Regulation of immune responses is central to an effective clearance of pathogens. An effective immune response is also necessary for preventing the development of cancer and autoimmune diseases and for maintaining homeostasis. Although the thymus is the central lymphoid organ that regulates immune responses for self-tolerance during the maturation of T cells, regulatory immune cells are still required for the proper functioning of mature immune cells in the periphery. Regulatory cells are a subpopulation of immune cells that suppress proliferation and cytokine production by other immune cells in response to antigenic stimulation. Although cells with regulatory functions have been identified in almost all of the major immune cell populations, regulatory T cells are still the dominant and most intensely studied type. Several types of regulatory T cells have been identified, including CD4+CD25+ T regulatory cells (Tregs), which are also positive for Foxp3, the master regulator of Treg development.1
Tregs are the most prominent regulatory cell type with established functions. Natural Tregs develop in the thymus, and inducible or adaptive Tregs develop in the periphery. The use of Tregs is potentially an attractive therapeutic option in the clinical management of autoimmune diseases or rejection of organ transplantation. However, a lack of stable and specific markers2 and a lack of antigen specificity has prevented the development of this cell type into a safe and effective cellular therapeutic. Therefore, the identification of novel Tregs that have manageable markers and antigen specificity has important theoretical and practical implications. The number of newly identified Tregs has been increasing.3 Bandala-Sanchez et al.4 reported in the May nineteenth issue of Nature Immunology the identification of a novel pancreatic islet autoantigen-specific CD4+ Treg that expressed high levels of cell surface marker CD52. Soluble CD52 was also used by the cell as an effector molecule for suppression.4 Therefore, the CD52 molecule is both a surface marker and an effector molecule of this novel regulatory T-cell subset.
This group had previously reported the generation of human CD4+ regulatory T-cell clones against the pancreatic islet autoantigen glutamic acid decarboxylase (GAD65). These regulatory T-cell clones did not share markers (CD25 and Foxp3) or inhibitory mechanisms with typical Tregs.5 In the current study, they demonstrated that the suppressive clones differed from non-suppressive clones through increased expression of CD52. Bandala-Sanchez et al. first performed a solid-phase antibody array. They then confirmed analysis of individual clones by flow cytometry. To confirm that high CD52 identified suppressive antigen-specific T cells in the peripheral blood mononuclear cells (PBMC) of healthy donors, carboxyfluorescein diacetate succinimidyl ester-labeled PBMC were stimulated with GAD65 for 7 days. Single-cell clones were generated from T cells separated into four groups based on the expression levels of CD52 (top 5, 10, 20 or bottom 80%) and tested for suppressive functions. Out of a total of 327 clones generated, only 29 were suppressive. Twenty-four clones (83%) were in the top 10% of CD52 high expressing CD4+ T cells. These T-cell clones were defined as regulatory CD52hiCD4+ T cells, and the rest of the non-suppressive clones with low CD52 as CD52loCD4+ T cells.
This finding was further corroborated by experiments that directly demonstrated the suppressive effect of CD52hiCD4+ T cells, but not CD52loCD4+ T cells, from GAD65-stimulated PBMC that were co-incubated with tetanus toxoid-specific human responder T cells labeled with carboxyfluorescein diacetate succinimidyl ester. The proliferation of T cells specific for tetanus toxoid was inhibited by CD52hi but not by CD52lo GAD65-specific CD4+ T cells. Additionally, GAD65-responsive CD52loCD4+ T cells produced low levels of IFN-γ in the presence of CD52hiCD4+ T cells from the same donor, and depletion of CD52hi cells from PBMC increased the antigen-stimulated proliferation of residual T cells. Expression of CD25 and Foxp3, prototypic markers for Tregs, was not found on this novel regulatory T-cell population, and depletion of CD25+ T cells from PBMC did not affect the generation of CD52hiCD4+ suppressive T cells. Importantly, the Foxp3 locus was highly methylated in CD52hiCD4+ T cells, suggesting that the Foxp3 gene in CD52hiCD4+ T cells was inactive and that this population likely did not originate from CD25+CD4+ Tregs. Thus, these results collectively suggested the identification of a novel T-cell population with regulatory activity.
The physiological significance of these novel regulatory T cells was supported by data from both human and mouse studies. Human studies demonstrated that the number of CD52hiCD4+ T cells specific for GAD65, but not for tetanus toxoid, in PBMC from preclinical and type 1 diabetic patients, was lower than that in PBMC from healthy donors or type 2 diabetic patients. The CD52hiCD4+ T cells from healthy donors, but not from preclinical and diabetic patients, were suppressive after reactivation by either tetanus toxoid or GAD65. Additionally, CD52hiCD4+ T cells from diabetic patients were suppressive only in response to tetanus toxoid, suggesting an antigen-specific reduction in the activity of this regulatory T cell in preclinical and clinical autoimmune disease. This finding was further confirmed by studies using a mouse model of type 1 diabetes. There was an enhanced onset of diabetes in non-obese diabetic (NOD)–severe combined immunodeficiency mice after an adoptive transfer of splenic cells depleted the CD52hiCD4+ T cells from 8-week-old NOD mice. This result was also observed with irradiated young NOD mice that received an adoptive transfer of splenic cells that lacked CD52hiCD4+ T cells from 8-week-old NOD mice, suggesting that CD52hiCD4+ T cells inhibit pathogenic effector T cells in NOD mice regardless of age. Together, these results established a role for CD52hiCD4+ regulatory T cells in the prevention of autoimmune diseases, despite the fact that the association of changes in the quantity and/or quality of CD52hiCD4+ T cells with disease severity remains to be addressed.
