Abstract
Cigarette smoking can harm fertility, but the existing research has targeted primarily on ovarian follicles, embryos or sex hormone. In this study, we tested cigarette smoke extract on ovulation, oocyte morphology and ovarian gene expression associated with inhibition of oxidative stress using C57BL/6 mice. Mice in the experimental group were administered a cigarette smoke extract (CSE) solution (2 mg/ml) orally daily, while the blank control group was given dimethylsulfoxide (DMSO). A positive control group (menadione) was used that received an intraperitoneal injection of 15 mg/kg menadione in oil solution daily. We found that the CSE group manifested a reduced diameter of zona pellucida-free oocyte (ZP-free OD) and a morphologically misshapen first polar body (PB). Our results suggest that CSE exposure is associated with a shrink size and poor quality of oocytes. Quitting smoking is a wise choice to ensure good fertility.
Introduction
The prevalence of smoking among women of reproductive age has increased worldwide over the last several years [1], [2]. There is evidence that 90% of smokers start this behavior during adolescence [3], and young women constitute the fastest-growing population of smokers [4]. It has been reported that cigarette smoking harms the reproductive system in many aspects [5], [6]. Cigarette smoke contains polycyclic aromatic hydrocarbons (PAHs) [e.g. benzo(a)pyrene (B[a]P)], aromatic amine, N-nitroso compounds, heavy metals [e.g. cadmium (Cd)], and so forth [7], [8]. Some studies have indicated a significantly higher level of smoking toxicants in reproductive tissues or fluids than in serum [9], [10], which suggested that the toxicants accumulated in reproductive organs [11]. And smoking may cause deleterious effects on ovary and abnormal sex steroid hormone concentrations [12], [13]. The adverse effects of cigarette smoking on fertility and their relation to premature ovarian failure have also been demonstrated [14]. Smoking is correlated to higher infertility risk [5], [15], lower fecundity rate [16], [17], lower in-vitro fertilization (IVF) success rates [18]–[20] and increased rate of spontaneous abortion [21]–[24].
Many of the studies investigating the mechanisms underlying cigarette smoking and fertility concerned the effects of the inherent toxicant molecules on follicles: e.g. B[a]P, a component of cigarette smoke, caused few of ovarian follicles [25], PAHs reduced numbers of primordial and primary follicles in rats and mice [26], and the cigarette toxicants stimulated reproductive organs in a way that was harmful to ovarian follicles, causing follicle depletion [27]–[29] and inhibition of follicle growth [13]. Huang focused much more on the embryos and found that cigarette smoke induces compromises to embryo development in vivo [11]. In addition, there are several studies about the effect of smoke on oocytes, such as thicker ZP, higher incidences of chromosomal abnormalities [30], [31] and shrink size [4]. Also, the number of retrived oocyte had been studied, without consistent opinion [32], [33]. However, there were few studies published simultaneously regarding ovulation number, oocyte morphology and ovarian gene expression to reflect the effect of cigarette smoke on oocyte or ovary before fertilization.
Besides, production of reactive oxygen species (ROS), which include superoxide anion [O2] and hydrogen peroxide [H2O2], is a physiological process and occurs in the cell mainly during the mitochondrial energy metabolism. O2 is transformed into a more stable ROS, H2O2 [34]. When H2O2 concentrations in the cytoplasm reach above the physiological threshold, it can be removed by cytosolic antioxidant systems of the cell. These antioxidant defense mechanisms may include both enzymatic such as catalase, glutathione peroxidase (GPx) [35], and superoxide dismutase (SOD) [36]. Oxidative stress reflects an imbalance between production of ROS and cellular antioxidant defense mechanisms [37], which may have serious consequences, for instance, enzymatic inactivation, DNA fragmentation, and irreversible damage of mitochondrial DNA, membrane lipids, and proteins, resulting in mitochondrial dysfunction and ultimately cell death [38], [39]. It has been found that the initiation of apoptotic cell death in ovarian follicles and granulosa cells by various stimuli is due to increased ROS [40]. SOD2 encodes the mitochondrial isoform of SOD and detoxifies ROS [41]. Heme oxygenase-1(HMOX1) can catalyze a biochemical reaction and the products of the HMOX reaction have an important effect, such as antioxidation [42]. Nuclear factor erythroid 2-related factor 2 (NRF2) regulates transcription of genes that encode enzymes important for protection against ROS [43]. Glutathione-s-transferases (GSTs) can catalyze the conjunction between intermediate metabolites of xenobiotic metabolism and glutathione (GSH), achieving detoxification [44]. As one of the GSTs, glutathione-S-transferase P1 (GSTP1) enzyme selectively detoxifies the carcinogenic epoxide of B[a]P, a highly carcinogenic metabolite of PAHs [45]. Glutathione-s-transferase Mu 1 (GSTM1), Mu 2 (GSTM2) and glutathione-s-transferase Alpha3 (GSTA3) also belong to the GSTs. One of the rate-limiting enzymes of GSH synthesis, glutamate cysteine ligase (GCL), is composed of modifier (GCLM) and catalytic subunits (GCLC) [46], which effect the detoxification directly.
Materials and Methods
Ethic statement
The study was approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University, and all animal studies were performed under an institutionally approved protocol according to the guidelines and the criteria from the committee.
Experimental animal preparation
Twenty-four four-week-old female C57BL/6 mice were purchased from the Laboratory Animal Centre of Zhongshan School of Medicine of Sun Yat-sen University. The number of the mice refers to the study from Sobinoff [47]. C57BL/6 mice has many advantages such as strain stability and easy-to-bred, and the sequencing of their genome has completed. So, this strain is always considered as a standard inbred strain, widely used in the genetics, immunology and pathology study. The mice were randomly divided into three groups, 8 for each, and maintained on a controlled light cycle schedule of 12∶12 h (light/dark) at 25°C with food ad libitum.
Preparation of cigarette smoke extract and menadione oil solution
We obtained cigarette smoke extract using the SHZ IIID-type, multi-use recycled water system. Joint the SHZ IIID-type, multi-use recycled water system with a filter flask, which contained 100 ml dimethylsulfoxide (DMSO, Sigma-Aldrich® D2650-100 ML; St. Louis Missouri USA). The filter with cigarette was inserted into the glass tube of the filter flask, and then the cigarette was lighted up under 0.1 MPa vacuum pressure. Changing the cigarette one by one after being burned out, we used 40 cigarettes for per 100 ml DMSO. The concentration was 8.767 g/100 ml DMSO.
Menadione powder (SIGMA M5625-100G) was dissolved in corn oil (Gold Arowana, China) to obtain a concentration of 2.55 mg/ml.
Animal dosing
We only gave a CSE oral solution to mice daily, and maturation of cumulus oocyte complexes (COCs) was allowed to occur in vivo; this would maintain stable absorbance of the CSE and a stable serum concentration of the inherent toxicants in smoke, unlike the smoke administered method via nose several times daily used by Huang [11], which may cause unstable serum concentration. Additionally, the effect of carbon monoxide was not tested.
