Abstract
Trichosanthin (TCS), extracted from the Chinese medicinal herb Trichosanthes kirilowi, has shown promise for the inhibition of tumor growth. However, its immunomodulatory effect on tumor–host interaction remains unknown. In this study, we focused on the effect of TCS on murine anti-tumor immune response in the 3LL Lewis lung carcinoma tumor model and explored the possible molecular pathways involved. In addition to inhibiting cell proliferation and inducing apoptosis in the 3LL tumor, TCS retarded tumor growth and prolonged mouse survival more significantly in C57BL/6 immunocompetent mice than in nude mice. This reflected the fact that the host immune system was involved in tumor eradication. Using FACS analysis, we found that TCS increased the percentage of effector T cells, particularly Interferon-gamma (IFN-γ) producing CD4+ and CD8+ T cells from tumor-bearing mice. TCS also promoted the vigorous proliferation of antigen-specific effector T cells, markedly increased Th1 cytokine secretion and elicited more memory T cells in tumor-bearing mice, consequently enhancing the anti-tumor response and inducing immune protection. Furthermore, we found that TCS upregulated the expression of tumor suppressor in lung cancer 1 (TSLC1) in 3LL tumor cells and the expression of its ligand, class I-restricted T cell-associated molecule (CRTAM), in effector T cells. Blocking TSLC1 expression with small interfering RNA (siRNA) significantly eliminated the effects of TCS on the proliferation and cytokine secretion of effector T cells, suggesting that TCS enhances anti-tumor immune response at least partially by boosting the interaction between TSLC1 and CRTAM. Collectively, our data demonstrate that TCS not only affects tumor cells directly, but also enhances anti-tumor immunity via the interaction between TSLC1 and CRTAM. These findings may lead to the development of a novel approach for tumor regression.
Keywords: anti-tumor immunity, CRTAM, immune protection, trichosanthin, TSLC1
Introduction
Several dietary components found in plant-derived foods have been recognized for their anti-carcinogenic properties in recent years.1, 2 Notably, these natural agents have fewer side effects than do many synthetic agents.3
Trichosanthin (TCS), isolated from the root tubes of Trichosanthes kirilowii Max,4, 5 is a single-chain peptide with 247 amino acid residues.6, 7 It has a broad spectrum of biological and pharmacological activities, including the stimulation of abortion,8 suppression of tumor growth9 and inhibition of HIV activity.10 TCS can inhibit tumor cell proliferation via suppression of the PKC/MAPK signaling pathway11 and induce apoptosis through downregulation of Bcl-2 and upregulation of Bax.12 Additionally, TCS can induce apoptosis of JAR cells via stimulating the production of reactive oxygen species.13
In addition to its direct effect on tumor cells, TCS shows immunomodulatory effects in naive mice. TCS has been found to be a T helper 2 (Th2)-type adjuvant that modulates the switching of immune responses to a Th2 pathway in a model of airway inflammation.14 It can also induce the expression of Th2 cytokines, such as IL-4, IL-10 and tumor-growth factor-β (TGF-β).15 In this way, TCS may induce Th2-type, rather than Th1-type, immunity in normal or inflammation conditions. However, the influence of TCS on immune response in tumors remains unknown.
Recent studies have reported that TCS has a demethylation function and can restore the activity of the tumor suppressor in lung cancer 1 (TSLC1), Syk and RASSF1A genes, indicating a possible mechanism for TCS inhibition of tumor growth.3, 16 TSLC1 is a tumor suppressor gene that is widely expressed on stromal cells, but it is always lost because of promoter hypermethylation in tumor tissue. Its ligand, class I-restricted T cell-associated molecule (CRTAM), is only expressed on activated T cells, and the interaction between TSLC1 and CRTAM may promote the proliferation of activated T cells and their secretion of interferon (IFN)-γ secretion, thereby enhancing the anti-tumor effectiveness of T cells.17, 18
In this study, we established an animal tumor model with the Lewis lung cancer cell line (3LL) in C57BL/6 mice in order to determine whether TCS is involved in the induction of anti-tumor immune response in tumor-bearing hosts.
Materials and methods
Mice
Female C57BL/6 (H-2b) mice and nude mice aged at 4–6 weeks were purchased from Shanghai Experimental Center, Chinese Academy of Science, and housed in a pathogen-free environment in center of Laboratory Animal, Fudan University. All animal experiments were performed according to the Guide for the Care and Use of Medical Laboratory Animals (Ministry of Health, China, 1998) and with the ethical approval of the Shanghai Medical Laboratory Animal Care and Use Committee as well as the Ethical Committee of Fudan University.
Cell culture
The 3LL cell line (mouse Lewis lung cancer cell line) and FBL3 cell line (erythroleukemia cells) were purchased from Chinese Academy of Science. Tumor cell lines were cultured at 37 °C under 5% CO2 in a RPMI 1640 (Gibco, Grand Island, NY, USA) medium containing 10% heat-inactivated fetal bovine serum and supplemented with 2 mM glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin sulfate.
Cell growth inhibitory activities
Cell growth-inhibitory activities of TCS on 3LL cells were evaluated by CCK-8 assay (Cell Counting Kit-8; Dojindo, Kumamoto, Japan). 3LL was seeded in 96-well plates (Corning, New York, USA) at a plating density of 1×104/well, 24 h later, cells were exposed to TCS (Shanghai Jinshan Pharmaceutical Factory, Shanghai, China) at various doses (0, 25, 50 and 100 µg/ml) in fresh RPMI 1640 medium. TCS was diluted by phosphate-buffered saline (PBS). Four replicate wells for each treatment dose were performed. The plate was placed at 37 °C in 5% CO2 for various time points (24, 48 and 72 h), and then the wells were added into 10 µl CCK-8 reagent for appropriate time at 37 °C, and measured at 450 nm by the Bio-Rad Microplate Reader 680. Absorbance of untreated cells was considered as 100%. Results are expressed as a calculated ratio of (A450 of cells cultured in the presence of TCS−A450 of blank)/(A450 of cells cultured in the absence of TCS−A450 of blank).