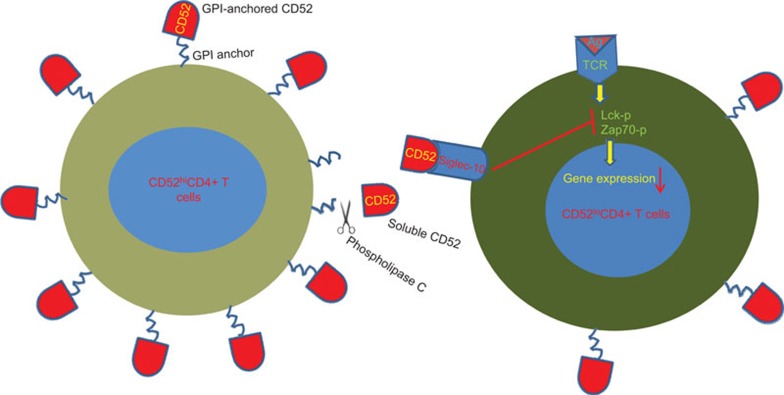
The transwell experiments with a 0.2-µm filter that separates regulatory cells from responder cells but allows soluble molecules to move freely from one side of the filter to the other suggested that the suppressive action of this regulatory cell is independent of cell–cell contact. Instead, it is likely mediated by soluble factors. After ruling out all of the known soluble factors required for the suppressive effect of CD25+CD4+ Tregs, including IL-10 and TGF-β, CD52 was examined as a potential effector molecule because CD52 is a glycosylphosphatidylinositol-anchored cell surface molecule. CD52 can be released into cell culture supernatants after cleavage by phospholipases. This action was confirmed by the presence of cell-free CD52 in the culture supernatants, which were free from exosomes or membrane particles, and was consistent with earlier reports that demonstrated the existence of soluble CD52 in the plasma.6 The release of soluble CD52 was inhibited by a phospholipase C inhibitor. Interestingly, this inhibitor also reversed the suppressive effect of CD52hiCD4+ T cells without affecting the viability of CD52hi or CD52loCD4+ T cells. The monoclonal antibody, CF1D12, which targets the terminal oligosaccharide moiety of CD52, blocked the suppressive activity of CD52hiCD4+ T cells by neutralizing soluble CD52 and enhancing the proliferative response of PBMC to tetanus toxoid and GAD65.
These results demonstrate a potential therapeutic option for the manipulation of regulatory T cells for the purpose of activating effector immune cells against cancer cells or infection. Interestingly, recombinant CD52 fused with Ig Fc inhibited T-cell responses to T-cell receptor (TCR) stimulation but not to phorbol ester PMA plus ionomycin, suggesting that CD52 targets proximal TCR signaling. This finding was further supported by data demonstrating that CD52 inhibits phosphorylation of Lck and Zap70, two critical kinases proximal to the TCR signaling pathways that are required for activation of T cells in response to antigenic stimulation.
The treatment of recombinant CD52 with endoglycosidase PNGase F, which cleaves the oligosaccharide adjacent to its N-linkage, abolished the suppressive activity of CD52hiCD4+ T cells, and a synthetic extracellular CD52 peptide with no carbohydrate modification had no effect on T cells. Furthermore, digestion of CD52-Fc with neuraminidase to remove the terminal sialic acids also abrogated the suppressive activity of CD52-Fc. Together, these data suggest that the terminal carbohydrate modification of the extracellular core of CD52 is required for T-cell suppression. The relevance of this mechanism in the manipulation of these Tregs during infection by pathogenic microorganisms that are equipped with such enzymes remains to be addressed.
Because sialoside structures, like CD52, are known to be recognized by cell surface sialic acid-binding immunoglobulin-like lectin (Siglec) proteins, which are the inhibitory receptors of the Ig superfamily and have two cytoplasmic immunoreceptor tyrosine-based inhibition motifs, the authors examined the role of Siglec-10 in the suppressive effect of CD52hiCD4+ T cells. Blocking Siglec-10 with an antibody against the extracellular domain of Siglec-10 or through a soluble Siglec-10-Fc fusion protein, reversed the inhibitory effect of CD52hiCD4+ T cells. Thus, a novel ligand for the suppressive action of CD52 on this novel regulatory subset was identified (Figure 1).
Figure 1.
