Abstract
Chemotherapeutic drugs eliminate tumor cells at relatively high doses and are considered weapons against tumors in clinics and hospitals. However, despite their ability to induce cellular apoptosis, chemotherapeutic drugs should probably be regarded more as a class of cell regulators than cell killers, if the dosage used and the fact that their targets are involved in basic molecular events are considered. Unfortunately, the regulatory properties of chemotherapeutic drugs are usually hidden or masked by the massive cell death induced by high doses. Recent evidence has begun to suggest that low dosages of chemotherapeutic drugs might profoundly regulate various intracellular aspects of normal cells, especially immune cells. Here, we discuss the immune regulatory roles of three kinds of chemotherapeutic drugs under low-dose conditions and propose low dosages as potential new chemotherapeutic weapons on the battlefield of immune-related disease.
Keywords: chemotherapeutic drug, immune-related disease, low dosage, mechanism, therapeutic weapon
Introduction
Chemotherapeutic drugs are regularly used as conventional therapeutic measures in clinical tumor treatment. The mechanisms involved in such therapy are both well studied and understood. Briefly, these drugs affect DNA synthesis or cell division and cause tumor cell death, or at least slow down malignant cell growth, as well as eliminating normal cells that undergo rapid division, such as bone marrow cells and skin cells. The history of such drugs goes back to two major wars of the twentieth century, when the therapeutic value of the military weapon mustard gas (now internationally banned) first became apparent to physicians. After exposure to mustard gas, people were diagnosed with very low white blood cell counts.1 Based on these observations, mustard gas was used in therapeutic trials in advanced lymphoma patients. The results appeared to show remarkable improvement in the disease due to the apoptosis or self-induced death of the lymphoma cells. Later, other chemotherapeutic agents were developed and put to clinical use against cancers, such as acute lymphoblastic leukemia, breast cancer, lung cancer, lymphoma, head and neck cancer, and skin cancer. Due to their diverse mechanisms of action, chemotherapeutic drugs were classified as alkylating agents, antimetabolites, anthracyclines, plant alkaloids, topoisomerase inhibitors and other antitumor agents.
Autoimmune diseases arise from an overactive immune response against self-substances and tissues. Currently, intense efforts are being made by medical and pharmaceutical researchers to develop more efficient and less toxic therapeutic agents to fight against such immune-related disorders. Not surprisingly, these efforts include re-examining old drugs for any new applications. Increasing numbers of research groups in this university as well as around the world, are reporting that low-dose chemotherapeutic drugs, relative to the high dosage hitherto used in cancer patients, are found to have potent and efficacious effects on both tumor- and non-tumor-related disorders.2, 3, 4, 5 Such low dosages can be reduced as much as 5- to 10-fold from the high dosages regularly used in cancer patients, without any of the usual side effects seen in cancer treatment. Studies have demonstrated that low-dose chemotherapeutic drugs have a positive effect on controlling the progress of immune-related disorders, such as rheumatoid arthritis (RA), Crohn's disease, psoriasis and multiple sclerosis, although their exact mechanisms of action have so far not been well understood.6, 7, 8, 9 However, massive cell death and the resultant side effects caused by high-dose chemotherapeutic drugs continuously overshadow the immune regulatory effects of low dosages, which have gone relatively unnoticed and are largely ignored. Here, we discuss three kinds of chemotherapeutic drugs: cyclophosphamide (CY), methotrexate (MTX) and cisplatin (Cis), focusing partly on their underlying immunoregulatory mechanisms but more on their potential clinical applications at low doses in autoimmune and infectious diseases and cancers. We propose that, in addition to these highlighted drugs, other chemotherapeutic drugs may also act as immune regulators under low-dose conditions.
Low-dose CY in immune regulation
CY is an alkylating agent that is widely used in cancer therapy. As a prodrug, CY undergoes a series of biological activation steps necessary for its cytotoxic effects.10 These include the following: (i) catalysis of CY to 4-hydroxycyclophosphamide by hepatic cytochrome P450 isozymes; (ii) interconversion of 4-hydroxycyclophosphamide with its tautomer, aldophosphamide; (iii) diffusion of aldophosphamide of hepatic cells into the circulation and subsequent uptake by other cells; and (iv) spontaneous degradation of aldophosphamide to phosphoramide mustard and acrolein. Finally, phosphoramide mustard mediates cell death by causing DNA crosslinking. To overcome drug resistance and kill as many tumor cells as possible, a very high dose of CY is usually used. For example, in B-cell chronic lymphocytic leukemia, 600 mg/m2 CY was used in combination with other chemotherapeutic drugs.11 In addition, high-dose CY can also be used to treat autoimmune disorders by killing activated immune cells. For instance, in pediatric multiple sclerosis, CY was generally administered at 600–1000 mg/m2 per dose.12 Interestingly, low doses of CY also appear to possess treatment value. Daily oral CY (50 mg, low dose) is now in phase II clinical trial in patients with advanced solid tumors, together with other chemo drugs, such as weekly vinblastine injection and oral rofecoxib.13 The effect of CY at low doses might be attributable to the fact that CY is capable of augmenting immune responses by reducing the suppressor function of regulatory T cells.14, 15 It has been demonstrated that low-dose CY selectively ablated CD4+CD25+ regulatory T (Treg) cells, leading to the enhancement of immune responses.16, 17 Based on this principle, low-dose CY has been successfully tested in the treatment of various types of tumor, as well as condylomata acuminata (CA).3, 18, 19, 20
