Abstract
Aim:
To investigate the effect of ginsenoside Rg1 on the migration, adhesion, proliferation, and VEGF expression of endothelial progenitor cells (EPCs).
Methods:
EPCs were isolated from human peripheral blood and incubated with different concentrations of ginsenoside Rg1 (0.1, 0.5, 1.0, and 5.0 μmol/L) and vehicle controls. EPC migration was detected with a modified Boyden chamber assay. EPC adhesion was determined by counting adherent cells on fibronectin-coated culture dishes. EPC proliferation was analyzed with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In vitro vasculogenesis was assayed using an in vitro vasculogenesis detection kit. A VEGF-ELISA kit was used to measure the amount of VEGF protein in the cell culture medium.
Results:
Ginsenoside Rg1 promoted EPC adhesion, proliferation, migration and in vitro vasculogenesis in a dose- and time-dependent manner. Cell cycle analysis showed that 5.0 μmol/L of ginsenoside Rg1 significantly increased the EPC proliferative phase (S phase) and decreased the resting phase (G0/G1 phase). Ginsenoside Rg1 increased vascular endothelial growth factor production.
Conclusion:
The results indicate that ginsenoside Rg1 promotes proliferation, migration, adhesion and in vitro vasculogenesis.
Keywords: endothelial progenitor cells, ginsenoside Rg1, proliferation, migration, angiogenesis
Introduction
Endothelial progenitor cells (EPCs) have the ability to circulate, proliferate and differentiate into mature endothelial cells, but have not yet acquired characteristic mature endothelial cell markers or formed a lumen1. Reduced numbers of EPCs reflect an impaired endogenous repair capacity and predict future cardiovascular events2, 3. Therefore, stimulation of mobilization and differentiation of EPCs may provide a novel therapeutic strategy for improvement of postnatal neovascularization and re-endothelialization in coronary heart disease (CHD) patients. Ricousse-Roussanne et al4 demonstrated that progenitors of both endothelial cells and smooth muscle cells were present in cord blood and could differentiate into mature endothelial and smooth muscle cells. In terms of cardiovascular diseases, the increase in the number of EPCs could be a potential benefit. Ginseng, the root of Panax ginseng CA Meyer, has been used for thousands of years in traditional Chinese medicine. Its name is derived from the Greek pan (all) and akos (healing)5. With the development of modern technology, more and more active ingredients have been isolated and purified from the herb. Ginsenoside Rg1 is panaxtriol with two sugars. The activity of ginseng extract has been studied extensively. Ginsenoside Rg1 is an active ingredient commonly found in ginseng root. Of more than 30 different ginsenosides, ginsenoside Rg1 is among the most abundant and active ingredients6. Ginsenoside Rg1, a stable angiogenic agent, successfully enhanced myocardial perfusion and preserved infarcted left ventricle function7. Following treatment with ginsenoside Rg1, the number of CD34+ cells increased significantly in peripheral blood, and the ability of bone marrow stem cells to repair infracted myocardium increased in rats with ischemic myocardia. Ginsenoside Rg1 was demonstrated to be a phytoestrogen that exerted estrogen-like activity without direct interaction with estrogen receptors in human breast cancer (MCF-7) cells8. Estrogen therapy was found to have a similar impact on the number of circulating EPCs9. Administration of estrogen could result in increased numbers of EPCs by anti-apoptotic effects9. Ginsenoside Rg1 exerts estrogen-like actions via ligand-independent activation of the ERalpha pathway10. In the present study, we aim to investigate the direct modulatory effect of ginsenoside Rg1 on the function of EPCs in vitro. We demonstrated that ginsenoside Rg1 promoted EPC proliferation, adhesion and angiogenesis. The results provide further insight into the possible therapeutic use of ginsenoside Rg1 for re-endothelialization and induction of angiogenesis, such as in atherosclerosis, restenosis and tissue regeneration.
Materials and methods
Isolation of mononuclear cells and cell culture
EPCs were cultured according to a previously described technique11, 12. Briefly, peripheral blood mononuclear cells (MNCs) were isolated from healthy volunteers by Ficoll density gradient centrifugation with Histopaque 1077 (Sigma, St Louis, MO, USA). Written informed consent was obtained from all volunteers involved in the study. None of the volunteers had risk factors for coronary heart disease, and they were free of wounds, inflammation, ulcers and malignant disease. All the procedures were performed in accordance with national and international laws and policies. Cells were plated on 6-well culture plates coated with human fibronectin (2 μg/cm2; Millipore Corporation, USA & Canada) and maintained in Medium 199 (M199; Sigma, USA) supplemented with 20% fetal bovine serum (FBS; GIBCO16000, USA), vascular endothelial growth factor (VEGF; 10 μg/L; Peprotech Inc, USA), bovine brain extract (12 μg/mL; Sigma, USA), penicillin (100 U/mL), and streptomycin (100 μg/mL). After 4 days of culture, non-adherent cells were removed by washing with phosphate-buffered saline (PBS). New medium was added and the cells were cultivated for 3 days. Before experiments, cells had been deprived of serum for 24 h. The solution of ginsenoside Rg1 (purity >98%, the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China) was prepared in sterile double distilled H2O. To demonstrate a concentration-dependent effect of ginsenoside Rg1 on EPCs, cells were incubated with different concentrations of ginsenoside Rg1 (0.1, 0.5, 1.0, and 5.0 μmol/L) and vehicle control for 24 h. Attached cells were stimulated with 5.0 μmol/L ginsenoside Rg1 for 6, 12, 24 and 48 h to determine the time-course of reaction.