Generation and treatment of tumor-bearing mice
Tumor-bearing mice were constructed as previously described.19 Briefly, immunocompetent C57BL/6 (H-2b) mice or nude mice were injected with 5×104 3LL tumor cells subcutaneously in the right anterior mammary region (day 0) and 4 days later, mice were randomly divided into two groups (n=6/group) and treated with (0.4 mg/kg/qod ×5 days, s.c.) or with equal volume of PBS on day 4, and continued to inject once every other day for total five times. Mice were monitored for evidence of tumor growth by palpation and inspection, and tumor size was measured with a digital caliper every 2 or 3 days. Tumor size was estimated according to the following formula: the maximal diameter×the perpendicular diameter. When tumor area was assessed to be ≥144 mm2, the mouse was regarded as dead. The survival of the tumor-bearing mice was also observed by daily assessment for over 100 days and rechallenged by 1×106 FBL3 and observed as above. All animals used in the experiments were treated humanely in accordance with Institutional Animal Care and Use Committee guidelines.
Flow cytometry
Nonspecific staining was blocked with fetal bovine serum (FBS) and stained with 1 µl /1×106 cells of FITC-CD3, PerCP-CD4, PE-CD8, FITC-CD44 and PE-CD62L (eBioscience, San Diego, CA, USA) and Annexin-V-FLUOS kit (Roche, Basel, Switzerland). Staining was performed at 4 °C for 30 min. Intracellular immuneofluorescent staining of FITC-IFN-γ and FITC-IL-4 and FITC-Foxp3 (eBioscience) were performed using Fixation/Permeabilization Kit (eBioscience) as instructed by the manufacturer. Cell-bound fluorescence was measured on a FACS FC500 and analyzed with CXP analysis software (Beckman, Miami, FL, USA).
Sorting of T cells
Splenocytes from naive or 3LL tumor-bearing C57BL/6 mice were obtained to isolate CD4+ cells and CD8+ cells by a Magnetic Activated Cell Sorting (MACS) system (Miltenyi Biotec, Aubum, CA, USA) according to the manufacturer's protocol. CD4+ and CD8+ lymphocytes were routinely 98% pure as determined by flow cytometry and were mixed as total T cells. Purified T cells were cultured at a density of 4×106 cells/ml in 24-well plates with RPMI 1640 medium supplemented with 10% FCS and CD3ε/28-Biotin MACSiBead Particles for 24 h (bead-to-cell ratio 1∶1), which was considered to be activated effector T cells.
Silencing of TSLC1 gene in 3LL cells
3LL cells were individually plated subconfluently onto each well of six-well tissue culture plates 24 h before transfection. Transient transfection of small interfering RNA (siRNA) pool of TSLC1 gene or non-targeting siRNA pool (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a final concentration of 50 nM was accomplished with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Culture medium was replaced with complete RPMI 1640 medium after overnight incubation and continued to culture for 24 h. The transfected or non-transfected cells were exposed to TCS (50 µg/ml) or PBS for another 24 h and were harvested for independent western blot analysis, RNA extraction and real-time RT-PCR or flow-based proliferation assay of T-cells in vitro.
Cell proliferation assay in vitro
T cell proliferation was assessed by BrdU incorporation using a BrdU ELISA colorimetric assay (Roche).20 Briefly, magnetic bead-purified mouse T cells were cultured at a 1∶10 ratio with inactivated 3LL cells in 96-well plates. T-cell proliferation was then measured after a 4-day culture period by analysis of BrdU incorporation according to the manufacturer's protocol. Results are expressed as the percentage of proliferation of responder T cells alone.
ELISA
MACS-sorted T cells from tumor-bearing mice were adjusted to 1×107 cells/ml with complete RPMI 1640 medium and added to each well of 24-well tissue culture plates coated with CD3 antibody. The transfected or non-transfected 3LL cells were treated with or without TCS (50 µg/ml) and were then inactivated by MMC, added (5×105 cells/ml) to each well of the appropriate plate. The cells were co-incubated at 37 °C in 5% CO2 for 4 days. Supernatants were collected for the cytokine level analysis of IL-2 and IFN-γ by the ELISA Kit (eBioscience) according to the manufacturer's protocol. In addition, the pelleted cells were harvested to extract total cellular RNA for real-time RT-PCR.
RNA extraction and real-time RT-PCR
The co-incubated T cells and sorted T cells from splenocytes were collected at indicated time points and total cellular RNA was prepared using Trizol reagent (Invitrogen) according to the manufacturer's protocol. One microgram RNA was reverse transcribed into single-stranded cDNA using PrimeScript RT reagent Kit (Perfect Real Time) (TaKaRa, Kyoto, Japan) according to the manufacturer's instructions. The real-time RT-PCR assays were performed using the 7500 Real-Time PCR System for quantitative mRNA detection and with FastStart Universal SYBR Green Master Kit (Roche, Basel, Switzerland). The primers for real-time PCR were: mouse CRTAM: 5′-GCCAAACGCTCACTCTAACG-3′ (forward) and 5′-GAAAGGAGTCACTAACACGGTCA-3′ (reverse); GAPDH: 5′-TGCACCACCAACTGCTTAGC-3′ and 5′-GCATGGACTGTGGTCATGAG-3′ (reverse). The relative level of the gene expression was obtained by calculating the ratio of threshold cycle numbers of the initial exponential amplification phase as determined by the Real-Time PCR System for the specific target gene and GAPDH. All experiments were performed in duplicate.