CA is a common sexually transmitted disease that results from infection with human papillomavirus, typically types 6 and 11.21, 22 Human papillomavirus infects primitive basal keratinocytes and causes genital warts with a highly variable latent period. An in-house study found that Foxp3+ Treg cells accumulate in large warts and play an important role in genital wart immune evasion. These findings suggest that Treg cells may act as a target for the treatment of CA. In another recently published in-house paper, low-dose CY was shown to efficiently deplete CD4+CD25+ Treg cells in patients with large CA, and prevented the recurrence of disease after laser therapy.3 In 78 patients recruited after laser therapy, 52 patients took 50 mg CY orally, once a day for 1 week, and showed complete clearance and no recurrence in the first 6 weeks. Although nine of them had a later recurrence, seven of nine patients recovered after another week of low-dose CY treatment. By contrast, six of eight patients who were taking a much higher concentration of CY (200 mg orally) did not receive such a curative effect. Interestingly, aside from depleting Treg cells, low-dose CY seems not to be capable of influencing other immune cells, such as other T-cell subsets and natural killer (NK) cells.3 Instead, low-dose CY treatment is also able to augment the proliferation of T cells and IFN-γ secretion by NK cells upon stimulation. By contrast, 200 mg high-dose CY treatment resulted in a decrease in the number of these two cell types.3 Similar effects of low-dose CY were also observed in cancer patients. Oral administration of low-dose CY (100 mg/day for 2 weeks or more) in advanced cancer patients not only selectively ablates circulating Treg cells but also recovers the function of conventional T and NK cells, which were suppressed by Treg cells, leading to the restoration of peripheral T-cell proliferation and innate killing activities.23 Also, there is no significant decrease in the number of total leukocytes, or any of the T-lymphocyte, CD3+ T-cell, CD8+ T-cell and CD3−CD56+ NK cell subsets.23 In addition, low-dose CY is capable of ameliorating the immune milieu of CA by upregulating IFN-γ and IL-2 and downregulating IL-10, transforming growth factor-β and Foxp3.3 Thus, although the potent immune regulatory role of CY has long been overshadowed by its killing effect at high doses, these new findings suggest that CY, under low-dose conditions, may act as positive immunoregulator, benefiting not only patients with cancer but also patients with infectious disease.
The mechanism underlying the augmentation of immunity by low-dose CY is at least partially explained by the selective depletion of Treg cells. However, the molecular basis of such Treg depletion needs to be addressed. Recently, our in-house studies found that differential ATP concentrations might explain the selective depletion of Treg cells by low-dose CY. It was found that CD4+CD25+ Treg cells from either human or mouse express much lower levels of intracellular ATP than conventional T cells. This may be due to the fact that Treg cells dramatically downregulate one microRNA, miR-142-3p, and upregulate ecto-nucleoside triphosphate diphosphohydrolase, CD39. The low levels of miR-142-3p lead to elevated synthesis of adenyl cyclase 9, which converts ATP to cyclic adenosine monophosphate (cAMP), and high levels of CD39 accelerate the degradation of extracellular ATP,24 which facilitates the efflux of cytosolic ATP.25 Thus, both biochemical reaction pathways lower the levels of intracellular ATP, leading to the attenuation of glutathione synthesis because ATP is required for the activity of glutamate cysteine ligase, the key rate-limiting enzyme for glutathione synthesis. Conjugation with glutathione is an important route for detoxification from CY, as well as phosphoramide mustard and other metabolites.26, 27 Therefore, the selective depletion of Treg cells by low-dose CY may be explained by reduced intracellular ATP levels. The elucidation of this mechanism provides assurance that metronomic, low-dose CY administration may be a safe way to deplete Treg cells for the treatment of cancers and infectious diseases.
Low-dose MTX in immune regulation
MTX is an antimetabolite drug that acts by competitively inhibiting the binding of dihydrofolate reductase to folate, inducing the subsequent blocking of tetrahydrofolate synthesis.28 The latter is essential for the de novo synthesis of DNA and RNA. As an antimetabolite chemotherapeutic drug, MTX has a strong, inhibitory effect on cell growth, especially for rapidly dividing cells, such as early hematopoietic cells and malignant cells. For a long time, MTX at very high doses has been considered an effective agent in the treatment of acute lymphoblastic leukemia.29, 30 It has been reported that a dose of 1 or 2 g/m2 MTX was administered to children with high-risk non-B acute lymphoblastic leukemia and that rescue by leucovorin, a 5-formyl derivative of tetrahydrofolic acid, could relieve the MTX-related toxicity.31 Another report indicated that the dosage of MTX could be increased from 0.5 g/m2 up to 5.0 g/m2 in the treatment of childhood and adult acute lymphoblastic leukemia with the help of leucovorin.32 Even higher doses were administered in childhood acute lymphoblastic leukemia by infusion at 5 or 8 g/m2 over 24 h, 2–9 times per patient, with high leucovorin doses for rescue.33 In addition to leukemia, MTX is also used alone or in combination with other chemotherapeutic drugs in regimens against breast cancer,34 lung cancer,35, 36 head and neck cancer,37 primary central nervous system lymphoma38 and osteosarcoma.39 In addition to the numerous applications of MTX to cancerous disorders, MTX appears to have a role in the treatment of other disorders. The most well-known use is to treat patients with RA, which was approved by the US Food and Drug Administration 30 years ago.40 To treat RA, the starting dose is usually 7.5–10 mg per week, and this can be increased to 20–25 mg per week, if a positive response has not occurred within 4–8 weeks after MTX initiation and there has been no toxicity.41 Aside from RA, MTX has also been tested in other inflammatory disorders, such as systemic lupus erythematosus,42 psoriasis43 and Crohn's disease.44 In a retrospective study of 12 cutaneous lupus erythematosus patients treated with weekly low-dose MTX at 10–25 mg, six and four of these patients showed complete and partial remission, respectively. Five of the 10 responding patients presented with a long-term remission. This beneficial effect of MTX was further confirmed by subsequent studies. Once again, the critical treatment difference between cancer and these autoimmune diseases lies in the dose. In contrast to the low doses used in autoimmune diseases, cancer patients usually receive high-dose MTX at 50–500 mg for treatment, and such high doses do not have a beneficial effect on autoimmune diseases. Therefore, different doses probably confer different properties on MTX, which raises the question of why MTX is efficacious in both cancer and autoimmune diseases, depending on the dose.
