Abstract
Inflammatory stimuli, such as a microbes or lipopolysaccharides, induce a rapid release of neutrophils from the bone marrow and promote neutrophil migration into inflamed sites to promote host defense. However, an excess accumulation and retention of neutrophils in inflamed tissue can cause severe tissue injuries in the later stages of inflammation. Recent studies have reported that both CXCL12 levels in injured lungs and its receptor, CXCR4, on accumulated neutrophils in injured lungs, increased; furthermore, these studies showed that the CXCL12/CXCR4 signaling pathway participated in neutrophil accumulation in the later stages of lipopolysaccharide (LPS)-induced lung injury. However, the mechanisms underlying this increase in surface CXCR4 expression in neutrophils remain unclear. In this study, we found that surface CXCR4 expression increased in extravascular, but not intravascular, neutrophils in the lungs of LPS-induced lung injury model mice. Furthermore, ex vivo studies revealed that CXCL12 acted not only as a chemoattractant, but also as a suppressor of cell death for the lung neutrophils expressing CXCR4. Sulfatide, one of the native ligands for L-selectin, induced the increase of surface CXCR4 expression on isolated circulating neutrophils, suggesting that the activation of L-selectin may be involved in the increase in surface CXCR4. Our findings show that surface CXCR4 levels on neutrophils increase after extravasation into injured lungs, possibly through the activation of L-selectin. The CXCL12/CXCR4 signaling pathway plays an important role in the modulation of neutrophil activity during acute lung injury, not only by promoting chemotaxis but also by suppressing cell death.
Keywords: CXCL12, CXCR4, lipopolysaccharides, lung injury, neutrophils
Introduction
During acute inflammation, neutrophils are released from the bone marrow and migrate into inflamed tissues.1, 2 In inflamed tissues, neutrophils extravasate from blood vessels to the site of tissue injury or infection. These extravasated neutrophils play an important role in host defense against pathogenic microorganisms, though the excess accumulation and activation of neutrophils can cause severe tissue injury. Therefore, the apoptosis of neutrophils and the proper processing of apoptotic neutrophils by macrophage phagocytosis are important for the resolution of inflammation to prevent tissue injury. It is well established that inflammatory cytokines (including TNF-α3 and IL-14) and neutrophil attractant CXC chemokines5, 6, 7 are critically involved in the accumulation of neutrophils within injured tissues during the acute phase of inflammation. However, the mechanisms of neutrophil retention and withdrawal during the later phase of inflammation are not well understood and are still being investigated.
The accumulated evidence published thus far suggests that CXC chemokine CXCL12/stromal cell-derived factor-1, which was first described as a strong chemotactic factor for lymphocytes,8, 9, 10, 11 contributes to the control of the neutrophil life cycle through the activation of CXCR4. It has been reported that the CXCL12/CXCR4 signaling system plays an important role in the regulation of neutrophil homeostasis, including both the release of neutrophils from bone marrow into blood12, 13, 14, 15 and the homing of the circulating neutrophils to the bone marrow.14, 16 In addition to the evidence supporting the role of CXCL12/CXCR4 in modulating neutrophil homeostasis, we and other investigators previously reported that both CXCL12 levels in the injured lungs and surface CXCR4 protein on accumulated neutrophils were increased; furthermore, the in vivo administration of an anti-CXCL12 blocking antibody suppressed airspace neutrophilia in the lungs in the later stages of lipopolysaccharide (LPS)-induced lung injury.17, 18, 19 These findings suggested that this chemokine participated in the accumulation of neutrophils in the injured tissue, particularly in the later stages of inflammation. However, the mechanisms underlying the increase in surface CXCR4 expression on neutrophils during LPS-induced lung injury and the CXCL12-promoted accumulation of neutrophils remain unclear.