Western blot
Total proteins were extracted for western blot analyses in order to determine the expression levels of TSLC1. Western blot were done as described previously.2 Briefly, total cell protein was extracted using RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) including Complete Protease Inhibitor Cocktail (Roche, Basel, Switzerland) and the concentration was determined using the BCA Kit (Wuxi, China). Then, 20 µg of protein was loaded and electrophoresed on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel, transferred to polyvinylidine fluoride (PVDF) membrane. Membranes were blocked for 1 h in 5% dried milk in TBST at room temperature and probed with first antibody TSLC1 (Santa Cruz Biotechnology; 1∶1000 dilution) and GAPDH (Cell Signal, Danvers, MA, USA; 1∶5000 dilution) overnight at 4 °C with gently rotation. After extensive washing with TBST, the membranes were incubated with a horseradish peroxidase-conjugated species-specific secondary antibody (Cell Signal; 1∶50 000 dilution) for 1 h at 37 °C with gently rotation. After four additional washes, the immunoreactive bands were visualized by enhanced chemiluminescence (Thermo Scientific, Rockford, IL, USA) detection and quantitated by densitometry scanning.
Statistical analysis
Statistical analyses of the data were done using a one-way analysis of variance or Kruskal–Wallis test with PRISM 5.0 (GraphPad Software Inc., San Diego, CA, USA). P values less than 0.05 were considered statistically significant.
Results
TCS inhibited tumor proliferation in vitro
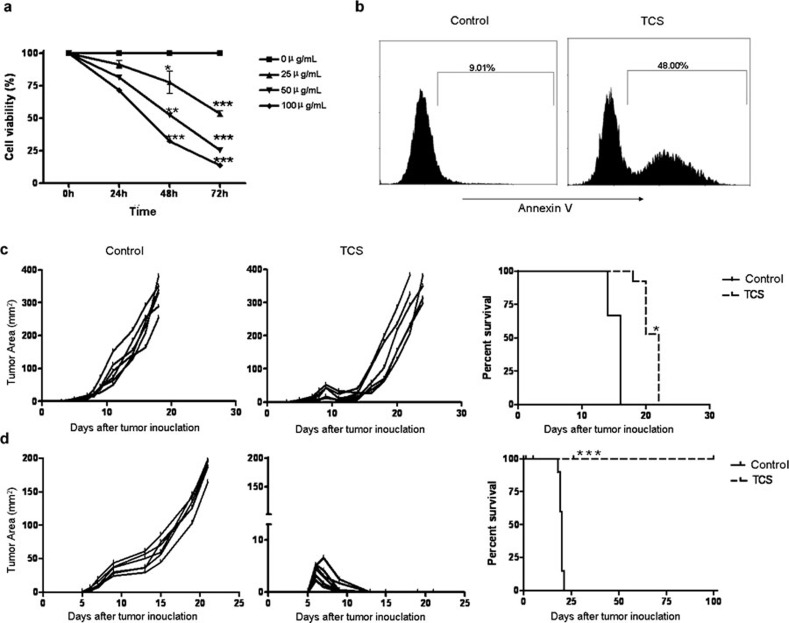
To investigate the direct effect of TCS on tumor cells in vitro, 3LL cells were treated with different doses of TCS (25, 50 and 100 µg/ml). Cell viability was then detected with a CCK-8 assay which is a redox assay similar to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. Our data showed that TCS inhibited the proliferation of 3LL cells in a time- and dose-dependent manner (Figure 1a). In addition, PI/Annexin V analysis revealed that a high percentage of 3LL cells underwent apoptosis after treatment with TCS for 48 h. The percentage of apoptotic 3LL cells increased from 9.01% to 48.00% (Figure 1b).
Figure 1.
TCS inhibited 3LL tumor growth both in vitro and in vivo. (a) 3LL tumor cells were treated for 24, 48 and 72 h in a 96-well plate in the presence of different doses of TCS (0, 25, 50 and 100 μg/ml). CCK-8 was added to the wells at the appropriate time and then detected at an optical density of 450 nm. (b) 3LL tumor cells were cultured in the absence or presence of 50 µg/ml TCS (IC50 was about 50 µg/ml). After 48 h, cells were harvested, stained with PI/Annexin V and then analyzed by flow cytometry. Nude mice (c) and C57BL/6 mice (d) were injected subcutaneously in the right inguinal region with 5×104 3LL tumor cells per mouse. TCS was injected subcutaneously on day 4 and then once every other day for a total of five injections. PBS was injected subcutaneously as a control. Mice with a tumor area reaching 144 mm2 were put to death. Data represent the mean value±s.e.m. from three independent experiments. *P<0.05, **P<0.01 and ***P<0.001. PBS, phosphate-buffered saline; PI, propidine iodide; TCS, trichosanthin.
TCS inhibited tumor growth and prolonged survival more significantly in immunocompetent mice than in nude mice
To further investigate the effect of TCS on tumor cells in vivo, both C57BL/6 immunocompetent mice and nude mice were injected subcutaneously into the right flank with 5×104 3LL tumor cells in 100 µL PBS. Beginning 4 days later, TCS (0.4 mg/kg) was injected next to the tumor site every 2 days, for a total of five injections. The mice were killed when the tumor area reached 144 mm2, and the survival times (in days) were recorded. Interestingly, we found that TCS inhibited tumor growth and prolonged survival more significantly in C57BL/6 immunocompetent mice than in nude mice. Tumor growth in the TCS-treated C57BL/6 mice was completely inhibited after day 13, and the mouse survival rate reached 100% (Figure 1d). However, tumor growth in the TCS-treated nude mice slowed temporarily during the first 12 days but then quickened in the following days (Figure 1c). These results suggested that the anti-tumor effect of TCS was mediated not only through a direct effect on tumor cells but also by the host immune system.