It is easy to take for granted that MTX exerts an anti-inflammatory effect through interfering with tetrahydrofolate pathway because MTX inhibits dihydrofolate reductase. However, there is debate about whether this is the true active pathway and the results from different laboratories are controversial. Administration of folic acid led to either little or mild-to-moderate reduction in the anti-inflammatory effects of MTX treatment in patients with RA.45, 46 On the other hand, reports showed that a decrease in folate polyglutamate level, a reduction in both the number and reactivity of antigen-specific T lymphocytes and the suppression of pathogenic rheumatoid factor in RA may be due to folate-related effects of low-dose MTX prescription.47, 48, 49 This inconsistency suggests the existence of other mechanism(s).50 Recently, adenosine, the nucleotide derivative, has been reported to function as an important immunosuppressor to mediate the inhibition of inflammation. By engaging with the adenosine A2A receptor, adenosine effectively inhibits the production of IL-2 and IFN-γ by T cells, which could be partially explained by its influence on the intracellular cAMP pathway.51 It is known that, when taken up by cells, MTX is metabolized to MTX polyglutamates. The latter inhibit a critical enzyme, aminoimidazole carboxamide ribonucleotide (AICAR) transformylase, leading to the elevation of intracellular AICAR52 and subsequently increasing adenosine concentration in different ways: (i) AICAR inhibits the metabolism of AMP by inhibiting AMP deaminase. The increase in AMP levels may prevent the transition of adenosine to AMP; on the other hand, the enzyme ecto-5'-nucleotidase (CD73) may catalyze the production of more adenosine from AMP; (ii) AICAR blocks the metabolism of adenosine by inhibiting adenosine deaminase. Besides inhibiting T-cell activation, adenosine may enhance endothelial barrier function by altering the expression of adhesion molecules, such as L-selectin and β2 integrin, thus inhibiting vascular permeability in the inflammatory milieu.53 In our unpublished data, in vitro treatment with MTX at low dose (0.1 µg/ml) was found to effectively downregulate the expression of IL-2 but upregulate the expression of Foxp3 in T cells in response to stimulation with concanavalin A or phorbol 12-myristate 13-acetate plus ionomycin. The in vivo administration of low-dose MTX (5 µg) also showed a potential therapeutic effect against concanavalin A-induced liver damage. However, such dosages impaired host defenses against bacterial infection in a mouse model, whereas high-dose MTX (500 µg) did not show such effects, suggesting that MTX under low-dose conditions has an immunoregulatory role. Whether the adenosine pathway mediates such effects needs further clarification. In addition, low-dose MTX may induce the production of polyamine, leading to the inhibition of inflammation. Low-dose MTX treatment affects a number of inflammatory mediators, such as lipid derivatives, cytokines, chemokines and growth factors. For example, MTX has been reported to suppress tumor-necrosis factor-α levels in RA patients and a mouse model54, 55 and other inflammatory mediators, such as IL-1 and leukotriene B4, are also modulated by MTX therapy.56, 57 MTX at low doses can act via numerous pathways to downregulate inflammatory responses.
Low-dose Cis in immune regulation
Cis is a broad-spectrum, cell cycle nonspecific, platinum-based chemotherapeutic drug used to treat various types of tumors, including sarcoma, lymphoma, small cell lung cancer, bladder cancer, ovarian cancer and testicular cancer.58, 59, 60 The therapeutic effect of Cis comes from its ability to bind and cause irreversible DNA crosslinking, ultimately triggering cellular apoptosis.61 To achieve the best treatment efficiency against tumors, high-dose Cis is required in clinical treatment. For example, platinum- and taxane-based chemotherapy is the standard program for the treatment of advanced-stage ovarian cancer after cytoreductive surgery. Patients with advanced-stage III/IV epithelial ovarian cancer received six cycles of an intense dose of Cis (75 mg/m2) in combination with other chemo drugs, such as CY, paclitaxel and filgrastim; 89% experienced complete clinical remission. The median progression-free survival and median survival were 18.9 months and 5.4 years, respectively.62 Despite the efficacy of high-dose Cis in cancer treatment, low concentration Cis treatment for non-cancer disorders has been attempted in recent years. Pan et al. reported that low-dose Cis therapy attenuated the lethality of cecal ligation and puncture (CLP) in a murine model.63 Interestingly, they showed that, when treated with low-dose Cis at 0.1 mg/kg, the survival rates of CLP mice were improved, whereas treatment with high-dose Cis at 1 mg/kg did not have this beneficial effect. Such protection was demonstrated to be due to the prevention of systemic release of high-mobility group box 1 protein (HMGB1). In addition, low-dose Cis was also reported to sequester HMGB1 inside the nucleus of redox-stressed hepatocytes.64 Therefore, it is assumed that targeting HMGB1 might be a mechanism involved in the therapeutic effects of low-dose Cis.
