Abstract
The major limitation for the maturation of dendritic cells (DCs) using Toll-like receptor (TLR) agonists is their decreased ability to migrate into lymph nodes compared with conventional DCs. CD38 can be used as a multifunctional marker to modulate migration, survival and Th1 responses of DCs. CD74 has been shown to negatively regulate DC migration. The goal of this study was to investigate the combinations of TLR agonists and interferons (IFNs) that most effectively regulate CD38 and CD74 expression on DCs. Synergistic TLR agonist stimulation in combination with IFN-α and IFN-γ was the best method for regulating CD38 and CD74 expression and inducing the highest secretion of IL-12p70. An in vitro migration assay showed that DCs treated with this combination had significantly enhanced migratory ability, similar to that observed in cells expressing CD38, CD74 and CCR7. The results of this study suggest that an alternative maturation protocol in which two TLR ligands are combined with type I and II IFNs generates potent DCs that have both a high migratory capacity and high IL-12p70 production.
Keywords: CD38, CD74, dendritic cells, interferon, Toll-like receptor
Introduction
For dendritic cell (DC)-based cancer immunotherapy, effective induction of tumor-specific immune responses requires that DCs secrete high concentrations of IL-12p70 in order to have a Th1 polarizing effect and to migrate through lymphatic vessels to interact with T cells. Conventional DCs expressing the specific receptor CCR7 can migrate toward chemokines such as CCL19 and CCL21;1, 2 conventional DCs that mature in response to prostaglandin E2 can polarize naive T cells toward a Th2 phenotype via increased secretion of IL-10 and inhibition of IL-12p70 production in response to CD40 ligand (CD40L) stimulation.3, 4 Toll-like receptor (TLR) agonists are well known to trigger DC maturation.5 TLR agonists alone induce DCs to produce small amounts of IL-12p70, whereas selected combinations of TLR agonists synergistically enhance the Th1 polarizing capacity of DCs by inducing the production of large amounts of IL-12p70, not only in adult DCs but also in human neonatal DCs.6, 7 However, a major limitation of using DCs activated by combinations of TLR agonists is their reduced ability to migrate into lymph nodes compared with conventional DCs.
CD38 is a signaling receptor that triggers a wide range of responses in various types of blood cell.8, 9, 10, 11, 12 CD38 is downregulated during the differentiation of monocytes into immature DCs and then expressed again upon maturation. The level of CD38 expression on DCs is dependent on the activating stimulus. CD38 activation can upregulate CD83 expression and IL-12 secretion, which suggests that CD38 is a genuine marker for maturation.13 In addition, CD38 serves as a novel multifunctional marker that is involved in migration, survival and the Th1 immune response.14 The migration-inducible activities of CD38 in mature DCs are driven by CCL21.14 Recently, the major histocompatibility complex class II-associated invariant chain (CD74 or Ii) has been reported to negatively regulate DC migration in vivo.15 The absence of CD74 causes activated DCs that migrate from the periphery to accumulate in lymph nodes. Regulation of DC migration by CD74 is not due to altered responses to the CCL21 and CCL19 chemokines or differential secretion of soluble factors.15
Interferon (IFN) has been shown to play an important role in DC function. Treatment of immature DCs with IFN-α results in the upregulation of surface markers, T-cell stimulatory capacities and migration-related chemokines.16, 17 IFN-γ also upregulates IL-12 production, CCR7-driven migration, CD38 expression and activated Th1 cell recruitment.18 Although a combination of IFN and a TLR3 agonist can generate potent DCs,19, 20 optimization of the combination of these stimuli is needed to produce high-quality DCs that can generate potent cytotoxic T lymphocytes and have the ability to migrate to lymph nodes. DCs with these characteristics may be used as an effective tool for cancer immunotherapy.
In this study, the optimal combination of TLR agonists and IFNs for inducing expression of CD38 and CD74 by DCs was investigated. In addition, the ability of DCs to produce large quantities of the Th1 polarized cytokine IL-12p70 and migrate toward CCL21 was examined.
Materials and methods
Generation of DCs
Monocytes were isolated from peripheral blood from healthy donors as described previously.21 Adherent monocytes were cultured in RPMI-1640 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (all from Gibco-BRL, Grand Island, NY, USA) in the presence of 50 ng/ml GM-CSF (LG Biochemical, Daejeon, Korea) and 10 ng/ml IL-4 (R&D Systems, Minneapolis, MN, USA). DCs were matured with one or more of the following TLR agonists: 1 µg/ml of lipopolysaccharide (LPS) (Sigma-Aldrich, St Louis, MO, USA), 20 µg/ml of poly(I:C) (Sigma-Aldrich), 1 µg/ml of flagellin (kindly provided by Dr Rhee at Chonnam National University, Gwangju, Korea) and 1 µg/ml of imidazoquinoline (InvivoGen, San Diego, CA, USA). When indicated, combinations of two TLR agonists were prepared in the presence or absence of 3000 U/ml of IFN-α (LG Life Science, Chonbuk, Korea) and/or 1000 U/ml of IFN-γ (Strathmann Biotech, Hamburg, Germany).