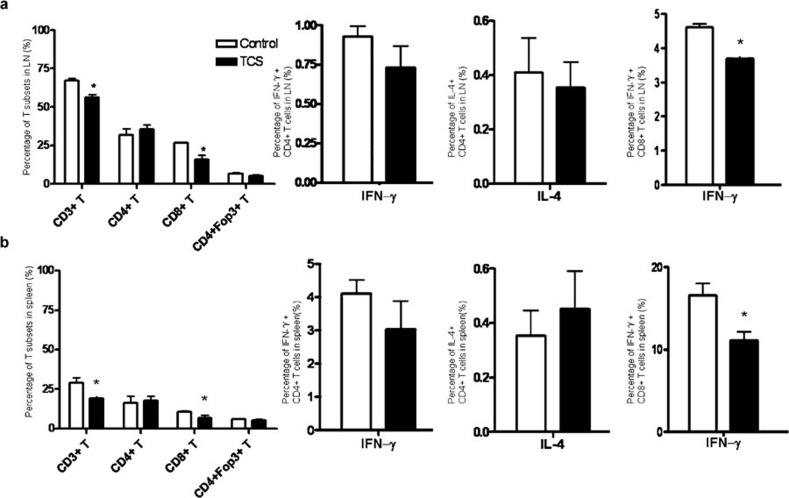
TCS decreased the percentage of CD8+ T cells and their IFN-γ production in naive mice
In order to investigate the immunomodulatory effect of TCS, we focused on its influence in a normal host and analyzed the changes of lymphocyte subsets by FACS. As shown in Figure 2a and b, TCS decreased the percentage of CD3+/CD8+ T cells in both the lymph nodes and the spleen, but the percentage of CD3+/CD4+ T cells, as well as that of regulatory T cells, was not significantly changed. In addition, the percentages of other immune cells, such as B cells, natural killer (NK) cells and dendritic cells (DCs), showed no significant change (data not shown). Next, we detected the cytokine production of T cells from naive mice treated with TCS. The percentage of CD8+/IFN-γ+ cells was markedly decreased in both the lymph node and the spleen. These data indicated that the therapeutic dose of TCS slightly inhibited the naive immune response, a result similar to those of other studies.21, 22
Figure 2.
TCS reduced the percentage of CD8+ T cells and their IFN-γ production in naive mice. T cells in (a) the LNs and (b) spleen from naive C57BL/6 mice following treatment with TCS or PBS were stained with fluorescent antibodies and detected by FACS. Foxp3 levels and cytokine production of CD4+ and CD8+ T cells derived from LN and spleen tissue were also analyzed by detecting intercellular cytokine and Foxp3 expression. *P<0.05. IFN, interferon; LN, lymph node; PBS, phosphate-buffered saline; TCS, trichosanthin.
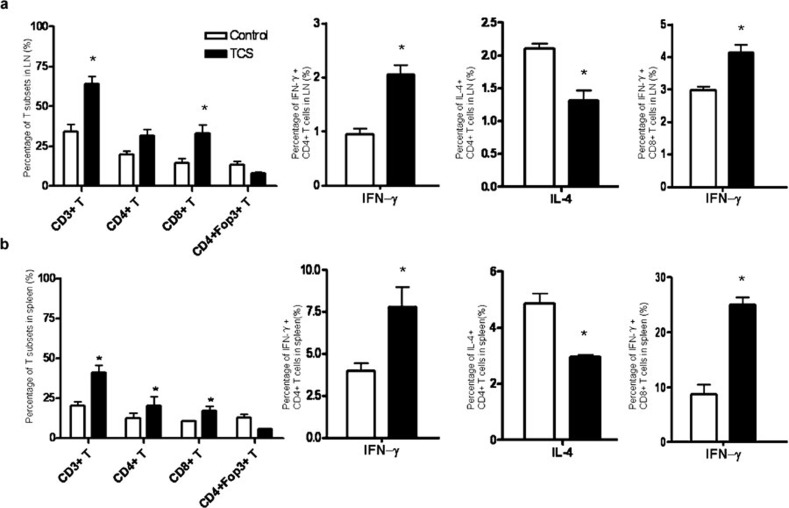
TCS increased the percentage of CD8+ T cells and their IFN-γ production in tumor-bearing mice
In naive mice, TCS slightly decreased the percentage of CD8+ T cells and their cytokine production. However, immunocompetent mice were more resistant to tumor growth following treatment with TCS than nude mice, indicating that the immune response was involved in the tumor regression (Figure 1d). Therefore, we analyzed the changes in the immune pattern in the 3LL Lewis tumor model following TCS treatment. Lymphocytes from lymph nodes (Figure 3a) and spleen (Figure 3b) were analyzed by FACS. Compared with the control group, the TCS-treated tumor-bearing mice had significantly elevated percentages of CD3+ T cells, CD4+ T cells and CD8+ T cells. The percentage of regulatory T cells was also downregulated slightly, but not to a statistically significant extent. However, the percentages of other immune cells—such as B cells, natural killer cells and dendritic cells—showed no significant change (data not shown).
Figure 3.
TCS increased the percentage of T cells and the cytokine production in tumor-bearing mice. T cells derived from (a) the LNs and (b) spleen of TCS-treated tumor-bearing mice were stained with fluorescent antibodies. Foxp3 and cytokine production of CD4+ and CD8+ T cells were also analyzed by detecting intercellular expression. *P<0.05. LN, lymph node; TCS, trichosanthin.
Unlike the naive mice, the tumor-bearing mice showed both a marked increase in the percentage of CD4+/IFN-γ+ cells and a significant decrease in IL-4-producing CD4+ T cells, in both the lymph nodes (Figure 3a) and the spleen (Figure 3b). The percentage of IFN-γ-producing CD8+ T cells was also elevated significantly. These data suggested that TCS induced a Th1-type pattern of cell-mediated immune response in the tumor-bearing mice that benefited the anti-tumor activity.
