Abstract
Highly pathogenic avian influenza H5N1 epidemics are a significant public health hazard. Genetically engineered H5N1 viruses with mammalian transmission activity highlight the potential risk of a human influenza H5N1 pandemic. Understanding the underlying principles of the innate immune system in response to influenza H5N1 viruses will lead to improved prevention and control of these potentially deadly viruses. γδ T cells act as the first line of defense against microbial infection and help initiate adaptive immune responses during the early stages of viral infection. In this study, we investigated the molecular mechanisms of γδ T cells in response to influenza H5N1 viral infection. We found that recombinant hemagglutinin (rHA) derived from three different strains of influenza H5N1 viruses elicited the activation of γδ T cells cultured in peripheral blood mononuclear cells (PBMCs). Both the cell surface expression of CD69, an early activation marker on γδ T cells, and the production of interferon-γ (IFN-γ) were significantly increased. Notably, the rHA protein-induced γδ T-cell activation was not mediated by TCRγδ, NKG2D or pattern recognition receptors (PRRs) or NKp46 receptors. The interaction of rHA proteins with sialic acid receptors may play a critical role in γδ T-cell activation. Our data may provide insight into the mechanisms underlying γδ T-cell activation in response to infection with H5N1 viruses.
Keywords: γδ T cells, hemagglutinin, highly pathogenic avian influenza H5N1 virus
Introduction
Because of the high mortality in poultry and several outbreaks of influenza in China caused by H5N1 viruses transmitted to humans directly from poultry, highly pathogenic avian influenza H5N1 epidemics are a significant public health hazard.1,2,3 Two recent studies demonstrated that engineered H5N1 viruses could move between mammals, further emphasizing the risk of a human influenza H5N1 pandemic.4,5 Therefore, understanding the pathogenicity, immunogenicity and transmissibility of H5N1 viruses is imperative. The disease phenotypes of H5N1 viruses are associated with mutations in the hemagglutinin (HA) gene, which encodes the most important protein in the influenza viral particle.6 Frequent mutation of HA is a major mechanism of viral escape.7,8 HA is essential for triggering the host immune response to viral influenza infection for the production of neutralizing antibodies.9,11 Therefore, understanding the immunogenicity of the H5N1 viral HA proteins is highly important for the development of immune therapeutics against influenza H5N1 viral infection.
γδ T cells are innate-like T cells that act as the first line of defense against microbial infection and help initiate adaptive immune responses during the early stages of viral infection.12,13,14 Recent studies demonstrated that γδ T cells can kill both human and avian influenza virus-infected monocyte-derived macrophages.15,16 γδ T cells from human peripheral blood mononuclear cells (PBMCs) can be activated by influenza A infection.17 Human Vγ9δ2 T cells express both type 1 cytokines and chemokine receptors in response to influenza A virus infection and display cytolytic activity against pandemic H1N1 virus-infected cells.15 These findings suggest that γδ T cells play critical roles in the host defense against influenza infection. However, little is known regarding the mechanisms underlying the activation of γδ T cells in response to viral influenza infection.
In this study, we investigated the molecular mechanisms of γδ T-cell activation in response to H5N1 viral infection. The results showed that recombinant HA (rHA) proteins derived from different H5N1 strains activated human γδT cells in PBMCs. As a result, CD69 expression and interferon-γ (IFN-γ) secretion were significantly increased. We found that γδ T-cell activation is not dependent on TCRγδ, NKG2D or pattern recognition receptors (PRRs), such as Toll-like receptor 2 (TLR2), TLR3, TLR4 and Nkp46. Sialic acid receptors may play critical roles in mediating γδ T- cell activation in response to influenza H5N1 virus infection.
Materials and methods
Expression of rHA proteins
rHA proteins were expressed and purified using a baculovirus/insect cell system (Invitrogen, BD Biosciences, San Diego, CA, USA) as described previously.18,19 Briefly, HA ectodomain DNA fragments from three H5N1 strains were cloned into the transfer vector PacGP67b (BD Biosciences, San Diego, CA, USA) and co-transfected with linearized baculovirus DNA into Sf-9 cells for the production of recombinant baculoviruses containing the HA genes. The transfected Sf-9 cells were cultured at 27 °C in Sf-900 II SFM for 4 h before replacement with fresh medium. The viral supernatant was collected at 72 h post-infection and incubated on a Ni+ column (GE Healthcare, Pittsburgh, PA, USA) for the purification of rHA proteins with a 6-His tag at the C-terminus. A western blot was performed with either anti-His antibodies or anti-HA antibodies to identify the rHA proteins.