Immunophenotyping
The immunophenotype of the DCs was analyzed by flow cytometry using commercially available FITC- and PE-labeled mAb against CD83, CD38, CD74 and CCR7. The samples were acquired on a FACSAria cell sorter (BD Biosciences, San Jose, CA, USA), and the data were analyzed with Win MDI Version 2.9 (Bio-Soft Net). Fluorescence data are reported as the percentage of positive cells when treatment induced the expression of the marker in cells that were double-stained; median fluorescence intensity was used when treatment increased the expression of the marker in cells that were single-stained.
IL-12p70 production
IL-12p70 concentrations in supernatants were measured using the BD OptEIA ELISA Set (BD Biosciences). Supernatants were collected from cultured DCs during differentiation and after subsequent stimulation with 5×104 CD40L-transfected J558 cells for 24 h (kindly provided by Dr Kalinski at University of Pittsburgh, Pittsburgh, PA, USA), which mimics the interaction with CD40L-expressing Th cells.
Migration assay
The chemotactic effects of CCL21 on DCs were measured by migration through a polycarbonate filter with 5 µm pores in 24-well transwell chambers (Corning Costar, Cambridge, MA., USA), as described previously.14 Briefly, the lower chamber was filled with 600 µl of medium containing the indicated dose (250 ng/ml) of CCL21 (BD Biosciences) or medium alone as the control. The DCs (1×105) were added to the upper chamber in 100 µl of medium. The migration chambers were incubated for 3 h at 37 °C. A 500 µl aliquot of the cells that migrated to the bottom chamber was counted by flow cytometry in a FACSAria cell sorter (BD Biosciences); events were acquired for a fixed time period of 60 s using CellQuest software (BD Biosciences). The results were expressed as the number of migrated cells calculated as the difference between the number of cells that migrated into the lower chamber containing chemokines and the number of cells that migrated in medium alone.
Statistical analysis
All statistical analyses were performed using the SPSS 13.0 program for Windows. The Mann–Whitney U test was used to calculate the statistical significance of non-parametric differences between groups. P values <0.05 were considered statistically significant.
Results
Combining TLR agonists had a synergistic effect on DC maturation
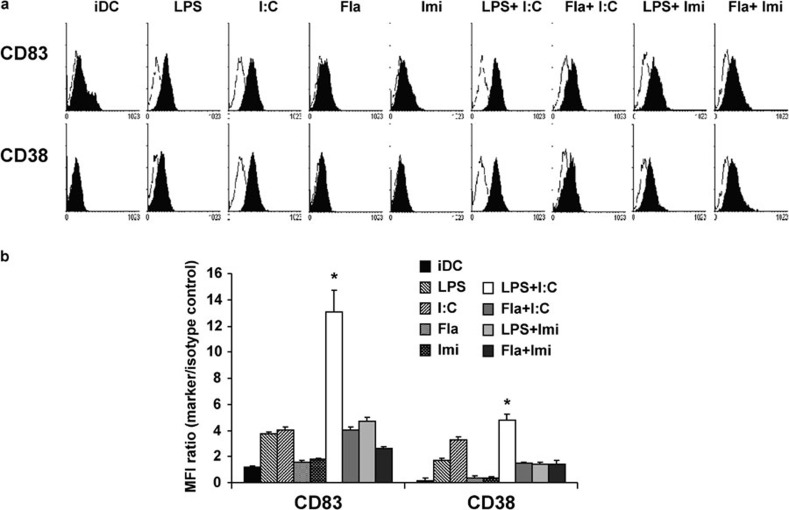
TLR-specific signals can induce the maturation of DCs and generate different responses to stimulation via different TLRs.22, 23 The optimal combination of TLR ligands for inducing synergistic effects was determined by evaluating IL-12p70 production by mature DCs in the presence of TLR ligands (TLR3, 4, 5 and 7/8) alone and in various combinations. All TLR agonists used in this study upregulated the expression of CD80 and CD86 in DCs (data not shown). As shown in Figure 1, the TLR3 agonist (poly(I:C)) and the TLR4 agonist (LPS) induced higher expression of maturation markers (CD83 and CD38) in DCs than the TLR5 agonist (flagellin) and the TLR7/8 agonist (imidazoquinoline), when used at a concentration of 1 µg/ml. However, these TLR agonists could induce full DC maturation when the concentration was increased to more than 1 µg/ml (data not shown). When the TLR agonists were combined, the phenotypic expression of CD83 and CD38 in the DCs was increased. Of the combinations tested, stimulation with an endosomal TLR3 agonist (poly(I:C)) and a membrane-associated TLR4 agonist (LPS) significantly enhanced the expression of CD83 and CD38 compared to the other combinations (P<0.05) (Figure 1). In addition, the extent of CD38 and CD83 expression was similar under all conditions.