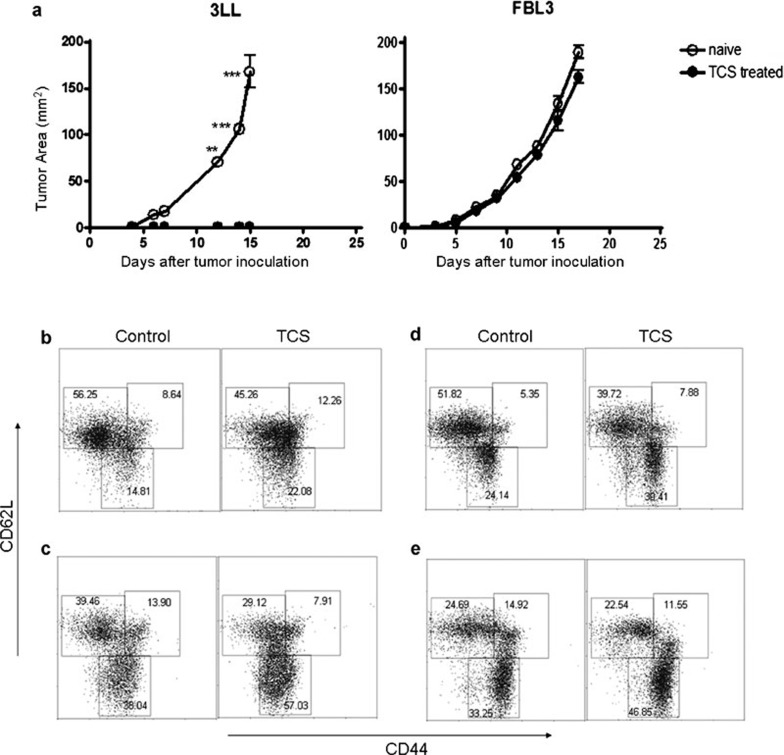
TCS induced tumor-specific immune protection in tumor-bearing mice
To assess whether TCS can induce a long-term anti-tumor immunity against parent tumor re-challenge, 3LL cells were injected into the left inguinal region of TCS-treated mice that had survived over 100 days. As a control, FBL3 tumor cells were inoculated into the right inguinal region. Naive mice treated in the same manner were used as another control. The TCS-treated mice were able to completely reject the second attack of 3LL tumor cells, whereas 3LL tumors grew persistently in the normal mice (Figure 4a). Meanwhile, the FBL3 tumors grew rapidly in both groups of mice without any significant difference—an indication that a specific anti-tumor effect had been elicited. Furthermore, the proportion of memory T cells (CD44+CD62L−) in the TCS-treated group was markedly elevated in both CD4+ (Figure 4b and c) and CD8+ (Figure 4d and e) T cells in both the lymph nodes (Figure 4b and d) and the spleen (Figure 4c and e). These results indicated that TCS-treated tumor-bearing mice bore a specific immune protection against 3LL tumors.
Figure 4.
Tumor-bearing mice treated with TCS were resistant to rechallenge with 3LL tumor cells. (a) Mice bearing 3LL tumors that had been treated with TCS and remained tumor-free for 100 days were rechallenged with 3LL tumor cells (1×105) implanted subcutaneously in the left inguinal region and FBL3 tumor cells (1×106) implanted subcutaneously in the right inguinal region. T cells from the (b, d) lymph node and (c, e) spleen were collected and stained with (b, c) anti-CD4 or (d, e) anti-CD8 antibody combined with anti-CD44 and anti-CD62L antibody. CD44+CD62L– cells are known to be memory cells.35 **P<0.01 and ***P<0.001. TCS, trichosanthin.
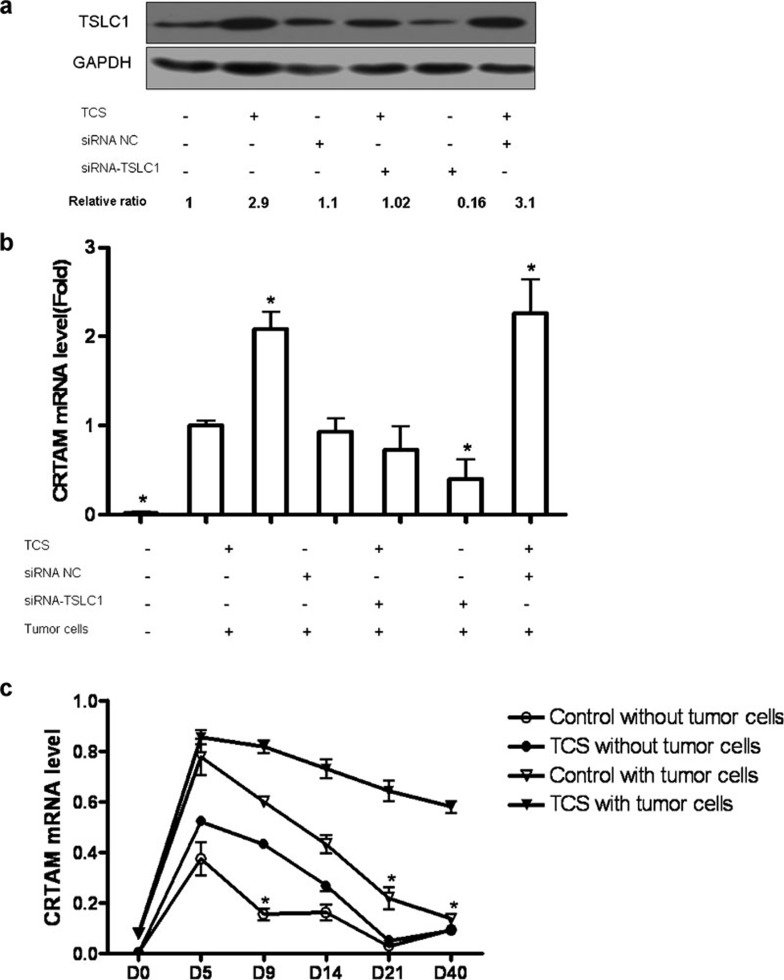
TCS upregulated the expression of TSLC1 on 3LL cells and CRTAM on T cells
To further elucidate the underlying mechanisms for the effects of TCS, we wished to test whether the tumor suppressor protein TSLC1 and its ligand, CRTAM, were involved in the enhancement of the immune response by TCS. We first used siRNA of the TSLC1 gene to block the expression of TSLC1. Western blot analysis indicated that TCS significantly upregulated the expression of TSLC1. However, this effect was eliminated when it was silenced with siRNA (Figure 5a). Moreover, the expression of CRTAM by T cells was augmented when T cells were cocultured with TCS-treated and MMC-inactivated 3LL cells and was downregulated when T cells were cocultured with TSLC1-silenced and MMC-inactivated 3LL cells (Figure 5b). Furthermore, in vivo experiments showed that TCS prolonged the expression of CRTAM on T cells acquired from tumor-bearing mice (Figure 5c). These results indicated that TCS could upregulate the expression of TSLC1 and CRTAM and may boost the interaction between these molecules.
