Abstract
Aim:
To investigate the protective effects of ginsenoside Rb3, a triterpenoid saponin isolated from the leaves of Panax notoginseng, on ischemic and reperfusion injury model of PC12 cells and elucidate the related mechanisms.
Methods:
PC12 cells exposed to oxygen and glucose deprivation (OGD) and restoration (OGD-Rep) were used as an in vitro model of ischemia and reperfusion. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and lactate dehydrogenase (LDH) leakage were used to evaluate the protective effects of ginsenoside Rb3. Cellular apoptosis and mitochondrial membrane potential (MMP) were analyzed using flow cytometry. Intracellular calcium ion concentration ([Ca2+]i) was detected using fluorophotometer system. Caspase-3, -8, and -9 activities were measured using assay kits with an ELISA reader. Western blotting assay was used to evaluate the release of cytochrome c and expression of caspase-3, Bcl-2 and Bax proteins.
Results:
It was shown that ginsenoside Rb3 (0.1–10 μmol/L) significantly increased cell viability and inhibited LDH release in a dose-dependent manner on the ischemic model. In addition, ginsenoside Rb3 also significantly inhibited ischemic injury-induced apoptosis, [Ca2+]i elevation, and decrease of MMP. Meanwhile, pretreatment with ginsenoside Rb3 significantly induced an increase of Bcl-2 protein expression and a decrease of cytosolic cytochrome c, cleaved-caspase 3 and Bax protein expression, the caspase-3, -8, and -9 activity were also inhibited.
Conclusion:
The results indicated that ginsenoside Rb3 could markedly protected OGD-Rep induced ischemic injury and the mechanisms maybe related to its suppression of the intracellular Ca2+ elevation and inhibition of apoptosis and caspase activity. Ginsenoside Rb3 could be a promising candidate in the development of a novel class of anti-ischemic agent.
Keywords: ginsenoside Rb3, PC12 cells, ischemic injury, apoptosis, oxygen and glucose deprivation
Introduction
Nowadays, focal cerebral ischemia is a leading cause of death and disability in the aged population all around the world. Focal cerebral ischemia is a disease characterized by obstructing of blood flow to the brain, resulting in deficient supply of glucose, oxygen, serum, and nutrient that are indispensable for the energy generation1. Neuronal injury induced by cerebral ischemia/reperfusion, is a very complex process with multiple mechanism, such as excitotoxicity, oxidative stress, apoptosis, and variations in gene expression or the activation of kinase2, 3. Mitochondria are vital cell organs in the process of transmitting apoptosis signs through releasing apoptotic factors into the cytosol4, 5, ischemia/reperfusion-induced mitochondrial dysfunction plays a central role in cell death through controlling cellular energy metabolism. Mitochondrium was recently believed to be a good therapeutic target for the cerebral ischemia6.
PC12 cell line is initially derived from a rat adrenal medullary pheochromocytoma, which expressed electrical excitability and calcium channels, possessing the phenotypic and genotypic properties of sympathetic neurons when differentiated by NGF7, 8. Therefore, NGF-differentiated PC12 cell line was commonly and widely used as a replacement for neuronal cells in vitro cerebral ischemia/reperfusion and neuronal protection study9, 10.
Panax notoginseng, one of the family of Acanthopanax gracilistylus, is a popular medicinal plant and displays a wide variety of pharmacological properties, such as sedation, anti-inflammatory and analgesia activities11. Ginsenoside Rb3 (Figure 1) is a major component of protopanoxadiol triterpenoid saponin extracted from the leaves of Panax notoginseng. Ginsenoside Rb3 was recently reported to exhibit the potential neuroprotective effects against ischemic damage in vivo, which decreased cerebral infarction and edema and improved neurological behavioral score in rats induced by transient cerebral ischemia12, 13.
Figure 1.
Chemical structure of ginsenoside Rb3. The molecular formula of ginsenoside Rb3 is C53H90O22 and the molecular weight is 1079.27.
Notwithstanding the widespread study of ginsenoside Rb3, its related mechanism of neuroprotective effects against ischemic brain damage have remained unexplored. In order to further investigate the neuronal protective mechanisms of ginsenoside Rb3, the present study was designed to use NGF-differentiated PC12 cells as in vitro ischemia/reperfusion model, the initial phase of OGD mimics the obstruction of oxygen and glucose supply, the second phase of OGD-Rep reflects the reperfusion of oxygen and glucose supply to the injured brain.
Materials and methods
Drugs and reagents
Ginsenoside Rb3 with a purity of 98% was obtained from Kunming Tongchi Pharmaceutical R&D Co, Ltd (Kunming, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), Propodium iodide (PI), Fura-2 acetoxymethyl ester (Fura-2AM) and α-tocopherol, rhodamine 123 (Rh123) were purchased from Sigma Chemical Co (MO, USA). Dulbecco's modified Eagle's medium (DMEM), glucose-free DMEM, fetal bovine serum (FBS) were obtained from Gibco (Carlsbad, CA USA). Annexin V-fluorescein isothioncyanate (FITC) apoptosis detection kit was obtained from Backman Coulter Inc (IM, USA). The chemical kits for the measurement of lactate dehydrogenase (LDH) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Caspase-3, -8, -9 Activity Kits were acquired from Beyotime Institute of Biotechnology (Jiangsu Province, China). Antibody of cytochrome c, cleaved-caspase-3, Bcl-2, Bax, and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). All other chemicals were of analytic grade and commercially available.