Figure 1.
Phenotypic expression of DC maturation markers in response to different TLR agonists. (a) iDCs were matured with agonists for TLR3 (poly(I:C)), TLR4 (LPS), TLR5 (Fla) or TLR7/8 (Imi), or with a combination of two TLR agonists. Expression of CD83 and CD38 was analyzed by flow cytometry. CD83 and CD38 expression after stimulation with the combination of poly(I:C) and LPS was higher than that observed with the other agonists. A representative histogram indicating the expression of markers (shaded) compared to isotype controls (black line) is shown. (b) Data are shown as the increase in MFI over the isotype control±s.d. from five independent experiments (*P<0.05). DC, dendritic cell; Fla, flagellin; iDC, immature DCs; Imi, imidazoquinoline; LPS, lipopolysaccharide; MFI, mean fluorescence intensity; TLR, Toll-like receptor.
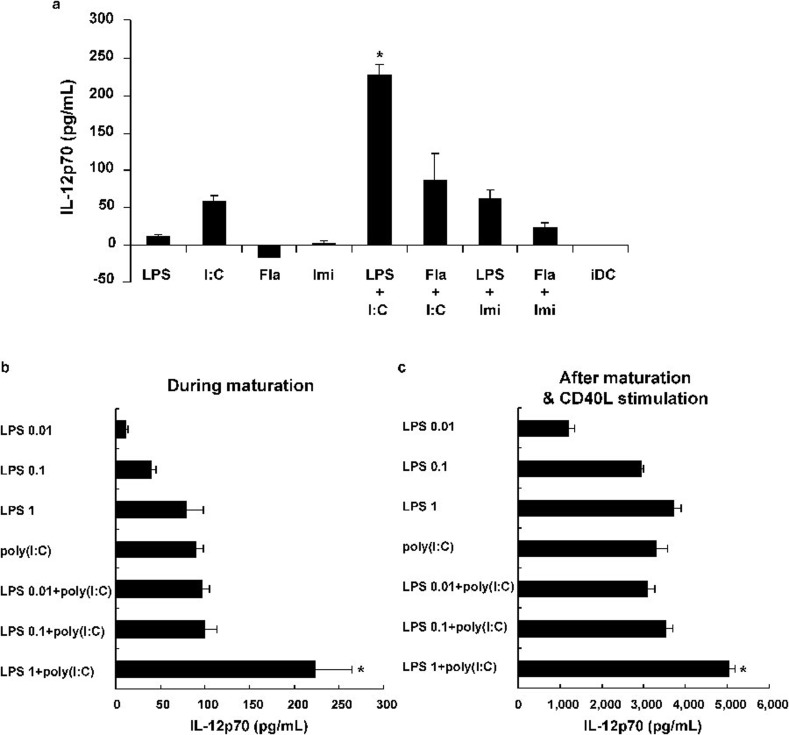
IL-12p70 is not efficiently secreted in DCs stimulated by microbial pathogens alone; however, it is induced at high levels after CD40 on activated DCs interacts with CD40L on activated Th cells.24, 25 In this study, the production of IL-12p70 was generally low in primary culture supernatants from DCs during maturation with a TLR agonist alone. However, IL-12p70 production was significantly increased when the TLR3 and TLR4 agonists were combined to induce DC maturation relative to the other combinations of TLR agonists (P<0.05) (Figure 2a). These results, based on phenotypic expression and IL-12p70 production, suggest that an endosomal TLR3 agonist and a membrane-associated TLR4 agonist could act synergistically to enhance DC maturation.
Figure 2.