Figure 5.
TCS upregulated TSLC1 expression on 3LL tumor cells and CRTAM expression on T cells. (a) 3LL tumor cells were transfected with TSLC1 siRNA pool or siRNA-NC pool. After 48 h, the medium was refreshed, and cells were treated with TCS (50 µg/ml) or PBS. After a further 24-h culture, a group of 3LL cells was harvested, and the total cell protein was extracted for an independent western blot analysis of TSLC1. (b) Another group of 3LL cells was inactivated with MMC and cocultured with T cells isolated from the spleens of tumor-bearing mice. After 12 h, we collected the cocultured T cells and extracted the total RNA for analysis of the expression of CRTAM by real-time RT-PCR. (c) T cells from tumor-bearing mice treated with TCS or PBS were collected and cocultured with or without MMC-treated 3LL tumor cells for 6 h. The mRNA level of CRTAM was also detected by real-time RT-PCR. *P<0.05. CTRAM, class I-restricted T cell-associated molecule; MMC, mitomycin C; PBS, phosphate-buffered saline; TCS, trichosanthin; TSLC1; tumor suppressor in lung cancer 1.
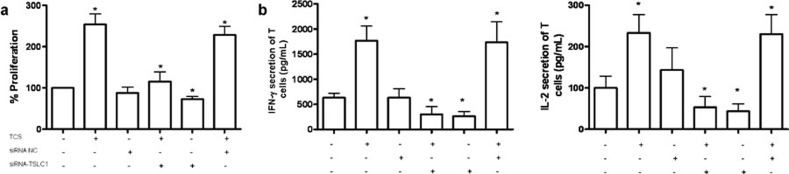
TCS enhanced the proliferation and cytokine secretion of T cells
To confirm the possibility that enhancing the interaction between TSLC1 and CRTAM could augment the activity of T cells purified from tumor-bearing mice, we used BrdU ELISA colorimetric assay to assess T-cell proliferation under the conditions indicated in Figure 5. The specific cell proliferation capacity was markedly increased compared with the control group and was decreased when TSLC1 was silenced with TSLC1 siRNA (Figure 6a). In addition, the secretion of Th1-type cytokines, such as IFN-γ and IL-2, was significantly elevated, and this effect was eliminated when TSLC1 was silenced (Figure 6b). Therefore, TCS may enhance the anti-tumor immune response in this model at least partly by strengthening the interaction between TSLC1 on 3LL cells and CRTAM on T cells.
Figure 6.
The proliferation of T cells and related cytokine secretion after coculture with TCS- or PBS-treated 3LL cells. MMC-inactivated 3LL cells were cocultured for 4 days with T cells acquired from tumor-bearing mice. (a) The specific proliferation of T cells was measured by BrdU incorporation. (b) The secretions of IFN-γ and IL-2 were detected by ELISA. *P<0.05. IFN, interferon; MMC, mitomycin C; PBS, phosphate-buffered saline; siRNA, small interfering RNA; TCS, trichosanthin; TSLC1; tumor suppressor in lung cancer 1.
Discussion
Increasing evidence over the past 20 years has indicated that TCS is cytotoxic in a variety of tumor cell lines both in vitro and in vivo.4 Thus, TCS is considered to be a potential biological agent for cancer treatment.23 It is capable of inhibiting protein synthesis and consequently inducing necrosis or apoptosis. However, the molecular basis of this effect has not yet been elucidated. TCS may stimulate the production of reactive oxygen species and induce apoptosis of tumor cells.24, 25 Mutation of the mistletoe lectin A chain (E166N and R169N) causes a concomitant decrease in ribosome-inactivating activity and apoptosis, suggesting that apoptosis may be a result of ribosome inactivation.26 In addition, TCS-induced tumor cell apoptosis can be enhanced by dexamethasone through inhibition of the NF-kappaB signaling pathway.27 In the present study, we demonstrated that TCS inhibited 3LL Lewis lung cancer growth both in vitro and in vivo. Moreover, TCS inhibited tumor growth and prolonged mouse survival more significantly in immunocompetent mice than in immunodeficient nude mice.