Human EPC staining and identification
After 7 d in culture, fluorescent chemical detection of EPCs was performed on attached MNC. Direct fluorescent staining was used to detect dual binding of fluorescein isothiocyanate (FITC)-labeled Ulex europaeus agglutinin (UEA-1; Sigma, USA) and dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (Dil)-labeled acetylated low-density lipoprotein (Dil-acLDL; Molecular Probes, USA). Cells were first incubated with Dil-acLDL at 37 °C for 1 h and later fixed with 2% paraformaldehyde for 10 min. After being washed, the cells were treated with UEA-1 (10 μg/mL) for 1 h. Following staining, samples were then viewed with an inverted fluorescent microscope (Leica, Germany) and a laser scanning confocal microscope (LSCM; Leica, Germany). Cells that were doubly fluorescent were identified as differentiating EPC13, 14, 15. Two or three independent investigators evaluated the number of EPCs per well by counting 15 randomly selected high-power fields (×200) with an inverted fluorescent microscope. The cells were further analyzed by immunostaining with goat polyclonal anti-CD31 antibody (Santa Cruz Biotechnology, USA) and goat polyclonal anti-von Willebrand factor antibodies (vWF; Sigma, USA). The cells were plated on 6-well chamber slides for 7 d, then washed and fixed with acetone at 4 °C for 10 min. The cells were blocked with 5% horse serum for 30 min and incubated overnight at 4 °C in 1:100 dilutions of goat polyclonal anti-vWF and CD31 antibodies, followed by incubation with horseradish peroxidase-conjugated anti-goat IgG for an additional 30 min. The slides were visualized with DAB-based color development.
Migration assay
EPC migration was evaluated using a modified Boyden chamber assay. Briefly, isolated EPCs were detached using 1 mmol/L EDTA in PBS, harvested by centrifugation at 400×g for 10 min at room temperature, resuspended in 500 μL M199 and counted. Then, 5×104 EPCs were placed in the upper chamber of a modified Boyden chamber (Jiangsu Qilin Medical Equipment Factory, China). M199 and human recombinant VEGF (50 ng/mL) were placed in the lower compartment of the chamber. After a 24 h incubation at 37 °C, the lower side of the filter was washed with PBS and fixed with 2% paraformaldehyde. For quantification, cells were stained with Giemsa solution. Cells migrating into the lower chamber were counted manually in three random microscopic fields (×200)14, 15.
Cell adhesion assay
EPCs were washed with PBS and gently detached with 0.25% trypsin. After centrifugation at 225×g for 10 min at room temperature and resuspension in M199 with 5% FBS, identical cell numbers were replated onto fibronectin-coated culture dishes and incubated for 30 min at 37 °C. Adherent cells were counted manually in five random microscopic fields (×200) by independent blinded investigators16.
EPC proliferation assay
The effect of ginsenoside Rg1 on EPC proliferation was determined by 3-(4,5-dimethyl-2thiazol)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. EPCs cultured for 7 d were digested with 0.25% trypsin and then cultured in serum-free medium in 96-well culture plates (200 μL per well) to which different concentrations of ginsenoside Rg1 were added (n=6 wells/concentration). The serum-free medium served as a vehicle control. After being cultured for 24 h, EPCs were supplemented with 10 μL MTT (5 g/L; Ameresco, Framingham, MA, USA) and incubated for another 4 h. Then, the supernatant was discarded by aspiration, and the EPC preparation was shaken with 200 μL DMSO for 10 min, before the optical density (OD) value was measured at 490 nm.
In vitro vasculogenesis assay
The in vitro vasculogenesis assay was performed with the in vitro Angiogenesis Assay Kit (Chemicon International, USA & Canada), according to the manufacturer's instructions. Briefly, ECMatrix™ solution was thawed on ice overnight, then mixed with 10× ECMatrix™ diluent and placed in a 96-well tissue culture plate at 37 °C for 1 h to allow the matrix solution to solidify. EPCs were harvested as described above and replated (1×104 cells per well) on top of the solidified matrix solution. Cells were grown with ginsenoside Rg1 or vehicle control and incubated at 37 °C for 24 h. Tubule formation was inspected under an inverted light microscope at 200×magnification. Tubule formation was defined as development of a structure with a length at least 4 times its width17, 18. Five independent fields were assessed for each well, and the average number of tubules per 200×field was determined.
Cell cycle analysis
EPCs were treated with ginsenoside Rg1 for 24 h, and cells were isolated into conical tubes, washed twice with PBS and fixed in 75% ice-cold ethanol. For DNA analysis, cells were centrifuged at 400×g for 10 min at room temperature and washed twice with PBS. After incubation at 37 °C in the dark for 30 min, the DNA content of the nuclei was determined by staining nuclear DNA with propidium iodide solution (50 μg/mL, sigma, USA) containing 50 μg/mL ribonuclease A. The DNA content was measured by a flow cytometer (Becton Dickinson, USA).