The CSE group was only administered a 2 mg/ml CSE solution (with distilled water as solvent) orally daily ad libitum, while the control group was given an equal concentration of the DMSO solution (in distilled water) ad libitum. Those from CSE received 4.01 ml/d for each, while 5.86 ml/d in control group. The menadione group, as a positive control, was given an ip injection of 15 mg/kg of menadione oil solution daily and water ad libitum. The dosage and route of administration for menadione were based on several studies and were chosen with the intention of inducing partial ovotoxicity with minimal cytotoxicity [47],[48]. The procedures mentioned above were administrated for four weeks in four-week-old mice.
All mice were superovulated at eight weeks of age via ip injection of 5 IU of equine chorionic gonadotropin (eCG, Zhengjiang Modern Biotechnology, Tianjin, China) followed by ip administration of 5 IU of human chorionic gonadotropin (hCG, Yantai Northern pharmaceutical Co. Ltd, China) 48 h later.
Ovary removal and oocyte retrieval
Fourteen hours after hCG injection, all the mice were sacrificed. The COCs were isolated from oviducts followed by granulosa cell digestion with HYASE-10X (Vitrolife; Göteborg Sweden), cultured in G-1 PLUS (Vitrolife; Göteborg Sweden), and then observed microscopically.
Ovaries were surgically removed, placed in the cryopreservation tubes, and stored in the liquid nitrogen.
Oocyte observation and measurement
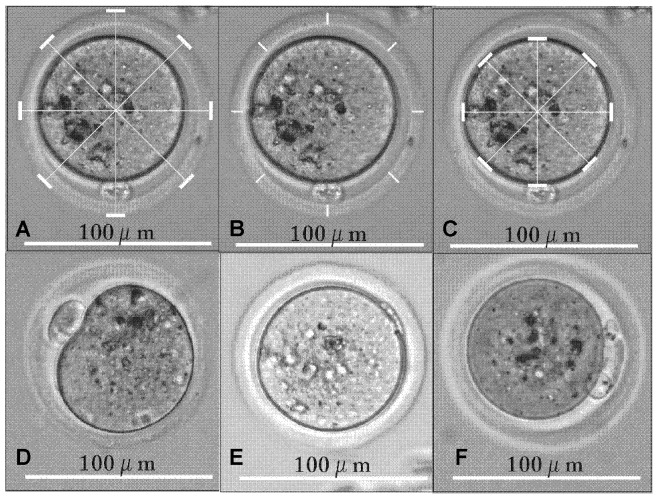
We counted the number of ovulations for every mouse. Measurements of oocyte diameter (OD), ZP thickness and ZP-free OD were taken from digital photos using a LEICA inverted microscope (LEICA DM IL LED; Wetzlar, Germany) at ×200 magnification mounted with a camera (LEICA DM6000 B; Wetzlar, Germany). Diameter was measured at four different locations to obtain a mean, while thickness was measured at eight different locations to obtain a mean (Figure A, B and C in Figure 1). The size of the perivitelline space (PVS) was calculated (PVS = OD–ZP-free OD–ZP thickness×2). All the measurements were performed with Corel Draw edition 12.0.0525. Additionally, we counted the numbers of the first PB in different types as shown in Figure D, E and F in Figure 1.
Figure 1. The measurement of OD, ZP and ZP-free OD and different types of first polar body.
These microscopy pictures were taken from digital photos using a LEICA inverted microscope (LEICA DM IL LED; Wetzlar, Germany) at ×200 magnification mounted with a camera (LEICA DM6000 B; Wetzlar, Germany). All the measurements were performed with Corel Draw edition 12.0.0525. Figure A. The white lines show the diameter of oocyte (OD). Figure B. The white lines show the thickness of zona pellucida (ZP). Figure C. The white lines show the diameter of ZP-free oocyte (ZP-free OD). Figure D. The first polar body with appropriate size, round shape and smooth surface (ARS-PB). Figure E. The first polar body with small size, strip-like shape and rough surface (SSR-PB). Figure F. Broken PB.
RNA extraction from ovaries
Total RNA was extracted from ovarian tissue samples and preserved in TRIzol reagent (Invitrogen; Carlsbad California USA) according to the manufacturer's instructions. Briefly, samples were thawed, placed at room temperature for 10 min, and 0.2 ml of chloroform was added per 1 ml of TRIzol reagent. Sample tubes were securely capped, briefly vortexed, placed at room temperature for 5 min, and then centrifuged at 12 000×g for 15 min at 4°C. The aqueous phase was then transferred to a fresh tube and RNA was precipitated by mixing with 0.5 ml isopropyl alcohol, and placed at −20°C for 30 min. Centrifugation was repeated before removing the supernatant. The RNA was washed twice with 0.5 ml 70% ethanol followed by repeated centrifugation before removing the supernatant. The final RNA pellet was dried and then dissolved in 50 µl of diethyl pyrocarbonate (DEPC)-reated water and placed in a bath at 65°C for 10 min.
Quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was used to assess the expression of ten genes (ACTIN, SOD2, GSTP1, HMOX1, GSTA3, NRF2, GSTM1, GSTM2, GCLM, GCLC) in the ovarian samples. First, cDNA was synthesized using the Reverse Transcription System (Promega A3500; Madison USA) according to the manufacturer's instructions. Briefly, 2 µl of total RNA was reverse transcribed by adding 4 µl of MgCl2 (25 mM), 2 µl of RT 10× Buffer, 2 µl of dNTP Mixture, 0.5 µl of Recombinant Rnasin Ribonuclease Inhibitor, 15 u of AMV Reverse Transcriptase (HC; Promega M9004; Madison USA), 0.5 µg of Oligo(dT)15 Primer, and nuclease-free water to a final volume of 20 µl.
The cDNA was prepared in a Mastercycler nexus flat PCR system (Eppendorf; Hamburg Germany) using the following program: 1 cycle at 42°C for 15 min, 1 cycle at 95°C for 5 min and 1 cycle at 4°C for 5 min. At the end of the run, samples were stored at 4°C. The GoTaq® q PCR Master Mix (Promega A6001; Madison USA) was used according to the manufacturer's instructions to perform qPCR analysis of the genes mentioned above and Actin transcript frequency. Briefly, 10 µl of GoTaq qPCR Master Mix, 10 µl of the appropriate primer designed against published mRNA sequences (Table 1) at a concentration of 0.4 µM, and 50-100 ng of cDNA template were added for a final reaction volume of 20 µl. The reaction was performed in the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories #185-5195; Hercules California USA) using the following program: 1 cycle at 95°C for 30 sec, and 40 cycles of 95°C for 5 sec followed by 60°C for 30 sec. After cycling, the temperature was increased starting from 60°C to 95°C at a rate of 0.5°C every 5 sec to generate a melting curve. Samples were amplified in triplicate and a melting curve was completed after each PCR reaction to ensure fluorescence quantification was specific to a single PCR product. The amplification data obtained with qRT-PCR for individual genes was expressed as cycle threshold (Ct), which subtracted the Ct for ACTIN to obtain △Ct (△Ct = Ct [specific gene]-Ct [ACTIN]) followed by 2−△Ct.