Cell culture
NGF-differentiated PC12 cells, obtained from China Pharmaceutical University (Nanjing, China) were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The medium was changed every two days, and the confluent cells were passaged by trypsinization weekly.
Drug treatment
Cells were plated at a density of 1.0×105 cells/mL 2 days before each experiment. To initiate OGD14, the cell culture medium was removed and replaced with the glucose-free DMEM, then the cells were incubated at 37 °C in an oxygen-free chamber (95% N2 and 5% CO2) for 4 h (OGD), and the change in oxygen levels of the culture medium were monitored during incubation in oxygen-free chamber. Following OGD, glucose was added to normal levels (final concentration: 4.5 mg/mL) and cells were incubated under normal growth conditions (95% air and 5% CO2) for additional 24 h as OGD-reperfusion (OGD-Rep). Ginsenoside Rb3 was freshly prepared as stock solution with dimethyl sulfoxide (DMSO) and diluted with phosphate buffer solution (PBS, pH 7.3), the final concentration of DMSO added to cells never exceeded 0.1%. Ginsenoside Rb3 (final concentrations: 0.1, 1, 10 μmol/L) was added to the culture 24 h before OGD treatment and throughout the OGD reperfusion. The control culture was always maintained in normal DMEM and put in the incubator under normal conditions.
Assay for cell viability
The cell viability was evaluated by two methods: morphological observation with reversed-microscope and MTT reduction. Following the above cell treatment protocol, the morphology of the cells was observed and recorded as a photo under an Olympus Microscope (Tokyo, Japan). The medium was collected for MTT assay, which was quantified as previously described15. MTT stock solution in PBS was added to each well with a final concentration of 5 mg/mL, and the incubation continued for 4 h. Then, the medium with MTT was removed and the blue MTT-formazan was dissolved in DMSO. Optical density (OD) values were measured by spectrophotometry at 570 nm using an ELISA reader (ELx800uv, Bio Tek Instruments, USA). Cell viability was expressed as a percentage of OD value in the control cultures.
LDH release assay
Cytotoxicity was quantitatively assessed by measuring the activity of LDH released from the damaged cells into the culture medium16. At the end of treatments, PC12 cells were treated with 0.5% Triton X-100, and the media which contained detached cells were collected and centrifuged. The supernatant was used for the assay of LDH activity. The enzyme was determined by using an assay kit according to the manufacturer's instructions. LDH leakage was expressed as the percentage of the total LDH activity (LDH in the medium+LDH in the cell), according to the equation LDH released (%)=(LDH activity in the medium/total LDH activity)×100. Cultures under normal conditions (control group) represent basal LDH release.
Flow cytometric apoptosis assay
Apoptosis was assayed by annexin V-FITC and PI staining followed by analysis with fluorescene-activated cell sorting (FACS, BD FACScanto™, San Jose, CA) equipped with the analysis software. The cultured PC12 cells were harvested after the treatment, and then the concentration was adjusted to 1.0×106 cells/mL with PBS. After that the PC12 cells were washed with 1×annexin V-FITC binding buffer before stained with annexin V-FITC and PI for 15 min at room temperature in the dark. Then the cells were analyzed by flow cytometry. Apoptotic and necrotic cells were quantitated by annexin V binding and PI uptake. The cell populations of annexin V-FITC+/PI− were calculated to represent apoptotic cells.
Determination of intracellular Ca2+ concentration ([Ca2+]i)
The intracellular Ca2+ concentration was determined by the intensity of fluorescent Ca2+-sensitive dye, Fura 2-acetoxymethyl ester (Fura-2-AM) according to the method by Lenart 17. The PC12 cells were loaded with Fura-2-AM (final concentration 5 mmol/L), 0.1% DMSO and 1% BSA for 30 min at room temperature in dark conditions, then in incubator for 30 min at 37 °C. Fluorescence measurement was carried out with an F-4500 fluorescence spectrophotometer (Hitachi, Japan). Fura-2-AM-loaded PC12 cells were exposed sequentially to an excitation wavelength of 340 nm and 380 nm (bandwidth 10 nm), and the emission signal was monitored at a wavelength of 510 nm (bandwidth 10 nm). Fluorescence ratios were converted into calcium concentrations by using the following equation: [Ca2+]i=Kd×[(R–Rmin)/(Rmax–R)]×FD/Fs18, 19, where Kd was 224 nmol/L, R(F340/F380) was fluorescence intensity. Rmax was determined by adding tritonX-100 (The final concentration was 0.1%) and Rmin by EGTA (The final concentration was 5 mmol/L). Values of FD and Fs were the fluorescence of Fura-2 at 380 nm in Ca2+-free and saturation with Ca2+, respectively.