In this study, we investigated when and how surface CXCR4 expression levels increased on neutrophils in the lungs during LPS-induced lung injury. We further examined the effects of CXCL12 on isolated neutrophils to evaluate how this chemokine contributes to neutrophil accumulation in the injured tissue. In our investigation of the mechanism underlying the increase in surface CXCR4 expression levels, we focused on L-selectin because previous reports have shown that the activation of L-selectin induced the expression of surface CXCR4 in both human and mouse lymphocytes;20, 21 furthermore, the shedding of L-selectin, which resulted from the activation of L-selectin by its ligands,22 was observed in extravasated neutrophils in inflamed tissues of mice.23, 24
Materials and methods
Animals
C57BL/6J mice were purchased from CLEA Japan Inc. (Tokyo, Japan). All mice were 7- to 8-week-old males and were housed under specific pathogen-free conditions for 1 week prior to experimental use. All animal experiments were permitted by the Institutional Animal Care and Use Committee of the Tohoku University Environmental and Safety Committee and were performed in accordance with the Regulations for Animal Experiments and Related Activities at Tohoku University.
Reagents
The reagents used in this study were obtained from the following sources: mouse monoclonal anti-CXCL12 blocking antibody was purchased from R&D Systems (Minneapolis, MN, USA); control mouse immunoglobulin G1 (IgG1) was purchased from Sigma (St Louis, MO, USA); a specific CXCR4 antagonist, 4F-benzoyl-TE14011, was synthesized as previously described;25, 26 a rabbit anti-mouse CXCL12 antibody was purchased from BioVision (Mountain View, CA, USA) for immunohistochemical experiments; mouse monoclonal anti-mouse/human CXCL12 antibody was purchased from R&D Systems for immunoblotting analysis; phycoerythrin (PE)-conjugated rat anti-mouse CXCR4 monoclonal antibody (clone 2B11/CXCR4) and an isotype control antibody (clone A95-1), PE-conjugated rat IgG2b), fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse Ly-6G monoclonal antibody (clone 1A8) and purified rat anti-mouse CD16/CD32 (Fcγ III/II receptor) monoclonal antibody (clone 2.4G2, Mouse BD Fc Block) were purchased from BD Pharmingen (San Diego, CA, USA); FITC-conjugated anti-mouse neutrophil antibody (clone 7/4) was purchased from Serotec (Raleigh, NC, USA); Alexa Fluor 647-conjugated anti-mouse Ly-6G (Gr-1) monoclonal antibody (clone RB6-8C5), 7-aminoactinomycin D (7-AAD) viability staining solution and an Annexin V apoptosis detection kit APC were purchased from eBioscience (San Diego, CA, USA); Alexa Fluor 647 anti-mouse CD62L antibody and Alexa Fluor 647 rat IgG2a, κ isotype control antibody were purchased from Biolegend (San Diego, CA, USA); recombinant mouse CXCL12α was purchased from R&D Systems; sulfatide sodium salt from bovine spinal cord was purchased from Wako Chemicals (Tokyo, Japan); TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA); anti-p44/42 MAPK (ERK1/2) rabbit monoclonal antibody, anti-phospho-p44/42 MAPK (ERK1/2, Thr202 and Tyr204) rabbit monoclonal antibody, anti-Akt rabbit monoclonal antibody, anti-phospho-Akt (Thr308) rabbit monoclonal antibody, goat anti-rabbit IgG, HRP-linked antibody, cell lysis buffer, U0126 (MEK1/2 inhibitor) and LY294002 (PI3 kinase inhibitor) were purchased from Cell Signaling Technology (Danvers, MA, USA).
LPS-induced lung injury
LPS from Escherichia coli serotype 055:B5 was obtained from Sigma. Mice were anesthetized by ketamine hydrochloride. While anesthetized, mice intranasally inhaled LPS (20 µg/mouse in phosphate-buffered saline (PBS)) that was placed on one nostril. Control mice received only PBS.
Preparation of single lung cells from lungs
Mice received an overdose of inhaled halothane, and their lungs were perfused with PBS via the right ventricles. PBS-perfused lungs were isolated with other mediastinal organs. Dispase II solution was instilled into the lungs through the trachea, which was ligated with a silk suture. After incubation at 37 °C for 50 min, the lungs were separated from the other mediastinal organs. The lungs were then thoroughly minced and digested in PBS with 0.1% collagenase, 0.01% deoxyribonuclease I and 5-mM CaCl2 at 37 °C for 20 min. The cells were then suspended in red blood cell lysing buffer to remove red blood cells and subsequently washed with PBS. The cells were then centrifuged and resuspended in PBS.
