Abstract
Aim:
To investigate the synergistic action of L-carnitine (LC) and taurine (TAU) on the proliferation and osteoblastic differentiation of vascular smooth muscle cells (VSMCs).
Methods:
DNA and protein synthesis of VSMCs were assessed using scintillation counting. Alkaline phosphatase (ALP) activity and calcium content were determined to investigate the effects of LC and TAU on the osteoblastic differentiation and mineralization of VSMCs. TAU uptake by VSMCs was assayed. RNA interference was used to down-regulate the expression of the TAU transporter (TAUT) in rat VSMCs.
Results:
LC and TAU synergistically inhibited the proliferation and β-glycerophosphate (β-GP)-induced osteoblastic differentiation of VSMCs as evidenced by the decreased [3H]thymidine incorporation, ALP activity and calcium deposition. Furthermore, LC stimulated the TAU uptake and TAUT expression in VSMCs. Suppression of TAUT with short hairpin RNA (shRNA) abolished the synergistic action of LC and TAU in VSMCs.
Conclusion:
The synergistic inhibitory action of LC and TAU on the proliferation and osteoblastic differentiation of VSMCs is attributable to the up-regulation of TAUT expression and TAU uptake by LC.
Keywords: L-carnitine, taurine, vascular smooth muscle cells, proliferation, osteoblastic differentiation
Introduction
Atherosclerosis is one of the leading causes of cardiovascular disease. In human atherosclerosis cases, thickening of the vessel wall intima has been observed, and the constituents of atherosclerotic lesions, which are composed of lipid deposits, macrophages and vascular smooth muscle cells (VSMCs), have been described. VSMCs represent the principal mesenchymal cell in human atherosclerotic plaques1. The accelerated proliferation of VSMCs has been suggested to be a central and characteristic feature of atherogenesis2, 3, 4. Vascular calcification, a prominent feature of atherosclerosis, has been considered an organized and regulated process, similar to mineralization in bone tissue5, 6. The osteoblastic differentiation of VSMCs is currently considered responsible for the formation of vascular calcification.
Taurine (TAU; 2-aminoethanesulfonic acid) is a sulfur-containing free β-amino acid in mammals. Various physiological roles of TAU have been suggested, including calcium modulation, membrane stabilization, intracellular osmotic regulation and the regulation of protein phosphorylation7, 8, 9, 10, 11, 12. TAU is necessary for normal development, and its deficiency leads to defects in growth, tissue differentiation, and immune development. A worldwide epidemiological study revealed a strong inverse association between the levels of TAU excretion in a population and their mortality due to ischemic heart disease. This result suggested that TAU intake could be effective in preventing cardiovascular disease13. Murakami et al14 showed that TAU prevented the progression of atherosclerosis in rabbits, indicating its potential therapeutic role in atherosclerosis. Moreover, our previous study demonstrated that TAU transporter (TAUT) was expressed in VSMCs15. Furthermore, previous evidence suggested that TAU plays an important role in the function of VSMCs by inhibiting the proliferation and preventing the β-glycerophosphate (β-GP)-induced calcification of these cells16, 17, 18.
L-carnitine (LC), a trimethylated amino acid, roughly similar to choline in structure, is a cofactor required for the transformation of free long-chain fatty acids into acylcarnitines and for their subsequent transport into the mitochondrial matrix, where they undergo β-oxidation for cellular energy production19, 20. The conditions that appear to benefit from exogenous supplementation of LC include cardiovascular disease, anorexia, chronic fatigue, diphtheria, hypoglycemia, male infertility, muscular myopathies, Rett syndrome, dialysis, preterm infants and HIV-positive individuals21. Previous data documented that LC has a strong anti-atherogenic potential22, 23, 24, 25.
The present study was undertaken to determine whether LC and TAU can synergistically regulate VSMC proliferation and calcification and to examine the possible mechanisms.
Materials and methods
Materials
[3H]TAU (specific activity 1.11×1012 Bq/mmol) was purchased from Amersham International (Buckinghamshire, UK). TAUT polyclonal affinity-purified IgG was purchased from ADI (Alpha Diagnostic Intl, Inc, USA). Three-month-old male Sprague-Dawley rats (Grade II, weighing 200–250 g) were purchased from the Animal Center, the Second Xiang-Ya Hospital of Central South University. All animal experiments were conducted according to the National Research Council's guidelines. The certificate number of the animal breeder is 20020047.
Cell culture
Rat VSMCs were obtained by an explant method described by Campbell and Campbell26. Briefly, a fragment of rat thoracic aorta was stripped of intima and adventitia. The remaining medial layer was cut into small pieces, placed in Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g/L glucose, 10 mmol/L sodium pyruvate, and 20% FBS supplemented with 100 U/mL of penicillin and 100 μg/mL of streptomycin, and incubated at 37 °C in a humidified atmosphere containing 5% CO2. After 5−7 d, cells migrated from the explants, and the explant fragments were removed after 15 d of culture. Vascular smooth muscle cells were passaged every 3 or 4 d, and all experiments were performed between passages 3 and 6 from the primary culture. The VSMC phenotype was confirmed by positive immunostaining of α-smooth muscle actin.
VSMCs do not spontaneously calcify in culture27. Therefore, at about 80% confluence, cells were placed into the calcifying medium consisting of the growth medium described above supplemented with 10 mmol/L β-GP28. The media were replaced with fresh media every 2 or 3 d. The transformation to calcifying cells was characterized by the appearance of the multilayer nodules undergoing calcification detected by Alizarin Red S staining.
DNA and protein synthesis assay
DNA and protein synthesis were assessed using scintillation counting to measure the incorporation of [3H]thymidine (7.4×104 Bq/mL) or [3H]leucine into DNA or protein, respectively, as previously described29, 30, 31. Briefly, cells were plated at a density of 2×104/well in 24-well plates, cultured in growth medium and treated with vehicle controls, 5−20 mmol/L TAU, 1−10 mmol/L LC or 20 mmol/L TAU+10 mmol/L LC for 48 h. Then, the plates were washed with PBS, and 10% trichloroacetic acid solution was added to the wells to precipitate the DNA and protein. Incorporated [3H]thymidine was released by washing with 0.2 mol/L of NaOH, and radioactivity was measured using a β-scintillation counter. The results were expressed as counts per minute per μg protein.