Table 1. Sequences of 10 Relevant mRNA.
| Primer name | Gene Bank Accession Number | Sequence(5'to3') |
| ACTIN-f* | NM_001148849.1 | TTGCTGACAGGATGCAGAAG |
| ACTIN-r* | NM_001148849.1 | ACATCTGCTGGAAGGTGGAC |
| SOD2-f | NM_013671.3 | CAGACCTGCCTTACGACTATGG |
| SOD2-r | NM_013671.3 | CTCGGTGGCTTGAGATTGTT |
| GSTP1-f | NM_013541.1 | ATGCCACCATACACCATTGTC |
| GSTP1-r | NM_013541.1 | GGGAGCTGCCCATACAGAC |
| HMOX1-f | NM_010442.2 | AAGCCGAGAATGCTGAGTTC |
| HMOX1-r | NM_010442.2 | GCCGTGTAGATATGGTACAAGGA |
| GSTA3-f | NM_001077353.1 | AAGAATGGAGCCTATCCGGTG |
| GSTA3-r | NM_001077353.1 | AGGTCATCCCGAGTTTTCAGAA |
| NRF2-f | NM_010902.3 | CAGCATGTTACGTGATGAGG |
| NRF2-r | NM_010902.3 | GCTCAGAAAAGGCTCCATCC |
| GSTM1-f | NM_010358.5 | AGCACCCTGGCCTTCTGCACT |
| GSTM1-r | NM_010358.5 | TTCGCAGAAACGGGCTGTGAG |
| GSTM2-f | NM_008183.3 | TACACCATGGGGGACGCTCCT |
| GSTM2-r | NM_008183.3 | TGGCCAACTGTATGCGGGTGT |
| GCLM-f | NM_008129.4 | GCCACCAGATTTGACTGCCTTT |
| GCLM-r | NM_008129.4 | CAGGGATGCTTTCTTGAAGAGCTT |
| GCLC-f | NM_010295.2 | ATGTGGACACCCGATGCAGTATT |
| GCLC-r | NM_010295.2 | TGTCTTGCTTGTAGTCAGGATGGTTT |
*f: forword primer. r: reverse primer.
SOD2: superoxide dismutase; GSTP1: glutathione-S-transferase P1; HMOX1: heme oxygenase-1; NRF2: nuclear factor erythroid 2-related factor 2; GSTM1: glutathione S-transferase Mu 1; GSTM2: glutathione S-transferase Mu 2; GSTA3: glutathione S-transferase alpha3; GCLM: glutamate cysteine ligase modifier subunit; GCLC: glutamate cysteine ligase catalytic subunit.
Statistical analysis
All statistical analyses were performed using SPSS Statistics 17.0. Differences were considered to be significant at P<0.05. The Shapiro-Wilk test was used to determine whether the data were normally distributed (P>0.05). One-way Analysis of variance (ANOVA), the non-parametric Tamhane's T2 test, Kruskal-Wallis, Mann-Whitney U tests or Pearson chi-square test were used in the analysis of gene expression, ovulation number and oocyte morphology.
Results
Ovulation quantity and oocyte morphology
The number of oocytes ovulated from the CSE group showed an increase compared with the control group (CSE 21.86±3.70, control 12.00±2.05, P = 0.061, ANOVA, LSD) (Table 2) without statistical significance. One mouse didn't ovulate, which happened in all the three groups (Dataset S1).
Table 2. The comparison of ovulation quantity and oocyte morphology of the 3 groups.
| Results | Control | CSE | Menadione | |
| Ovulation Quantitya (/mouse mean±SE) | 12.00±2.05 | 21.86±3.70 | 17.43±4.31 | |
| Oocyte Morphology | ZP thicknessa (µm mean±SE) | 7.59±0.07* | 7.43±0.05 | 7.31±0.06 |
| ZP-free ODa (µm mean±SE) | 76.56±0.12# | 76.10±0.13 | 76.41±0.12 | |
| PVSb (µm median) | 9.1328* | 9.2818* | 10.0474 | |
| ODc (µm median) | 100.7656 | 100.6098 | 101.1314 | |
| ARS-PB rate (%) | 15.19%# | 6.62% | 7.62% | |
| SSR-PB rate (%) | 7.59%# | 24.26%* | 13.33% | |
| Immature oocyte rate (%) | 3.57% | 3.27%* | 9.84% | |
| The rate of broken PB (%) | 2.47% | 8.11% | 4.55% | |
ZP: zona pellucida. PVS: perivitelline space. OD: oocyte diameter. ARS-PB: the first polar body with appropriate size, round shape and smooth surface. SSR-PB: the first polar body with small size, strip-like shape and rough surface. CSE: cigarette smoke extract.
The data was normally distributed (P>0.05, Shapiro-Wilk), with equal variances (P>0.05, ANOVA).
The data from at least one group was not normally distributed (P<0.05, Shapiro-Wilk test).
The data was not normally distributed (P<0.05, Shapiro-Wilk test).
*<0.05 versus menadione.
<0.05 versus CSE.
The ZP thickness non-significantly reduced in the CSE group (CSE 7.43±0.05, control 7.59±0.07, P = 0.082, LSD), while it was significantly decreased in the menadione group (menadione 7.31±0.06, control 7.59±0.07, P = 0.004, LSD) compared with the control group (Table 2, Dataset S2).
There was a significant reduction in ZP-free OD in the CSE group compared with the control group (CSE 76.10±0.13, control 76.56±0.12, P = 0.018, LSD). Also, there was a decrease in the CSE group, compared with the menadione group (CSE 76.10±0.13, menadione 76.41±0.12, P = 0.068, LSD) (Table 2), though with no significance (Dataset S3).
The PVS of the CSE group appeared visually to be larger than in the control group, but not to a statistically significantly extent (CSE 9.2818, control 9.1328, P = 0.379, Mann-Whitney test). However, that of the menadione group was significantly larger than the PVS of either the CSE (menadione 10.0474, CSE 9.2818, P = 0.024, Mann-Whitney test) or control groups (menadione 10.0474, control 9.1328, P = 0.002, Mann-Whitney test) (Table 2, Dataset S4).