Evaluation of surface CXCR4 expression on neutrophils
The surface CXCR4 expression levels of neutrophils isolated from bone marrow, circulating blood, single lung cell suspensions and BAL fluid were evaluated. Mouse neutrophils were identified by their forward scatter and side scatter characteristics and positive Ly-6G staining. The samples were stained with a PE-labeled anti-CXCR4 antibody or a PE-labeled isotype-matched control antibody and an FITC-labeled anti-Ly6G (1A8) antibody. Dead cells were excluded based on 7-AAD staining. The samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Identification of intravascular and extravascular neutrophils in the lungs
The identification of intravascular and extravascular neutrophils in the lungs was performed as previously described with some modifications.27 An Alexa Fluor 647-conjugated anti-mouse Ly-6G (Gr-1) antibody (1 µg) was injected intravenously and allowed to circulate for 5 min. After 5 min, the mice were killed. The lungs were then digested as described above in the presence of an excess of unlabeled anti-mouse Gr-1 antibody to prevent possible binding of Alexa Fluor 647-conjugated anti-Gr-1 antibody to extravascular neutrophils. The lung cell suspensions were then stained with an FITC-conjugated anti-mouse neutrophil antibody (7/4). Intravascular (7/4+Gr-1+) and extravascular (7/4+Gr-1−) neutrophil populations were assessed using flow cytometry.
In vivo blocking the CXCL12/CXCR4 signaling pathway using a specific CXCR4 antagonist
A specific CXCR4 antagonist (4F-benzoyl-TE14011) was used for in vivo inhibition of the CXCL12/CXCR4 signaling pathway as described previously.28 Briefly, 4F-benzoyl-TE14011 was dissolved in PBS and subcutaneously administered using ALZET osmotic pumps (Durect Corp., Cupertino, CA, USA) that were implanted dorsally under the skin 1 day before administration of LPS. 4F-benzoyl-TE14011 was infused at a rate of 120 µg/day for 3 days after implantation. For the control study, ALZET osmotic pumps filled with the same volume of PBS were subcutaneously implanted in the same manner.
Bronchoalveolar lavage (BAL)
First, mice were killed by administering an overdose of halothane. Lavage tubes were then implanted into the mice according to the following procedure: a median sternotomy was performed, the trachea were dissected and isolated from the underlying soft tissues and a 0.8-mm lavage tube was inserted through a small incision in the trachea. BAL was performed by instilling 0.5 ml of ice cold PBS into the lungs and then gently aspirating the fluid. BAL was then repeated two times using fresh 0.5-ml aliquots of PBS. These three fluid samples were pooled and centrifuged. Cell counts and differentials were then performed. BAL protein in cell-free BAL fluid was assayed as an index of lung injury and capillary leakage. Protein quantification was performed using a BCA Protein Assay Reagent Kit (Pierce Biotechnology Inc., Rockford, IL, USA).
Histological analysis
The lungs were fixed by inflation with 10% neutral-buffered formalin at a transpulmonary pressure of 20 cm H2O, embedded in paraffin and cut into 5-µm thick sections. Sections were stained with hematoxylin and eosin for histological assessment. Images were taken with Nikon Eclipse E80i Microscope (Nikon, Tokyo, Japan). These images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
The lung injury scores were calculated using the previously published scoring system.29 Two sections per animal were scored independently. Alveolar wall thickness was quantified in a blinded fashion by measuring of all septa along a crosshair placed on each image. At least 200 septa per animal were measured. The number of emigrated neutrophils was quantified by counting the number neutrophils present within all alveolar spaces in randomly selected fields. At least 200 alveolar spaces were counted.