Bradford assay
The protein concentration in extracts was determined by the Bradford assay. Briefly, the Bradford reagent (BioRad Protein Assay Dye Reagent; cat# 500-0006) was diluted 1:5 with dH2O. The diluted reagent was filtered through Whatman 540 paper and stored at 4 °C. To measure the protein concentration, 10−20 μL of the protein extract was added to 1 mL of the diluted reagent and mixed. The blue color formed was measured at the wavelength of 595 nm. A standard curve was prepared using a serial dilution (0.1−1.0 mg/mL) of BSA.
Analysis of ALP activity
VSMCs were cultured in either non-calcifying medium or calcifying medium and treated with TAU and LC for 12 d. Cells were then washed three times with PBS, scraped into 1 mL of 10 mmol/L Tris-Cl buffer (pH 7.6) containing 0.1% Triton-X-100 on ice, and centrifuged. The lysates were homogenized. Then ALP activity was assayed by spectrophotometric measurement of p-nitrophenol release at 37 °C. The protein content was measured by the Bradford assay. ALP activity was normalized to the total protein content of the cell layer.
Quantification of calcium deposition
VSMCs were cultured in non-calcifying medium or calcifying medium and treated with TAU and LC for 12 d. Cells were then decalcified with 0.6 mol/L HCl for 24 h. The calcium content was determined by measuring the concentration of calcium in the HCl supernatant by atomic absorption spectroscopy. After decalcification, cells were washed three times with PBS and solubilized with 0.1 mol/L NaOH/0.1% SDS. The protein content was measured by the Bradford assay. The calcium content of the cell layer was normalized to the protein content.
TAU uptake assay
TAU uptake by VSMCs was assayed basically as previously described32, 33. [3H]TAU uptake experiments were performed in the absence (total uptake) or presence (non-specific uptake) of 20 mmol/L unlabeled TAU, allowing the specific uptake to be calculated by subtraction. Briefly, VSMCs were grown to 80% confluence in six-well polyethylene dishes (9.6 cm2 per well). Cells were washed three times by gentle aspiration/addition of 2 mL aliquots of TAU uptake buffer (HEPES 20 mmol/L, NaCl 140 mmol/L, KCl 5.4 mmol/L, CaCl2 1.8 mmol/L, MgSO4 0.8 mmol/L and glucose 5 mmol/L) at 37 °C and equilibrated in 2 mL TAU uptake buffer at 37 °C. TAU uptake assay was initiated by adding the uptake buffer containing [3H]TAU (3.7×106 Bq/mL, 1 mL), with or without extra TAU (20 mmol/L), at 37 °C for 20 min. Cells were then quickly washed four times with ice-cold buffer, solubilized with 0.1 mol/L NaOH and sonicated; then, TAU uptake was measured by liquid scintillation spectrometry. After the final wash, the sixth well was used for the estimation of the average protein content (g protein per well) by the Bradford assay.
Western blot
Western blot was performed as previously described34. The LC treated monolayers of VSMCs in 25-cm2 culture flasks were rinsed twice with 1 mmol/L EDTA in PBS, lysed with Triton lysis buffer (50 mmol/L Tris–HCl, pH 8.0, containing 150 mmol/L NaCl, 1% Triton X-100, 0.02% sodium azide, 10 mmol/L EDTA, 10 mg/mL aprotinin and 1 mg/mL aminoethylbenzenesulfonyl fluoride) and then saved after centrifugation. Total protein content was determined by the Bradford assay. Fifty micrograms of the total protein from each sample was loaded onto a polyacrylamide gel (Bio-Rad) and separated by electrophoresis. The protein was transferred to a polyvinylidene difluoride (PVDF) membrane by the semidry method (Bio-Rad). The blot was blocked with 2.5% nonfat milk in PBS for a minimum of 1 h. The membrane was incubated with 1 μg/mL of a rabbit anti-TAUT polyclonal antibody or an anti-β-actin monoclonal antibody (Sigma) in PBS at room temperature for 3 h. The membrane was washed three times in PBS and incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody (Amersham International) for 1 h. Detection was performed with a chemiluminescence detection kit according to the manufacturer's protocol (ECL Detection, Amersham International).
RNA interference of TAUT
RNA interference was used to down-regulate the expression of TAUT in rat VSMCs. The short hairpin RNA (shRNA) of TAUT was synthesized by Genesil Biotechnology Co (Wuhan, China), of which the target sense sequence is 5′-GATCCAGACTTCCACAAAGACATCTTCAAGAG AGATGTCTTTGTGGAAGTCTTTTTTTGGAAA-3′, while the corresponding antisense sequence is 5′-AGCTTTTCCAAAAAAAGACTTCCACAAAGACATC TCTCTTGAAGATGTCTTTGTGGAAGTCTG-3′. The base pairs underlined in target sequences are the restriction sites BamH I and Hind III. The pSilencer™ 2.1-U6 neo plasmid (Ambion Inc, Austin, TX, USA) was chosen to express the TAUT shRNA. For gene knockdown experiments, VSMCs were plated in 6-well dishes, cultured for 24 h in the medium without antibiotics and then transfected with shRNAs using Lipofectamine 2000 (Invitrogen Inc, Carlsbad, CA, USA) according to the manufacturer's instructions. After 24 h of culture, the transfection media were replaced with selection media containing 600 μg/mL G418. The resulting G418-resistant cell colonies were picked and expanded and then the cell colonies, with the confirmed TAUT expression knockdown, were maintained in medium containing 300 μg/mL G418 and used for further analysis.
Statistical analysis
SPSS 13.0 was used for the statistical analysis. The results are presented as means±SD. Statistical comparison between groups and treatments was performed using Student's t-test. P<0.05 was considered significant. All in vitro experiments were repeated at least three times, and representative experiments are shown.