IL-12p70 production after DC maturation with different TLR agonists. (a) iDCs were matured with different TLR agonists as described in Figure 1. IL-12p70 secretion was measured in culture supernatants by ELISA. Only (LPS+poly(I:C))-DCs showed significantly higher production of IL-12p70 than the other DCs. (b, c) The optimal concentration of LPS to induce the synergistic effects was determined. iDCs were matured with various concentrations of LPS (0.01, 0.1 or 1 µg/ml) alone and in various combinations with poly(I:C) at 20 µg/ml. IL-12p70 was measured in supernatants during maturation and after CD40L stimulation. Only a concentration of 1 µg/ml LPS in combination with poly(I:C) enhanced the production of IL-12p70 during maturation as much as CD40L stimulation. The data are shown as the mean±s.d. (pg/ml) of triplicate cultures from two representative data sets from four independent experiments (*P<0.05). CD40L, CD40 ligand; DC, dendritic cell; Fla, flagellin; iDC, immature DC; Imi, imidazoquinoline; LPS, lipopolysaccharide; TLR, Toll-like receptor.
The optimal concentration of LPS for inducing the synergistic effects when combined with poly(I:C) was determined. The production of IL-12p70 by mature DCs in the presence of various concentrations of LPS (0.01, 0.1 and 1 µg/ml) alone and in combination with poly(I:C) at 20 µg/ml was evaluated. We found that the combination of lower concentrations of LPS (up to 0.1 µg/ml) and poly(I:C) could not increase the production of IL-12p70 during maturation or upon CD40L stimulation compared with LPS or poly(I:C) alone. However, when used at 1 µg/ml, LPS in combination with poly(I:C) enhanced the production of IL-12p70 during maturation and after CD40L stimulation (Figure 2b and c).
Both IFN-α and IFN-γ synergistically enhanced the maturation of DCs in the presence of TLR agonists
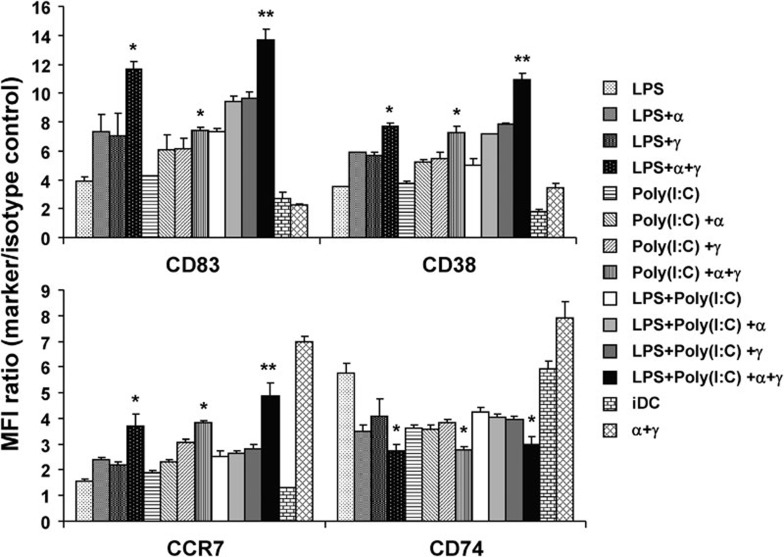
IFN-α and IFN-γ have been shown to play important roles in DC function and cytokine production. The effects of IFN, when combined with TLR agonists, were studied by examining the regulation of expression of several DC markers (CD83, CD38, CD74 and CCR7) and the production of IL-12p70 during maturation and after CD40L stimulation. Immature DCs underwent maturation with poly(I:C) or LPS alone, with both stimuli, or with both in combination with IFN-α and/or IFN-γ. As shown in Figure 3, the DCs treated with IFN-α plus IFN-γ showed lower expression of CD83 and higher expression of CD74 compared with the TLR agonist-treated DCs. These results suggest that IFN could not fully induce DC maturation despite the upregulation of CCR7. The addition of IFN-α or IFN-γ to the TLR stimulation further enhanced the expression of CD83, CD38, and CCR7 compared to TLR stimulation alone. The simultaneous addition of IFN-α plus IFN-γ to the TLR stimulation had a synergistic effect, resulting in a further increase in CD83, CD38 and CCR7 expression compared to TLR stimulation alone (for each marker, P<0.05). The synergistic effects were clearly observed with the combination of both TLR agonists, poly(I:C) and LPS, with IFN-α plus IFN-γ (P<0.02). The negative regulation of CD74 expression upon DC activation modifies the capacity of DCs to migrate.15 In this study, the question of whether CD74 expression was regulated by TLR agonists and/or IFN was investigated. Immature DCs and IFN-induced DCs showed high levels of CD74 expression. As shown in Figure 3, TLR agonists alone slightly downregulated CD74 expression compared to the immature DCs. The addition of IFN-α or IFN-γ to the DCs that were matured with a TLR agonist did not change CD74 expression compared with the TLR agonists alone. However, the addition of both IFN-α and IFN-γ resulted in significant downregulation of CD74 expression in the TLR-stimulating DCs (P<0.05). These results indicate that selected TLR agonists synergistically act with IFN-α and IFN-γ to regulate CD74 expression. These findings suggest that IFN synergistically acts with TLR agonists to enhance the expression of DC maturation markers and the regulation of migrating DC molecules.