Isolation of lung neutrophils
Neutrophils were separated from bone marrow cells, circulating blood leukocytes and single lung cell suspensions using an anti-Ly6G (1A8) MicroBead Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. The purity of the isolated neutrophils was evaluated using both microscopic and flow cytometric analyses and was determined to be greater than 98%.
Neutrophil migration assay
Neutrophils were placed on a modified Boyden chamber with 3-µm pores (Chemotaxcell; Kurabo Industries Ltd, Osaka, Japan) to evaluate the migration stimulating activity of CXCL12. Splenocytes, which can migrate toward CXCL12,26 were used as a positive control. A total of 5×105 neutrophils or splenocytes in 200 µl of RPMI 1640 medium containing 0.25% bovine serum albumin were placed in the upper chambers. The upper chambers were then placed in individual wells of a 24-well cell culture plate containing 500 µl of assay buffer either with or without mouse CXCL12α (50 nM). An equal number of neutrophils or splenocytes were added to some of the lower wells without a top chamber to provide a standard count of total cells. In some experiments, the cells were pre-incubated with 100-nM 4F-benzoyl-TE14011 at 37 °C for 30 min. The chambers were then incubated for 2 h at 37 °C. The cells in the lower chamber were collected, and the percentage of migration was determined from the original cell input.
Cell death analysis
Lung neutrophils (2×106) in RPMI 1640 containing 10% FCS were either untreated or preincubated with a specific CXCR4 antagonist for 1 h at 37 °C. The neutrophils were then mixed with 100 ng/ml CXCL12α alone or in combination with a MEK1/2 inhibitor (U0126, 1 μM) or a PI3K inhibitor (LY294002, 10 µM) and incubated for an additional 24 h at 37 °C. After this incubation, the neutrophils were counted and the percentage of dead cells was calculated using trypan blue staining. Neutrophils were also stained with Annexin V and 7-AAD, and then analyzed using a FACSCalibur flow cytometer.
Western blotting
Lung neutrophils isolated from the injured lung were left untreated or incubated at 37 °C with 100 ng/ml CXCL12α for 30 s. Some neutrophils were pre-incubated with 100-nM 4F-benzoyl-TE14011 at 37 °C for 30 min. Cells were lysed in 1× cell lysis buffer (Cell Signaling Technology). Whole-cell lysate was run on an any kD Mini-PROTEAN TGX Precast SDS-PAGE gel (Bio-Rad, Hercules, CA, USA) and the proteins were transferred by electroblotting onto polyvinylidene fluoride membrane (Invitrogen). The blots were probed with antibodies specific for ERK1/2 phosphorylation at Thr202 and Tyr204 or Akt phosphorylation at Thr 308. Membranes were stripped with Restore Western Blot Stripping Buffer (Thermo Fisher Scientific, Rockford, IL, USA) and then reblotted with anti-ERK1/2 or anti-Akt.
L-selectin stimulation
Neutrophils were resuspended in RPMI 1640 (Invitrogen) with 0.1% bovine serum albumin (Sigma). The neutrophils were then incubated with sulfatide at a final concentration of 100 µg/ml for 1 h at 37 °C.
Data presentation and statistical analysis
Unless otherwise noted, all data presented are expressed as mean±standard error of the mean (s.e.m.). Statistical analyses were performed using Statistica software (StatSoft Inc., Tulsa, OK, USA). Data were assessed for significance by ANOVA with Scheffé's post hoc method for multiple comparisons. Statistical significance was defined as P<0.05.