Isolation of human PBMCs and γδ T cells
Fresh PBMCs were isolated from adult healthy donors by Ficoll-Hypaque (Pharmacia, TBD, Tianjin, China) density gradient centrifugation as described previously.20 The PBMCs were cultured and maintained in RPMI-1640 medium (Gibco BRL, Gibco, Gaithersburgh, MD, USA) with 10% fetal calf serum. The γδ T cells were purified by negative selection using a TCRγδ T-cell isolation kit (Miltenyi Biotec, Miltenyi, Bergisch Gladbach, Germany) in accordance with the manufacturer's instructions.
Flow cytometry analysis
Cultured or freshly isolated human PBMCs were resuspended in phosphate-buffered saline (PBS) containing 1% bovine serum albumin. For cell surface marker staining, the cells were incubated with FITC-, PE- and APC-conjugated monoclonal antibodies or isotype control antibodies for 20 min at 4 °C. For intracellular IFN-γ staining, the cells were stimulated with rHA or control proteins in the presence of Brefeldin A Solution (BioLegend, San Diego, CA, USA) for 6 h before fixation and permeabilization according to the manufacturer's instructions. The cells were then stained with cytokine specific antibodies. The following antibodies were used: FITC-anti-human TCR Panγδ (IMMU510) from Immunotech, PE-anti-NKG2D (1D11), FITC-anti-NKp46 (9E2), APC-anti-CD69 (FN50), PE-anti-CD25 (BC96), PE-anti-TLR2 (TL2.1), PE-anti-TLR3 (TLR-104), PE-anti-TLR4 (HTA125) and PE-anti-IFN-γ (B27) and the respective isotypes from Biolegend, as well as SNA-FITC and MAA-FITC from Vector. The cells were washed with PBS and fixed with methanol before analysis on an Accuri C6 flow cytometer. The data are represented as the percentage positive or the mean fluorescence intensity.
Expression of the TCRγ9/δ2 (OT3)–Fc fusion protein
TCRγ9/δ2 (OT3)–Fc fusion proteins were expressed and purified by Sino Biological Inc. (Beijing, China). Briefly, the TCRγ9δ2 (OT3)-Fc heterodimer was constructed by fusing the extracellular domains of the γ9 and δ2 chains of TCRγ9/δ2 (OT3) with the hinge region, CH2 and CH3 domains of the human IgG1 H chain.
ELISA assay
TCRγ9/δ2 (OT3)–Fc fusion proteins (1 µg/well) were coated onto polystyrene 96-well plates at 4 °C overnight. The plates were blocked with 5% bovine serum albumin (Sigma) at 37°C for 2 h before the addition of His-tagged rHAs (10 µg/ml), control protein (10 µg/ml) or anti-TCRγ/δ mAb (5 µg/ml). After 1 h of incubation at 37 °C, horseradish peroxidase-conjugated anti-His mouse monoclonal antibodies (CWbiotech, Beijing, China) or horseradish peroxidase-conjugated anti-mouse IgG (Fab-specific) goat polyclonal antibodies (Sigma, St Louis, MO, USA) were added to each well for color development with OPD/H2O2. The reaction was halted by the addition of H2SO4. The plates were read on a microplate reader (Labsystem, Thermo Scientific, Waltham, MA, USA) at 450/630 nm.
Statistical analysis
The data are presented as the mean±s.e.m. Comparisons of the quantitative data between two groups were performed using Student's t-test. A P value less than 0.05 was considered statistically significant.
Results
The expression and identification of rHA proteins
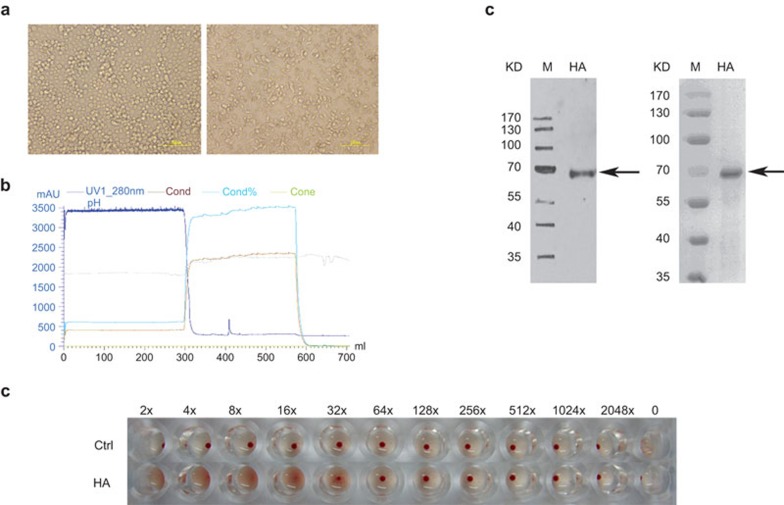
HA is a major antigen on the surface of influenza viruses and is a critical protein for inducing the majority of neutralizing antibodies and for cross-protection against influenza viruses.21,22 HA is also the primary component of the currently licensed influenza virus vaccines.23 Therefore, in this study, we used rHA proteins to investigate the response of γδ T cells to H5N1 infection. The following HA genes from three H5N1 strains were obtained from Hong Kong University, China's Center for Disease Control and the Chinese Academy of Sciences: A/Bar-headed Goose/Qinghai/2005 H5N1 HA (QH-HA), A/Xinjiang/2006 H5N1 HA (XJ-HA) and A/Hongkong/2003 H5N1 HA (HK-HA). The HA ectodomains are responsible for the primary immunogenicity of the HAs and were expressed using a baculovirus expression system and purified with an AKTA chromatography system (Figure 1a and b). Western blot analysis and Coomassie blue staining confirmed that the rHA proteins were successfully expressed and purified (Figure 1c). The hemagglutination test showed that the rHA proteins possessed hemagglutination activity (Figure 1d).
Figure 1.
Expression of recombinant H5N1 HA proteins. (a) Representative images of Sf-9 cells before (left) and after (right) transfection of the recombinant baculovirus. (b) The elution curve of rHA proteins from an AKTA affinity chromatography system. (c) Purified rHA proteins were analyzed by western blot (left) with anti-HA antibodies and Coomassie blue staining of SDS–PAGE (right). M: protein marker. The arrows show the locations of the rHA proteins. (d) Hemagglutination activity analysis of the rHA proteins. HA, hemagglutinin; rHA, recombinant hemagglutinin.
rHA proteins trigger the activation of γδ T cells in PBMCs in vitro
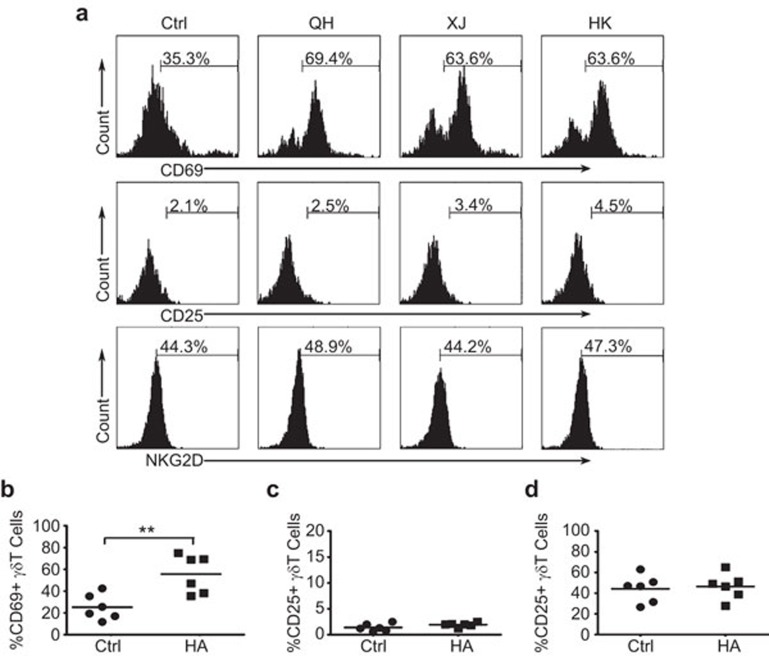
Recent studies showed that influenza A infection can induce the rapid activation of γδ T cells in PBMCs.17 To determine whether HA proteins from the H5N1 strains could induce γδ T-cell activation, PBMCs from healthy adults were incubated with the rHAs from three different influenza A H5N1 strains, A/Hongkong/2003 H5N1, A/Xinjiang/2006 H5N1 and A/Bar-headed Goose/Qinghai/2005 H5N1, or the control protein. The cell surface expression of the C-type lectin-like glycoprotein CD69 on γδ T cells was determined by flow cytometry. CD69 is a sensitive and very early marker of leukocyte activation.24,25 Following exposure to the different rHA proteins from different strains, the percentages of CD69+ γδ T cells reached approximately 60%–70%, representing a twofold increase compared to the control (Figure 2a and b). The expression of CD25 was also slightly upregulated following stimulation with the rHA proteins (Figure 2a and c). However, no obvious change was observed for the expression of NKG2D on the surface of γδ T cells after incubation with the rHA proteins (Figure 2a and d).
Figure 2.
Expression of early activation markers on γδ T cells in response to stimulation with rHAs from different H5N1 strains. (a) Flow cytometry analysis of the expression of the early activation markers CD69, CD25 and NKG2D on human peripheral γδ T cells in response to rHA stimulation. Representative images from at least three independent experiments. Human PBMCs were stimulated with recombinant QH-HA (A/Bar-headed Goose/Qinghai/2005 H5N1), XJ-HA (A/Xinjiang/2006 H5N1) or HK-HA (A/Hongkong/2003 H5N1). The cells were stained with antibodies specific for TCRγδ, CD69, CD25 and NKG2D. The TCRγδ-positive cells were gated, and the expression of CD69, CD25 or NKG2D was analyzed by flow cytometry. (b) The average percentage of CD69+γδ T cells from six independent experiments. (c) The average percentage of CD25+γδ T cells from six independent experiments. (d) The average percentage of NKG2D+γδ T cells from six independent experiments. **P<0.01. The horizontal lines represent the mean values. HA, hemagglutinin; PBMC, peripheral blood mononuclear cell; rHA, recombinant hemagglutinin.
The production of IFN-γ is also an important index of T-cell functional activation during viral infection. IFN-γ plays a key role in degrading antigens, inhibiting viral proliferation and promoting the differentiation of lymphocytes. To determine whether H5N1 viral HA proteins can rapidly induce the production of IFN-γ in γδ T cell, intracellular IFN-γ staining was performed to detect the percentage of IFN-γ-producing γδ T cells in PMBCs incubated with rHAs. As expected, flow cytometry analysis showed that the H5N1 viral rHA (A/Bar-headed Goose/Qinghai/2005 H5N1) stimulated the production of IFN-γ in γδ T cells. The percentage of IFN-γ-producing γδ T cells was increased nearly threefold after rHA stimulation compared to the control (Figure 3a and b). Taken together, these results demonstrate that human peripheral blood γδ T cells are activated by rHAs from the H5N1 strains.
Figure 3.
Production of IFN-γ in γδT cells in response to stimulation with rHA. (a) PBMCs were stained with antibodies specific for TCRγδ and IFN-γ after incubation with the recombinant QH-HA proteins (A/Bar-headed Goose/Qinghai/2005 H5N1). The TCRγδ-positive cells were gated, and the IFN-γ-secreting γδT cells were analyzed by flow cytometry. (b) The average percentages of IFN-γ+ γδ T cells from six independent experiments. *P<0.05. The horizontal lines represent the mean values. HA, hemagglutinin; IFN, interferon; PBMC, peripheral blood mononuclear cell; rHA, recombinant hemagglutinin.
rHAs directly bind to the surface of γδ T cells
The finding that peripheral blood γδ T cells are activated by rHA stimulation led us to seek the underlying molecular mechanism. Therefore, we examined whether rHA proteins bind directly to the surface of γδ T cells. γδ T cells were freshly sorted from human PBMCs by flow cytometry and were incubated with the three H5N1 rHA proteins or the control protein for 30 min at 4 °C. Flow cytometry showed that the majority of γδ T cells were HA-positive (Figure 4), suggesting that the rHA proteins bind directly to the surface of γδ T cells.
Figure 4.
rHA proteins directly bind to the surface of γδ T cells. Flow cytometry analysis of the binding of rHAs (QH-HA, XJ-HA and HK-HA) to the surface of γδ T cells freshly sorted from human PBMCs using anti-His-FITC antibodies. The percentages of FITC-positive cells represent the binding activities of the rHA proteins to γδ T cells. HA, hemagglutinin; PBMC, peripheral blood mononuclear cell; rHA, recombinant hemagglutinin.
TCRγδ and NKG2D are not necessary for rHA protein recognition by γδ T cells
TCRγδ is the most important receptor on the surface of γδ T cells and is responsible for recognition of the majority of antigen ligands, such as major histocompatibility complex class I-related proteins A and B (MICA/B), phosphoantigens and several glycoproteins and lipoproteins.26,27,28,29,30,31 Antigen recognition by TCRγδ is not major histocompatibility complex-restricted and is more similar to an ‘antibody-like' response.32,33,34,35 Therefore, using a CDR3δ grafted TCRγ9/δ2–Fc fusion protein technology system, we constructed a TCRγ9/δ2 (OT3)–Fc fusion protein to determine whether rHA binds directly to TCRγδ. TCRγ9/δ2 (OT3)–Fc has a similar stereochemical structure to TCRγδ, with an OT3 fragment at the CDR3 region.36,37 We measured the binding activity of the rHA proteins to TCRγ9/δ2 (OT3)–Fc fusion proteins by ELISA. The results demonstrated that the three H5N1 strain rHA proteins did not bind the TCRγ9/δ2 (OT3)–Fc fusion protein (Figure 5a), indicating that rHA-induced γδ T-cell activation may be not mediated by TCRγδ.
Figure 5.
rHA-induced activation of γδ T cells is not TCRγδ- or NKG2D-dependent. (a) ELISA analysis of the binding of rHAs to TCRγ9δ2 (OT3)-Fc proteins. (b) Flow cytometry analysis of the binding of an anti-TCRγδ monoclonal antibody and an anti-NKG2D monoclonal antibody to γδ T cells. (c) Confocal microscope scanning analysis of the binding of an anti-TCRγδ monoclonal antibody and an anti-NKG2D monoclonal antibody to γδ T cells. FITC-labeled goat-anti-mouse IgG was used as the secondary antibody. Scale bar=100 µm. (d) Antibodies against TCRγδ or NKG2D did not block rHA binding to γδ T cells. HA, hemagglutinin; rHA, recombinant hemagglutinin.
NKG2D is an important costimulatory receptor on γδ T cells and plays a critical role in antigen recognition and cytotoxicity activation of γδ T cells.14,38,39,40,41,42 Therefore, we examined whether the binding of rHA proteins to NKG2D receptors mediates the rHA-induced activation of γδ T cells. As shown in Figure 5, although the binding of the anti-NKG2D antibody to the surface of γδ T cells was efficient (Figure 5b and c), the anti-NKG2D antibody did not block rHA binding to the γδ T cells (Figure 5d), suggesting that NKG2D is not necessary for the rHA-induced activation of γδ T cells. In addition, blocking with anti-TCRγδ antibodies did not change the median fluorescence intensity of HA-binding to the γδ T cells (Figure 5b–d), suggesting that TCRγδ may not mediate the binding of HAs with γδ T cells.
γδ T-cell activation is not mediated by PRRs during influenza H5N1 infection
PRRs, including TLR2, TLR3 and TLR4, have been reported to interact with the HA protein and elicit the activation of myeloid dendritic cells or alveolar macrophages during influenza viral infection.43,44,45 Therefore, we determined whether rHA-induced γδ T-cell activation is mediated by these receptors. We found no surface expression of TLR2, TLR3 or TLR4 on γδ T cells (Figure 6a). On natural killer (NK) cells, NKp46 is hypothesized to interact with the HA protein to mediate the activation of NK cells in response to influenza infection.46 However, our results indicated that NKp46 was not expressed on the surface of γδ T cells (Figure 6a). These findings suggest that γδ T-cell activation during viral influenza infection is not mediated by these receptors.
Figure 6.
Sialic acid receptors mediate the binding of rHA to γδ T cells. (a) Flow cytometry analysis showed that γδ T cells lacked expression of TLR2, TLR3, TLR4 and NKp46. (b) Flow cytometry analysis of α-2,3 and α-2,6 SA expression before (upper panel) and after (lower panel) neuraminidase digestion. (c) Flow cytometry analysis of rHA binding on γδ T cells before (left) and after (right) neuraminidase digestion. NK, natural killer; rHA, recombinant hemagglutinin; TLR, Toll-like receptor.
Sialic acid receptors mediate HA binding to human γδ T cells
Sialic acid receptors on the surface of host cells play a critical role in the binding of influenza HA and mediate viral entry into host bronchial epithelial cells.47,48 Therefore, we determined whether sialic acid receptors mediate the adhesion or binding of rHA proteins to the surface of γδ T cells and subsequently mediate rHA-induced activation of γδ T cells. Therefore, the expression of sialic acid receptors on the surface of γδ T cells was examined by flow cytometry. The results demonstrate that both α-2,3 and α-2,6 sialic acid receptors are highly expressed on γδ T cells (Figure 6b), which was also confirmed by digestion with neuramidase (Figure 6b). After neuramidase digestion, the rHA proteins no longer bound to the γδ T cells, indicating that the sialic acid receptors may also play critical roles in mediating the binding of HA to γδ T cells and γδ T cell activation (Figure 6c).