HMGB1 is found in the nucleus of all cells and is normally responsible for DNA transcription by binding chromosomes.65 In addition to such physiological effects, HMGB1 is also released into the extracellular space and acts as an alarm for both innate and adaptive immunity.66 In response to stress-induced necrosis, for example, HMGB1 may be passively released from necrotic cells. More significantly, HMGB1 can be secreted from activated immune cells such as lipopolysaccharide-stimulated macrophages, and the underlying mechanism involves the deacetylation of lysine residues of HMGB1. This extracellular, soluble HMGB1, in turn, functions as a pro-inflammatory cytokine.67 In vitro studies have shown that HMGB1 upregulates expression of the key pro-inflammatory cytokine tumor-necrosis factor-α. As expected, injection of anti-HMGB1 antibody attenuates the inflammatory response in animal models with liver disease or myocardial infarction.68, 69 HMGB1 has also been identified as a participant in lipopolysaccharide-induced sepsis in mice and the administration of neutralizing HMGB1 antibody decreased the lethality in mice.70, 71 Pan et al. found that the injection of 0.1 mg/kg Cis prevented systemic HMGB1 release and the administration of even small amounts of recombinant HMGB1 could restore the mortality of the CLP mouse model.63 Their experiments confirmed that HMGB1 upregulated inducible NO synthase gene expression. In line with the in vivo data, low-dose Cis in vitro inhibited the release of HMGB1 induced by lipopolysaccharide stimulation and instead retained it in the nucleus of macrophages. Moreover, in vitro studies showed that non-toxic concentrations of Cis can sequester HMGB1 inside the nucleus of hypoxic cells. The in vivo administration of non-toxic doses of Cis prevented liver damage in a murine model of hepatic ischemia/reperfusion, resulting in decreased levels of inflammatory cytokines, such as tumor-necrosis factor-α, IL-6 and inducible NO synthase, inhibition of mitogen-activated protein kinase activation, autophagy and ischemia/reperfusion-associated histopathological changes.64 Together, these findings suggest that the mechanism for the anti-inflammatory and therapeutic effects of low-dose Cis in non-cancer diseases may occur via its regulation of HMGB1 release as well as its ability to alter cell survival and stress signaling in the form of autophagy and mitogen-activated protein kinase activation.
Currently, the activities of HMGB1 are a focus of attention for the study of the induction and propagation of autoimmune conditions. Autoimmune diseases, like systemic lupus erythematosus, Sjögren's syndrome, juvenile idiopathic arthritis, myositis and systemic sclerosis, have already been reported to be associated with the presence of autoantibodies recognizing HMGB1 and the closely related protein HMGB2.72, 73, 74, 75, 76 These findings indicate that low-dose Cis has great potential for the treatment of inflammatory autoimmune disease by regulating the release of HMGB1. Recently, a report from Shen et al. showed that low-dose, metronomic chemotherapy with Cis (0.6 mg/kg/day) can dramatically inhibit the proliferation of human umbilical vascular endothelial cells in a dose- and time-dependent manner.77 Under such a low dose in a mouse model, tumor growth was delayed without apparent body weight loss, compared with mice that received Cis at the maximum tolerated dose. The expression of genes such as vascular endothelial growth factor and matrix metallopeptidase 2 was also much lower in those mice that were treated with low-dose, metronomic Cis compared with the control and maximum tolerated dose groups. Moreover, continuous low-dose Cis was shown to suppress angiogenesis in a chicken chorio-allantoic membrane model. The anti-angiogenic effect of low-dose Cis chemotherapy provides a new strategy with few toxic side effects and little drug resistance, and the same rule might also be applicable to certain other chemotherapeutic drugs. However, the underlying mechanisms behind the efficacy of low-dose chemotherapy may be very complicated.78 Low-dose Cis can lead to significant upregulation of Fas (CD95) mRNA and protein in SW480 colon cancer cells and oral cancer cell lines,79, 80 thus facilitating apoptosis of the tumor cells. This could be explained by the fact that Cis could trigger the redistribution of Fas into the plasma membrane rafts by activating acid sphingomyelinase, which contributed to cell death and sensitized tumors to Fas-mediated apoptosis.81 Therefore, it is possible for low-dose Cis to attenuate inflammatory responses by downregulating the expression of vascular endothelial growth factor and matrix metallopeptidase 2 and even by inducing Fas expression on effector T cells. The latter is an interesting hypothesis and worthy of investigation.