Dysfunction of the host's immune system can result from T-cell anergy, the existence of regulatory T cells, systemic defects of dendritic cells, deficient expression of immunomodulatory molecules and secretion of immunosuppressive cytokines by tumor cells.28 All of these can lead to a failure to mount a proper and specific anti-tumor immune response and are thus the key factors inhibiting the success of cancer immunotherapy. Unfortunately, previous studies indicated that TCS induced an immunosuppressive response. TCS augmented a Th2, rather than Th1, immune response in an inflammatory condition.14 Also, TCS induced HLA-associated immune suppression by activating IL-4/IL-10-secreting T cells, which might belong to the CD8+ Th2 subset.29 Such a suppression effect of TCS was evoked only if bone marrow-derived dendritic cells—instead of purified T cells—were treated with TCS in an ovalbumin (OVA)-specific T–BDC interaction.14 In our study, we observed a TCS-induced decrease in the percentage of CD3+CD8+ T cells in the lymph nodes and spleen of naive mice. By contrast, TCS increased the percentage of CD3+ and CD8+ T cells and the production of Th1-type cytokines in tumor-bearing mice, resulting in an enhancement of the anti-tumor immune response.
In addition, we looked at the changes in some important immune-related molecules on 3LL tumor cells after TCS treatment and found that levels of MHC class I and costimulatory molecules (such as CD86, H-2K and H-2D) were obviously raised compared with the control group (data not shown). These results suggested that alteration of immunogenicity and antigenicity of tumor cells might be involved in the enhancement of the anti-tumor immune response by TCS in tumor-bearing mice.
Furthermore, the apoptosis of HeLa cells by TCS has been reported to be closely related to the methylation of tumor suppressor genes, such as TSLC1, Sky, p16 and RASSF1A.3, 30 The universal demethylation role of TCS may be the mechanism behind its ability to induce apoptosis and inhibit tumor growth. Our data showed that TCS upregulated the expression of the tumor suppressor gene TSLC1 on tumor cells, along with the expression of its ligand, CRTAM, on activated T cells.31, 32 Blocking TSLC1 expression with siRNA eliminated the effect of TCS on T cells. The interaction between TSLC1 and CRTAM may promote the proliferation of activated T cells and the secretion of IFN-γ thereby enhancing the anti-tumor effects of T cells.33, 34 We speculate that the increase of CRTAM on T cells was caused by the high expression of TSLC1 on TCS-treated tumor cells. TCS boosted the interaction between TSLC1 and CRTAM, thereby causing both an enhancement of the anti-tumor immune response in tumor-bearing mice and a Th2-like immune pattern in naive mice. It remains to be seen whether the presentation of dendritic cells and the activation of natural killer (NK) cells are involved in this process.
In summary, our data indicate that, in addition to having direct toxic effects on tumor cells, TCS can enhance the anti-tumor immune response. This is done, at least partially, by boosting the interaction between TSLC1 and CRTAM. These findings provide a theoretical and experimental basis for the potential application of TCS as an anti-tumor drug and will also aid the development of new strategies using immune-based therapeutic interventions or the combination of chemotherapy and immunotherapy against cancers.
Acknowledgments
This work was supported by the National Key Technologies R&D Program of China during the Eleventh Five-Year Plan Period (2009ZX10004-104 and 2009ZX09301-011), National Science Foundation of China (30872378 and 81072408), the Science and Technology Commission of Shanghai Municipality (10JC1401100) in China and National 973 Project (2010CB912603 and 2011CB910400) in China. We thank the editors of Editage Company for professional editing of the article.
References
- Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- Xiong SD, Yu K, Liu XH, Yin LH, Kirschenbaum A, Yao S, et al. Ribosome-inactivating proteins isolated from dietary bitter melon induce apoptosis and inhibit histone deacetylase-1 selectively in premalignant and malignant prostate cancer cells. Int J Cancer. 2009;125:774–782. doi: 10.1002/ijc.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Huang LM. The effect of the trichosanthin on the methylation and expression of TSLC1 gene in cervical carcinoma HeLa cells. Lett Biotechnol. 2008;19:217–220. [Google Scholar]
- Shaw PC, Lee KM, Wong KB. Recent advances in trichosanthin, a ribosome-inactivating protein with multiple pharmacological properties. Toxicon. 2005;45:683–689. doi: 10.1016/j.toxicon.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Collins EJ, Robertus JD, LoPresti M, Stone KL, Williams KR, Wu P, et al. Primary amino acid sequence of alpha-trichosanthin and molecular models for abrin A-chain and alpha-trichosanthin. J Biol Chem. 1990;265:8665–8669. [PubMed] [Google Scholar]
- Chow TP, Feldman RA, Lovett M, Piatak M. Isolation and DNA sequence of a gene encoding alpha-trichosanthin, a type I ribosome-inactivating protein. J Biol Chem. 1990;265:8670–8674. [PubMed] [Google Scholar]
- Shaw PC, Yung MH, Zhu RH, Ho WK, Ng TB, Yeung HW. Cloning of trichosanthin cDNA and its expression in Escherichia coli. . Gene. 1991;97:267–272. doi: 10.1016/0378-1119(91)90061-f. [DOI] [PubMed] [Google Scholar]
- Ding GS. Important Chinese herbal remedies. Clin Ther. 1987;9:345–357. [PubMed] [Google Scholar]