Measurement of VEGF protein in the culture medium
To assess secretion of the vascular endothelial growth factor (VEGF) protein, EPCs (1×106) treated with or without ginsenoside Rg1 were switched at d 14 to growth factor-free medium M199 with 5% FBS for 24 h. VEGF protein in the cell culture medium was measured using a commercially available VEGF-Elisa kit (R&D systems, Minneapolis, MN, USA), according to the manufacturer′s instructions. The assay system has no cross-reactivity with platelet-derived growth factor and basic fibroblast growth factor.
Statistical analysis
Data analysis was performed using the Statistical Package for Social Science (SPSS for Windows, version 10.0, 1999, SPSS Inc, Chicago, USA). Data are expressed as mean±SD. We used one-way ANOVA and the independent-samples t-test to analyze the differences in variables. The differences were considered significant if P<0.05.
Results
Characteristics of human EPC
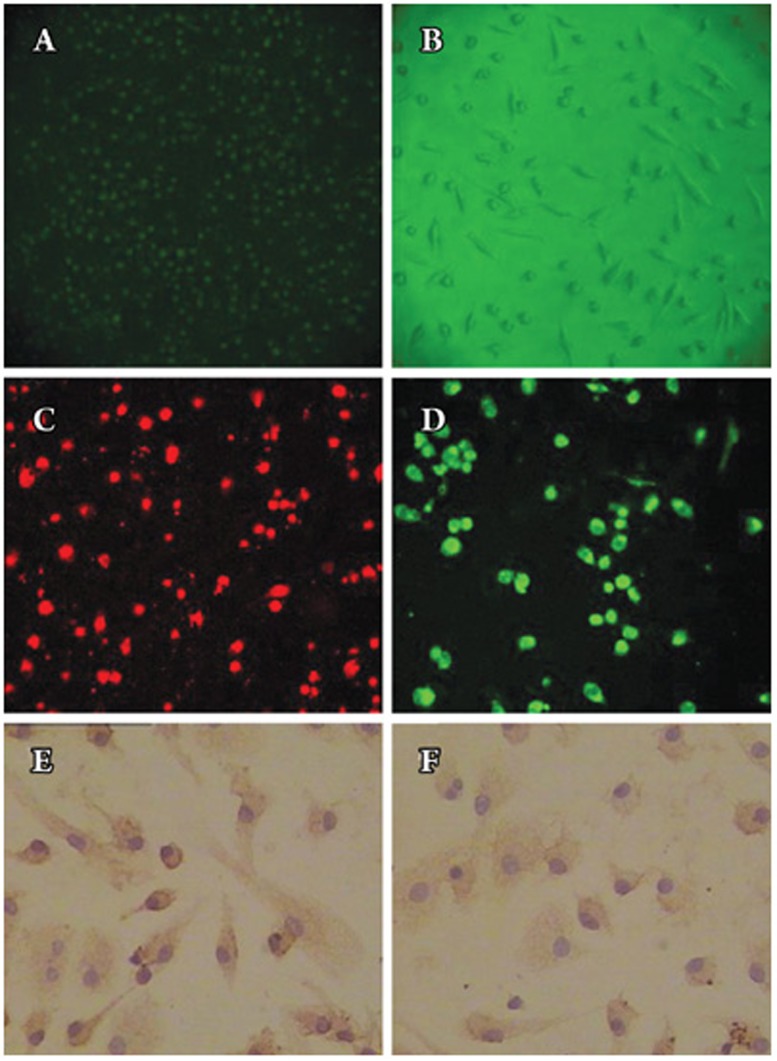
Mononuclear cells isolated from peripheral blood were immediately plated onto culture plates pre-coated with human fibronectin. They appeared small and round under an inverted light microscope (Figure 1A). After 7 days in culture, attached cells exhibited a spindle-shape, with an endothelial cell-like morphology (Figure 1B). The cells tested for DiI-labeled acetylated low-density lipoprotein (DiLDL) uptake, lectin binding and double labeling were stained. Double labeling cells represented about 80% of the culture cells. EPCs were characterized as adherent cells that were double-positive for DiLDL-uptake and lectin binding under a laser scanning confocal microscope (Figure 1C–1D). Immunohistochemistry showed that the cells were positive for endothelial cell surface marker antibodies, such as vWF and CD31 (Figure 1E–1F).
Figure 1.
Characterization of endothelial progenitor cell (EPC) number. (A) Mononuclear cells isolated from human peripheral blood appeared small and round under an inverted light microscope (original magnification×200). (B) After 7 days in culture, attached cells exhibited a spindle-shaped, endothelial cell-like morphology (original magnification×100). (C) DiLDL uptake (red, exciting wave-length 543 nm, original magnification×200) and (D) Adherent cells, lectin binding (green, exciting wave-length 477 nm, original magnification×200), were assessed under a laser scanning confocal microscope and by fluorescent microscopy. The cells were further analyzed by immunostaining with (E) vWF antibodies (original magnification×400) and (F) anti-CD31 (original magnification×400).