Potential applications of low-dose chemotherapeutic drugs in cancer treatment through immunological pathways
Routine high-dose chemotherapeutic drugs are widely used for the treatment of various tumors, especially for that of unresectable and/or extensively metastatic tumors.82 However, the intensive and widespread side effects, due to non-selective cytotoxic effects on both tumor cells and normal cells, have largely limited their applications. Currently, the search for more specific chemotherapeutic drugs is the focus of cancer therapeutic research. However, the success of tumor treatment is not achieved solely by killing tumor cells. It is now widely accepted that the tumor problem is not only about seeds (i.e., the tumor cells themselves) but also about the soil (i.e., the tumor microenvironment) and that targeting the tumor microenvironment can also have therapeutic effects. Cellular components and mediators in the tumor microenvironment aid the proliferation and survival of tumor cells, facilitate angiogenesis and metastasis, subvert antitumor immune responses and modify the responses of tumor cells to hormones and chemotherapeutic agents. Thus, the tumor microenvironment is vital for the initiation, promotion and progression of cancer. Immune cells and immune-associated molecules are major components of the tumor microenvironment, and regulating their function is considered a promising strategy against cancer. In this regard, low-dose chemotherapeutic drugs may have potential as cancer treatments due to their immune regulatory properties. Chemotherapeutic drugs can kill cancer cells not only via cytotoxic effects, but also by acting as adjuvants for antitumor immune responses.82 The latter function might be more important for the complete eradication of any remaining cancer cells and the prevention of relapse after surgical treatment. The advantages of low-dose chemotherapeutic drugs in cancer treatment are evident: (i) low-dose chemotherapeutic drugs have fewer effects on normal cells, thereby avoiding the side effects usually seen in routine high-dose chemotherapy; and (ii) by modulating the immune microenvironment, low-dose chemotherapy may have a profound impact on the progression of cancer and could promote relief and even recovery from cancer.82, 83, 84 As reported, the low-dose CY regimen can selectively deplete or inhibit CD4+CD25+ Treg cells by reducing intracellular ATP and glutathione levels, thus restoring the activities of cytotoxic T and NK cells in end-stage cancer patients.17, 18, 23 Such an effect has also been confirmed in other cases, such as lymphoma, sarcoma and metastatic melanoma.85, 86 Despite the widespread use of low-dose CY in cancer treatment for targeting Treg cells, data on the use of other low-dose chemotherapeutic drugs to enhance antitumor immunity are still rare. Therefore, testing the dosages of other chemotherapeutic drugs and screening for candidates that are able to upregulate antitumor immunity under low-dose conditions is highly desirable and also clinically significant.
In addition to the inherent ability of some low-dose chemotherapeutic drugs to augment antitumor immunity, chemotherapeutic drugs may use inflammatory pathways or be combined with antibody-based therapy to achieve antitumor immunotherapy. Although it is thought that chemotherapy exerts its effects via the direct elimination of tumor cells, the success of some chemotherapeutic protocols depends on innate and adaptive antitumor immune responses, indicating that some chemotherapeutic drugs play an immune adjuvant role. Studies from Zitvogel's group have provided evidence for this hypothesis and suggest that inflammatory signaling molecules derived from dead tumor cells after chemotherapy are potential arms for antitumor immunotherapy.87 We here suggest that a better antitumor benefit may be generated by lowering the dosage of some of the adjuvant-like chemotherapeutic drugs to reduce side effects while still keeping the inflammatory–immunological signals. A new study by Park et al. has shown that, although anti-HER2/neu antibody increased influx of both innate and adaptive immune cells into the tumor microenvironment, which resulted in enhanced antitumor immune responses and tumor eradication, the addition of the chemotherapeutic drugs CY or paclitaxel, could also abrogate antibody-initiated immunity, leading to decreased resistance to rechallenge or earlier relapse.88 The authors speculated that such a loss of protection from tumor rechallenge might be attributable to the reduced white blood cell count or early immune suppression that occurs after routine chemotherapy. However, as discussed above, the dosage might be critical in this case and low dosages, especially for CY, are worthy of testing. In short, low-dose chemotherapeutic drugs are potential weapons to augment antitumor immune responses by directly antagonizing regulatory pathways or indirectly expanding tumor-reactive T cells in the tumor microenvironment. Low-dosage chemotherapy plus other cancer regimens might generate more effective treatment protocols in the near future.
Conclusions
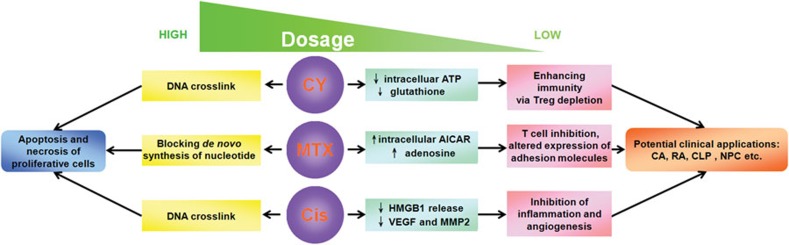
Despite their well-established role as direct killers, chemotherapeutic drugs may actually act as basic regulators of the fundamental intracellular events (Figure 1). This latter regulatory nature of chemotherapeutic drugs has long been hidden or masked by the overwhelming cell death brought about by the high dosages of the drugs that are normally administered. At low dosages, however, the regulatory nature may become more evident. To date, numerous chemotherapeutic drugs have been produced and used in the clinic. This provides a grand opportunity to do some gold panning in the slags of chemotherapeutic drugs administered in cancerous and non-cancerous diseases, such as autoimmune and infectious diseases. It should be noted that specific low-dose agents for cancer treatment may not be suitable for autoimmune diseases and vice versa, due to their different modes of action. Based on what has been reviewed here, one can be forgiven for hypothesizing that chemotherapeutic drugs, under low-dose conditions, may provide new vistas into the development of pharmacological therapies against non-cancerous diseases, perhaps even resulting in revolutionary breakthroughs in the treatment of one or more refractory diseases.
Figure 1.
Schematic representation of the effects and modes of action of CY, MTX and Cis. AICAR, aminoimidazole carboxamide ribonucleotide; CA, condylomata acuminata; Cis, cisplatin; CLP, cecal ligation and puncture; CY, cyclophosphamide; HMGB1, high-mobility group box 1 protein; MMP2, matrix metallopeptidase 2; MTX, methotrexate; NPC, nasopharygeal carcinoma; RA, rheumatoid arthritis; Treg, regulatory T; VEGF, vascular endothelial growth factor.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30871020 and 30972667), Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (30911120482), Program for New Century Excellent Talents in University (NCET-08-0219), Fundamental Research Funds for the Central Universities (HUST-2010JC024) and Scientific Research Foundation of Wuhan City Human Resource for Returned Scholars.