The OD in the CSE group appeared smaller than in the control group, but not significantly (CSE 100.6098, control 100.7656, P = 0.642, Mann-Whitney test). In the menadione group there was a contrary change, with the OD visually larger than in control group, but also not significant (menadione 101.1314, control 100.7656, P = 0.192, Mann-Whitney test), with a non-significantly greater size compared with the CSE group (menadione 101.1314, CSE 100.61, P = 0.084, Mann-Whitney test) (Table 2, Dataset S5).
The morphology of the first PB
The morphologic classification for the first PB is shown in Figure D, E and F in Figure 1. There was a significant reduction in the rate of the first PB with appropriate size, round shape and smooth surface (ARS-PB)(Table 2) in the CSE group compared with the control group (CSE 6.62%, control 15.19%, P = 0.041, Pearson Chi-Square) (Dataset S6).
The rate of the first PB with small size, strip-like shape and rough surface (SSR-PB) is shown in Table 2. There was a significant increase in the incidence of SSR-PB in the CSE group compared with the controls (CSE 24.26%, control 7.59%, P = 0.002, Pearson Chi-Square) and the menadione group (CSE 24.26%, menadione 13.33%, P = 0.034, Pearson Chi-Square) (Dataset S6).
The rate of broken PB in the CSE group was higher than for the menadione group, while the latter was higher than the control group, although these changes were not statistically significant (CSE 8.11%, control 2.47%, menadione 4.55%, P = 0.174, Pearson Chi-Square) (Table 2, Dataset S6).
Immature oocyte rate
The rate of immature oocyte in the menadione group was non-significantly higher than that in control (control 3.57%, menadione 9.84%, P = 0.089, Pearson Chi-Square) but significantly higher than the CSE group (CSE 3.27%, menadione 9.84%, P = 0.025, Pearson Chi-Square), while there was no significant difference between the latter two groups (control 3.57%, CSE 3.27%, P = 1.000, Pearson Chi-Square Continuous Correction) (Table 2, Dataset S6).
Ovarian gene expression
The expression of GSTM2 decreased in the CSE group, but not significantly, compared with the control group (CSE 0.3927±0.0897, control 0.5784±0.1210, P = 0.562, ANOVA post-hoc Tamhane's T2 test). GSTM2 expression in the menadione group was significantly lower than in the control group (menadione 0.1433±0.0246, control 0.5784±0.1210, P = 0.025, ANOVA post-hoc Tamhane's T2 test) and was attenuated compared with the CSE group (menadione 0.1433±0.0246, CSE 0.3927±0.0897, P = 0.093, ANOVA post-hoc Tamhane's T2 test) (Table 3) but not to a statistically significant extent (Dataset S9).
Table 3. The comparison of gene expression of the 3 groups.
| Gene Expression Result | Control | CSE | Menadione |
| ACTIN | 1 | 1 | 1 |
| GSTM1a (2−△Ct mean±SE) | 0.3153±0.0604* | 0.3396±0.0576* | 0.1216±0.0183 |
| GSTM2a (2−△Ct mean±SE) | 0.5784±0.1210* | 0.3927±0.0897 | 0.1433±0.0246 |
| GSTA3b (2−△Ct median) | 0.0057* | 0.0048 | 0.0024 |
| SOD2b (2−△Ct median) | 0.094 | 0.0821 | 0.0805 |
| GSTP1b (2−△Ct median) | 0.0562 | 0.049 | 0.0268 |
| HMOX1b (2−△Ct median) | 0.0093 | 0.0093 | 0.0073 |
| NRF2b (2−△Ct median) | 0.2749 | 0.2413 | 0.1339 |
| GCLM b (2−△Ct median) | 0.0766 | 0.0712 | 0.0538 |
| GCLC b (2−△Ct median) | 0.0291 | 0.0324 | 0.0209 |
CSE: cigarette smoke extract. SOD2: superoxide dismutase. GSTP1: glutathione-S-transferase P1. HMOX1: heme oxygenase-1. NRF2: nuclear factor erythroid 2-related factor 2. GSTM1: glutathione S-transferase Mu 1. GSTM2: glutathione S-transferase Mu 2. GSTA3: glutathione S-transferase alpha3. GCLM: glutamate cysteine ligase modifier subunit. GCLC: glutamate cysteine ligase catalytic subunit.
The data was normally distributed (P>0.05, Shapiro-Wilk), with unequal variances (P<0.05, ANOVA), and was analysed with Tamhane's T2 test.
The data from at least one group was not normally distributed (P<0.05, Shapiro-Wilk test).
*<0.05 versus menadione.
GSTM1 expression in the CSE group was insignificantly higher than in the control group (CSE 0.3396±0.0576, control 0.3153±0.0604, P = 0.989, ANOVA post-hoc Tamhane's T2 test); in contrast, there was a significant diminution in the expression in the menadione group compared to the control group (menadione 0.1216±0.0183, control 0.3153±0.0604, P = 0.044, ANOVA post-hoc Tamhane's T2 test), there was a similar significant difference between the CSE and menadione groups (CSE 0.3396±0.0576, menadione 0.1216±0.0183, P = 0.025, ANOVA post-hoc Tamhane's T2 test) (Table 3, Dataset S8).
GSTA3 expression in ovaries from the CSE group was lower than that in the control group (CSE 0.0048, control 0.0057, P = 0.271, Mann-Whitney test), and that of the menadione group was also changed (CSE 0.0048, menadione 0.0024, P = 0.141, Mann-Whitney test). Although neither of these two changes was significant, there was a significant decrease in expression in the menadione group compared with the control group (menadione 0.0024, control 0.0057, P = 0.049, Mann-Whitney test) (Table 3, Dataset S14). Expression of all other genes was unaffected by treatments (Table 3, Dataset S7, S10-S13, S15-S16).
Discussion
From our study we have concluded that exposure to CSE alters several reproductive parameters in mice: a reduction in the ZP-free oocyte size and the number of ARS-PB; more SSR-PB.
According to our data, a positive control (menadione) was successfully established with a significantly lower level of GSTM1, GSTM2 and GSTA3, a thinner ZP, larger PVS, and higher rate of immature oocyte.