Results
Effects of TAU and LC on the incorporation of [3H]thymidine and [3H]leucine into DNA and protein of VSMCs
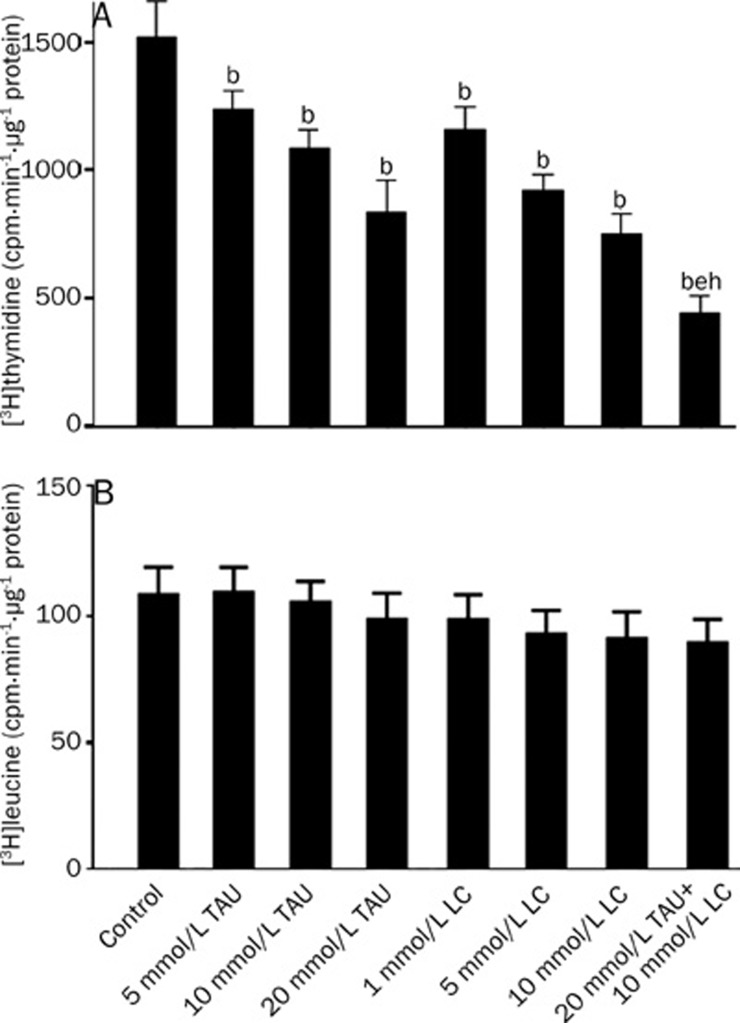
As shown in Figure 1A, 5–20 mmol/L TAU and 1–10 mmol/L LC, respectively, inhibited the [3H]thymidine incorporation (cpm·min−1·μg−1 protein) into VSMCs in a dose-dependent manner, and the combined treatment of TAU and LC synergistically suppressed the [3H]thymidine incorporation into VSMCs. However, the [3H]leucine incorporation (cpm·min−1·μg−1 protein) into newly synthesized protein was not affected by TAU or LC in any of the tested concentrations, and it was not affected by the combined treatment of TAU and LC either (Figure 1B). These data indicate that the treatment with TAU or LC alone or in combination inhibits VSMC proliferation without causing toxic effects on the cells.
Figure 1.
Effects of LC and TAU at various concentrations on the incorporation of [3H]thymidine (A) and [3H]leucine (B) into DNA and protein in VSMCs. Cells were exposed to vehicle control, 5−20 mmol/L TAU, 1−10 mmol/L LC or 20 mmol/L TAU+10 mmol/L LC for 48 h. Cell proliferation was determined by measuring [3H]thymidine incorporation. Cell toxic effect was determined by measuring [3H]leucine incorporation. The results are expressed as counts per minute per μg protein. The bars represent the mean±SD. n=6. bP<0.05 vs control; eP<0.05 vs the 20 mmol/L TAU-treated group; hP<0.05 vs the 10 mmol/L LC-treated group.
Effects of LC and TAU on the osteoblastic differentiation and mineralization of VSMCs
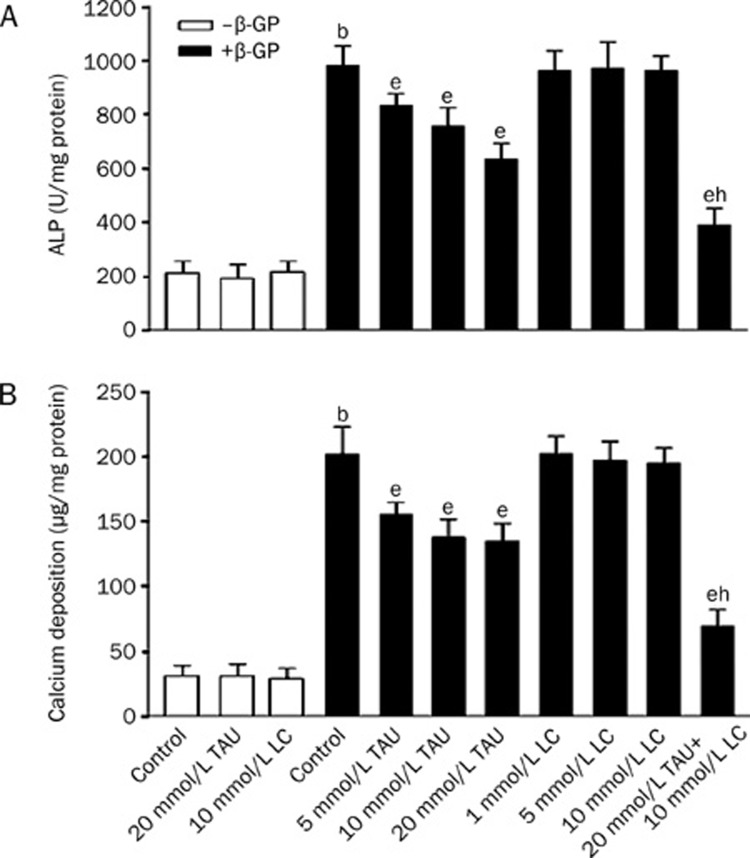
Recently, it has been shown that vascular calcification in vivo has many features in common with bone mineralization. Therefore, we investigated whether ALP activity and calcium deposition were altered during TAU and LC treatment for the mineralization of VSMCs. ALP is a well-established phenotypic marker of osteoblastic differentiation and a critical enzyme in calcification. After 12 d of culture, the ALP activity in the β-GP-induced calcifying VSMC group dramatically increased compared to that in the noncalcifying group, while TAU or LC treatment alone had no influence on the ALP activity in the noncalcifying VSMCs. The ALP activity of the cells treated with 5, 10, or 20 mmol/L TAU was lower than that of the calcifying controls. In contrast, the ALP activity of the cells treated with 1, 5, or 10 mmol/L LC had no difference compared with that of the calcifying controls. Furthermore, in the cells treated with TAU and LC in combination, the ALP activity was lower than that of the cells treated with TAU alone (Figure 2A). The effects of TAU and LC on calcium deposition in VSMCs were determined in parallel with the ALP activity (Figure 2B). These data indicate that LC by itself has no effect on the osteoblastic differentiation or mineralization of VSMCs, but it can enhance the inhibitory effects of TAU on the ALP activity and calcium deposition in VSMCs.
Figure 2.
Effects of LC and TAU on the ALP activity and calcium deposition in VSMCs. (A) Effect of LC and TAU on ALP activity. Cells were cultured in non-calcification or calcification medium with LC and TAU. ALP activity was measured, normalized to the cellular protein content, and presented as means±SD. n=3. bP<0.05 vs non-calcification control; eP<0.05 vs calcification control; hP<0.05 vs the 20 mmol/L TAU-treated group. (B) Effect of TAU and LC on calcium deposition. The calcium contents of the cell layers were assessed as described in materials and methods and presented as means±SD. n=3. bP<0.05 vs non-calcification control; eP<0.05 vs calcification control; hP<0.05 vs the 20 mmol/L TAU-treated group.
Dose and time-dependence of the LC-induced up-regulation of the TAU uptake by VSMCs
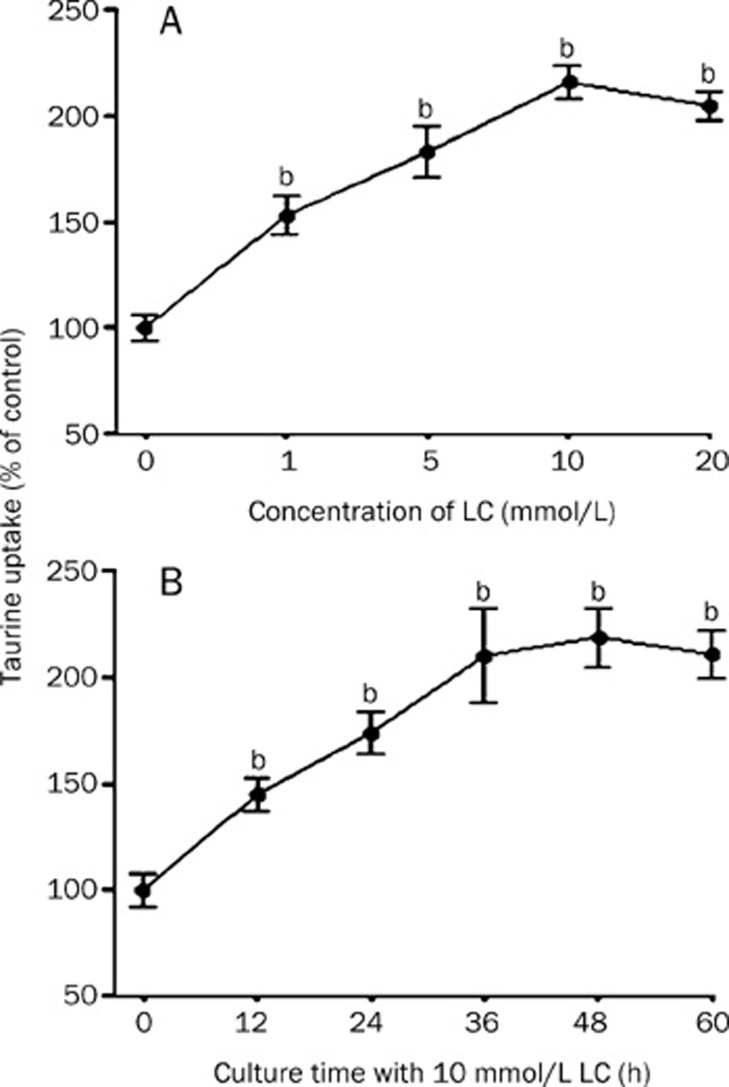
VSMCs were treated with 0, 1, 5, 10, or 20 mmol/L LC for 48 h before the uptake experiments were performed. The results showed that 1−10 mmol/L LC increased the TAU uptake activity by 150%−220% of the control value (Figure 3A).
Figure 3.
Time- and dose-dependence of the LC-induced up-regulation of TAU uptake in VSMC monolayers. (A) Cells were precultured for 48 h in medium containing various concentrations of LC as indicated. Uptake experiments were then performed as described in Materials and methods. Each value is the mean±SD. n=4. bP<0.05 vs control. (B) VSMCs were precultured in medium containing 10 mmol/L LC for various time (0−60 h) as indicated, and uptake experiments were performed as described in Materials and methods. Each value is the mean±SD. n=4. bP<0.05 vs control.
VSMC monolayers were also incubated with 10 mmol/L LC for 0−60 h before the uptake experiments were performed. As shown in Figure 3B, the TAU uptake activity increased in a time-dependent manner from 0 to 36 h and reached a plateau after 36 h.
Kinetics of the TAU uptake in VSMCs cultured with LC
A kinetic analysis of the TAU uptake was performed on the VSMCs cultured with or without 10 mmol/L LC for 48 h. The maximal velocity Vmax value of the control cells was 35.42±3.65 pmol·L−1·min−1·mg−1 protein, and the apparent affinity constant Km (Michaelis–Menten constant) value was 5.62±1.03 μmol/L (Table 1). For the LC-treated cells, the Vmax and Km values were 80.39±7.35 pmol·L−1·min−1·mg−1 protein and 6.18±1.70 μmol/L, respectively (Table 1). These results indicate that the LC-induced up-regulation of TAU uptake is associated with an increase in the maximal velocity of TAUT, while the affinity of TAUT remains unchanged.
Table 1. Kinetic analysis of the TAU uptake by LC-treated and control cells.bP<0.05 vs control.
| Control | LC-treated | |
|---|---|---|
| Vmax (pmol·L−1·min−1·mg−1 protein) | 35.42±3.65 | 80.39±7.35b |
| Km (μmol/L) | 5.62±1.03 | 6.18±1.70 |
Cells were precultured in a medium containing vehicle or 10 mmol/L of LC for 48 h. The TAU uptake by the LC-treated and control cells was then measured over the concentration range of 1–100 μmol/L TAU. Vmax: maximal velocity value; Km: apparent affinity constant.