References
- Hirsch J. An anniversary for cancer chemotherapy. JAMA. 2006;296:1518–1520. doi: 10.1001/jama.296.12.1518. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Ochsendorf F, Bonsmann G. Treatment of cutaneous lupus erythematosus. Lupus. 2010;19:1125–1136. doi: 10.1177/0961203310370345. [DOI] [PubMed] [Google Scholar]
- Cao Y, Zhao J, Yang Z, Cai Z, Zhang B, Zhou Y, et al. CD4+FOXP3+ regulatory T cell depletion by low-dose cyclophosphamide prevents recurrence in patients with large condylomata acuminata after laser therapy. Clin Immunol. 2010;136:21–29. doi: 10.1016/j.clim.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatol Int. 1998;18:59–62. doi: 10.1007/s002960050058. [DOI] [PubMed] [Google Scholar]
- Castano AP, Mroz P, Wu MX, Hamblin MR. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc Natl Acad Sci USA. 2008;105:5495–5500. doi: 10.1073/pnas.0709256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp L, Roberts R, Kennedy M, Barclay M, O'Donnell J, Chapman P. The use of low dose methotrexate in rheumatoid arthritis—are we entering a new era of therapeutic drug monitoring and pharmacogenomics. Biomed Pharmacother. 2006;60:678–687. doi: 10.1016/j.biopha.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Rampton DS. Methotrexate in Crohn's disease. Gut. 2001;48:790–791. doi: 10.1136/gut.48.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich SJ, Bello-Quintero CE. Advancements in the treatment of psoriasis: role of biologic agents. J Manag Care Pharm. 2004;10:318–325. doi: 10.18553/jmcp.2004.10.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadiq SA, Simon EV, Puccio LM. Intrathecal methotrexate treatment in multiple sclerosis. J Neurol. 2010;257:1806–1811. doi: 10.1007/s00415-010-5614-4. [DOI] [PubMed] [Google Scholar]
- Rooney PH, Telfer C, McFadyen MC, Melvin WT, Murray GI. The role of cytochrome P450 in cytotoxic bioactivation: future therapeutic directions. Curr Cancer Drug Targets. 2004;4:257–265. doi: 10.2174/1568009043333014. [DOI] [PubMed] [Google Scholar]
- Kay NE, Geyer SM, Call TG, Shanafelt TD, Zent CS, Jelinek DF, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhani N, Gorman MP, Branson HM, Stazzone L, Banwell BL, Chitnis T. Cyclophosphamide therapy in pediatric multiple sclerosis. Neurology. 2009;72:2076–2082. doi: 10.1212/WNL.0b013e3181a8164c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SD, Whissell M, Noble JC, Cano PO, Lopez PG, Germond CJ. Phase II clinical trial results involving treatment with low-dose daily oral cyclophosphamide, weekly vinblastine, and rofecoxib in patients with advanced solid tumors. Clin Cancer Res. 2006;12:3092–3098. doi: 10.1158/1078-0432.CCR-05-2255. [DOI] [PubMed] [Google Scholar]
- Awwad M, North RJ. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 1989;49:1649–1654. [PubMed] [Google Scholar]
- Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: reduction of T-suppressor function without depletion of the CD8+ subset. Cancer Res. 1987;47:3317–3321. [PubMed] [Google Scholar]
- Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- Lutsiak ME, Semnani RT, de Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. 2010;70:4850–4858. doi: 10.1158/0008-5472.CAN-10-0283. [DOI] [PubMed] [Google Scholar]
- Daenen LG, Shaked Y, Man S, Xu P, Voest EE, Hoffman RM, et al. Low-dose metronomic cyclophosphamide combined with vascular disrupting therapy induces potent antitumor activity in preclinical human tumor xenograft models. Mol Cancer Ther. 2009;8:2872–2881. doi: 10.1158/1535-7163.MCT-09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada S, Yoshimura K, Hipkiss EL, Harris TJ, Yen HR, Goldberg MV, et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin N. Genital warts. Clin Dermatol. 2004;22:481–486. doi: 10.1016/j.clindermatol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Dunne EF, Markowitz LE. Genital human papillomavirus infection. Clin Infect Dis. 2006;43:624–629. doi: 10.1086/505982. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Gamcsik MP, Dolan ME, Andersson BS, Murray D. Mechanisms of resistance to the toxicity of cyclophosphamide. Curr Pharm Des. 1999;5:587–605. [PubMed] [Google Scholar]
- Dirven HA, van OB, van Bladeren PJ. Involvement of human glutathione S-transferase isoenzymes in the conjugation of cyclophosphamide metabolites with glutathione. Cancer Res. 1994;54:6215–6220. [PubMed] [Google Scholar]
- Rajagopalan PT, Zhang Z, McCourt L, Dwyer M, Benkovic SJ, Hammes GG. Interaction of dihydrofolate reductase with methotrexate: ensemble and single-molecule kinetics. Proc Natl Acad Sci USA. 2002;99:13481–13486. doi: 10.1073/pnas.172501499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterba J, Valík D, Bajciová V, Kadlecová V, Gregorová V, Mendelová D. High-dose methotrexate and/or leucovorin rescue for the treatment of children with lymphoblastic malignancies: do we really know why, when and how. Neoplasma. 2005;52:456–463. [PubMed] [Google Scholar]
- Mantadakis E, Cole PD, Kamen BA. High-dose methotrexate in acute lymphoblastic leukemia: where is the evidence for its continued use. Pharmacotherapy. 2005;25:748–755. doi: 10.1592/phco.25.5.748.63584. [DOI] [PubMed] [Google Scholar]