Results
The expression of surface CXCR4 increased on extravascular neutrophils in the mouse lungs during LPS-induced lung injury
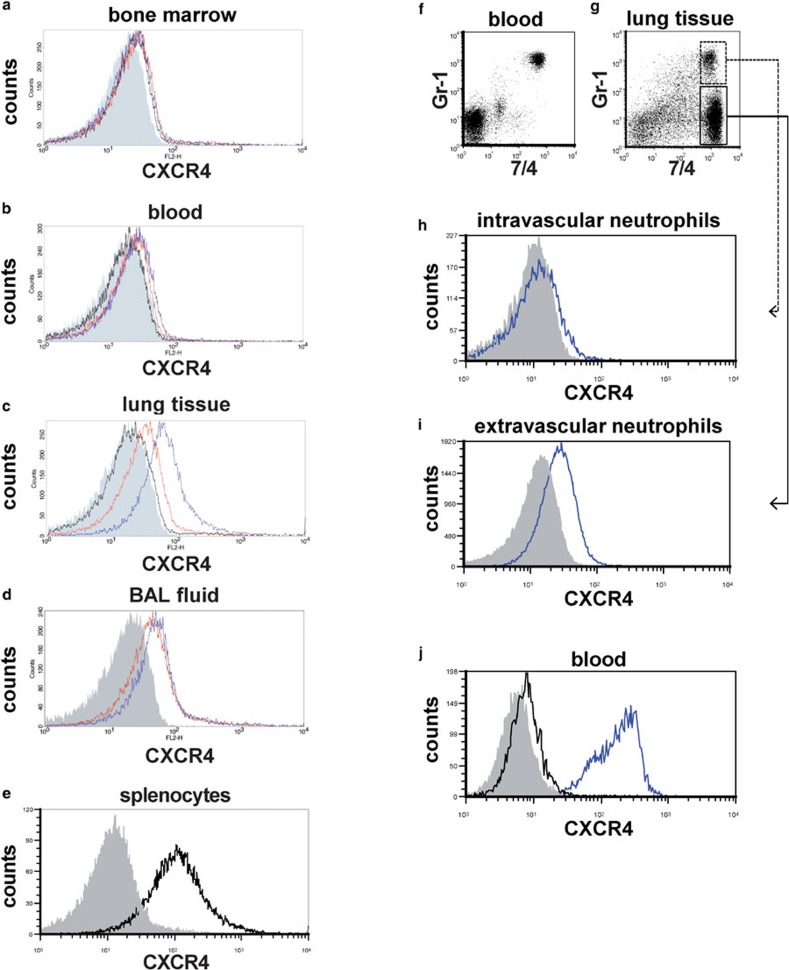
Neutrophils in bone marrow, peripheral blood and lung digests from untreated control mice expressed very low levels of surface CXCR4 (Figure 1a–c, black lines). Surface expression of CXCR4 did not clearly increased in neutrophils from the bone marrow or peripheral blood at 6 and 24 h after LPS instillation (Figure 1a and b, red and blue lines, respectively). However, neutrophils from lung digests and BAL fluids exhibited a significant increase in surface CXCR4 expression at both 6 and 24 h during LPS-induced lung injury (Figure 1c and d, red and blue lines, respectively). To investigate whether a subset of neutrophils with higher surface CXCR4 expression in the blood emigrates into the lungs or if neutrophil surface CXCR4 expression increases after the cells emigrate into the lungs, we examined the surface CXCR4 expression of both intravascular and extravascular neutrophils in the lungs during LPS-induced lung injury. We first labeled intravascular neutrophils in vivo by the intravascular administration of the Alexa 647-conjugated anti-mouse Gr-1 antibody and labeled all neutrophils in the lung digest using the FITC-conjugated anti-mouse 7/4 antibody (Figure 1f and g, also see the section on ‘Materials and methods'). Almost all circulating neutrophils in blood were stained with Gr-1 5 min after antibody injection (Figure 1f). Extravascular neutrophils, which were labeled with the anti-7/4 but not the anti-Gr-1 antibody (Figure 1g), exhibited clearly higher surface CXCR4 expression levels (Figure 1i) compared to intravascular neutrophils (Figure 1h). As previously reported in humans,16 intracellular staining revealed that the levels of CXCR4 expression were high in the intracellular compartments of neutrophils isolated from mouse blood (Figure 1j), suggesting that translocation of CXCR4 to the cell surface occurred in the neutrophils isolated from injured lungs. Taken together, these findings suggested that the cell surface CXCR4 expression levels increased after these cells emigrated into the lungs and extravasated during LPS-induced lung injury.
Figure 1.