- Chan WY, Huang H, Tam SC. Receptor-mediated endocytosis of trichosanthin in choriocarcinoma cells. Toxicology. 2003;186:191–203. doi: 10.1016/s0300-483x(02)00746-1. [DOI] [PubMed] [Google Scholar]
- Wang YY, Ouyang DY, Huang H, Chan H, Tam SC, Zheng YT. Enhanced apoptotic action of trichosanthin in HIV-1 infected cells. Biochem Biophys Res Commun. 2005;331:1075–1080. doi: 10.1016/j.bbrc.2005.03.230. [DOI] [PubMed] [Google Scholar]
- Wang P, Chen LL, Yan H, Li JC. Trichosanthin suppresses HeLa cell proliferation through inhibition of the PKC/MAPK signaling pathway. Cell Biol Toxicol. 2009;25:479–488. doi: 10.1007/s10565-008-9102-x. [DOI] [PubMed] [Google Scholar]
- Huang YL, Huang LM, Shi XL. Effect of trichosanthin on apoptosis induction in cervical carcinoma Hela cells and expression of Bax and Bcl-2. J Parct Train Med. 2007;35:32–35. [Google Scholar]
- Bronte V, Mocellin S. Suppressive influences in the immune response to cancer. J Immunother. 2009;32:1–11. doi: 10.1097/CJI.0b013e3181837276. [DOI] [PubMed] [Google Scholar]
- Wang QZ, Xu W, Habib N, Xu R. Potential uses of microRNA in lung cancer diagnosis, prognosis, and therapy. Curr Cancer Drug Targets. 2009;9:572–594. doi: 10.2174/156800909788486731. [DOI] [PubMed] [Google Scholar]
- Zhao S, Wang Y, Wei H. Trichosanthin induced Th2 polarization status. Cell Mol Immunol. 2006;3:297–301. [PubMed] [Google Scholar]
- Hua F, Shan BE, Zhao LM, Yao ZG, Peng H. Trichosanthin inhibited the proliferation of MDA-MB-231 cells and reversed the methylation of syk gene. Tumor. 2009;29:944–949. [Google Scholar]
- Boles KS, Barchet W, Diacovo T, Cella M, Colonna M. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood. 2005;106:779–786. doi: 10.1182/blood-2005-02-0817. [DOI] [PubMed] [Google Scholar]
- Yeh JH, Sidhu SS, Chan AC. Regulation of a late phase of T cell polarity and effector functions by Crtam. Cell. 2008;132:846–859. doi: 10.1016/j.cell.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Liu R, Xiong S, Zhang L, Chu Y. Enhancement of antitumor immunity by low-dose total body irradiation is associated with selectively decreasing the proportion and number of T regulatory cells. Cell Mol Immunol. 2010;7:157–162. doi: 10.1038/cmi.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratelli F, Lin Y, Zhu L, Yang SC, Heuzé-Vourc'h N, Zeng G. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- Xia XF, Wang F, Sui SF. Effect of phospholipid on trichosanthin adsorption at the air–water interface. Biochim Biophys Acta. 2001;1515:1–11. doi: 10.1016/s0005-2736(01)00348-0. [DOI] [PubMed] [Google Scholar]
- Gu YJ, Xia ZX. Crystal structures of the complexes of trichosanthin with four substrate analogs and catalytic mechanism of RNA N-glycosidase. Proteins. 2000;39:37–46. [PubMed] [Google Scholar]
- Li M, Li X, Li JC. Possible mechanisms of trichosanthin-induced apoptosis of tumor cells. Anat Rec (Hoboken) 2010;293:986–992. doi: 10.1002/ar.21142. [DOI] [PubMed] [Google Scholar]
- Zhang C, Gong Y, Ma H, An C, Chen D, Chen ZL. Reactive oxygen species involved in trichosanthin-induced apoptosis of human choriocarcinoma cells. Biochem J. 2001;355:653–661. doi: 10.1042/bj3550653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CY, Gong YX, Ma H, An CC, Chen DY. Trichosanthin induced calcium-dependent generation of reactive oxygen species in human choriocarcinoma cells. Analyst. 2000;125:1539–1542. doi: 10.1039/b005352j. [DOI] [PubMed] [Google Scholar]
- Langer M, Mockel B, Eck J, Zinke H, Lentzen H. Sitespecific mutagenesis of mistletoe lectin: the role of RIP activity in apoptosis. Biochem Biophys Res Commun. 1999;264:944–948. doi: 10.1006/bbrc.1999.1610. [DOI] [PubMed] [Google Scholar]
- Li M, Chen F, Liu CP, Li DM, Li X, Wang C, et al. Dexamethasone enhances trichosanthin-induced apoptosis in the HepG2 hepatoma cell line. Life Sci. 2010;86:10–16. doi: 10.1016/j.lfs.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Seliger B. Strategies of tumor immune evasion. Biodrugs. 2005;19:347–354. doi: 10.2165/00063030-200519060-00002. [DOI] [PubMed] [Google Scholar]
- Zhou H, Jiao Z, Pan J, Hong J, Tao J, Li N, et al. Immune suppression via IL-4/IL-10-secreting T cells: a nontoxic property of anti-HIV agent trichosanthin. Clin Immunol. 2007;122:312–322. doi: 10.1016/j.clim.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Xing XS, Huang LM, Huang YL, Chen MH, Shi XL. Detection of human cervical carcinoma Hela cell by methylation specific polymerase chain reaction. China J Mod Med. 2006;16:191–194. [Google Scholar]
- Kennedy J. A molecular analysis of NKT cells: indentification of a class-I restricted T cell-associated molecule (CRTAM) J Leukoc Biol. 2000;67:725–734. doi: 10.1002/jlb.67.5.725. [DOI] [PubMed] [Google Scholar]
- Fukami T, Fukuhara H, Kuramochi M, Maruyama T, Isogai K, Sakamoto M, et al. Promoter methylation of the TSLC1 gene in advanced lung tumors and various cancer cell lines. Int J Cancer. 2003;107:53–59. doi: 10.1002/ijc.11348. [DOI] [PubMed] [Google Scholar]
- Mao X, Seidlitz E, Truant R, Hitt M, Ghosh HP. Re-expression of TSLC1 in a non-small-cell lung cancer cell line induces apoptosis and inhibits tumor growth. Oncogene. 2004;23:5632–5642. doi: 10.1038/sj.onc.1207756. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Itoh Y, Takumi A, Ishihara C, Arase N. CRTAM confers late-stage activation of CD8+ T cells to regulate retention within lymph node. J Immunol. 2009;183:4220–4228. doi: 10.4049/jimmunol.0901248. [DOI] [PubMed] [Google Scholar]
- Takashi M, Koichi Y, Jean-David B, Manabu F, Thomas FT. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3260–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]