficking of nicotinic receptors on somatic spines. J Neurosci. 2005;25:1159–68. doi: 10.1523/JNEUROSCI.3953-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer K, Burli T, Huh KH, Wiesner A, Erb-Vogtli S, Gockeritz-Dujmovic D, et al. PICK1 interacts with alpha7 neuronal nicotinic acetylcholine receptors and controls their clustering. Mol Cell Neurosci. 2007;35:339–55. doi: 10.1016/j.mcn.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani N, Woll MP, Levenson R, Lindstrom JM, Changeux JP. Intracellular complexes of the beta2 subunit of the nicotinic acetylcholine receptor in brain identified by proteomics. Proc Natl Acad Sci USA. 2007;104:20570–5. doi: 10.1073/pnas.0710314104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ, Cohen MW. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977;268:757–73. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D, Ravdin PM, Podleski TR. Control of acetylcholine receptor mobility and distribution in cultured muscle membranes. A fluorescence study. Biochim Biophys Acta. 1978;511:23–38. doi: 10.1016/0005-2736(78)90062-7. [DOI] [PubMed] [Google Scholar]
- Young SH, Poo MM. Rapid lateral diffusion of extrajunctional acetylcholine receptors in the developing muscle membrane of Xenopus tadpole. J Neurosci. 1983;3:225–31. doi: 10.1523/JNEUROSCI.03-01-00225.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaaboune M, Grady RM, Turney S, Sanes JR, Lichtman JW. Neurotransmitter receptor dynamics studied in vivo by reversible photo-unbinding of fluorescent ligands. Neuron. 2002;34:865–76. doi: 10.1016/s0896-6273(02)00739-0. [DOI] [PubMed] [Google Scholar]
- McCann CM, Tapia JC, Kim H, Coggan JS, Lichtman JW. Rapid and modifiable neurotransmitter receptor dynamics at a neuronal synapse in vivo. Nat Neurosci. 2008;11:807–15. doi: 10.1038/nn.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]