Surface CXCR4 expression increased in extravascular neutrophils in the mouse lungs during LPS-induced lung injury. (a–e) Flow cytometric analyses were performed to determine the surface CXCR4 expression levels of neutrophils isolated from bone marrow (a), peripheral blood (b), lung tissue (c) and BAL fluid (d). Neutrophils were analyzed before LPS administration (black line) and at 6 h (red line) and 24 h (blue line) afterward. The filled images show the staining using an isotype-matched control antibody. We were unable to analyze the surface CXCR4 expression levels of neutrophils isolated from BAL fluid before LPS administration because there were an insufficient number of neutrophils in these samples. Splenocytes from untreated control mice were used as a positive control for CXCR4 staining (e). (f–i) The surface CXCR4 expression levels of intravascular neutrophils (g; GR-1+7/4+) and extravascular neutrophils (g; GR-1−7/4+) in the lungs at 24 h during LPS-induced lung injury were examined. Note almost all circulating neutrophils (7/4+ cells) in blood were stained with Gr-1 5 min after antibody injection (f). The blue lines show the surface CXCR4 expression levels of intravascular neutrophils (h) or extravascular neutrophils (i). The filled images show the staining using an isotype-matched control antibody. (h) The levels of intracellular CXCR4 (blue line) in neutrophils isolated from mouse blood were analyzed by staining with permeabilization. The filled image shows the staining using an isotype-matched control antibody. The black line shows the surface CXCR4 expression levels. Representative histograms or dot plots from one of three experiments that showed similar results are presented. BAL, bronchoalveolar lavage; LPS, lipopolysaccharide.
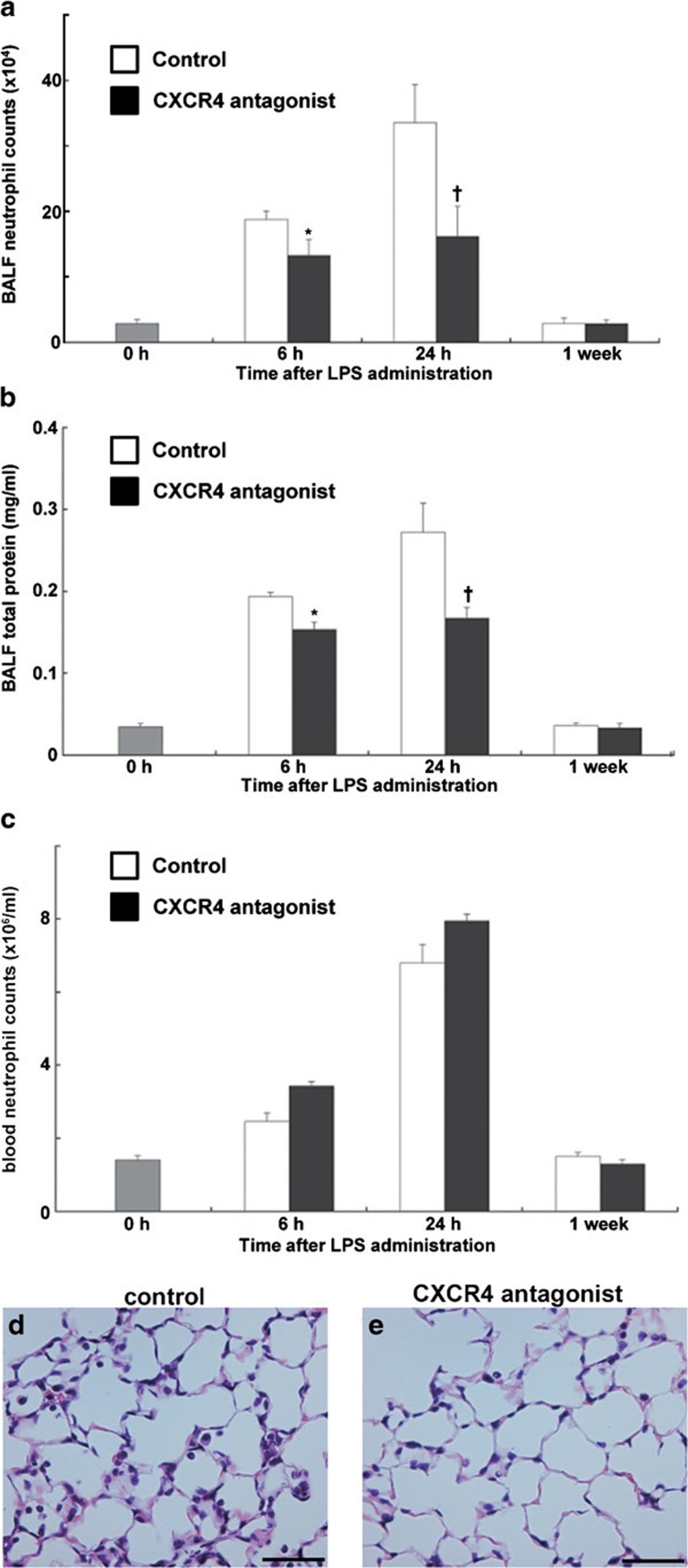
Blocking the CXCL12/CXCR4 signaling pathway inhibited neutrophil accumulation into the air space and attenuated the increase in lung permeability during LPS-induced lung injury.