Discussion
Influenza is a leading prevalent infectious disease that periodically causes pandemics that end with the death of millions of people.6 The potential emergence of a new pandemic strain through natural re-assortment is a major public health concern. Highly pathogenic avian influenza H5N1 viruses have been of particular interest because of their unusual pathogenicity in domestic poultry and their extremely high mortality in humans. According to the World Health Organization, H5N1 viruses have infected 608 people and killed 359 since 2003, representing a 59% pandemic case-fatality rate. Over the past 500 years of observation, no influenza pandemic is believed to have caused a case-fatality rate greater than approximately 2%.6 Therefore, among many other important research areas related to H5N1 viruses, it is necessary to study the immunogenicity of the H5N1 virus with an emphasis on H5N1 viral infection mechanisms leading to hypercytokinemia in humans. These areas support the development of immune therapeutics such as vaccines.
In our study, we found that rHA proteins derived from different H5N1 strains could activate human γδ T cells in PBMCs, indicating that HA immunogenicity may be required for γδ T cell activation in response to influenza H5N1 viruses. This finding supports the hypothesis that HA proteins may play an essential role in the initiation of innate immunity against viral influenza infection.49,50
We also found that recombinant H5N1 HA proteins could specifically bind to the surface of human γδ T cells. However, the binding activity was not dependent on TCRγδ and NKG2D, suggesting that H5N1 HA-induced activation of γδ T cells may not be mediated via the TCRγδ or NKG2D signaling pathway.
PRRs play critical roles in eliciting innate immune responses in the host against viral infections.45 Myeloid dendritic cells and alveolar macrophages in the lung express high levels of TLRs, such as TLR2, TLR3 and TLR4, which are important for the recognition of influenza viruses.43,44 However, cell surface expression of TLRs in human peripheral blood γδ T cells was nearly undetectable by flow cytometry, indicating that TLRs may not be involved in the response of γδ T cells to influenza virus and that the PRR-mediated signaling pathway may not play an essential role in the activation of human γδ T cells in influenza H5N1 virus infection. NK cells, which represent another important cell population in the innate immune response to viral infections, are activated by influenza virus HA proteins through interaction with the NKp46 receptor.46 However, we found that NKp46 receptors were undetectable on the surface of human γδ T cells by flow cytometry. Our findings suggest that the mechanisms of influenza virus-induced cell activation may be variable in different cell types.
Previous studies demonstrated that the HA from influenza A virus interacts with sialic acid receptors on the cell surface and mediates the entry of viral particles.50,51,52 In this study, we found that human γδ T cells expressed high levels of sialic acid receptors that were required for the binding of H5N1 HA to the surface of human γδ T cells. These findings indicate that H5N1 virus-induced activation of γδ T cells may be mediated by the interaction of H5N1 virus HA with sialic acid receptors on the surface of γδ T cells. Sialic acid receptors are usually attached by α-2,3 or α-2,6 linkages to the terminal galactose of the underlying sugar chains of glycoproteins on the cell surface.45 Furthermore, HA from the avian influenza H5N1 virus preferentially binds to α-2,3 sialic acid receptors, whereas HA from human isolates preferentially binds to α-2,6 sialic acid receptors.51,52 Therefore, the identification of specific glycoproteins that interact with H5N1 HA will be critical for understanding the mechanism of HA-induced γδ T-cell activation.
In conclusion, our findings confirm the importance of HA protein immunogenicity in initiating the immune response to influenza virus infection, which supports the development of vaccines or immune therapeutics based on HA proteins. Our data may also provide insights into the mechanisms underlying γδ T-cell activation in response to H5N1 virus infection.
Acknowledgments
This work was supported by two grants, No. CHB1-31056-BE-11 from the US Civilian Research & Development Foundation from the National Institute of Allergy and Infectious Diseases and No. 31070785 from the National Natural Science Foundation of China. We thank Dr Jianmin Zhang and Dr Austin Cape for critical reading of the manuscript.