Expression level of TAUT protein in VSMCs cultured with LC
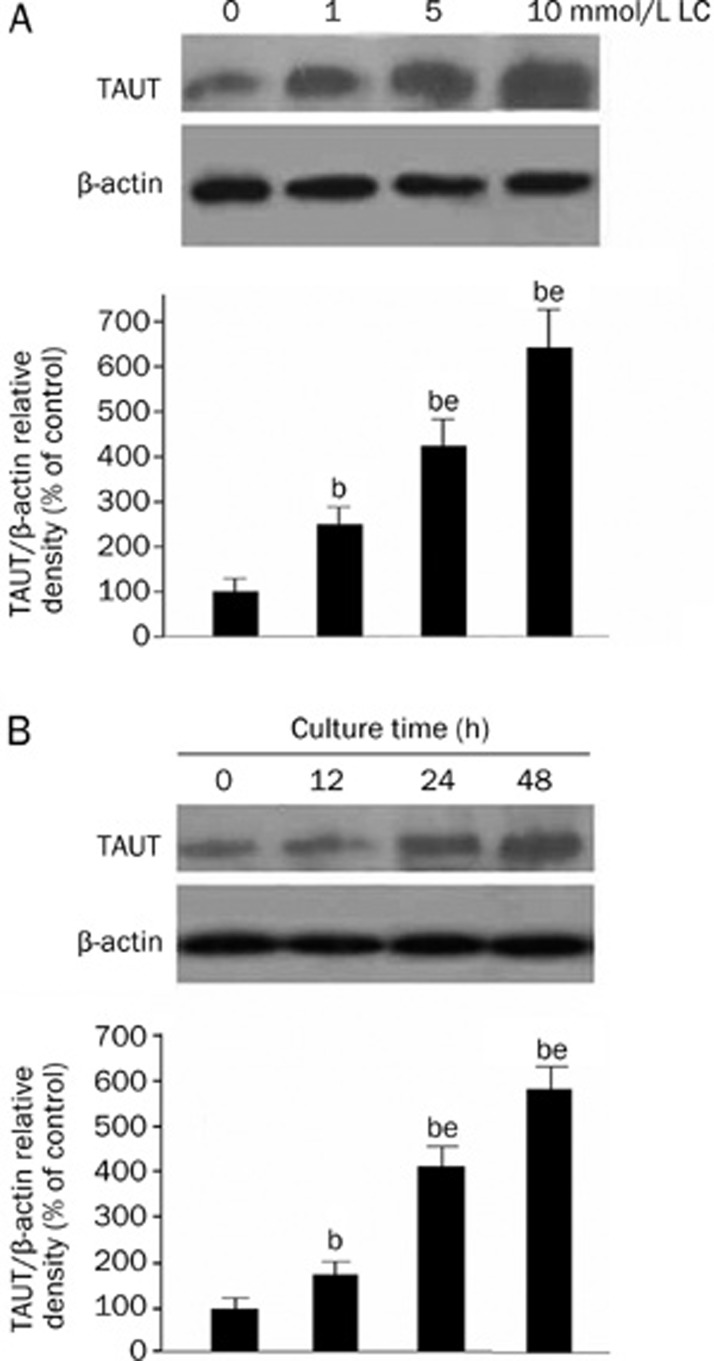
Western blot was performed to determine whether the up-regulation of TAU uptake by LC was associated with the change in the expression level of TAUT protein. VSMCs were cultured in medium with 1, 5, or 10 mmol/L LC for 48 h. VSMCs were also cultured in medium with 10 mmol/L LC for 0, 12, 24, or 48 h. As shown in Figure 4, the expression level of TAUT protein was markedly increased by LC treatment in a dose- and time-dependent manner, suggesting that the up-regulation of TAU uptake by LC is attributable to the increased expression level of TAUT protein.
Figure 4.
Western blot analysis of TAUT protein in VSMCs cultured with LC. (A) VSMCs were cultured with different concentrations (0, 1, 5, and 10 mmol/L) of LC for 48 h and then subjected to Western blot analysis. The levels of 70 kDa TAUT and 43 kDa β-actin were quantified by densitometric analysis of the three autoradiograph pictures, and the relative densities of TAUT/β-actin were determined. The bars represent the mean±SD. n=3. bP<0.05 vs the control group; eP<0.05 vs the previous adjacent group. (B) VSMCs were cultured with 10 mmol/L LC for different time (0, 12, 24, and 48 h) and then subjected to Western blot analysis. The levels of 70 kDa TAUT and 43 kDa β-actin were quantified by densitometric analysis of the three autoradiograph pictures, and the relative densities of TAUT/β-actin were determined. The bars represent the mean±SD. n=3. bP<0.05 vs the control group; eP<0.05 vs the previous adjacent group.
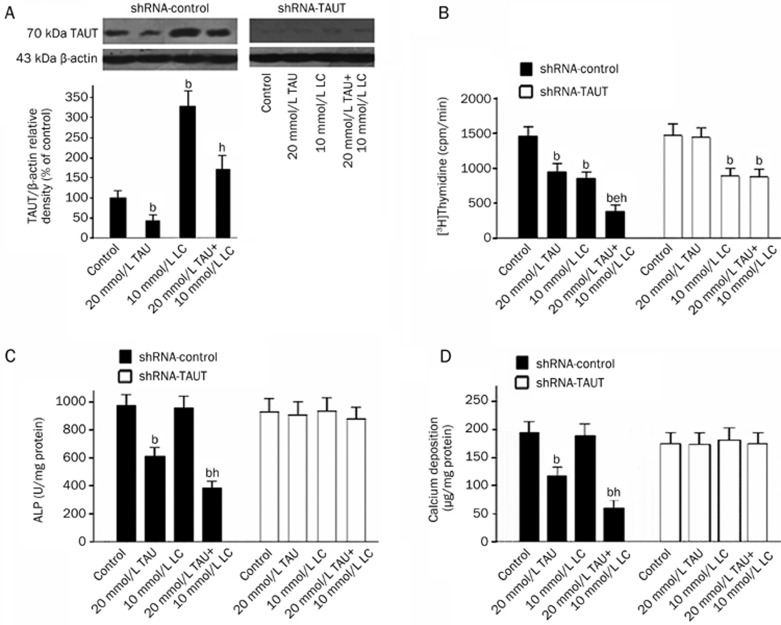
Impact of TAUT expression knockdown on the anti-mitogenic and anti-osteogenic effects of LC and TAU
In the shRNA-mock cell cultures, the intensity of the 70 kDa TAUT band of the cells treated with 20 mmol/L TAU was lower than that of controls, while the intensity of the band of the cells treated with 10 mmol/L LC was remarkably higher than that of controls. Furthermore, the intensity of the band from the cells treated with 20 mmol/L TAU and 10 mmol/L LC in combination was dramatically higher than that of the 20 mmol/L TAU-treated cells. In contrast, in the shRNA-TAUT cell cultures, no TAUT protein could be detected (Figure 5A).
Figure 5.
Impact of TAUT expression knockdown on the anti-mitogenic and anti-osteogenic effects of LC and TAU. (A) Effect of LC and TAU on TAUT expression in the shRNA-transfected cells. Cells were exposed to different agents as indicated for 48 h and then subjected to Western blot analysis. The levels of TAUT and β-actin were quantified by densitometric analysis of the three autoradiograph pictures, and the relative densities of TAUT/β-actin were determined. The bars represent the mean±SD. n=3. bP<0.05 vs the control group; eP<0.05 vs the 20 mmol/L TAU-treated group. (B) Impact of shRNA-mock or shRNA-TAUT on the anti-mitogenic effect of LC and TAU. Cells were exposed to different agents as indicated for 48 h. The results are expressed as counts per minute. The bars represent the mean±SD. n=6. bP<0.05 vs control; eP<0.05 vs 20 mmol/L TAU-treated group; hP<0.05 vs the 10 mmol/L LC-treated group. (C) and (D) Impact of shRNA-mock or shRNA-TAUT on the anti-osteogenic effect of LC and TAU. Cells were cultured in calcification medium with different agents as indicated for 12 d. ALP activity and calcium content were measured, normalized to the cellular protein contents and presented as mean±SD. n=6. bP<0.05 vs control; eP<0.05 vs the 20 mmol/L TAU-treated group.
Knockdown of TAUT with shRNA in VSMCs not only abolished the anti-mitogenic and anti-osteogenic effects of TAU, but also eliminated the synergistic inhibitory action of LC and TAU on the proliferation, ALP activity and calcium deposition of VSMCs (Figure 5B, 5C, and 5D). These data indicate that LC enhances the effects of TAU on reducing the proliferation and osteoblastic differentiation of VSMCs via the up-regulation of TAUT in these cells.
Discussion
LC and TAU can exert therapeutic effects on atherosclerosis14, 16, 17, 18, 22, 23, 24, 25. Our present data indicate that LC and TAU synergistically inhibit the proliferation and β-GP-induced osteoblastic differentiation of VSMCs. In addition, LC stimulates the TAUT expression and TAU uptake in VSMCs. Suppression of TAUT with shRNA can abolish the synergistic action of LC and TAU.
Atherosclerosis has been classically associated with an increased level of cellular proliferation. In the case of atherosclerosis, it is generally accepted that the abnormal proliferation of VSMCs plays an important role in the pathophysiology of the disease2, 3, 4.
Mineralization of soft tissues occurs under pathological conditions and has detrimental consequences, particularly when it occurs in blood vessels and heart valves. Calcification of arterial plaques decreases vessel elasticity, augments plaque brittleness and leads to increased plaque rupture during angioplasty procedures35. It is, therefore, associated with an increased risk of myocardial infarction and death. Despite its clinical significance, the molecular mechanisms underlying the regulation of vascular calcification are unclear. However, some evidence has recently emerged in support of the concept that ectopic calcification, such as bone mineralization, is a cell-regulated process. Steitz et al6 reported that VSMCs undergo a phenotypic transition into osteoblast-like cells in response to mineralization, evidenced by an increase in the expression of ALP, osteocalcin, Cbfa1 and osteopontin.
TAU, a metabolite of methionine and cysteine, has been shown to have antihypertensive and antiatherogenic effects in animal models. Epidemiological studies also revealed that TAU uptake inversely correlates with the incidence of coronary heart disease13. The molecular mechanism underlying this protective effect is obscure; some researchers have attributed this effect to its cholesterol-lowering properties36. However, others reported that TAU could prevent atherosclerosis without significantly affecting the serum cholesterol level14. Currently, TAU and its analogues are reported to suppress the proliferation of VSMCs16. Furthermore, it was reported that TAU significantly suppresses the cell proliferation induced by the platelet-derived growth factor (PDGF)-BB via the protein tyrosine phosphatase (PTPase)-mediated suppression of the PDGF-β receptor phosphorylation and by decreasing the activation of its downstream signaling molecules in VSMCs37. In addition, Li et al17 demonstrated that TAU treatment alleviated the calcification in VSMCs induced by β-GP. Our previous studies showed that TAUT is expressed in VSMCs15, and TAU can inhibit the osteoblastic differentiation of VSMCs via ERK activation18.
Recently, LC has been introduced into the therapeutic approaches of peripheral arterial disease. Carnitine is a vital component in lipid metabolism for the production of adenosine triphosphate through β-oxidation and subsequent oxidative phosphorylation19. The concentrations of carnitine in blood and tissues decrease under hyperlipidemic conditions38. Lipoprotein concentration is generally related to coronary artery disease (CAD) and cerebrovascular disease39. LC can lower the lipoprotein serum levels of patients with type 2 diabetes mellitus40. Moreover, LC treatment (300 mg/kg body weight per day) for 7 and 14 d caused significant reduction of the tissue lipid peroxidation and marked improvement of the antioxidant status in atherosclerotic rats41, and the long-term oral administration of LC was associated with a decrease of plaque cell proliferation and the severity of aortic atherosclerotic lesions in a rabbit model22. Furthermore, previous data documented the fact that LC prevented the progression of atherosclerotic lesions in rabbits in association with a significant reduction of VSMC proliferation and plasma triglycerides22, 23, 24, 25.
In the present study, TAU decreased VSMC proliferation and osteoblastic differentiation markers (including ALP activity and calcium deposition), while LC inhibited only proliferation but not osteoblastic differentiation in VSMCs. The combined treatment of LC and TAU was more effective than that of LC or TAU alone on suppressing the proliferation and osteoblastic differentiation in VSMCs. However, the treatment of TAU or LC in any of the tested concentrations, or the combination of the highest tested concentration of TAU (20 mmol/L) and LC (10 mmol/L) did not inhibit normal cellular protein synthesis, indicating they have no cell toxic effects.
TAU is transported by a specific transporter, TAUT. In the intracellular space, TAU is present in millimolar concentrations, whereas TAU is found at the concentration of 20–100 nmol/L in plasma7, suggesting that TAUT plays an important role in maintaining a high concentration of TAU in cells. Recently, a TAUT knockout mouse model has been established42. The animals exhibited retinal degeneration and marked impairment of reproduction. These phenomena suggest that TAUT is a functional protein in maintaining cell physiological function in vitro and in vivo. Our previous studies demonstrated that the transcription and translation of TAUT occur in cultured VSMCs15. Additionally, the TAUT protein expressed in VSMCs is functional in terms of taking up [3H]TAU15.
Our present data showed a higher level of TAUT protein in the LC-treated cells compared to that in the control cells, indicating that LC markedly increases the TAU uptake by VSMCs, resulting in an increased intracellular TAU level. Furthermore, knockdown of TAUT with shRNA in VSMCs not only abolished the anti-mitogenic and anti-osteogenic effects of TAU, but also eliminated the synergistic inhibitory action of LC and TAU on the proliferation and osteoblastic differentiation of VSMCs. These data indicate that LC enhances the effects of TAU on reducing the proliferation and osteoblastic differentiation of VSMCs via the up-regulation of TAUT in these cells.
In conclusion, we found for the first time that LC and TAU have synergistic inhibitory effects on the proliferation and calcification of VSMCs and that the up-regulation of TAUT expression and TAU uptake by LC in VSMCs may be involved in the synergistic action. Our findings provide evidence for a possible role of the combined treatment of LC and TAU in inhibiting the development of atherosclerotic lesions in atherosclerosis patients, rather than the treatment with either of them alone. Further investigation is warranted on the effects of the combined treatment of LC and TAU in an atherosclerotic animal model.
Author contribution
Xiao-bo LIAO and Hui XIE conceived and designed the experiments; Hui XIE, Bing YANG, Xin-min ZHOU, Feng-lin SONG, Jian-ming LI, Kang ZHOU, Wen HU and Yi-qun PENG performed the experiments; Si-yuan TANG, Ling-qing YUAN and Si-yuan XIONG analyzed the data; Hui XIE wrote the paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30600661, 30872708, 30801174, 30871191 and 30900622), the Scientific Research Foundation of the Health Bureau of Hunan Province (B2007058), the Natural Science Foundation of Hunan Province (05C0163) and the Post-doctorate Scientific Research Special-purpose Project of Hunan Province (2008RS4012).
References
- Haust MD, More RH, Movat HZ. The role of smooth muscle cells in the fibrogenesis of arteriosclerosis. Am J Pathol. 1960;37:377–89. [PMC free article] [PubMed] [Google Scholar]
- Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell. Science. 1973;180:1332–9. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Hegele RA. The pathogenesis of atherosclerosis. Clin Chim Acta. 1996;246:21–38. doi: 10.1016/0009-8981(96)06224-9. [DOI] [PubMed] [Google Scholar]
- Trion A, van der Laarse A. Vascular smooth muscle cells and calcification in atherosclerosis. Am Heart J. 2004;147:808–14. doi: 10.1016/j.ahj.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, et al. Smooth muscle cell phenotypic transition associated with calcification upregulation of cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–54. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–63. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Lombardini JB. The inhibitory effects of taurine on protein phosphorylation: comparison of various characteristics of the taurine-affected phosphoproteins present in rat retina, brain and heart. Adv Exp Med Biol. 1994;359:9–17. doi: 10.1007/978-1-4899-1471-2_2. [DOI] [PubMed] [Google Scholar]
- Schaffer SW, Ballard C, Azuma J. Mechanisms underlying physiological and pharmacological actions of taurine on myocardial calcium transport. Adv Exp Med Biol. 1994;359:171–80. doi: 10.1007/978-1-4899-1471-2_18. [DOI] [PubMed] [Google Scholar]
- Militante JD, Lombardini JB. Taurine stimulation of calcium uptake in the retina: mechanism of action. Adv Exp Med Biol. 2003;526:547–54. doi: 10.1007/978-1-4615-0077-3_65. [DOI] [PubMed] [Google Scholar]
- Yuan LQ, Xie H, Luo XH, Wu XP, Zhou HD, Lu Y, et al. Taurine transporter is expressed in osteoblasts. Amino Acids. 2006;31:157–63. doi: 10.1007/s00726-005-0313-7. [DOI] [PubMed] [Google Scholar]
- Yuan LQ, Lu Y, Luo XH, Xie H, Wu XP, Liao EY. Taurine promotes connective tissue growth factor (CTGF) expression in osteoblasts through the ERK signal pathway. Amino Acids. 2007;32:425–30. doi: 10.1007/s00726-006-0380-4. [DOI] [PubMed] [Google Scholar]
- Yamori Y, Liu L, Ikeda K, Miura A, Mizushima S, Miki T, et al. Distribution of twenty-four hour urinary taurine excretion and association with ischemic heart disease mortality in 24 populations of 16 countries: results from the WHO-CARDIAC study. Hypertens Res. 2001;24:453–7. doi: 10.1291/hypres.24.453. [DOI] [PubMed] [Google Scholar]
- Murakami S, Kondo Y, Sakurai T, Kitajima H, Nagate T. Taurine suppresses development of atherosclerosis in Watanab heritable hyperlipidemic (WHHL) rabbits. Atherosclerosis. 2002;163:79–87. doi: 10.1016/s0021-9150(01)00764-x. [DOI] [PubMed] [Google Scholar]
- Liao XB, Zhou XM, Li JM, Tan ZP, Liu LM, Zhang W, et al. Taurine transporter is expressed in vascular smooth muscle cells. Amino Acids. 2007;33:639–43. doi: 10.1007/s00726-006-0486-8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tenner TE, Lombardini JB. Inhibition of rat vascular smooth muscle cell proliferation by taurine and taurine analogues. Biochem Pharmacol. 1999;57:1331–9. doi: 10.1016/s0006-2952(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang B, Huang Z, Wang S, Tang C, Du J. Taurine prevents beta-glycerophosphate-induced calcification in cultured rat vascular smooth muscle cells. Heart Vessels. 2004;19:125–31. doi: 10.1007/s00380-003-0744-6. [DOI] [PubMed] [Google Scholar]