Measurement of mitochondrial membrane potential (MMP)
Diversities of mitochondrial membrane potential were assessed by using the fluorescent cationic dye Rh12320, which accumulates in mitochondria as a direct function of the membrane potential and is released upon membrane depolarization. The PC12 cells (1.0×106 cells/mL) were incubated with 2 μmol/L Rh123 for 30 min at 37 °C. Then the Rh123 fluorescence intensity was measured with flow cytometry, with the excitation filter wave set at 488 nm, and the emission filter wave set at 535 nm. The mean fluorescence intensity (MFI) in the cells represented the state of depolarization of MMP. The decreased percentage of MMP was compared with that of the control group.
Western blot analysis
PC12 cells were subjected to Western blot analysis for cleaved-caspase-3, Bcl-2, and Bax protein expression. Briefly, cells were lysed with lysis buffer [10 mmol/L Tris-HCl (pH 7.5), 0.5% CHAPS, 1 mmol/L EGTA, 5 mmol/L β-2-mercaptoethanol, 10% (v/v) glycerol, and 0.1 mmol/L phenylmethylsulfonyl fluoride] and incubated on ice for 30 min. After centrifugation at 10 000×g at 4 °C for 20 min, the supernatant extracts were quantified for protein using a BCA Protein Assay Kit. Cell proteins were separated by electrophoresis on 8% SDS-polyacrylamide gel (SDS–PAGE). After transferring to PVDF membranes, the membrane was blocked with nonfat milk and incubated overnight with primary antibody of cleaved -caspase-3, Bcl-2, and Bax at 4 °C. Antibody recognition was detected with the respective secondary antibody, anti-mouse or anti-rabbit IgG1 conjugated to horseradish peroxidase at room temperature for 2 h. Antibody-bound proteins were detected by Pierce's SupeSignal West Pico chemiluminescent substrate. β-actin served as an internal control.
Mitochondrial cytochrome c release assay
After the treatment, the cells were harvested by trypsinization followed by centrifugation. Then, cells were alysed with lysis buffer (75 mmol/L NaCl, 8 mmol/L Na2HPO4, 1 mmol/L NaH2PO4, 250 mmol/L sucrose, 1 mmol/L PMSF, 5 μg/mL, leupeptin, 12.5 μg/mL digitonin and 21 μg/mL aprotinin). After centrifugation at 10 000×g for 10 min, supernatant was collected and resolved in 15% denaturing SDS-PAGE minigel. Level of cytochrome c release from mitochondria was detected by probing with anti-cytochrome c antibody using Western blot analysis protocol as described above.
Measurement of caspase-3, -8, and -9 activities
Caspases-3, -8, and -9 activities were measured using Caspase Activity Kit according to the manufacturer's instructions, using substrate peptides Ac-DEVD-pNA, Ac-IETD-pNA, and Ac-LEHD-pNA, respectively. Briefly, cells were lysed, then the supernatant was mixed with buffer containing the substrate peptides for caspase attached to p-nitroanilide (pNA). The release of pNA was qualified by determining the absorbance with an ELISA reader (Bio-Tek, USA) at 405 nm. The caspase activities were expressed as percentage compared to control.
Statistical analysis
All the data obtained in the experiments were represented as mean±standard deviation (SD). Statistical analysis was performed with the software package SPSS 10.0. Differences were determined using one-way analysis of variance (ANOVA). P values of less than 0.05 and 0.01 were regarded as statistically significant.
Results
Protective effects of ginsenoside Rb3 on PC12 cells against OGD/OGD-Rep induced injury
OGD/OGD-Rep induced significant cell injury as indicated by morphological observation and MTT assay. PC12 cells exposed to OGD for 4 h and OGD-Rep for 24 h exhibited typical swelling and a marked decrease in the cell number, with most cells losing their neurites and showing a round shape (Figure 2B). However, the situation appeared much better in the groups pretreated with ginsenoside Rb3 (0.1, 1, 10 μmol/L) (Figure 2C, 2D, and 2E). It indicated some degree of protection against the OGD/OGD-Rep by ginsenoside Rb3. As shown in Figure 3A, PC12 cell viability as determined by MTT reduction was also markedly decreased after the cell was exposed to OGD/OGD-Rep. But, when the cells were pretreated with ginsenoside Rb3 (0.1, 1, and 10 μmol/L), OGD/OGD-Rep induced cell toxicity was significantly attenuated, which was concentration-dependently attenuated by ginsenoside Rb3 treatment. The viabilities were raised to 52.8%±5.6%, 64.6%±5.7%, and 76.4%±8.8%, respectively, compared with the control group.
Figure 2.
Effects of ginsenoside Rb3 on PC12 cell injury induced by OGD/OGD-Rep. (A) PC12 control cells. (B) PC12 cells exposed to OGD for 4 h followed by OGD-R for 24 h with no treatment of ginsenoside Rb3. There is a significant decrease in the cell number, and most of the cells lose neurites and demonstrate a round shape. (C, D, E) PC12 cell were pre-incubated with 0.1, 1, 10 μmol/L ginsenoside Rb3, respectively, and then exposed to OGD for 4 h followed by OGD-R for 24 h. All photos (Magnification, 200×) were taken 24 h after exposure to OGD-Rep.