References
- CDC Update: Influenza activity-United States and worldwide, 2006–07 season, and composition of the 2007–08 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:789–794. [PubMed] [Google Scholar]
- WHO . Geneva: WHO; 2012. Cumulative Number of Confirmed Human Cases for Avian Influenza A(H5N1) Reported to WHO, 2003–2012. [Google Scholar]
- WHO Update on human cases of highly pathogenic avian influenza A(H5N1) virus infection, 2011. Wkly Epidemiol Rec. 2012;13:117–128. [PubMed] [Google Scholar]
- Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336:1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486:420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens DM, Subbarao K, Taubenberger JK. Engineering H5N1 avian influenza viruses to study human adaptation. Nature. 2012;486:335–340. doi: 10.1038/nature11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglesby TV, Cicero A, Henderson DA. The risk of engineering a highly transmissible H5N1 virus. Biosecur Bioterror. 2012;10:151–152. doi: 10.1089/bsp.2011.1214. [DOI] [PubMed] [Google Scholar]
- Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 2010;7:440–451. doi: 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Jin L, Zhao G, Sun S, Li J, Yu H, et al. Identification and structural characterization of a broadly neutralizing antibody targeting a novel conserved epitope on influenza H5N1 hemagglutinin. J Virol. 2013;87:2215–2225. doi: 10.1128/JVI.02344-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M, Hu H, Zuo T, Wang G, Zhang L, Zhou P. Unravel a neutralization mechanism by two human antibodies against conserved epitopes in the globular head of H5 hemagglutinin. J Virol. 2013;87:3571–3577. doi: 10.1128/JVI.01292-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chan CC, Yang M, Deng J, Poon VK, Leung VH, et al. A critical role of IL-17 in modulating the B-cell response during H5N1 influenza virus infection. Cell Mol Immunol. 2011;8:462–468. doi: 10.1038/cmi.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18:539–546. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Born WK, Reardon CL, O'Brien RL. The function of gammadelta T cells in innate immunity. Curr Opin Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Zheng J, Liu Y, Lau YL, Tu W. gammadelta-T cells: an unpolished sword in human anti-infection immunity. Cell Mol Immunol. 2013;10:50–57. doi: 10.1038/cmi.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Liu Y, Zheng J, Ng IH, Xiang Z, Lam KT, et al. Type 1 responses of human Vgamma9Vdelta2 T cells to influenza A viruses. J Virol. 2011;85:10109–10116. doi: 10.1128/JVI.05341-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Mao H, Zheng J, Sia SF, Liu Y, Chan PL, et al. Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis. 2009;200:858–865. doi: 10.1086/605413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson JM, Cruz J, Costanzo A, Terajima M, Ennis FA. A role for the mevalonate pathway in the induction of subtype cross-reactive immunity to influenza A virus by human gammadelta T lymphocytes. Cell Immunol. 2010;264:71–77. doi: 10.1016/j.cellimm.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu Z, Ma C, Zhang L, Su Y, Gao GF, et al. Identification of amino acids in highly pathogenic avian influenza H5N1 virus hemagglutinin that determine avian influenza species specificity. Arch Virol. 2011;156:1803–1812. doi: 10.1007/s00705-011-1056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ma C, Liu Z, He W. Serologic cross-reactivity among humans and birds infected with highly pathogenic avian influenza A subtype H5N1 viruses in China. Immunol Lett. 2011;135:59–63. doi: 10.1016/j.imlet.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kang N, Zhang X, Dong X, Wei W, Cui L, et al. Generation of human regulatory gammadelta T cells by TCRgammadelta stimulation in the presence of TGF-beta and their involvement in the pathogenesis of systemic lupus erythematosus. J Immunol. 2011;186:6693–6700. doi: 10.4049/jimmunol.1002776. [DOI] [PubMed] [Google Scholar]
- Terajima M, Cruz J, Co MD, Lee JH, Kaur K, Wrammert J, et al. Complement-dependent lysis of influenza a virus-infected cells by broadly cross-reactive human monoclonal antibodies. J Virol. 2011;85:13463–13467. doi: 10.1128/JVI.05193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, He W. The recognition pattern of gammadelta T cells. Front Biosci. 2005;10:2676–2700. doi: 10.2741/1729. [DOI] [PubMed] [Google Scholar]