- Liao XB, Zhou XM, Li JM, Yang JF, Tan ZP, Hu ZW, et al. Taurine inhibits osteoblastic differentiation of vascular smooth muscle cells via the ERK pathway. Amino Acids. 2008;34:525–30. doi: 10.1007/s00726-007-0003-8. [DOI] [PubMed] [Google Scholar]
- Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983;63:1420–80. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Xie H, Tang SY, Li H, Luo XH, Yuan LQ, Wang D, et al. L-carnitine protects against apoptosis of murine MC3T3-E1 osteoblastic cells. Amino Acids. 2008;35:419–23. doi: 10.1007/s00726-007-0598-9. [DOI] [PubMed] [Google Scholar]
- [No authors listed]. Monograph. L-carnitine Altern Med Rev 20051042–50. [PubMed] [Google Scholar]
- Spagnoli LG, Orlandi A, Marino B, Mauriello A, De Angelis C, Ramacci MT. Propionyl-L-carnitine prevents the progression of atherosclerotic lesions in aged hyperlipemic rabbits. Atherosclerosis. 1995;114:29–44. doi: 10.1016/0021-9150(94)05460-z. [DOI] [PubMed] [Google Scholar]
- Orlandi A, Marcellini M, Pesce D, Calvani M, Spagnoli LG. Propionyl-L-carnitine reduces intimal hyperplasia after injury in normocholesterolemic rabbit carotid artery by modulating proliferation and caspase 3-dependent apoptosis of vascular smooth muscle cells. Atherosclerosis. 2002;160:81–9. doi: 10.1016/s0021-9150(01)00568-8. [DOI] [PubMed] [Google Scholar]
- Orlandi A, Francesconi A, Marcellini M, Di Lascio A, Spagnoli LG. Propionyl-L-carnitine reduces proliferation and potentiates Bax-related apoptosis of aortic intimal smooth muscle cells by modulating nuclear factor-kappaB activity. J Biol Chem. 2007;282:4932–42. doi: 10.1074/jbc.M606148200. [DOI] [PubMed] [Google Scholar]
- Sayed-Ahmed MM, Khattab MM, Gad MZ, Mostafa N. L-carnitine prevents the progression of atherosclerotic lesions in hypercholesterolaemic rabbits. Pharmacol Res. 2001;44:235–42. doi: 10.1006/phrs.2001.0852. [DOI] [PubMed] [Google Scholar]
- Campbell JH, Campbell GR. Culture techniques and their applications to studied of vascular smooth muscle. Clin Sci. 1993;85:501–12. doi: 10.1042/cs0850501. [DOI] [PubMed] [Google Scholar]
- Balica M, Bostrom K, Shin V, Tillisch K, Demer LL. Calcifying subpopulation of bovine aortic smooth muscle cells is responsive to 17beta-estradiol. Circulation. 1997;95:1954–60. doi: 10.1161/01.cir.95.7.1954. [DOI] [PubMed] [Google Scholar]
- Beck GR Jr, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci USA. 2000;97:8352–7. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Tang SY, Cui RR, Huang J, Ren XH, Yuan LQ, et al. Apelin and its receptor are expressed in human osteoblasts. Regul Pept. 2006;134:118–25. doi: 10.1016/j.regpep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, et al. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201–10. doi: 10.1007/s00223-006-0155-7. [DOI] [PubMed] [Google Scholar]
- Tang SY, Xie H, Yuan LQ, Luo XH, Huang J, Cui RR, et al. Apelin stimulates proliferation and suppresses apoptosis of mouse osteoblastic cell line MC3T3-E1 via JNK and PI3-K/Akt signaling pathways. Peptides. 2007;28:708–18. doi: 10.1016/j.peptides.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Brandsch M, Miyamoto Y, Ganapathy V, Leibach FH. Regulation of taurine transport in human colon carcinoma cell lines (HT-29 and Caco-2) by protein kinase C. Am J Physiol. 1993;264:G939–46. doi: 10.1152/ajpgi.1993.264.5.G939. [DOI] [PubMed] [Google Scholar]
- Satsu H, Miyamoto Y, Shimizu M. Hypertonicity stimulates taurine uptake and transporter gene expression in Caco-2 cells. Biophys Acta. 1999;1419:89–96. doi: 10.1016/s0005-2736(99)00058-9. [DOI] [PubMed] [Google Scholar]
- Xie H, Yuan LQ, Luo XH, Huang J, Cui RR, Guo LJ, et al. Apelin suppresses apoptosis of human osteoblasts. Apoptosis. 2007;12:247–54. doi: 10.1007/s10495-006-0489-7. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty: an observational study using intravascular ultrasound. Circulation. 1992;86:64–70. doi: 10.1161/01.cir.86.1.64. [DOI] [PubMed] [Google Scholar]
- Murakami S, Kondo-Ohta Y, Tomisawa K. Improvement in cholesterol metabolism in mice given chronic treatment of taurine and fed a high-fat diet. Life Sci. 1999;64:83–91. doi: 10.1016/s0024-3205(98)00536-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Nariai Y, Terashima M, Mitani T, Tanigawa Y. Taurine suppresses platelet-derived growth factor (PDGF) BB-induced PDGF-beta receptor phosphorylation by protein tyrosine phosphatase-mediated dephosphorylation in vascular smooth muscle cells. Biochim Biophys Acta. 2005;1745:350–60. doi: 10.1016/j.bbamcr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Seccombe DW, James L, Hahn P, Jones E. L-carnitine treatment in the hyperlipidemic rabbit. Metabolism. 1987;36:1192–6. doi: 10.1016/0026-0495(87)90247-2. [DOI] [PubMed] [Google Scholar]
- Karalis DG. Intensive lowering of low-density lipoprotein cholesterol levels for primary prevention of coronary artery disease. Mayo Clin Proc. 2009;84:345–52. doi: 10.1016/S0025-6196(11)60544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfrizzi V, Capurso C, Colacicco AM, D'Introno A, Fontana C, Capurso SA, et al. Efficacy and tolerability of combined treatment with L-carnitine and simvastatin in lowering lipoprotein (a) serum levels in patients with type 2 diabetes mellitus. Atherosclerosis. 2006;188:455–61. doi: 10.1016/j.atherosclerosis.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Dayanandan A, Kumar P, Panneerselvam C. Protective role of L-carnitine on liver and heart lipid peroxidation in atherosclerotic rats. J Nutr Biochem. 2001;12:254–7. doi: 10.1016/s0955-2863(00)00151-0. [DOI] [PubMed] [Google Scholar]
- Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, et al. Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J. 2002;16:231–3. doi: 10.1096/fj.01-0691fje. [DOI] [PubMed] [Google Scholar]