- Joannon P, Oviedo I, Campbell M, Tordecilla J. High-dose methotrexate therapy of childhood acute lymphoblastic leukemia: lack of relation between serum methotrexate concentration and creatinine clearance. Pediatr Blood Cancer. 2004;43:17–22. doi: 10.1002/pbc.20032. [DOI] [PubMed] [Google Scholar]
- Gokbuget N, Hoelzer D. High-dose methotrexate in the treatment of adult acute lymphoblastic leukemia. Ann Hematol. 1996;72:194–201. doi: 10.1007/s002770050160. [DOI] [PubMed] [Google Scholar]
- Skärby TV, Anderson H, Heldrup J, Kanerva JA, Seidel H, Schmiegelow K, et al. High leucovorin doses during high-dose methotrexate treatment may reduce the cure rate in childhood acute lymphoblastic leukemia. Leukemia. 2006;20:1955–1962. doi: 10.1038/sj.leu.2404404. [DOI] [PubMed] [Google Scholar]
- Bocci G, Tuccori M, Emmenegger U, Liguori V, Falcone A, Kerbel RS, et al. Cyclophosphamide-methotrexate “metronomic” chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann Oncol. 2005;16:1243–1252. doi: 10.1093/annonc/mdi240. [DOI] [PubMed] [Google Scholar]
- Gonzalez Baron M, Garcia Giron C, Zamora P, Garcia de Paredes ML, Feliu J, Ordoñez A, et al. Non-small-cell lung cancer (NSCLC): chemotherapy in advanced disease. Our experience in ten years. Am J Clin Oncol. 1992;15:23–28. doi: 10.1097/00000421-199202000-00005. [DOI] [PubMed] [Google Scholar]
- Giaccone G. Teniposide alone and in combination chemotherapy in small cell lung cancer. Semin Oncol. 1992;19:75–80. [PubMed] [Google Scholar]
- Specenier PM, Vermorken JB. Current concepts for the management of head and neck cancer: chemotherapy. Oral Oncol. 2009;45:409–415. doi: 10.1016/j.oraloncology.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Algazi AP, Kadoch C, Rubenstein JL. Biology and treatment of primary central nervous system lymphoma. Neurotherapeutics. 2009;6:587–597. doi: 10.1016/j.nurt.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat Res. 2009;152:239–262. doi: 10.1007/978-1-4419-0284-9_12. [DOI] [PubMed] [Google Scholar]
- Korn S, DeHoratius RJ. Methotrexate in the treatment of rheumatoid arthritis. Am Fam Physician. 1989;40:243–246. [PubMed] [Google Scholar]
- Swierkot J, Szechinski J. Methotrexate in rheumatoid arthritis. Pharmacol Rep. 2006;58:473–492. [PubMed] [Google Scholar]
- Sato EI. Methotrexate therapy in systemic lupus erythematosus. Lupus. 2001;10:162–164. doi: 10.1191/096120301666080831. [DOI] [PubMed] [Google Scholar]
- Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol. 2009;60:824–837. doi: 10.1016/j.jaad.2008.11.906. [DOI] [PubMed] [Google Scholar]
- Sun JH, Das KM. Low-dose oral methotrexate for maintaining Crohn's disease remission: where we stand. J Clin Gastroenterol. 2005;39:751–756. doi: 10.1097/01.mcg.0000177249.46130.a3. [DOI] [PubMed] [Google Scholar]
- Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev. 2005;57:163–172. doi: 10.1124/pr.57.2.3. [DOI] [PubMed] [Google Scholar]
- Khanna D, Park GS, Paulus HE, Simpson KM, Elashoff D, Cohen SB, et al. Reduction of the efficacy of methotrexate by the use of folic acid: post hoc analysis from two randomized controlled studies. Arthritis Rheum. 2005;52:3030–3038. doi: 10.1002/art.21295. [DOI] [PubMed] [Google Scholar]
- Dervieux T, Furst D, Lein DO, Capps R, Smith K, Caldwell J, et al. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Ann Rheum Dis. 2005;64:1180–1185. doi: 10.1136/ard.2004.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102:322–328. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen NJ, Murray LM. Antiproliferative effects of methotrexate on peripheral blood mononuclear cells. Arthritis Rheum. 1989;32:378–385. doi: 10.1002/anr.1780320404. [DOI] [PubMed] [Google Scholar]
- Chan ES, Cronstein BN. Methotrexate—how does it really work. Nat Rev Rheumatol. 2010;6:175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- Alam MS, Kurtz CC, Wilson JM, Burnette BR, Wiznerowicz EB, Ross WG, et al. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunol. 2009;2:232–242. doi: 10.1038/mi.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra CJ, Drake JC, Jolivet J, Chabner BA. Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc Natl Acad Sci USA. 1985;82:4881–4885. doi: 10.1073/pnas.82.15.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel M, Chambers JD, Chouker A, Fischer S, Zourelidis C, Bardenheuer HJ, et al. Effect of adenosine on the expression of beta2 integrins and L-selectin of human polymorphonuclear leukocytes in vitro. . J Leukoc Biol. 1996;59:671–682. doi: 10.1002/jlb.59.5.671. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Hildner K, Becker C, Schlaak JF, Barbulescu K, Germann T, et al. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): a mechanism for methotrexate-mediated immunosuppression. Clin Exp Immunol. 1999;115:42–55. doi: 10.1046/j.1365-2249.1999.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolhain RJ, Tak PP, Dijkmans BA, de Kuiper P, Breedveld FC, Miltenburg AM. Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Br J Rheumatol. 1998;37:502–508. doi: 10.1093/rheumatology/37.5.502. [DOI] [PubMed] [Google Scholar]