- Du N, Zhou J, Lin X, Zhang Y, Yang X, Wang Y, et al. Differential activation of NK cells by influenza A pseudotype H5N1 and 1918 and 2009 pandemic H1N1 viruses. J Virol. 2010;84:7822–7831. doi: 10.1128/JVI.00069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D, He W. The multifunctionality of human Vgamma9Vdelta2 gammadelta T cells: clonal plasticity or distinct subsets. Scand J Immunol. 2012;76:213–222. doi: 10.1111/j.1365-3083.2012.02727.x. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- Chen ZW. Multifunctional immune responses of HMBPP-specific Vgamma2Vdelta2 T cells in M. tuberculosis and other infections. Cell Mol Immunol. 2013;10:58–64. doi: 10.1038/cmi.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie JJ, Sicard H, Poupot M, Bezombes C, Blanc A, Romagne F, et al. What lessons can be learned from gammadelta T cell-based cancer immunotherapy trials. Cell Mol Immunol. 2013;10:35–41. doi: 10.1038/cmi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo N, Todaro M, Sireci G, Meraviglia S, Stassi G, Dieli F. Mechanisms underlying lineage commitment and plasticity of human gammadelta T cells. Cell Mol Immunol. 2013;10:30–34. doi: 10.1038/cmi.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born WK, Kemal Aydintug M, O'Brien RL. Diversity of gammadelta T-cell antigens. Cell Mol Immunol. 2013;10:13–20. doi: 10.1038/cmi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, Hinz T. gamma delta T cells, their T cell receptor usage and role in human diseases. Springer Semin Immunopathol. 1999;21:55–75. [PubMed] [Google Scholar]
- Li H, Lebedeva MI, Llera AS, Fields BA, Brenner MB, Mariuzza RA. Structure of the Vdelta domain of a human gammadelta T-cell antigen receptor. Nature. 1998;391:502–506. doi: 10.1038/35172. [DOI] [PubMed] [Google Scholar]
- Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison TJ, Winter CC, Fournie JJ, Bonneville M, Garboczi DN. Structure of a human gammadelta T-cell antigen receptor. Nature. 2001;411:820–824. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]
- Xi X, Cui L, He W. The recognition of gammadelta TCR to protein antigen does not depend on the hydrophobic I97 residue of CDR3delta. Int Immunol. 2010;22:299–306. doi: 10.1093/intimm/dxq011. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang T, Hu H, Zhang H, Yang Z, Cui L, et al. Targeting solid tumors via T cell receptor complementarity-determining region 3delta in an engineered antibody. Cancer Lett. 2008;272:242–252. doi: 10.1016/j.canlet.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Nedellec S, Sabourin C, Bonneville M, Scotet E. NKG2D costimulates human V gamma 9V delta 2 T cell antitumor cytotoxicity through protein kinase C theta-dependent modulation of early TCR-induced calcium and transduction signals. J Immunol. 2010;185:55–63. doi: 10.4049/jimmunol.1000373. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Terao S, Acharya B, Naoe M, Yamamoto S, Okamura H, et al. The antitumour effect of {gamma}{delta} T-cells is enhanced by valproic acid-induced up-regulation of NKG2D ligands. Anticancer Res. 2010;30:4509–4513. [PubMed] [Google Scholar]
- Kuroda H, Saito H, Ikeguchi M. Decreased number and reduced NKG2D expression of Vdelta1 gammadelta T cells are involved in the impaired function of Vdelta1 gammadelta T cells in the tissue of gastric cancer. Gastric Cancer. 2012;15:433–439. doi: 10.1007/s10120-011-0138-x. [DOI] [PubMed] [Google Scholar]
- Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- Zhou J, Kang N, Cui L, Ba D, He W. Anti-gammadelta TCR antibody-expanded gammadelta T cells: a better choice for the adoptive immunotherapy of lymphoid malignancies. Cell Mol Immunol. 2012;9:34–44. doi: 10.1038/cmi.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris NA, Dessing MC, de Vos AF, Bresser P, van der Zee JS, Jansen HM, et al. Toll-like receptor mRNA levels in alveolar macrophages after inhalation of endotoxin. Eur Rrespir J. 2006;28:622–626. doi: 10.1183/09031936.06.00010806. [DOI] [PubMed] [Google Scholar]
- Kovach MA, Standiford TJ. Toll like receptors in diseases of the lung. Int Immunopharmacol. 2011;11:1399–1406. doi: 10.1016/j.intimp.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos I, Fernandez-Sesma A. Innate immunity to H5N1 influenza viruses in humans. Viruses. 2012;4:3363–3388. doi: 10.3390/v4123363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- Oshansky CM, Pickens JA, Bradley KC, Jones LP, Saavedra-Ebner GM, Barber JP, et al. Avian influenza viruses infect primary human bronchial epithelial cells unconstrained by sialic acid alpha2,3 residues. PloS ONE. 2011;6:e21183. doi: 10.1371/journal.pone.0021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer GF, Schwick HG, Fletcher MA. The relationship of the influenza virus inhibitory activity of glycoproteins to their molecular size and sialic acid content. Proc Natl Acad Sci USA. 1969;64:634–641. doi: 10.1073/pnas.64.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988;333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Ruigrok RW, Aitken A, Calder LJ, Martin SR, Skehel JJ, Wharton SA, et al. Studies on the structure of the influenza virus haemagglutinin at the pH of membrane fusion. J Gen Virol. 1988;69 Pt 11:2785–2795. doi: 10.1099/0022-1317-69-11-2785. [DOI] [PubMed] [Google Scholar]
- van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, et al. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]