CD52hiCD4+ T cells inhibit CD52loCD4+ T-cell activation through the release of cell surface CD52. GPI-anchored, membrane-bound CD52 core molecules from CD52hiCD4+ T cells are released by phospholipase C cleavage. Free CD52 interacts with Siglec-10 on responder CD52loCD4+ T cells and inhibits the activation of the responder cells by blocking Lck and Zap70 phosphorylation. GPI, glycosylphosphatidylinositol; Siglec, sialic acid-binding immunoglobulin-like lectin.
CD52 is only 12-amino acid long, and it is a heavily glycosylated glycopeptide that is tethered into the cell membrane through a glycosylphosphatidylinositol anchor (Figure 1).7 This peptide is abundantly expressed on all human lymphocytes and male reproductive tissues, including mature sperm cells. Alemtuzumab, a humanized monoclonal antibody against CD52, has been widely used for the depletion of T cells to prevent graft-versus-host diseases in bone marrow transplantation and in the treatment of lymphoma.8 Interestingly, CD52 was also shown to be costimulatory for the induction of adaptive Tregs.9 Phospholipase is required for the cleavage and release of soluble CD52, but its preference for cleaving elevated versus normally expressed CD52 on T cells remains to be addressed. It is also unknown how Tregs replenish CD52 after cleavage to maintain their inhibitory functions.
One might hypothesize that the cleavage and release of CD52 from normal cells may provide an inhibitory function in this setting. It is possible that the loss of CD52 may render regulatory T cells into conventional T cells at least transiently, and this transition may regulate the feedback mechanism for the regulatory cells. Inhibitors of phospholipase C may have a therapeutic effect by targeting immunosuppressive T cells. This action may release the suppression of effector memory cells, resulting in a defense against cancer cells or pathogenic microorganisms. Another interesting question that remains to be addressed is why there were no CD25+CD4+ Tregs identified in the suppressive T-cell clones, despite previous studies demonstrating that CD4+CD25+ Tregs play a critical role in the prevention of type 1 diabetes.10,11 A possible explanation is that the GAD65 antigen may preferentially activate non-conventional Tregs. It was previously reported that GAD65-specific TCR transgenic T cells prevented the development of diabetes in NOD mice in a CD4+CD25+ T cell-independent manner.12 Thus, this group identified a novel T-cell population with regulatory functions, and this study may lead to novel therapeutic options for autoimmune diseases, cancer and infectious diseases.
Acknowledgments
This work was supported in part by grants (AI099345 and AI097667) from the National Institutes of Health, USA.
References
- Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai V, Karandikar NJ. Human regulatory T cells: a unique, stable thymic subset or a reversible peripheral state of differentiation. Immunol Lett. 2007;114:9–15. doi: 10.1016/j.imlet.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Bandala-Sanchez E, Zhang YX, Reinwald S, Dromey JS, Lee B, Qian JY, et al. T cell regulation mediated by interaction of soluble CD52 with the inhibitory receptor Siglec-10. Nat Immunol. 2013;14:741–748. doi: 10.1038/ni.2610. [DOI] [PubMed] [Google Scholar]
- Dromey JA, Lee BH, Yu H, Young HE, Thearle DJ, Jensen KP, et al. Generation and expansion of regulatory human CD4+ T-cell clones specific for pancreatic islet autoantigens. J Autoimmun. 2011;36:47–55. doi: 10.1016/j.jaut.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Albitar M, Do KA, Johnson MM, Giles FJ, Jilani I, O'Brien S, et al. Free circulating soluble CD52 as a tumor marker in chronic lymphocytic leukemia and its implication in therapy with anti-CD52 antibodies. Cancer. 2004;101:999–1008. doi: 10.1002/cncr.20477. [DOI] [PubMed] [Google Scholar]
- Xia MQ, Hale G, Lifely MR, Ferguson MA, Campell D, Packman L, et al. Structure of the CAMPATH-1 antigen, a glycosylphosphatidylinositol-anchored glycoprotein which is an exceptionally good target for complement lysis. Biochem J. 1993;293 Pt 3:633–640. doi: 10.1042/bj2930633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treumann A, Lifely MR, Schneider P, Ferguson MA. Primary structure of CD52. J Biol Chem. 1995;270:6088–6099. doi: 10.1074/jbc.270.11.6088. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Masuyam J, Sohma Y, Inazawa H, Horie K, Kokima K, et al. CD52 is a novel costimulatory molecule for induction of CD4+ regulatory T cells. Clinl immunol. 2006;120:247–259. doi: 10.1016/j.clim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Hao J, Zhang Y, Tian L, Yi H, O'Brien TD, et al. Upregulating CD4+CD25+FOXP3+ regulatory T cells in pancreatic lymph nodes in diabetic NOD mice by adjuvant immunotherapy. Transplantation. 2009;87:198–206. doi: 10.1097/TP.0b013e3181933261. [DOI] [PubMed] [Google Scholar]
- Tarbell KV, Lee M, Ranheim E, Chao CC, Sanna M, Kim SK, et al. CD4+ T cells from glutamic acid decarboxylase (GAD)65-specific T cell receptor transgenic mice are not diabetogenic and can delay diabetes transfer. J Exp Med. 2002;196:481–492. doi: 10.1084/jem.20011845. [DOI] [PMC free article] [PubMed] [Google Scholar]