Figure 3.
Phenotypic expression of DC maturation markers in response to different TLR agonists in combination with IFN. iDCs were matured with poly(I:C), LPS or both in the presence or absence of IFN-α and/or IFN-γ. The expression of CD83, CD38, CCR7 and CD74 was analyzed by flow cytometry. The combination of poly(I:C) and LPS with type I and II IFN upregulated the expression of CD83, CD38 and CCR7, but downregulated the expression of CD74 compared with DCs matured with other stimuli. Data are shown as the increase in MFI over the isotype control±s.d. from five independent experiments (*P<0.05; **P<0.02). DC, dendritic cell; iDC, immature DC; IFN, interferon; LPS, lipopolysaccharide; MFI, mean fluorescence intensity.
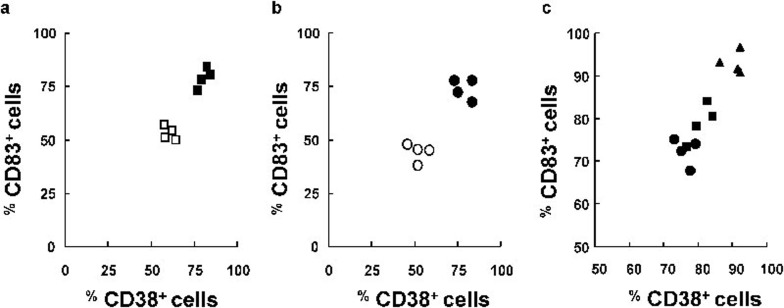
Expression of CD38 and the well-established maturation marker, CD83, was studied in DCs to confirm that the activated cell population was positive for both CD38 and CD83. The comparative analysis of the percentage of CD38+ and CD83+ cells among mature DC after 48 h maturation is shown in Figure 4. The simultaneous addition of IFN-α plus IFN-γ to the stimulation with LPS had a synergistic effect, resulting in a significantly higher percentage of CD83+ and CD38+ mature DC than in samples stimulated with LPS alone (80.8±2.9% and 79.6±3.2% versus 60.7±2.6% and 53.1±4.7% mean±s.e., P<0.05) (Figure 4a). Similar results were observed when poly(I:C) was combined with IFN-α plus IFN-γ (76.1±4.6% and 72.4±2.8% versus 62.6±1.3% and 45.4±2.8% mean±s.e., P<0.05) (Figure 4b). The synergistic effects were clearly observed when both TLR agonists, poly(I:C) and LPS, were combined with IFN-α and IFN-γ (90.4±3.4% CD83+ and 95.3±1.2% CD38+; mean±s.e., P<0.02) (Figure 4c).
Figure 4.
Comparative analysis of CD83 and CD38 surface expression. Immature DCs were treated for 48 h in the presence of the indicated stimuli, and the expression of CD83 and CD38 was assessed by FACS analysis of DCs by double-staining with CD83 and CD38-specific mAb or control isotype-matched mAb. The percentages of cells expressing CD83+ and CD38+ are shown for TLR-stimulated DCs (open symbols; n=4) or DCs treated with a combine of IFN and TLR agonists (filled symbols; n=4). The TLR agonists are indicated as follows: (a) LPS (▪), (b) poly(I:C) (•) or (c) LPS + poly(I:C) (▴). DC, dendritic cell; IFN, interferon; LPS, lipopolysaccharide; mAb, monoclonal antibody; TLR, Toll-like receptor.
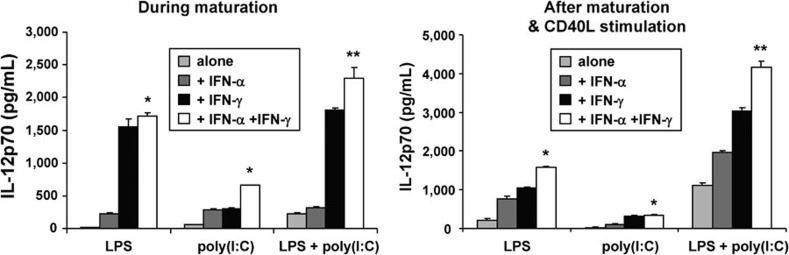
The effect of IFN, in combination with TLR agonists, on IL-12p70 production by DCs, was investigated. The addition of IFN-α or IFN-γ to TLR-stimulated DCs alone or in combination significantly enhanced the ability of maturing DCs to produce IL-12p70, compared to DCs stimulated with a TLR agonist alone (P<0.05). The effects with IFN-γ were more dramatic than with IFN-α, consistent with prior reports.24, 25 Furthermore, the addition of both IFN-α and IFN-γ to a combination of the TLR agonists, poly(I:C) and LPS, significantly enhanced the production of IL-12p70 in DCs compared with the addition of IFN-α or IFN-γ alone (P<0.02). The synergistic effects on IL-12p70 production were observed not only during maturation but also during subsequent CD40L stimulation (Figure 5).
Figure 5.
IL-12p70 production after DC maturation with different TLR agonists in combination with IFN. iDCs underwent maturation for 48 h, as described in Figure 3. IL-12p70 secretion was measured in culture supernatants (left panel) and from DCs further stimulated with CD40L-transfected J558 cells (right panel). The addition of IFN-α and/or IFN-γ to TLR agonist stimulation enhanced IL-12p70 production during maturation as well as after CD40L stimulation. The data are shown as mean±s.d. (pg/ml) of triplicate cultures from three representative data sets from five independent experiments (*P<0.05; **P<0.02). CD40L, CD40 ligand; DC, dendritic cell; iDC, immature DC; IFN, interferon; LPS, lipopolysaccharide; TLR, Toll-like receptor.
Both IFN-α and IFN-γ had synergistic effects on the regulation of DC migration in the presence of TLR agonists
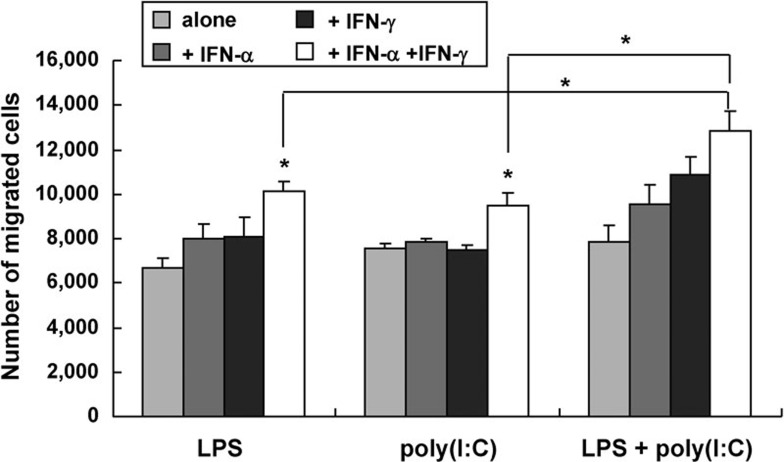
By regulating CD38, CD74 and CCR7 expression, type I and II IFNs had synergistic effects in the presence of TLR agonists on the regulation of DC migration. Migration to secondary lymph node organs is required for the induction of antigen-specific T-cell responses by DCs. The first important mediator in the mobilization of DCs to lymph nodes is CCR7; however, upregulation of CCR7 alone by DCs is insufficient to drive DC migration toward CCL19 and CCL21.1 Recently, the upregulation of CD38 and downregulation of CD74 have been shown to regulate DC migration in vitro and in vivo.12, 13, 14, 15 Figure 3 shows that the TLR agonists poly(I:C) and LPS had synergistic effects on the upregulation of CD38 and CCR7 and the downregulation of CD74 in DCs in the presence of IFN-α and IFN-γ. To confirm the migratory capacity of DCs in response to CCL21, a direct migration assay was used. As shown in Figure 6, the number of DCs that migrated toward CCL21 was similar in the DCs that underwent maturation with TLR ligands alone or in combination. Although IFN-α and IFN-γ could further enhance the production of IL-12p70 in the DCs, their effect on the migratory capacity of the DCs remains unknown. In this study, the addition of IFN-α or IFN-γ to a single TLR agonist had a minor effect on the migratory capacity of DCs, whereas the addition of IFN-α or IFN-γ alone to the combination of poly(I:C) plus LPS increased migration. Migration was greater following stimulation with IFN-γ than with IFN-α. The combination of IFN-α and IFN-γ significantly increased the migration of poly(I:C)- or LPS-stimulating DCs, which was dramatically enhanced when poly(I:C) was combined with LPS (P<0.05), which is consistent with the phenotypic expression profiles shown in Figure 3.
Figure 6.
Migration of TLR agonist-stimulated DCs was enhanced in the presence of both type I and II IFNs. Immature DCs underwent maturation with different TLR agonists in combination with IFN and were tested for migration in response to CCL21 as a ligand for CCR7 in a transwell assay. The addition of IFN-α and IFN-γ to the TLR agonist combination markedly enhanced the migration of DCs. The results represent the mean number of migrated cells±s.d. from three independent experiments (*P<0.05). DC, dendritic cell; IFN, interferon; LPS, lipopolysaccharide; TLR, Toll-like receptor.
The addition of IFN-α and IFN-γ in the presence of the TLR agonists, poly(I:C) and LPS, upregulated CCR7 and CD38 in DCs and downregulated CD74. This phenomenon enabled the DCs to efficiently migrate to the lymph nodes in response to CCL21. Type I and II IFN have synergistic effects in the presence of TLR agonists on the regulation of DC migration by modulating the expression of CD38, CD74 and CCR7.
Discussion
Functionally potent DCs for effective immunotherapy should have a strong Th1-polarizing capacity, characterized by IL-12 production, and the ability to migrate into lymph nodes for the cross-presentation of antigens to effector T cells. In this study, combinations of TLR ligands were studied that significantly enhanced DC maturation with regard to the phenotypic expression of maturation-related molecules and production of the IL-12p70 cytokine. Each member of the TLR family recognizes a specific ligand derived from bacteria or viruses that activates a specific cellular response. For the TLR agonists used in this study, poly(I:C) is recognized by TLR3; the recognition of LPS is mediated by TLR4, flagellin is recognized by TLR5 and imidazoquinoline is recognized by TLR7 and TLR8 in humans.22 When the immune system responds to a pathogen, TLRs can recognize several TLR agonists that trigger TLR signaling in distinct cellular compartments. Although overlap exists among the signaling pathways induced by the various TLRs, the differences between cells activated by individual TLR-specific signals suggest that DCs are able to differentially respond to stimulation with different TLRs.22, 23 Synergistic effects of TLR agonists have been reported in adults and neonates, leading to enhanced production of inflammatory cytokines and Th1-polarizing capacity.6, 7 Although several TLR agonists could induce the maturation of DCs to different degrees, the combination of an endosomal TLR3 agonist, poly(I:C), with a membrane-associated TLR4 agonist, LPS, synergistically affected DC maturation. This synergistic effect may be due to priming by one TLR agonist, resulting in increased stimulation by a second TLR agonist, which reinforces the maturation and activation process.
Type I or II IFN alone cannot fully induce DC maturation. However, as potent cofactors with other maturation signals, type I and II IFNs have synergistic effects that enhance DC maturation.18 Treating immature DCs with a combination of IFN and a TLR 3 agonist has been reported to generate mature type 1-polarized DCs with high levels of IL-12p70 and potent cytotoxic T lymphocyte generating capacity. DCs activated in this manner have increased cytotoxicity in vitro against tumors compared with conventional DCs that undergo maturation with prostaglandin E2.19 However, a major limitation of such DCs is their reduced ability to migrate to primary lymph organs due to their relatively low expression of CCR7 compared with conventional prostaglandin E2-stimulated DCs. The results of this study show that both IFN-α and IFN-γ enhanced the maturation of DCs, especially in the presence of a combination of TLR3 and TLR4 agonists. DCs stimulated with type I & II IFN plus TLR agonists showed increased expression of maturation-related molecules and high production of IL-12p70. Furthermore, both IFN-α and IFN-γ significantly enhanced the migratory capacity of DCs when combined with poly(I:C) and LPS, resulting in the upregulation of CD38 and CCR7, downregulation of CD74 and increased migration toward CCL21. The ability of CD38+ DCs to migrate in response to chemokines appears to be due to the ability of these cells to mobilize Ca2+ in response to chemokine receptor engagement.12 CD74 regulates DC movement by controlling the activity of myosin II, which is the motor protein that controls actomyosin contractility, thus providing a mechanism to coordinate antigen processing and migration.15 The results of this study suggest that type I and II IFNs have synergistic effects in the presence of TLR agonists on the regulation of DC migration by controlling the expression of CD38, CCR7 and CD74 in DCs.
DCs generated in vitro that can home to a local lymph node are highly sought after for vaccination protocols but are difficult to produce in practice. This study and other recent reports offer new insight into how the migratory capacity of DCs is enhanced and may provide a novel approach for improving vaccination efficacy. The results of this study suggest that, at the optimal concentration used to stimulate DCs, the combination of the two TLR agonists poly(I:C) and LPS with type I and II IFNs can be used to generate fully mature DCs that have high migratory capacity and maintain the ability to produce IL-12p70. Regulation of CD38 and CD74 could, in turn, enhance the migration of DCs in the presence of a combination of two TLR agonists and IFNs.
Acknowledgments
This study was financially supported by a grant from the Korea Healthcare Technology R&D Project (A080489), Ministry of Health & Welfare, Korea, and by Grant No. RTI05-01-01 from the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy, Korea.
References
- Luft T, Jefford M, Luetjens P, Toy T, Hochrein H, Masterman KA, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E2 regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–1372. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Thomson AW. Dendritic cells under the spell of prostaglandins. Trends Immunol. 2003;24:108–111. doi: 10.1016/s1471-4906(03)00023-1. [DOI] [PubMed] [Google Scholar]
- Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–2809. [PubMed] [Google Scholar]
- Kaisho T, Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr Mol Med. 2003;3:373–385. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007;68:813–822. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Malavasi F, Funaro A, Roggero S, Horenstein A, Calosso L, Mehta K. Human CD38: a glycoprotein in search of a function. Immunol Today. 1994;15:95–97. doi: 10.1016/0167-5699(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. . Nat Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- Gasser A, Bruhn S, Guse AH. Second messenger function of nicotinic acid adenine dinucleotide phosphate revealed by an improved enzymatic cycling assay. J Biol Chem. 2006;281:16906–16913. doi: 10.1074/jbc.M601347200. [DOI] [PubMed] [Google Scholar]
- Bruzzone S, de Flora A, Usai C, Graeff R, Lee HC. Cyclic ADP-ribose is a second messenger in the lipopolysaccharide-stimulated proliferation of human peripheral blood mononuclear cells. Biochem J. 2003;375:395–403. doi: 10.1042/BJ20030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida-Sanchez S, Goodrich S, Kusser K, Oppenheimer N, Randall TD, Lund FE. Regulation of dendritic cell trafficking by the ADP-ribosyl cyclase CD38: impact on the development of humoral immunity. Immunity. 2004;20:279–291. doi: 10.1016/s1074-7613(04)00048-2. [DOI] [PubMed] [Google Scholar]
- Fedele G. Frasca L, Palazzo R, Ferrero E, Malavasi F, Ausiello CM. CD38 is expressed on human mature monocyte-derived dendritic cells and is functionally involved in CD83 expression and IL-12 induction. Eur J Immunol. 2004;34:1342–1350. doi: 10.1002/eji.200324728. [DOI] [PubMed] [Google Scholar]
- Frasca L, Fedele G, Deaglio S, Capuano C, Palazzo R, Vaisitti T, et al. CD38 orchestrates migration, survival, and Th1 immune response of human mature dendritic cells. Blood. 2006;107:2392–2399. doi: 10.1182/blood-2005-07-2913. [DOI] [PubMed] [Google Scholar]
- Faure-Andre G, Vargas P, Yuseff MI, Heuze M, Diaz J, Lankar D, et al. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science. 2008;322:1705–1710. doi: 10.1126/science.1159894. [DOI] [PubMed] [Google Scholar]
- Luft T, Luetjens P, Hochrein H, Toy T, Masterman KA, Rizkalla M, et al. IFN-alpha enhances CD40 ligand-mediated activation of immature monocyte-derived dendritic cells. Int Immunol. 2002;14:367–380. doi: 10.1093/intimm/14.4.367. [DOI] [PubMed] [Google Scholar]
- Padovan E, Spagnoli GC, Ferrantini M, Heberer M. IFN-alpha2a induces IP-10/CXCL10 and MIG/CXCL9 production in monocyte-derived dendritic cells and enhances their capacity to attract and stimulate CD8+ effector T cells. J Leukoc Biol. 2002;71:669–676. [PubMed] [Google Scholar]
- Frasca L, Nasso M, Spensieri F, Fedele G, Palazzo R, Malavasi F, et al. IFN-gamma arms human dendritic cells to perform multiple effector functions. J Immunol. 2008;180:1471–1481. doi: 10.4049/jimmunol.180.3.1471. [DOI] [PubMed] [Google Scholar]
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, et al. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W, et al. Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother. 2009;58:1329–1336. doi: 10.1007/s00262-008-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Akira S. Microbial recognition by Toll-like receptors. J Dermatol Sci. 2004;34:73–82. doi: 10.1016/j.jdermsci.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Huang Q, Liu D, Majewski P, Schulte LC, Korn JM, Young RA, et al. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]