Figure 3.
Effects of ginsenoside Rb3 on cell survival (A) and LDH release (B) in PC12 cells under OGD/OGD-Rep. PC12 cultures treated with several concentrations (0.1, 1, 10 μmol/L) of ginsenoside Rb3 (added upon 24 h before OGD initiation of the insult) or untreated (OGD/OGD-Rep alone), were exposed to OGD for 4 h followed by OGD-Rep for 24 h. Each independent experiment was carried out in three replicates. Values were expressed as mean±SD. bP<0.05, cP<0.01 vs cells exposed to OGD/OGD-Rep alone. fP<0.01 vs control group.
Another indicator of cell toxicity was performed to further investigate the protective effect of ginsenoside Rb3. LDH is a stable cytoplasmic enzyme present in most cells, and it is rapidly released into the cell culture supernatant upon the plasma membrane damage. An increase in the number of dead or plasma membrane-damaged cells results in an increase in LDH activity in the culture supernatant. As shown in Figure 3B, LDH leakage increased to 37.1%±4.9% compared with the control group after treatment with OGD/OGD-Rep. Ginsenoside Rb3 (0.1, 1, and 10 μmol/L) significantly attenuated OGD/OGD-Rep-induced cell death, reducing the LDH leakage to 27.6%±3.2%, 18.4%±3.0%, and 11.3%±2.1%, respectively.
Protective effects of ginsenoside Rb3 on PC12 cells against OGD/OGD-Rep-induced apoptosis
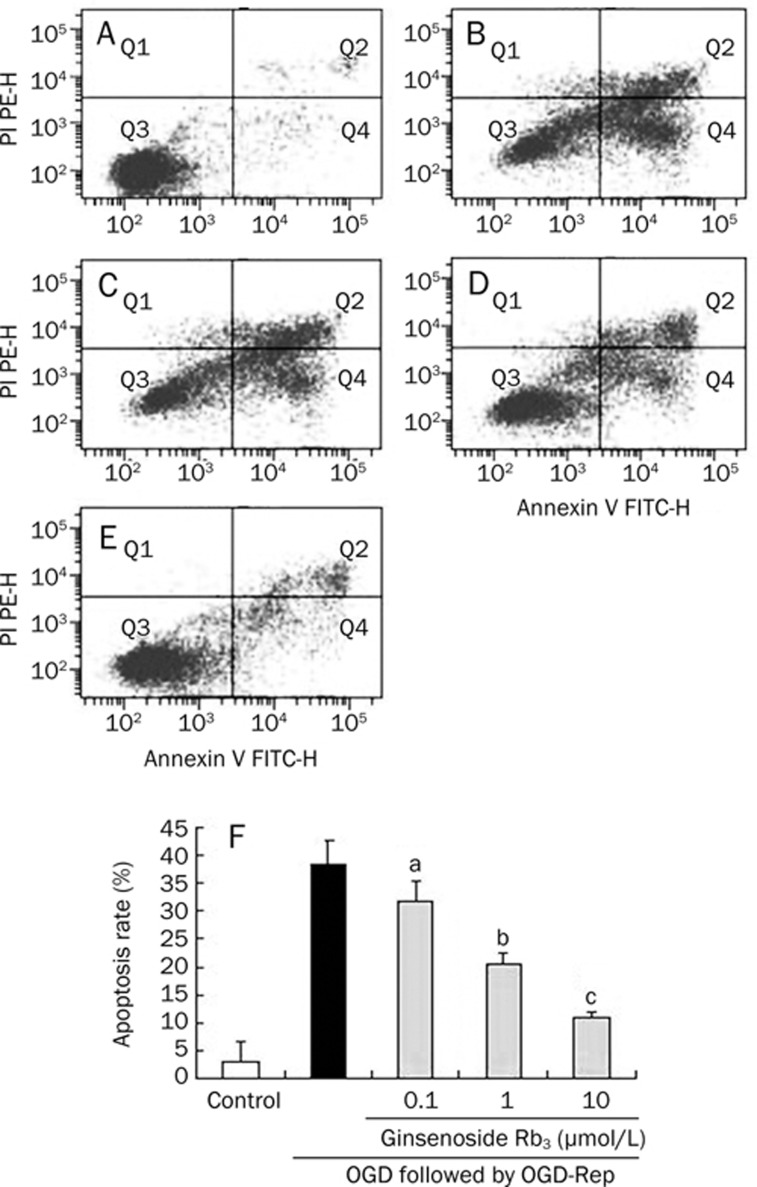
To test whether OGD/OGD-Rep-induced cell death and apoptosis, PC12 cells were stained with both propidium iodide (PI) and FITC-labeled Annexin V (AV-FITC), then analyzed by flow cytometry. Necrotic cells are demonstrated by AV−/PI+ staining, since PI enters cells when membrane integrity is lost and binds to nucleic acids. Apoptotic cells are demonstrated by AV+/PI− staining, since annexin V binds to phosphatidylserine that translocates to the outer leaflet of the plasma membrane during apoptosis. AV+/PI+ stained cells are likely to be late apoptotic or necrotic and AV−/PI− cells represent viable cells. The cell populations of AV+/PI− and AV+/PI+ were calculated respectively to represent apoptotic and necrotic cells and the cell without AV and PI as control. The data was presented as the percentage of apoptotic cells. Treatment of cells with OGD/OGD-Rep significantly induced apoptosis of PC12 cells, indicating an apoptotic cell accumulation of 38.6%±4.1%. While ginsenoside Rb3 at the concentration of 0.1, 1, and 10 μmol/L significantly attenuated OGD-Rep-induced apoptosis (31.7±3.8%, 20.4±2.1%, and 10.9±1.1%, Figure 4).
Figure 4.
Effects of ginsenoside Rb3 on apoptotic rate by flow cytometry. (A) PC12 control cells. (B) PC12 cells exposed to OGD for 4 h followed by OGD-R for 24 h with no treatment of ginsenoside Rb3. (C, D, E) PC12 cells were pre-incubated with 0.1, 1, 10 μmol/L ginsenoside Rb3, respectively 24 h before OGD, and then exposed to OGD for 4 h followed by OGD-R for 24 h. Cells in the lower-left quadrant (Q3), unstained for both Annexin V-FITC and PI, are defined as viable cells. Cells in the lower-right quadrant (Q4), stained for Annexin V-FITC but negative for PI, are defined as early-medium apoptotic cells. Cells in the upper-right quadrant (Q2), positive for both Annexin V-FITC and PI, are defined as late apoptotic and necrotic populations. Apoptosis rate are presented as the mean±SD for three independent experiments performed in triplicate. bP<0.05, cP<0.01 as comparison with cells exposed to OGD/OGD-Rep alone. fP<0.01 as comparison with control group.
Ginsenoside Rb3 inhibited OGD/OGD-Rep-induced increase of concentration of cytosolic calcium ion ([Ca2+]i) in PC12 cells
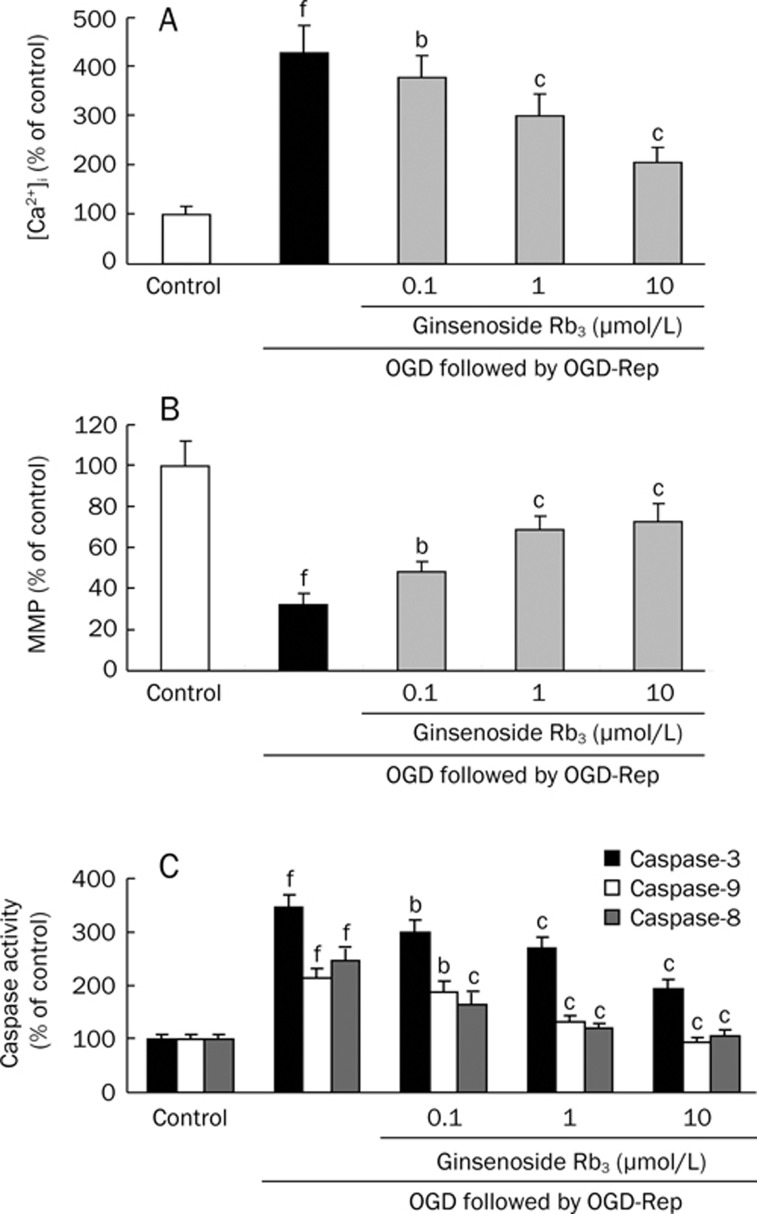
After exposure of the cells to OGD for 4 h followed by OGD-R for 24 h, the intracellular Ca2+ concentration in PC12 cells was increased to 437%±84% of the control value. However, pretreatment of the cells with 0.1, 1, 10 μmol/L ginsenoside Rb3 could dose-dependently decrease the intracellular Ca2+ concentration to 359%±43%, 299%±46%, and 205%±31% of the control value respectively (Figure 5A).
Figure 5.
Effect of ginsenoside Rb3 on [Ca2+]i elevation (A), decrease of MMP (B) and activities of caspase-3, -8, -9 (C) induced by OGD/OGD-Rep in PC12 cells. PC12 cell were pre-incubated with 0.1, 1, and 10 μmol/L ginsenoside Rb3, respectively 24 h before OGD, and then exposed to OGD for 4 h followed by OGD-R for 24 h. Each independent experiment was carried out in three replicates. The values presented are the mean±SD. bP<0.05, cP<0.01 as comparison with cells exposed to OGD/OGD-Rep alone. fP<0.01 as comparison with control group.
Ginsenoside Rb3 attenuated OGD/OGD-Rep-induced dissipation of the MMP
Disruption of the mitochondrial membrane potential is one of the earliest intracellular events that occur following induction of apoptosis. We further assessed the effect of ginsenoside Rb3 on the mitochondrial depolarization induced by OGD/OGD-Rep in PC12 cells. Flow cytometric analyses were carried out using Rh123, uptake of which by mitochondria is proportional to the MMP (Figure 5B). After exposure to OGD for 4 h followed by OGD-R for 24 h alone, the MMP of PC12 cells was significantly decreased to 32.48%±5.26% of that of control. However, the MMP decrease were dose-dependently recovered significantly after being pre-incubated with 0.1, 1, 10 μmol/L ginsenoside Rb3 24 h before OGD, up to 48.35%±4.98%, 68.65%±6.93%, 72.59%±9.11%, respectively.
Effects of ginsenoside Rb3 on expressions of Bcl-2, Bax, cleaved-caspase 3 proteins and cytochrome c release
The expression of proteins including cleaved-caspase 3, Bcl-2, and Bax may be involved in an intrinsic mitochondrial caspase pathway. In our case, an increase in the expression of the pro-apoptotic Bax, cleaved-caspase 3, and a decrease in the expression of the anti-apoptotic Bcl-2 after treatment of PC12 cells with OGD/OGD-Rep. Moreover, cytochrome c release from mitochondria into cytosol was also observed. Pretreatment with ginsenoside Rb3 at the concentration of 1, 10 μmol/L antagonized all the above regulation induced by OGD/OGD-Rep. In addition, they significantly attenuated the decrease of the intracellular ratio of Bcl-2 to Bax (Figure 6).
Figure 6.
Effect of ginsenoside Rb3 on the levels of cytochrome c release in the cytosolic fractions (Figure 6A), and the expression of cleaved caspase-3, Bcl-2, and Bax expression (Figure 6B) by Western blot analysis. PC12 cells were pre-incubated with 1 and 10 μmol/L ginsenoside Rb3 for 24 h, respectively, and then exposed to OGD for 4 h followed by OGD-R for 24 h. At the end of treatment, cells were harvested for Western blotting with ß-actin as a protein loading control. Densitometric analyses of Western blot and the ratio of Bcl-2 to Bax are presented as the mean±SD for three independent experiments performed in triplicate, and the data are presented as the fold induction over control cells (Figure 6C, 6D). bP<0.05, cP<0.01 as comparison with cells exposed to OGD/OGD-Rep alone, fP<0.01 as comparison with control group.
Ginsenoside Rb3 inhibited the activities of caspase-3, -9, and -8
To investigate whether the blocking OGD/OGD-Rep-induced apoptosis by ginsenoside Rb3 is dependent on caspase activation, we examined the activities of caspase-3, -9, and -8, which are initiating caspases in mitochondria-mediated apoptosis pathway. As shown in Figure 5C, after treatment of cells with OGD/OGD-Rep, the activities of caspase-3, -9, and -8 were enhanced by 3.4, 2.1, and 2.5-fold, as compared to control group respectively. However, pretreatment with ginsenoside Rb3 (0.1, 1, 10 μmol/L) significantly attenuated these increase of caspase activities in a dose-dependent manner.
Discussion
It has been demonstrated that ischemic cell death signaling pathways was at least partly related to intrinsic mitochondria-dependent and extrinsic receptor-mediated apoptosis pathways in the past few years21, 22. Although the mechanisms of how OGD-Rep worsen neural functions are not radically understood, cellular calcium dysregulation appears to be a common endpoint in the development of cerebral ischemia injury23. Energy failure in oxygen and glucose deprivation-induced ischemia can evoke the accumulation of intracellular Ca2+ by enhancing the entry of Ca2+ through NMDA receptors or release of Ca2+ from the mitochondria through the Na+-Ca2+ exchanger, and interfering with the ATP-dependent extrusion and sequestration of Ca2+ 24. Stained elevated Ca2+ levels induced by OGD-Rep may impair mitochondrial function preceded by the opening of a permeability transition pore in the inner mitochondrial membrane, excessive release of glutamate, activate phospholipase, protease and endonucleases, eventually leading to neuronal apoptosis and death25, 26.
Apoptosis is driven from the activation of a family of cysteine protease called caspases, which modulate the mitochondria- and death receptor-mediated pathways, respectively. In mitochondria mediated pathway, it is activation of the initiator caspase-9 that is responsible for subsequently activating caspase-3. Caspase-8 is a key initiating caspase involved in neuronal apoptosis through the death receptor-dependent. In addition, recent studies also suggested that caspase-8 was not always activated early in the death receptor-dependent pathway, but also in turn activated caspase-9 initiating the processing of caspase-3 in the mitochondria-mediated pathway27.
In the process of ischemia, the Bcl-2 family of proteins, which consists of anti-apoptotic proteins of Bcl-2 and pro-apoptotic proteins of Bax, could mediate apoptosis by opening of mitochondrial permeability transition pore marked with the collapse of MMP, and down-regulation of the ratio of Bcl-2 to Bax has been reported to be correlated with the collapse of MMP28, 29. The opening of the mitochondrial permeability transition pore causes a release of pro-apoptotic proteins such as cytochrome c from mitochondria into cytoplasm30, and then, cytochrome c will bind to apoptotic protease-activating factor-1, and support the catalytic activation of caspase-9, which further cleaves and activates the effector caspase-3 resulting in the subsequent apoptosis event.
Recently, attention has been focused on finding the nature compounds with advantages of anti-apoptotic activity and low toxicity for neuroprotective agents31, 32. In this study, we used the oxygen and glucose deprivation-induced insult in PC12 cells to partially model the pathological process of cerebral ischemia. The present results showed that exposure of PC12 cells to OGD-Rep was followed by decreased cell viability, an increase in LDH leakage and elevation of the level of intracellular Ca2+, which was attenuated by ginsenoside Rb3 in a concentration-dependent manner.
Pretreatment with ginsenoside Rb3 significantly reduced apoptotic cells, the further results show that ginsenoside Rb3 inhibited the increase in caspase-9, caspase-8, and caspase-3 activities which correlated with the increase in cytosolic cytochrome c levels induced by OGD. These suggested that protection of PC12 cells against OGD-Rep-induced apoptosis by ginsenoside Rb3 involved mitochondria mediated pathways and caspase-8 may be the direct targets of ginsenoside Rb3 on cell protection. Furthermore, ginsenoside Rb3 upregulating the ratio of Bcl-2 to Bax may alter MMP, trigger the decrease of mitochondrial cytochrome c release into cytosol, and finally inhibited apoptosis via a mitochondria-mediated pathway.
In summary, ginsenoside Rb3 protected PC12 cells against OGD-Rep induced ischemia/reperfusion injury. Such protective effects are mediated by its action on anti-apoptosis and suppression of the intracellular Ca2+ elevation. Although a more precise mechanism of ginsenoside Rb3 on these molecular targets and whether a death receptor-mediated pathway involved in the protection should be investigated in further studies, the present findings provided a preliminary pharmacological basis for the therapeutic efficacy of ginsenoside Rb3 for cerebral ischemia.
Author contribution
Jun-rong ZHU and Yi-fu TAO designed the study, performed the research and wrote the paper. Shen LOU provided assistance in experimental methods and performed partial research. Zi-mei WU revised the paper.
Acknowledgments
This work was supported by the Clinical Pharmacy Fund of Science and Technology Department Foundation supported by Jiangsu Health Department (No P200802). The authors are appreciated for the help of Dr Ling HE and Dr Jian-guo SUN for their thoroughly reviews of the manuscript.