Tobacco smoke as a source of exogenous pro-oxidants, such as ROS and free radical generators, can cause oxidative damage [49]–[51], and smoking may increase ROS and the depletion of redox scavengers in peripheral blood [49], [52]. Shifting the dynamic balance between pro-oxidation and antioxidation can lead to oxidative stress [53], [54]. Active smoking affects the pro-oxidant/antioxidant balance inside the pre-ovulatory ovarian follicle in women undergoing ovulation induction for IVF [55]. Siddique [56] assessed the influence of cigarette smoke condensate (CSC) and B[a]p on the levels of oxidative stress biomarkers, in in-vitro spent media of follicle cells and concluded that CSC and B[a]p exposure could induce oxidative stress in ovarian follicles. Similarly, Gannon [29] reported that exposure to smoke components caused oxidative stress with increased heat shock protein 25 (Hsp25), a kind of stress protein, and decreased SOD activities. In our study, SOD2 expression had a slight decline in CSE just like the positive control group, though non-significantly. In addition, similar to the remarkable decrease in the menadione positive control group, the expression of GSTM2 and GSTA3 in CSE exposure ovary had a mildly reduced level compared with control group. GSTs, a super-gene family composed of multifunctional enzyme systems [57], [58], catalyze the conjunction between intermediate metabolites of xenobiotic metabolism and GSH. The conjugates gain a reduced toxicity and are then easy to be expelled. This process exerts an important effect on the cellular detoxification of electrophilic compounds and the antioxidative reactions protecting lipids [40]. Both GSTM2, a cell-type GSTs, and GSTA3, belonging to the α class GSTs [42], [44], are involved in antioxidative reactions. Lim [40] demonstrated that the expression of GSTM2 in the ovary may be significant in protecting oocytes from toxic substances and the decrease in mRNA expression of the cytosolic antioxidant GSTM2 is involved in ovarian oxidative damage to lipids, proteins, DNA, and other cellular components vital for maintaining ovarian function and fertility.
The effects of cigarette smoke on oxidative stress are well known as are the effects of smoke on cellular apoptosis [59]–[61]. Increased lipid peroxidation, reduced glutathione contents, increased catalase activity, decreased SOD activity, cytoplasmic retractions and fewer intercellular junctions were observed in granulosa cells exposed to Cd [62], [63], a heavy metal compound in cigarette smoke. In vitro, B[a]P was shown to inhibit gap junction formation [64], and junctions being indispensable for oocyte-granulosa cell cross-talk [65]. So we can hypothesis that cigarette smoke may have detrimental effects on oocyte through inducing oxidative stress and injuring granulosa cells.
The studies about the effect of smoke on oocyte are few. Smokers present a lower estradiol (E2) level during ovarian stimulation in IVF [66], [67]. Inhibition of follicle growth and decreased E2 synthesis were demonstrated [13], [68], which was associated with the oocyte of poor quality. Sobinoff's study [69] concluded that B[a]P exposure caused mitochondrial leakage resulting in reduced oolemma fluidity and impaired fertilization in adulthood, resulting in oocyte aging and dysfunction, which was supported by Gruber's finding [70]. In our study, OD in the CSE group was non-significantly less than that in the control group, in contrast to the positive control (menadione); and there was a notable reduction in the ZP-free OD in the CSE group compared with the control group. Similarly, a smaller OD in incipient antral follicles was found in mice after nicotine exposure and ex-smoking mice showed an increase in OD compared to smoking mice [4]. Some researchers have concluded that OD was clearly relevant to meiotic maturation and the developmental potential exhibited by embryos after in-vitro maturation, IVF, or in-vitro culture [71]. The reduction in oocyte size has been widely accepted to be one of the apparent characteristics of apoptosis [72]. Some investigators considered that it was related to Bax gene expression and oocyte destruction mediated by PAH correlated with activation of relevant genes governing programmed cell death (PCD) [73], [74]. There are also several studies about the effect of smoke on oocytes morphology, such as thicker ZP [30], [31], leading to difficult fertilization, though the alteration in our study was not significant.
Investigators have considered the morphology of PB to be one of the indices to be used for evaluation of overall oocyte viability [75] and an indicator of aging in ovulated oocytes [76]. However, studies on the relationship between cigarette smoke and first PB morphology are few. We demonstrated that the CSE group showed a noticeably lower rate of ARS-PB and a higher rate of SSR-PB than the control group, and exhibited a stronger effect than that observed in menadione group. Additionally, the incidence of broken PB after CSE exposure was greater, although it was not statistically significant. It was considered that the oocytes with smooth and intact PB are expected to engender a higher fertilization rate and better embryo quality [76]; and this type of PB has been correlated with an increase in development to the blastocyst stage and overall pregnancy rate [77], [78]. According to our study, though ZP thickness, PVS, immature oocyte rate and the rate of broken PB were non-significantly altered, we can conclude that following CSE exposure, the mouse oocyte is affected negatively.
Synthesizing all the researches mentioned above, it's reasonable to suppose that cigarette smoking may potentially emerge a lower rate of fertility and successful pregnancy, producing oocyte of poor quality, through oxidative stress. For further study, we will perform in-vitro fertilization or intracytoplasmic sperm injection (ICSI) in each group to prove this standpoint in the future.
In the research from Whitcomb [79], compared with non-smokers, smokers had higher levels of follicle-stimulating hormone (FSH) in the early follicular phase (7.9 mIU/mL versus 6.3 mIU/mL) after adjusting for potential confounding factors, such as age, similar to that from Cooper [80]. Freour [81] found that anti-Mullerian hormone (AMH) was significantly lower in smokers (3.06 versus 3.81 mg/l). Higher FSH and lower AMH, we know that, were associated with lower reserve and aging of ovary. Many studies involving IVF procedures provided evidence that cigarette smoke had deleterious effects on ovaries: lower sensitivity [82], [83] and fewer retrieved oocytes [67], [84]-[86]. The number of ovulation of our study had no statistically significant alteration, similar to the data from other researchers [19], [33]. Maybe more thorough and large studies are needed for a consistent consequence.
Combined with many evidences that smoke and its component causing follicle depletion [25]-[29] and the inhibition of follicle growth [13], it is reasonable to suppose that cigarette smoke may do harm to ovary, causing impaired ovary function, fewer follicles, oocyte of poor quality, through inducing oxidative stress.
Conclusion
According to our study, we suggested that CSE exposure was associated with a shrink size and poor morphology of oocytes and oxidative stress maybe the underlying mechanism. We certainly recommend that quitting smoking is a wise choice to ensure good fertility.
Supporting Information
Data of ovulation quantity.
(SAV)
Data of ZP thickness.
(SAV)
Data of ZP-free OD.
(SAV)
Data of PVS.
(SAV)
Data of OD.
(SAV)
Data of PB.
(XLS)
Ct of 3 replication of ACTIN.
(SAV)
Ct of 3 replication of GSTM1.
(SAV)
Ct of 3 replication of GSTM2.
(SAV)
Ct of 3 replication of GSTP1.
(SAV)
Ct of 3 replication of HMOX1.
(SAV)
Ct of 3 replication of NRF2.
(SAV)
Ct of 3 replication of SOD2.
(SAV)
Ct of 3 replication of GSTA3.
(SAV)
Ct of 3 replication of GCLC.
(SAV)
Ct of 3 replication of GCLM.
(SAV)
Acknowledgments
We thank all current and previous members of the Institute of Human Virology of Zhongshan School of Medicine of Sun Yet-san University and Pro. Zhaofeng Wang for guidance, support and administrative support.
Funding Statement
The source of funding that has supported the authors' study is from a scientific research project, "the study of the key technology of increasing the pregnancy rate and security of IVF-ET", of the Technological and Information Bureau of Guangzhou. The grant number is 2012Y2-00022. The URL of the funder's website is http://www.gzsi.gov.cn/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Europe WHOROf (2007) The European tobacco control report: 2007. WHO Regional Office Europe.
- 2.Organization WH (2008) WHO report on the global tobacco epidemic, 2008: the MPOWER package.
- 3. Sims TH (2009) From the American Academy of Pediatrics: Technical report—Tobacco as a substance of abuse. Pediatrics 124: e1045–1053. [DOI] [PubMed] [Google Scholar]
- 4. Paixao LL, Gaspar-Reis RP, Gonzalez GP, Santos AS, Santana AC, et al. (2012) Cigarette smoke impairs granulosa cell proliferation and oocyte growth after exposure cessation in young Swiss mice: an experimental study. J Ovarian Res 5: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Augood C, Duckitt K, Templeton AA (1998) Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod 13: 1532–1539. [DOI] [PubMed] [Google Scholar]
- 6. Cooper AR, Moley KH (2008) Maternal tobacco use and its preimplantation effects on fertility: more reasons to stop smoking. Semin Reprod Med 26: 204–212. [DOI] [PubMed] [Google Scholar]
- 7. Giovannucci E, Martinez ME (1996) Tobacco, colorectal cancer, and adenomas: a review of the evidence. J Natl Cancer Inst 88: 1717–1730. [DOI] [PubMed] [Google Scholar]
- 8. Potter JD, Bigler J, Fosdick L, Bostick RM, Kampman E, et al. (1999) Colorectal adenomatous and hyperplastic polyps: smoking and N-acetyltransferase 2 polymorphisms. Cancer Epidemiol Biomarkers Prev 8: 69–75. [PubMed] [Google Scholar]
- 9. McLachlan JA, Dames NM, Sieber SM, Fabro S (1976) Accumulation of nicotine in the uterine fluid of the six-day pregnant rabbit. Fertil Steril 27: 1204–1213. [PubMed] [Google Scholar]
- 10. Paszkowski T (1998) [Concentration gradient of cotinine between blood serum and preovulatory follicular fluid]. Ginekol Pol 69: 1131–1136. [PubMed] [Google Scholar]
- 11. Huang J, Okuka M, McLean M, Keefe DL, Liu L (2009) Effects of cigarette smoke on fertilization and embryo development in vivo. Fertil Steril 92: 1456–1465. [DOI] [PubMed] [Google Scholar]
- 12. Soldin OP, Makambi KH, Soldin SJ, O'Mara DM (2011) Steroid hormone levels associated with passive and active smoking. Steroids 76: 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neal MS, Zhu J, Holloway AC, Foster WG (2007) Follicle growth is inhibited by benzo-[a]-pyrene, at concentrations representative of human exposure, in an isolated rat follicle culture assay. Hum Reprod 22: 961–967. [DOI] [PubMed] [Google Scholar]
- 14. Roth LK, Taylor HS (2001) Risks of smoking to reproductive health: assessment of women's knowledge. Am J Obstet Gynecol 184: 934–939. [DOI] [PubMed] [Google Scholar]
- 15. de Mouzon J, Belaisch-Allart J (2005) [Consequences on women's fecundity and on assisted reproductive technology]. J Gynecol Obstet Biol Reprod (Paris) 34 Spec No 1: 3S112–118. [PubMed] [Google Scholar]
- 16. Bolumar F, Olsen J, Boldsen J (1996) Smoking reduces fecundity: a European multicenter study on infertility and subfecundity. The European Study Group on Infertility and Subfecundity. Am J Epidemiol 143: 578–587. [DOI] [PubMed] [Google Scholar]
- 17. Hull MG, North K, Taylor H, Farrow A, Ford WC (2000) Delayed conception and active and passive smoking. The Avon Longitudinal Study of Pregnancy and Childhood Study Team. Fertil Steril 74: 725–733. [DOI] [PubMed] [Google Scholar]
- 18. Neal MS, Hughes EG, Holloway AC, Foster WG (2005) Sidestream smoking is equally as damaging as mainstream smoking on IVF outcomes. Hum Reprod 20: 2531–2535. [DOI] [PubMed] [Google Scholar]
- 19. Wright K, Trimarchi J, Allsworth J, Keefe D (2006) The effect of female tobacco smoking on IVF outcomes. Hum Reprod 21: 2930–2934. [DOI] [PubMed] [Google Scholar]
- 20. Waylen AL, Metwally M, Jones GL, Wilkinson AJ, Ledger WL (2009) Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Hum Reprod Update 15: 31–44. [DOI] [PubMed] [Google Scholar]
- 21. Harrison KL, Breen TM, Hennessey JF (1990) The effect of patient smoking habit on the outcome of IVF and GIFT treatment. Aust N Z J Obstet Gynaecol 30: 340–342. [DOI] [PubMed] [Google Scholar]
- 22. Zenzes MT (2000) Smoking and reproduction: gene damage to human gametes and embryos. Hum Reprod Update 6: 122–131. [DOI] [PubMed] [Google Scholar]
- 23. Winter E, Wang J, Davies MJ, Norman R (2002) Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod 17: 3220–3223. [DOI] [PubMed] [Google Scholar]
- 24. Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, et al. (1999) Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med 340: 333–339. [DOI] [PubMed] [Google Scholar]
- 25. Kristensen P, Eilertsen E, Einarsdottir E, Haugen A, Skaug V, et al. (1995) Fertility in mice after prenatal exposure to benzo[a]pyrene and inorganic lead. Environ Health Perspect 103: 588–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mattison DR PB, Meadows MJ, Miller MM, Malek A, London S (1989) The effect of smoking on oogenesis, fertilization, and implantation. Seminars in reproductive endocrinology 7: 291–304. [Google Scholar]
- 27.Madden JA, Hoyer PB, Devine PJ, Keating AF (2014) Acute 7,12-dimethylbenz[a]anthracene exposure causes differential concentration-dependent follicle depletion and gene expression in neonatal rat ovaries. Toxicol Appl Pharmacol. [DOI] [PMC free article] [PubMed]
- 28. Sobinoff AP, Beckett EL, Jarnicki AG, Sutherland JM, McCluskey A, et al. (2013) Scrambled and fried: cigarette smoke exposure causes antral follicle destruction and oocyte dysfunction through oxidative stress. Toxicol Appl Pharmacol 271: 156–167. [DOI] [PubMed] [Google Scholar]
- 29. Gannon AM, Stampfli MR, Foster WG (2012) Cigarette smoke exposure leads to follicle loss via an alternative ovarian cell death pathway in a mouse model. Toxicol Sci 125: 274–284. [DOI] [PubMed] [Google Scholar]