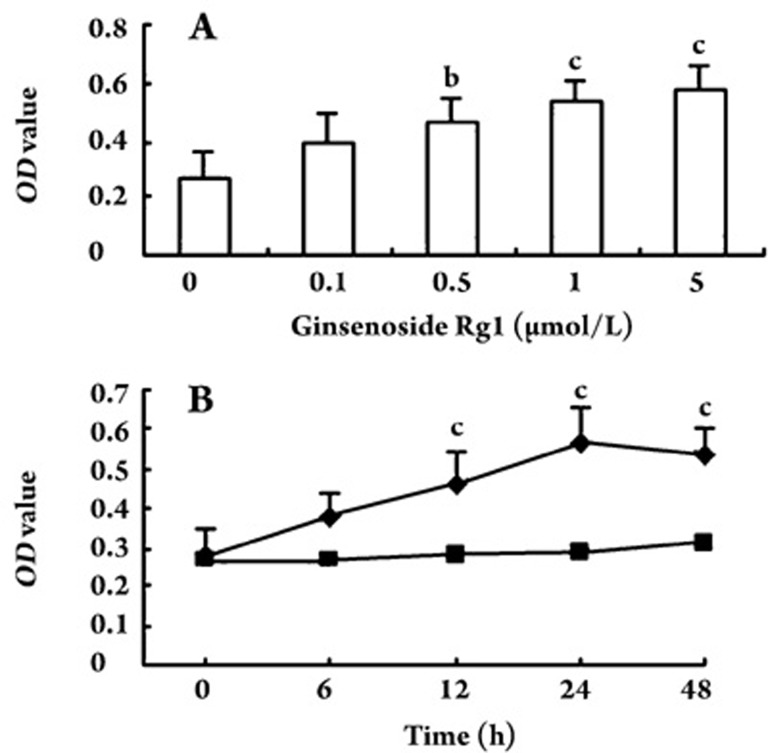
Effect of ginsenoside Rg1 on EPC proliferation
The MTT assay was used to demonstrate the effect of ginsenoside Rg1 on EPC proliferation. Incubation of isolated human MNCs with ginsenoside Rg1 increased EPC proliferative capacity in a concentration- and time-dependent manner (Figure 2). In dose-dependent experiments, the EPC proliferative activity was greatest at 5.0 μmol/L ginsenoside Rg1 [0.27±0.09 vs 0.58±0.08 (light absorbance at 490 nm) for vehicle control and 5.0 μmol/L ginsenoside Rg1, respectively; P<0.01; Figure 2A]. In time-course experiments with EPCs treated with 5.0 μmol/L ginsenoside Rg1, increases in EPC proliferation became apparent after 12 h (P<0.01) and reached the maximum at 24 h (P<0.01; Figure 2B).
Figure 2.
Effect of ginsenoside Rg1 on endothelial progenitor cell (EPC) proliferation (490 nm light absorbance) in dose- and time-course experiments. EPCs incubated with different concentrations of ginsenoside Rg1 dose-dependently increased proliferation (A); EPCs incubated with 5.0 μmol/L ginsenoside Rg1 time-dependently increased proliferation of EPCs (B). Increase of EPC proliferative activity became apparent after 12 h and reached the maximum at 24 h; ▪ vehicle control, ♦ 5.0 μmol/L ginsenoside Rg1; OD, optical density, light absorbance at 490 nm. Data are presented as the mean±SD. n=5. bP<0.05, cP<0.01 compared with vehicle control.
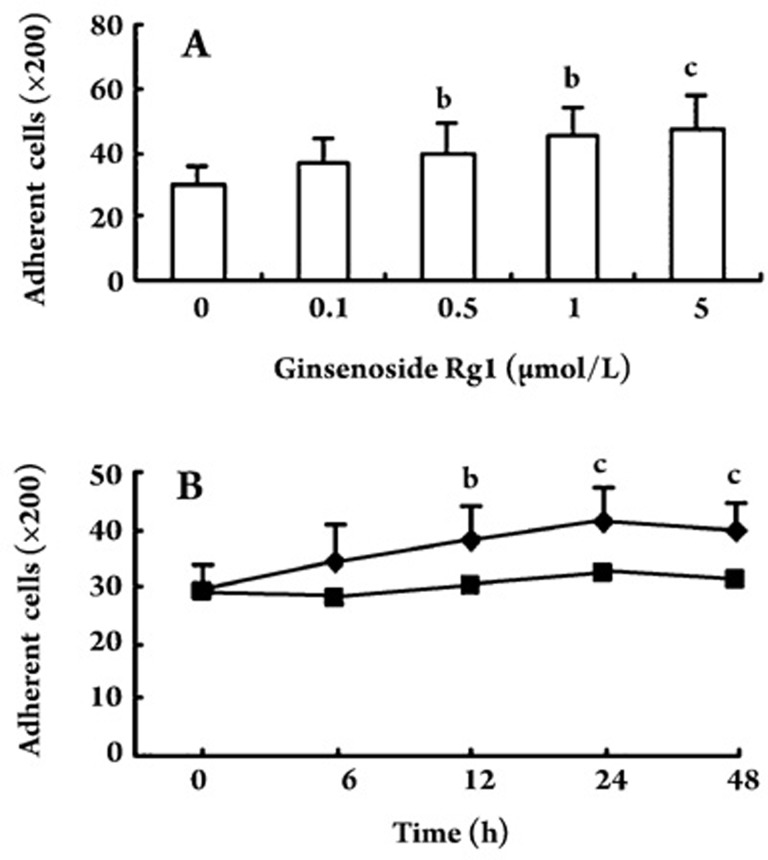
Effect of ginsenoside Rg1 on EPC adhesiveness
EPCs were incubated with different concentrations (0, 0.1, 0.5, 1.0, and 5.0 μmol/L) of ginsenoside Rg1 for 24 h. EPCs were then placed on fibronectin-coated dishes for 30 min. EPCs pretreated with ginsenoside Rg1 exhibited a significant increase in the number of adhesive cells after 30 min. The change in the number of adhesive cells was dose-dependent with the maximal effect at 5.0 μmol/L ginsenoside Rg1. In the time-course study, the increase in EPC adhesive activity became apparent at 6 h and reached its maximum at 24 h (Figure 3).
Figure 3.
Effect of ginsenoside Rg1 on adhesion of endothelial progenitor cells (EPCs) in dose- and time-dependent experiments. In EPC cells treated with ginsenoside Rg1, there was a dose-dependent increase in the number of adhesive cells (A); In EPCs treated with ginsenoside Rg1 (5.0 μmol/L), there was a time-dependent increase in the number of adhesive cells (B). ▪ vehicle control, ♦ 5.0 μmol/L ginsenoside Rg1. Data are presented as the mean±SD. n=5. bP<0.05 , cP<0.01 compared with vehicle control.
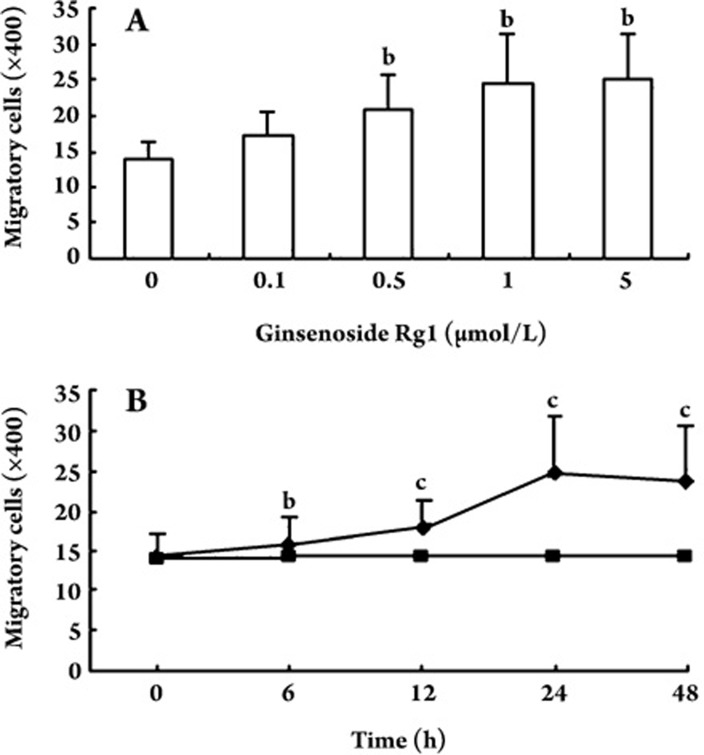
Effect of ginsenoside Rg1 on EPC migration EPCs were incubated with different concentrations of ginsenoside Rg1, and effects on EPC migratory activity were analyzed in a modified Boyden chamber assay. In dose-response experiments, ginsenoside Rg1 enhanced cell migration, and this effect was greatest at 5.0 μmol/L ginsenoside Rg1 (13.80±2.77 vs 25.20±6.14 for vehicle control and ginsenoside Rg1 5.0 μmol/L , respectively; P<0.05; Figure 4A). When EPCs were treated with 5.0 μmol/L ginsenoside Rg1, migratory activity was enhanced after 6 h (P<0.05) and reached a maximum at 24 h (P<0.01; Figure 4B).
Figure 4.
Effect of ginsenoside Rg1 on migratory activity of endothelial progenitor cells (EPCs) in dose- and time-course experiments using a modified Boyden chamber assay. In EPCs treated with ginsenoside Rg1, there was a dose-dependent increase in EPC migratory activity (A). In cells treated with 5.0 μmol/L ginsenoside Rg1, there was a time-dependent increase in EPC migratory activity (B). Enhanced EPC migratory activity became apparent after 6 h and reached its maximum at 24 h. ▪ vehicle control, ♦ 5.0 μmol/L ginsenoside Rg1. Data are presented as the mean±SD. n=5. bP<0.05, cP<0.01 compared with vehicle control.
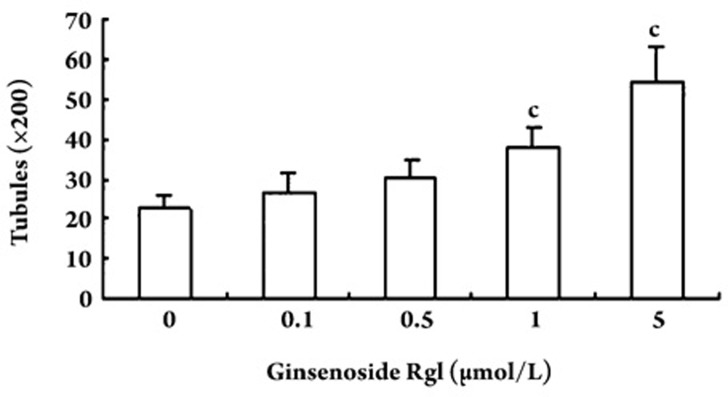
Effect of ginsenoside Rg1 on EPC vasculogenesis in vitro
An in vitro vasculogenesis assay kit was used to investigate the ability of EPCs to undergo neovascularization following ginsenoside Rg1 treatment. Tubule number increased in a concentration-dependent manner in response to Rg1 after 24 h of incubation (Figure 5). The peak tubule number resulted following treatment with 5.0 μmol/L ginsenoside Rg1, and the tubules were more complex than that in control cells.
Figure 5.
Effect of ginsenoside Rg1 on in vitro vasculogenesis of EPCs. EPCs were replated on top of the solidified matrix solution. Typical vascular tubes could be seen in some fields after 24-h incubation. EPCs incubated with ginsenoside Rg1 for 24-h exhibited a dose-dependent increase in the number of tubules, with a peak number at 5.0 μmol/L ginsenoside Rg1. Data are presented as the mean±SD. n=5. cP<0.01 compared with vehicle control.
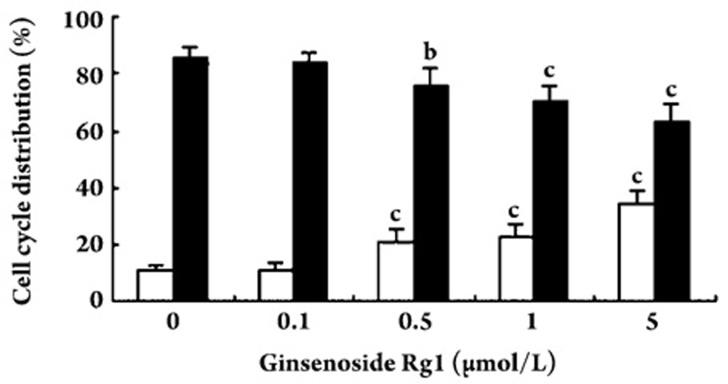
Effect of ginsenoside Rg1 on cell cycle
Effects of ginsenoside Rg1 on the cell cycle in EPCs were determined by flow cytometry. Ginsenoside Rg1 induced a significant increase in the proliferative phase (S phase) and decrease in the resting phase (G0/G1) of the cell cycle (Figure 6).
Figure 6.
Effect of ginsenoside Rg1 on cell cycle distribution. Endothelial progenitor cells (EPCs) were treated with ginsenoside Rg1 for 24 h. Flow cytometric analysis showed that ginsenoside Rg1 significantly increased the proportion of cells in S phase (□) and decreased the proportion of cells in the G0/G1 phase (▪). Data are presented as the mean±SD. n=5. bP<0.05, cP<0.01 compared with vehicle control.
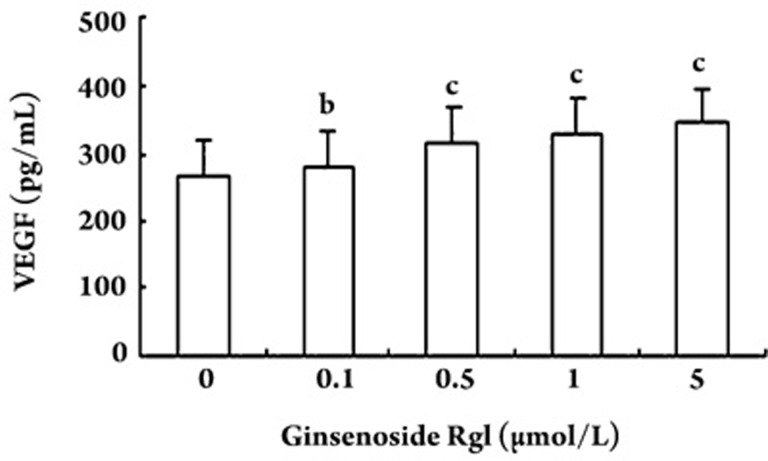
Effect of ginsenoside Rg1 on VEGF secretion
In the study, we evaluated the secretion of vascular endothelial growth factor (VEGF) by EPCs. The concentration of VEGF in the culture medium was greater following ginsenoside Rg1 treatment of EPCs when compared to untreated controls (Figure 7).
Figure 7.
Effect of different concentrations of ginsenoside Rg1 on vascular endothelial growth factor (VEGF) secretion. Secretion of VEGF by endothelial progenitor cells (EPCs) in the ginsenoside Rg1-treated group was significantly greater than that in vehicle control group. Data are presented as the mean±SD. n=5. bP<0.05, cP<0.01 compared with vehicle control.
Discussion
Endothelial progenitor cells (EPCs) play an important role in the regeneration of ischemic and damaged tissues via angiogenesis and repairing denuded endothelium in injured vessels13, 19, 20. It has been noted that EPCs contributed up to 25% of the endothelial cells in newly formed vessels21, 22. There is an inverse relationship between EPC number and family history of coronary artery disease23, age24, diabetes23, smoking25, and hypertension26. The number of EPCs is higher in subjects with low to moderate alcohol consumption . The mechanisms responsible for the increase in the number and activity of EPCs are not very clear. Standard cardiovascular drugs, such as statins and ACE inhibitor/angiotensin-receptor blockers, are capable of enhancing the number of EPCs27, 28, 29. Vascular endothelial growth factor (VEGF) is probably the most important angiogenesis inducer. It has the ability to regulate key steps in angiogenesis, including proliferation and migration of endothelial cells30. Over-expression of VEGF and its receptors promotes blood vessel formation, and VEGF inhibition blocks angiogenesis31. Leung KW et al showed that ginsenoside Rg1 promoted the synthesis of VEGF and hence angiogenic activity by activating the PI3K/Akt pathway32. Ginsenoside Rg1 could enhance nitric oxide production and the expression of eNOS mRNA in TNF-alpha stimulated HUVECs33. Ginsenoside Rg1 could mobilize more bone marrow stem cells to infarcted zones to differentiate to cardiomyocyte-like cells34. Accumulating evidence indicates the ability of blood EPCs to repair injured vessels of dying endothelial cells in animal models35. Thus, increasing the number of circulating EPCs by transplantation of hematopoietic stem cells or by injection of differentiated EPCs in vitro has been shown to improve neovascularization of ischemic hindlimbs36 and cardiac function37.
There are two major classes of ginsenosides: protopanaxatriols (Rg1, Rg2, Re, and Rf) and protopanaxadiols (Rb1, Rb2, Rc, and Rd). They both possess four trans-ring rigid steroid skeletons with a modified side-chain at C20, which is absent in estradiol8. Re and Rg1 may be a novel group of non-peptidic angiogenic agents with superior stability and may be used for the management of tissue regeneration38. BMSC proliferation stimulated by ginsenoside Rg1 can be completely blocked by ER antagonist ICI 182, 780, or ERα-specific antagonist methylpiperidinopyrazole, which indicates that ERα may be the key subunit that enables Rg1 to exert its action on BMSC39. Rg1 did not directly bind to ER or change the expression of ERα in BMSC, which was confirmed by RT-PCR and Western blotting39.
In this study, EPCs derived from human peripheral blood could differentiate into cells that display classical endothelial cell morphology and characteristics, such as the capacity to take up acetylated low-density lipoprotein and the expression of vWF and CD31. The present study shows that ginsenoside Rg1 could increase the number of EPCs and promote the proliferative, migratory and adhesive capacity of EPCs. The in vitro vasculogenesis capacity of EPCs was effectively elevated. Cell cycle analysis illustrated that the effect of ginsenoside Rg1 was achieved by stimulation of the G1/S transition in EPCs. Moreover, ginsenoside Rg1 increased EPC production of VEGF in a concentration-dependent manner. Ginsenoside Rg1 has been reported to trigger transcriptional activation of the glucocorticoid-responsive element-containing reporter gene, suggesting that ginsenoside Rg1 could activate the glucocorticoid receptor-240, 41, 42. In prior work, ginsenoside Rg1 has been demonstrated to promote functional neovascularization into a polymer scaffold in vivo, as well as the proliferation, chemoinvasion, and tubulogenesis of endothelial cell in vitro43. These data indicate that ginsenoside Rg1 can be used as a novel therapeutic modality for inducing angiogenesis.
In conclusion, ginsenoside Rg1 has direct effects on human EPCs in vitro. The results of the present study demonstrated that ginsenoside Rg1 promoted EPC proliferation, adhesion, migration and vasculogenesis. The levels of secreted VEGF were elevated after ginsenoside Rg1 treatment of EPCs, although the evidence did not indicate that all the effects of ginsenoside Rg1 on EPCs were because of VEGF. Further studies will determine whether the ginsenoside Rg1-induced EPC effects could be eliminated by removing the effect of VEGF. The findings provide important insight into the molecular and cellular mechanisms mediated by ginsenoside Rg1 in endothelial repair and protection against myocardial ischemia.
Author contribution
Ai-wu SHI designed and performed the research and wrote the paper; Xiao-bin WANG contributed new analytical tools and some reagents; Min-min ZHU analyzed data; Feng-xiang LU, Xiang-qing KONG and Ke-jiang CAO guided the work and revised the manuscript.
Acknowledgments
The authors gratefully acknowledge helpful support from Prof Chang-fen XU. We also thank Zhu-ping TANG and Bo FENG for excellent technical assistance.
References
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Kunz GA, Liang G, Cuculi F, Gregg D, Vata KC, Shaw LK, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J. 2006;152:190–5. doi: 10.1016/j.ahj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- Le Ricousse-Roussanne S, Barateaua V, Contreres JO, Boval B, Kraus-Berthier L, Tobelem G. Ex vivo differentiated endothelial and smooth muscle cells from human cord blood progenitors home to the angiogenic tumor vasculature. Cardiovasc Res. 2004;62:176–84. doi: 10.1016/j.cardiores.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Moldzio R, Saito H, Rausch WD. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004. 1021. pp. 41–53. [DOI] [PubMed]
- Wei HJ, Yang HH, Chen CH, Lin WW, Chen SC, Lai PH, et al. Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial injection in a rat model with infarcted myocardium. J Control Release. 2007;120:27–34. doi: 10.1016/j.jconrel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–9. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–65. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- Lau WS, Chan RY, Guo DA, Wong MS. Ginsenoside Rg1 exerts estrogen-like activities via ligand-independent activation of ERalpha pathway. J Steroid Biochem Mol Biol. 2008;108:64–71. doi: 10.1016/j.jsbmb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Saw Imanishi T, Hano T, Matsuo Y, Nishio I. Oxidized low-density lipoprotein inhibits vascular endothelial growth factor-induced endothelial progenitor cell differentiation. Clin Exp Pharmacol Physiol. 2003;30:665–70. doi: 10.1046/j.1440-1681.2003.03894.x. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JZ, Zhu JH, Wang XX, Zhu JH, Xie XD, Sun J, et al. Effects of homocysteine on number and activity of endothelial progenitor cells from peripheral blood. J Mol Cell Cardiol. 2004;36:233–9. doi: 10.1016/j.yjmcc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–46. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–7. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization. Exp Hematol. 2002;30:967–72. doi: 10.1016/s0301-472x(02)00867-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nishida M, Futami S, Fukino K, Amaki T, Aizawa K, et al. Neoendothelialization after peripheral blood stem cell transplantation in humans. A case report of a Tokaimura nuclear accident victim. Cardiovasc Res. 2003;58:487–92. doi: 10.1016/s0008-6363(02)00780-0. [DOI] [PubMed] [Google Scholar]
- George J, Herz I, Goldstein E, Abashidze S, Deutch V, Finkelstein A, et al. Number and adhesive properties of circulating endothelial progenitor cells in patients with instent restenosis. Arterioscler Thromb Vasc Biol. 2003;23:e57–60. doi: 10.1161/01.ATV.0000107029.65274.db. [DOI] [PubMed] [Google Scholar]
- Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–80. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, et al. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–7. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens. 2005;23:1831–7. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI3-kinase/Akt pathway. J Clin Invest. 2001;108:391–7. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyridopoulos I, Haendeler J, Urbich C, Brummendorf TH, Oh H, Schneider MD, et al. Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation. 2004;110:3136–42. doi: 10.1161/01.CIR.0000142866.50300.EB. [DOI] [PubMed] [Google Scholar]
- Levy BI. Beneficial effects of circulating progenitor endothelial cells activated by angiotensin receptor antagonists. Hypertension. 2005;45:491–2. doi: 10.1161/01.HYP.0000159196.10515.b5. [DOI] [PubMed] [Google Scholar]
- Blázquez C, González-Feria L, Alvarez L, Haro A, Casanova ML, Guzmán M. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004;64:5617–23. doi: 10.1158/0008-5472.CAN-03-3927. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Leung KW, Pon YL, Wong RN, Wong AS. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281:36280–8. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- Lü JP, Ma ZC, Yang J, Huang J, Wang SR, Wang SQ. Ginsenoside Rg1-induced alterations in gene expression in TNF-alpha stimulated endothelial cells. Chin Med J (Engl) 2004;117:871–6. [PubMed] [Google Scholar]
- Yang M, Chen GL, Chen C, Zhang Y, Yan CY. Therapeutic effects of ginsenoside Rg1 on rat with myocardial infarction. Chin J Integr Med Cardio/Cerebrovasc Dis. 2007;5:1075–7. [Google Scholar]
- Xu Q. Mouse models of arteriosclerosis: from arterial injuries to vascular grafts. Am J Pathol. 2004;165:1–10. doi: 10.1016/S0002-9440(10)63270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–36. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Yu LC, Chen SC, Chang WC, Huang YC, Lin KM, Lai PH, et al. Stability of angiogenic agents, ginsenoside Rg1 and Re, isolated from Panax ginseng: in vitro and in vivo studies. Int J Pharm. 2007;328:168–76. doi: 10.1016/j.ijpharm.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Lu XZ, Wang JH, Wu X, Zhou L, Wang L, Zhang XW, et al. Ginsenoside Rg1 promotes bone marrow stromal cells proliferation via the activation of the estrogen receptor-mediated signaling pathway. Acta Pharmacol Sin. 2008;29:1209–14. doi: 10.1111/j.1745-7254.2008.00874.x. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Chung E, Lee KY, Lee YH, Huh B, Lee SK. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol. 1997;133:135–40. doi: 10.1016/s0303-7207(97)00160-3. [DOI] [PubMed] [Google Scholar]
- Chung E, Lee KY, Lee YH, Lee YJ, Lee SK. Ginsenoside Rg1 down-regulates glucocorticoid receptor and displays synergistic effects with cAMP. Steroids. 1998;63:421–4. doi: 10.1016/s0039-128x(98)00043-9. [DOI] [PubMed] [Google Scholar]
- Leung KW, Cheng YK, Mak NK, Chan KK, Fan TP, Wong RN. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006;580:3211–6. doi: 10.1016/j.febslet.2006.04.080. [DOI] [PubMed] [Google Scholar]
- Liang HC, Chen CT, Chang Y, Huang YC, Chen SC, Sung HW. Loading of a novel angiogenic agent, ginsenoside Rg1 in an acellular biological tissue for tissue regeneration. Tissue Eng. 2005;11:835–46. doi: 10.1089/ten.2005.11.835. [DOI] [PubMed] [Google Scholar]