References
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–8. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Ekshyyan O, Aw TY. Apoptosis in acute and chronic neurological disorders. Front Biosci. 2004;9:1567–76. doi: 10.2741/1357. [DOI] [PubMed] [Google Scholar]
- Seok JW, Doo YK, Byoung JG. Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol. 2002;35:67–86. doi: 10.5483/bmbrep.2002.35.1.067. [DOI] [PubMed] [Google Scholar]
- Zhu YG, Chen XC, Chen ZZ, Zeng YQ, Shi GB, Su YH, et al. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol Sin. 2004;25:1606–12. [PubMed] [Google Scholar]
- Lopez-Neblina F, Toledo AH, Toledo-Pereyra LH. Molecular biology of apoptosis in ischemia and reperfusion. J Invest Surg. 2005;18:335–50. doi: 10.1080/08941930500328862. [DOI] [PubMed] [Google Scholar]
- Iijima T. Mitochondrial membrane potential and ischemic neuronal death. Neurosci Res. 2006;55:234–43. doi: 10.1016/j.neures.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer TJ, Atchison WD. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 1991;12:473–92. [PubMed] [Google Scholar]
- Abu RS, Trembovler V, Shohami E. A tissue culture ischemic device to study eicosanoid release by pheochromocytoma PC12 cultures. J Neurosci Methods. 1993;50:197–203. doi: 10.1016/0165-0270(93)90008-f. [DOI] [PubMed] [Google Scholar]
- Hillion JA, Takahashi K, Maric D, Ruetzler CA. Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab. 2005;25:154–62. doi: 10.1038/sj.jcbfm.9600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang QL, Chen JW, Ma RQ. Protective effect of panaxatriol saponins on cerebral ischemia. J Sun Yat-sen Univ (Medical Sciences) 2008;29:705–10. [Google Scholar]
- Hu Y, Chen HF, Zang LQ. Protective effects of ginsenoside Rb3 on focal cerebral ischemic injury in rats. J Guangdong Coll Pharm. 2008;24:590–4. [Google Scholar]
- Jiang WW, Jiang ZL, Ke KF. The effect of ginsenoside Rb3 on the persistent sodium current in ischemic and mormal neurons. J Pharm Clin Res. 2007;15:444–9. [Google Scholar]
- Tabakman R, Jiang H, Shahar I. Neuroprotection by NGF in the PC12 in vitro OGD model: involvement of mitogen-activated protein kinases and gene expression. J Ann N Y Acad Sci. 2005;1053:84–96. doi: 10.1196/annals.1344.008. [DOI] [PubMed] [Google Scholar]
- Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye methods for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–10. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg JC, Rush BF. An enzymatic-spectrophotometric determination of pyruvic and lactic acid in blood. Methodologic aspects. Clin Chem. 1966;12:299–307. [PubMed] [Google Scholar]
- Lenart B, Kintner DB, Shull GE, Sun D. Na-K-Cl cotransporter mediated intracellular Na+ accumulation affects Ca2+ signaling in astrocytes in an in vitro ischemic model. J Neurosci. 2004;24:9585–97. doi: 10.1523/JNEUROSCI.2569-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Callewaert G. How to measure intracellular [Ca2+] in single cardiac cells with Fura-2 or indo-1. Cardiovasc Res. 1995;29:717–26. [PubMed] [Google Scholar]
- Liu WB, Wang YP. Magnesium lithospermate B inhibits hypoxia-induced calcium influx and nitric oxide release in endothelial cell. Acta Pharmacol Sin. 2001;22:1135–42. [PubMed] [Google Scholar]
- Zamzami N, Maisse C, Metivier D. Measurement of membrane permeability and permeability transition of mitochondria. Methods Cell Biol. 2001;65:147–58. doi: 10.1016/s0091-679x(01)65009-x. [DOI] [PubMed] [Google Scholar]
- Liu XQ, Sheng R, Qin ZH. The neuroprotective mechanism of brain ischemic preconditioning. Acta Pharmacol Sin. 2009;30:1071–80. doi: 10.1038/aps.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochemi Res. 2004;11:1943–9. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- Saris NE, Carafoli EA. Historical review of cellular calcium handling with emphasis on mitochondria. Biochemistry (Moscow) 2005;70:187–94. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- Iijim TH, Kensuke T, Sachie M. Calcium loading capacity and morphological changes in mitochondria in an ischemic preconditioned model. Neurosci Lett. 2008;448:268–72. doi: 10.1016/j.neulet.2008.10.056. [DOI] [PubMed] [Google Scholar]
- Xiao XH, Liu JT, Hu JW. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca2+ antagonism and antioxidant mechanisms. Eur J Pharmacol. 2008;591:21–7. doi: 10.1016/j.ejphar.2008.06.045. [DOI] [PubMed] [Google Scholar]
- Schenk GJ, Engels B, Zhang YP, Fitzsimons CP, Schouten T, Kruidering M, et al. A potential role for calcium/calmodulin-dependent protein kinase-related peptide in neuronal apoptosis: in vivo and in vitro evidence. Eur J Neurosci. 2007;26:3411–20. doi: 10.1111/j.1460-9568.2007.05956.x. [DOI] [PubMed] [Google Scholar]
- Slee EA, Harte MT, Kluck RM, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–92. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koub D, Jiang H, Zhang L J. Role of Bcl-2 family of proteins in mediating apoptotic death of PC12 cells exposed to oxygen and glucose deprivation. Neurochem Res. 2005;46:73–81. doi: 10.1016/j.neuint.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Ravagnan L, Roumier T, Kroemer G. Mitochondria, the killer organelles and their weapons. J Cell Physiol. 2002;192:131–7. doi: 10.1002/jcp.10111. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–60. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Xing QL, Chen JW, Ma RQ. Protective effect of panaxatriol saponins on cerebral ischemia. J Sun Yat-sen Univ (Medical Sciences) 2008;29:705–10. [Google Scholar]
- Li GS, Tian JW, Feng FH, Yang JQ. Protective effect of ginsenoside Rb3 from rat cortex mitochondrial injuries due to cerebral ischemia. Chin J New Drug. 2006;15:518–22. [Google Scholar]