Previous reports by our group and other investigators have shown that CXCL12 levels are upregulated in injured lungs and that in vivo administration of anti-CXCL12 blocking antibodies suppresses airspace neutrophilia in the lungs at the later stages of LPS-induced lung injury,17, 18, 19 suggesting that the increase of surface CXCR4 expression on lung extravasated neutrophils cooperates with the increase of CXCL12 in the lungs to facilitate the accumulation of neutrophils. To confirm the in vivo role of the CXCL12/CXCR4 signaling system in the pathogenesis of acute lung injury, we administrated a specific CXCR4 antagonist to block the activation of CXCR4. Because it has been reported that single-dose administration of a CXCR4 antagonist rapidly induces neutrophilia,12, 30, 31 we administrated the antagonist continuously using an osmotic pump as previously we did.28 We compared white blood cell and neutrophil count between subcutaneous single-dose and continuous dosing administration of 4F-benzoyl-TE14011 in uninjured mice. As previously reported in human,30, 31 single-dose administration of 4F-benzoyl-TE14011 caused the significant neutrophilia with a peak increase at 6 h (Supplementary Table 1). However, continuous dosing administration did not cause the significant neutrophilia (Supplementary Table 1). Continuous administration of the antagonist also did not result in significant neutrophilia in comparison with control mice during LPS-induced lung injury (Figure 2c). The number of the neutrophils (Figure 2a) and the protein concentration (Figure 2b) in the BAL fluid were significantly reduced in CXCR4 antagonist treated mice at 6 and 24 h during LPS-induced lung injury in comparison with the control mice. Histological assessment also revealed that the treatment with a CXCR4 antagonist attenuated the lung injury induced by LPS at 24 h (Figure 2e and c and Table 1). These findings confirmed that both CXCL12 and its receptor CXCR4 contribute to neutrophil accumulation in the air space and an increase in lung permeability during LPS-induced lung injury.
Figure 2.
Blocking the CXCL12/CXCR4 signaling pathway inhibited neutrophil migration into the lung air space and the increase of lung permeability during LPS-induced lung injury. (a–c) Neutrophil counts (a), total protein concentration (b) in the BALF and neutrophil counts in the circulating blood (c) were determined in C57BL/6 mice treated with either PBS (white) or a CXCR4 antagonist (black) at indicated time points during LPS-induced lung injury. A total of six mice were used in each group. Values represent mean±s.e.m. *P<0.01, †P<0.05, versus PBS control mice using ANOVA with Scheffé's post hoc test. (d, e) Histological evaluation of the treatment with a CXCR4 antagonist on LPS-induced lung injury. Representative images of hematoxylin and eosin stained lung tissue sections from PBS- (d) or CXCR4 antagonist-treated (e) mice at 24 h during LPS-induced lung injury. Scale bar=50 µm. BALF, bronchoalveolar lavage fluid; LPS, lipopolysaccharide; PBS, phosphate-buffered saline.
Table 1. