Type 2 immune immunity evolved a unique system to protect against noxious xenobiotics at the epithelial barrier.1 Recently, epithelial cell-derived cytokines IL-25, IL-33 and TSLP have been shown to be critical in producing Th2 cytokines, including IL-4, IL-5, IL-9 and IL-13, by type 2 innate lymphoid cells (ILC2s) as well as Th2 cells. Even though numerous studies have demonstrated that ILC2s are able to initiate a variety of reactions associated with type 2 immune responses independently of Th2 cells, little is known about whether they control the activity of other immune cells. In a paper published in Nature Immunology, Roediger et al.2 have demonstrated that ILC2s have two different mechanisms that could be crucial for maintaining skin homeostasis under steady state conditions and mediating skin inflammation.
Innate lymphoid cells are differentiated from Id2-expressing common lymphoid cells, but the specification of subsets is determined by different transcription factors.3 Two transcript factors GATA-3 and RORα are required for ILC2 differentiation.3 ILC2s are heterogeneous in terms of their phenotypes and tissue distribution. However, their effector functions are commonly mediated through Th2 cytokines and other soluble factors. Given the intrinsic properties of ILC2s and their location in lymphoid organs and at mucosal sites, ILC2s are considered to be a first line of defense against noxious allergens and parasitic infections. However, in line with Th2 immunity that plays an essential role in tissue repair in organs that experience tissue damage resulting from overly exuberant immune responses, ILC2s have been found to participate in lung repair after infection with influenza virus.4 In addition, ILC2s maintain visceral adipose tissue eosinophils and M2 macrophages to regulate metabolic homeostasis.5 Roediger et al.2 have identified a unique dermal ILC2s that express CD103 (αEβ7 integrin expressed by skin-resident T cells) and constitutively express IL-13. Using reporter mice, they have convincingly shown that ILC2s patrol the skin under the steady state and interact with skin-resident mast cells. In in vitro experiments, IL-13 has been shown to inhibit the ability of primed mast cells to product IL-6 and TNF upon exposure to antigen. On the other hand, another independent research group has demonstrated that skin-resident ILC2s are indispensible for atopic dermatitis (AD)-like disease induced by the vitamin D3 analog MC903.6 This chemical is a potent inducer of TSLP (but not IL-25 and IL-33) production by keratinocytes. Which cytokine is produced among IL-25, IL-33 and TSLP at mucosal barriers seems to be determined by types of allergens, parasites and other injury inducers, and responding parenchymal and stromal cells. The vitamin D3 analog MC903 has been shown to result in AD-like disease by inducing TSLP production by skin keratinocytes.7 One important finding by Roediger et al.2 is that treatment with complexes of IL-2 and anti-IL-2 monoclonal antibody (potently stimulate the IL-2 receptor) in Rag1−/− mice elicits AD-like disease with characteristics of eosinphil infiltrates and activated mast cells through the expansion of IL-5-producing dermal ILC2s. It remains to be determined whether IL-2 produced by allergen-primed Th2 T cells can participate in the expansion of ILC2s during allergic responses in T cell-replete mice. Although IL-2 can potentiate the proliferation and activation of ILC2s in IL-25- and/or IL-33-rich milieu, IL-2 may directly act on ILC2s in such conditions that these acute phase cytokines are no longer present in inflamed sites. There is compelling evidence that during papain-induced lung inflammation, IL-2 from adaptive immune cells elicits a transit production of IL-9 by ILC2s and IL-9 subsequently results in their production of IL-5 and IL-13 in an autocrine fashion.8 This observation suggest that once adaptive immunity is initiated, it can recruit all types of innate immune cells available, including ILC2s, to reinforce allergic responses.9
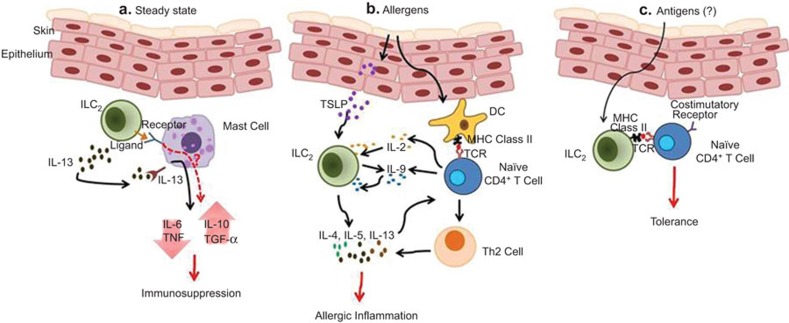
Dermal ILC2s may have a tropism to the skin due to their expression of CD103 on the cell surface and their abundance in the skin. If this turns out to be the case, ILC2s may continuously circulate the body for immunosurveillance in the skin. Roediger et al.2 suggested that extensive interactions with mast cells and provision of IL-13 signals might be crucial for a mechanism of immunosurveillance by ILC2s which are capable of suppressing the activity of mast cells (Figure 1a). But what is the meaning of the immunosupression by ILC2s in the naïve state? ILC2s may sensitize mast cells through ligand–receptor interactions and the secretion of IL-13 and this sensitization may raise the threshold for mast cell activation, which can function as a safety brake to prevent an unnecessary inflammation.10 It is also possible that ILC2s promote the production of immunomodulatory molecules such as IL-10 and TGF-β by mast cells (Figure 1a). There is a report showing that mast cell-derived IL-10 is critical in inhibiting skin inflammation.11 Therefore, overall consequences of this regulatory networking seem to prevent uncontrollable excessive tissue inflammation.
Figure 1.
Roles of ILC2 in the skin under the steady state and during inflammation. (a) ILC2s interact with mast cells through unknown ligand–receptor pair interactions and suppress mast cell activation through the secretion of IL-13. IL-13-primed mast cells produce lower levels of pro-inflammatory cytokines (IL-6 and TNF) in response to allergens. ILC2s may also stimulate mast cells to produce immunomodulatory cytokines (IL-10 and TGF-β) through unknown receptor and IL-13R signaling. (b) Allergens induce keratinocyte to produce TSLP which results in the production of IL-4, IL-5, IL-9 and IL-13 effector cytokines for allergic responses. ILC2s may affect CD4+ T-cell differentiation through Th2 cytokine secretion. Cytokines (IL-2 and IL-9) of CD4+ T cells can induce ILC2s to produce Th2 cytokines. (c) ILC2s may uptake antigens and present them to CD4+ T cells in the context of MHC class II–peptide complex. If this occurs in the absence of costimulation, tolerogenic CD4+ T cells may be generated. ILC2, type 2 innate lymphoid cell.
The observations by Roediger et al.2 indicate that ILC2s having regulatory functions in the naive state can mediate skin inflammation after exposure to allergens. As mentioned previously, TSLP is the major ILC2 stimulator in the skin during the progression of AD-like disease, presumably as keratinocytes and other skin-resident cells do not produce IL-25 and IL-33 in response to allergens (Figure 1b). Although ILC2s alone are sufficient to induce type 2 inflammation, they may critically affect Th2 T-cell responses by instructing their differentiation through the secretion of IL-4, IL-5, IL-6 and IL-13 (Figure 1b). Given that ILC2s express MHC class II8 and that their numbers are increased in the draining lymph node during skin inflammation,6 ILC2s may regulate CD4+ T-cell activation through the antigen presentation in the priming phase or during the effector response. Indeed, this type of regulation has been reported in ILC3s:12 antigen presentation of ILC2s lacking appropriate costimualtory ligands results in the generation of tolerogenic CD4+ T cells.12 It will be interesting to investigate whether ILC2s have a similar mechanism that is crucial for the negative regulation of CD4+ T cells (Figure 1c).
Recently, it has been clear that lineage marker-negative innate cells derived from common lymphoid progenitors can be divided into three groups based upon their ability to produce Th1, Th2 and Th17 cytokines. Allergens and parasites can mount both ILC2 and Th2 responses. In a similar context, antigens that induce ILC1 or ILC3 responses may preferentially mount Th1 or Th17 responses, respectively. This type of effector mechanism has a merit in that if a first line of defense against antigens by ILCs is successful, it can prevent the initiation of adaptive Th responses. Then do regulatory ILCs corresponding to Foxp3+CD4+ T cells exist? Or is there a mechanism of cross-regulation of one ILC subset to another subset? These issues will be an exciting area to be solved in near future.
Acknowledgments
Byungsuk Kwon's laboratory is supported by a grant (BRL 2009-0087350) from the National Research Foundation of Korea.
References
- Palm NW, Resenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waler JA, Barlow JL, McKenizie NJ. Innate lymphoid cells—how did we miss them. Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm C, Keiji H, Stieglitz B, van Snick J, Tolaini M, Lahl K, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- St John AL, Abraham SN. Innate immunity and its regulation by mast cells. J Immunol. 2013;190:4458–4463. doi: 10.4049/jimmunol.1203420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. . Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]