- 30. Jennings PC, Merriman JA, Beckett EL, Hansbro PM, Jones KT (2011) Increased zona pellucida thickness and meiotic spindle disruption in oocytes from cigarette smoking mice. Hum Reprod 26: 878–884. [DOI] [PubMed] [Google Scholar]
- 31. Shiloh H, Lahav-Baratz S, Koifman M, Ishai D, Bidder D, et al. (2004) The impact of cigarette smoking on zona pellucida thickness of oocytes and embryos prior to transfer into the uterine cavity. Hum Reprod 19: 157–159. [DOI] [PubMed] [Google Scholar]
- 32. Van Voorhis BJ, Dawson JD, Stovall DW, Sparks AE, Syrop CH (1996) The effects of smoking on ovarian function and fertility during assisted reproduction cycles. Obstet Gynecol 88: 785–791. [DOI] [PubMed] [Google Scholar]
- 33. Hannoun A, Nassar AH, Usta IM, Abu Musa A (2010) Effect of female nargile smoking on in vitro fertilization outcome. Eur J Obstet Gynecol Reprod Biol 150: 171–174. [DOI] [PubMed] [Google Scholar]
- 34. Khalil WA, Marei WF, Khalid M (2013) Protective effects of antioxidants on linoleic acid-treated bovine oocytes during maturation and subsequent embryo development. Theriogenology 80: 161–168. [DOI] [PubMed] [Google Scholar]
- 35. Nordberg J, Arner ES (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31: 1287–1312. [DOI] [PubMed] [Google Scholar]
- 36. Guerin P, El Mouatassim S, Menezo Y (2001) Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update 7: 175–189. [DOI] [PubMed] [Google Scholar]
- 37. Feugang JM, de Roover R, Moens A, Leonard S, Dessy F, et al. (2004) Addition of beta-mercaptoethanol or Trolox at the morula/blastocyst stage improves the quality of bovine blastocysts and prevents induction of apoptosis and degeneration by prooxidant agents. Theriogenology 61: 71–90. [DOI] [PubMed] [Google Scholar]
- 38. Lopes S, Jurisicova A, Sun JG, Casper RF (1998) Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod 13: 896–900. [DOI] [PubMed] [Google Scholar]
- 39. Kowaltowski AJ, Vercesi AE (1999) Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med 26: 463–471. [DOI] [PubMed] [Google Scholar]
- 40. Lim J, Luderer U (2011) Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod 84: 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bermejo-Alvarez P, Rosenfeld CS, Roberts RM (2012) Effect of maternal obesity on estrous cyclicity, embryo development and blastocyst gene expression in a mouse model. Hum Reprod 27: 3513–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mazurek B, Amarjargal N, Haupt H, Fuchs J, Olze H, et al. (2011) Expression of genes implicated in oxidative stress in the cochlea of newborn rats. Hear Res 277: 54–60. [DOI] [PubMed] [Google Scholar]
- 43. Yu B, Lin H, Yang L, Chen K, Luo H, et al. (2012) Genetic variation in the Nrf2 promoter associates with defective spermatogenesis in humans. J Mol Med (Berl) 90: 1333–1342. [DOI] [PubMed] [Google Scholar]
- 44. Hayes JD, Strange RC (2000) Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology 61: 154–166. [DOI] [PubMed] [Google Scholar]
- 45. Eaton DL, Bammler TK (1999) Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol Sci 49: 156–164. [DOI] [PubMed] [Google Scholar]
- 46. Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830: 3143–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sobinoff AP, Pye V, Nixon B, Roman SD, McLaughlin EA (2010) Adding insult to injury: effects of xenobiotic-induced preantral ovotoxicity on ovarian development and oocyte fusibility. Toxicol Sci 118: 653–666. [DOI] [PubMed] [Google Scholar]
- 48. Radjendirane V, Joseph P, Lee YH, Kimura S, Klein-Szanto AJ, et al. (1998) Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem 273: 7382–7389. [DOI] [PubMed] [Google Scholar]
- 49. Frei B, Forte TM, Ames BN, Cross CE (1991) Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochem J 277 (Pt 1): 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Church DF, Pryor WA (1985) Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 64: 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stone KK, Bermúdez E, Pryor WA (1994) Aqueous extracts of cigarette tar containing the tar free radical cause DNA nicks in mammalian cells. Environ Health Perspect 102: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou J, Yan X, Guo F, Sun N, Qian Z, et al. (2000) Effects of cigarette smoking and smoking cessation on plasma constituents and enzyme activities related to oxidative stress. Biomed Environ Sci 13: 44–55. [PubMed] [Google Scholar]
- 53. Hulea S, Olinescu R, Nita S, Crocnan D, Kummerow F (1994) Cigarette smoking causes biochemical changes in blood that are suggestive of oxidative stress: a case-control study. J Environ Pathol Oncol 14: 173–180. [PubMed] [Google Scholar]
- 54. Fraga CG, Motchnik PA, Wyrobek AJ, Rempel DM, Ames BN (1996) Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat Res 351: 199–203. [DOI] [PubMed] [Google Scholar]
- 55. Paszkowski T, Clarke RN, Hornstein MD (2002) Smoking induces oxidative stress inside the Graafian follicle. Hum Reprod 17: 921–925. [DOI] [PubMed] [Google Scholar]
- 56. Siddique S, Sadeu JC, Foster WG, Feng Yl, Zhu J (2014) In vitro exposure to cigarette smoke induces oxidative stress in follicular cells of F1 hybrid mice. J Appl Toxicol 34: 224–226. [DOI] [PubMed] [Google Scholar]
- 57. Strange RC, Spiteri MA, Ramachandran S, Fryer AA (2001) Glutathione-S-transferase family of enzymes. Mutat Res 482: 21–26. [DOI] [PubMed] [Google Scholar]
- 58. Stella L, Pallottini V, Moreno S, Leoni S, De Maria F, et al. (2007) Electrostatic association of glutathione transferase to the nuclear membrane. Evidence of an enzyme defense barrier at the nuclear envelope. J Biol Chem 282: 6372–6379. [DOI] [PubMed] [Google Scholar]
- 59. Lee CK, Brown BG, Rice WY Jr, Doolittle DJ (1989) Role of oxygen free radicals in the induction of sister chromatid exchanges by cigarette smoke. Environ Mol Mutagen 13: 54–59. [DOI] [PubMed] [Google Scholar]
- 60. van der Vaart H, Postma DS, Timens W, ten Hacken NH (2004) Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax 59: 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim SJ, Jeong HJ, Myung NY, Kim MC, Lee JH, et al. (2008) The protective mechanism of antioxidants in cadmium-induced ototoxicity in vitro and in vivo. Environ Health Perspect 116: 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nampoothiri LP, Agarwal A, Gupta S (2007) Effect of co-exposure to lead and cadmium on antioxidant status in rat ovarian granulose cells. Arch Toxicol 81: 145–150. [DOI] [PubMed] [Google Scholar]
- 63. Paksy K, Rajczy K, Forgács Z, Lázár P, Bernard A, et al. (1997) Effect of cadmium on morphology and steroidogenesis of cultured human ovarian granulosa cells. J Appl Toxicol 17: 321–327. [DOI] [PubMed] [Google Scholar]
- 64. Sharovskaya J, Kobliakova I, Solomatina N, Kobliakov V (2006) Effect of some carcinogenic and non-carcinogenic polycyclic aromatic hydrocarbons on gap junction intercellular communication in hepatoma cell cultures. Eur J Cell Biol 85: 387–397. [DOI] [PubMed] [Google Scholar]
- 65. Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, et al. (2011) Effects of cigarette smoking on reproduction. Hum Reprod Update 17: 76–95. [DOI] [PubMed] [Google Scholar]
- 66. Van Voorhis BJ, Syrop CH, Hammitt DG, Dunn MS, Snyder GD (1992) Effects of smoking on ovulation induction for assisted reproductive techniques. Fertil Steril 58: 981–985. [PubMed] [Google Scholar]
- 67. Van Voorhis BJ, Dawson JD, Stovall DW, Sparks AE, Syrop CH (1996) The effects of smoking on ovarian function and fertility during assisted reproduction cycles. Obstet Gynecol 88: 785–791. [DOI] [PubMed] [Google Scholar]
- 68. Vidal JD, VandeVoort CA, Marcus CB, Lazarewicz NR, Conley AJ (2006) In vitro exposure to environmental tobacco smoke induces CYP1B1 expression in human luteinized granulosa cells. Reprod Toxicol 22: 731–737. [DOI] [PubMed] [Google Scholar]
- 69. Sobinoff AP, Pye V, Nixon B, Roman SD, McLaughlin EA (2012) Jumping the gun: smoking constituent BaP causes premature primordial follicle activation and impairs oocyte fusibility through oxidative stress. Toxicol Appl Pharmacol 260: 70–80. [DOI] [PubMed] [Google Scholar]
- 70. Gruber I, Just A, Birner M, Losch A (2008) Effect of a woman's smoking status on oocyte, zygote, and day 3 pre-embryo quality in in vitro fertilization and embryo transfer program. Fertil Steril 90: 1249–1252. [DOI] [PubMed] [Google Scholar]
- 71. Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T (1997) Bovine oocyte diameter in relation to developmental competence. Theriogenology 48: 769–774. [DOI] [PubMed] [Google Scholar]
- 72. Jin X, Xiao LJ, Zhang XS, Liu YX (2011) Apotosis in ovary. Front Biosci (Schol Ed) 3: 680–697. [DOI] [PubMed] [Google Scholar]
- 73. Perez G, Knudson C, Brown G, Korsmeyer S, Tilly J (1997) Resistance of BAX-deficient mouse oocytes to apoptosis induced by 7, 12-dimethylbenz [a] anthracene (DMBA) in vitro. Toxicologist 36: 250. [Google Scholar]
- 74. Tilly JL, Tilly KI, Perez GI (1997) The genes of cell death and cellular susceptibility to apoptosis in the ovary: a hypothesis. Cell Death Differ 4: 180–187. [DOI] [PubMed] [Google Scholar]
- 75. Balaban B, Urman B (2006) Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online 12: 608–615. [DOI] [PubMed] [Google Scholar]
- 76. Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, et al. (2000) Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod 15: 427–430. [DOI] [PubMed] [Google Scholar]
- 77. Ebner T, Moser M, Yaman C, Feichtinger O, Hartl J, et al. (1999) Elective transfer of embryos selected on the basis of first polar body morphology is associated with increased rates of implantation and pregnancy. Fertil Steril 72: 599–603. [DOI] [PubMed] [Google Scholar]
- 78. Ebner T, Moser M, Sommergruber M, Yaman C, Pfleger U, et al. (2002) First polar body morphology and blastocyst formation rate in ICSI patients. Hum Reprod 17: 2415–2418. [DOI] [PubMed] [Google Scholar]
- 79. Whitcomb BW, Bodach SD, Mumford SL, Perkins NJ, Trevisan M, et al. (2010) Ovarian function and cigarette smoking. Paediatr Perinat Epidemiol 24: 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cooper GS, Baird DD, Hulka BS, Weinberg CR, Savitz DA, et al. (1995) Follicle-stimulating hormone concentrations in relation to active and passive smoking. Obstet Gynecol 85: 407–411. [DOI] [PubMed] [Google Scholar]
- 81. Freour T, Masson D, Mirallie S, Jean M, Bach K, et al. (2008) Active smoking compromises IVF outcome and affects ovarian reserve. Reprod Biomed Online 16: 96–102. [DOI] [PubMed] [Google Scholar]
- 82. Elenbogen A, Lipitz S, Mashiach S, Dor J, Levran D, et al. (1991) The effect of smoking on the outcome of in-vitro fertilization-embryo transfer. Hum Reprod 6: 242–244. [DOI] [PubMed] [Google Scholar]
- 83. Hughes E, Yeo J, Claman P, YoungLai E, Sagle M, et al. (1994) Cigarette smoking and the outcomes of in vitro fertilization: measurement of effect size and levels of action. Fertil Steril 62: 807–814. [DOI] [PubMed] [Google Scholar]
- 84. El-Nemr A, Al-Shawaf T, Sabatini L, Wilson C, Lower A, et al. (1998) Effect of smoking on ovarian reserve and ovarian stimulation in in-vitro fertilization and embryo transfer. Hum Reprod 13: 2192–2198. [DOI] [PubMed] [Google Scholar]
- 85. Klonoff-Cohen H, Natarajan L, Marrs R, Yee B (2001) Effects of female and male smoking on success rates of IVF and gamete intra-Fallopian transfer. Hum Reprod 16: 1382–1390. [DOI] [PubMed] [Google Scholar]
- 86. Fuentes A, Muñoz A, Barnhart K, Argüello B, Díaz M, et al. (2010) Recent cigarette smoking and assisted reproductive technologies outcome. Fertil Steril 93: 89–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data of ovulation quantity.
(SAV)
Data of ZP thickness.
(SAV)
Data of ZP-free OD.
(SAV)
Data of PVS.
(SAV)
Data of OD.
(SAV)
Data of PB.
(XLS)
Ct of 3 replication of ACTIN.
(SAV)
Ct of 3 replication of GSTM1.
(SAV)
Ct of 3 replication of GSTM2.
(SAV)
Ct of 3 replication of GSTP1.
(SAV)
Ct of 3 replication of HMOX1.
(SAV)
Ct of 3 replication of NRF2.
(SAV)
Ct of 3 replication of SOD2.
(SAV)
Ct of 3 replication of GSTA3.
(SAV)
Ct of 3 replication of GCLC.
(SAV)
Ct of 3 replication of GCLM.
(SAV)