- Sperling RI, Coblyn JS, Larkin JK, Benincaso AI, Austen KF, Weinblatt ME. Inhibition of leukotriene B4 synthesis in neutrophils from patients with rheumatoid arthritis by a single oral dose of methotrexate. Arthritis Rheum. 1990;33:1149–1155. doi: 10.1002/art.1780330815. [DOI] [PubMed] [Google Scholar]
- Thomas R, Carroll GJ. Reduction of leukocyte and interleukin-1 beta concentrations in the synovial fluid of rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 1993;36:1244–1252. doi: 10.1002/art.1780360909. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol. 2007;19:341–346. doi: 10.1097/CCO.0b013e328122d73f. [DOI] [PubMed] [Google Scholar]
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- Sarosy GA, Hussain MM, Seiden MV, Fuller AF, Nikrui N, Goodman A, et al. Ten-year follow-up of a phase 2 study of dose-intense paclitaxel with cisplatin and cyclophosphamide as initial therapy for poor-prognosis, advanced-stage epithelial ovarian cancer. Cancer. 2010;116:1476–1484. doi: 10.1002/cncr.24861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Cardinal J, Dhupar R, Rosengart MR, Lotze MT, Geller DA, et al. Low-dose cisplatin administration in murine cecal ligation and puncture prevents the systemic release of HMGB1 and attenuates lethality. J Leukoc Biol. 2009;86:625–632. doi: 10.1189/JLB.1108713. [DOI] [PubMed] [Google Scholar]
- Cardinal J, Pan P, Dhupar R, Ross M, Nakao A, Lotze M, et al. Cisplatin prevents high mobility group box 1 release and is protective in a murine model of hepatic ischemia/reperfusion injury. Hepatology. 2009;50:565–574. doi: 10.1002/hep.23021. [DOI] [PubMed] [Google Scholar]
- Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia–reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, et al. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97:e73–e83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi H, Ozaki S, Sobajima J, Osakada F, Shirakawa H, Yoshida M, et al. Prevalence and characterization of novel pANCA, antibodies to the high mobility group non-histone chromosomal proteins HMG1 and HMG2, in systemic rheumatic diseases. J Rheumatol. 1998;25:703–709. [PubMed] [Google Scholar]
- Wittemann B, Neuer G, Michels H, Truckenbrodt H, Bautz FA. Autoantibodies to nonhistone chromosomal proteins HMG-1 and HMG-2 in sera of patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1990;33:1378–1383. doi: 10.1002/art.1780330910. [DOI] [PubMed] [Google Scholar]
- Ek M, Popovic K, Harris HE, Naucler CS, Wahren-Herlenius M. Increased extracellular levels of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in minor salivary glands of patients with Sjogren's syndrome. Arthritis Rheum. 2006;54:2289–2294. doi: 10.1002/art.21969. [DOI] [PubMed] [Google Scholar]
- Ulfgren AK, Grundtman C, Borg K, Alexanderson H, Andersson U, Harris HE, et al. Down-regulation of the aberrant expression of the inflammation mediator high mobility group box chromosomal protein 1 in muscle tissue of patients with polymyositis and dermatomyositis treated with corticosteroids. Arthritis Rheum. 2004;50:1586–1594. doi: 10.1002/art.20220. [DOI] [PubMed] [Google Scholar]
- Ayer LM, Senecal JL, Martin L, Dixon GH, Fritzler MJ. Antibodies to high mobility group proteins in systemic sclerosis. J Rheumatol. 1994;21:2071–2075. [PubMed] [Google Scholar]
- Shen FZ, Wang J, Liang J, Mu K, Hou JY, Wang YT. Low-dose metronomic chemotherapy with cisplatin: can it suppress angiogenesis in H22 hepatocarcinoma cells. Int J Exp Pathol. 2010;91:10–16. doi: 10.1111/j.1365-2613.2009.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Liu JY, Yang CM, Xu HW, Zhang AZ, Cui Y, et al. Influence of antitumor drugs on the expression of Fas system in SW480 colon cancer cells. Eur J Gastroenterol Hepatol. 2006;18:1071–1077. doi: 10.1097/01.meg.0000231750.68513.6c. [DOI] [PubMed] [Google Scholar]
- Sundelin K, Roberg K, Grenman R, Hakansson L. Effects of cisplatin, alpha-interferon, and 13-cis retinoic acid on the expression of Fas (CD95), intercellular adhesion molecule-1 (ICAM-1), and epidermal growth factor receptor (EGFR) in oral cancer cell lines. J Oral Pathol Med. 2007;36:177–183. doi: 10.1111/j.1600-0714.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, et al. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593–3598. doi: 10.1158/0008-5472.CAN-03-2787. [DOI] [PubMed] [Google Scholar]
- Ménard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57:1579–1587. doi: 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- Rozados VR, Sánchez AM, Gervasoni SI, Berra HH, Matar P, Graciela Scharovsky O. Metronomic therapy with cyclophosphamide induces rat lymphoma and sarcoma regression, and is devoid of toxicity. Ann Oncol. 2004;15:1543–1550. doi: 10.1093/annonc/mdh384. [DOI] [PubMed] [Google Scholar]
- Borne E, Desmedt E, Duhamel A, Mirabel X, Dziwniel V, Mortier L, et al. Oral metronomic cyclophosphamide in elderly with metastatic melanoma. Invest New Drugs. 2010;28:684–689. doi: 10.1007/s10637-009-9298-5. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The Therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]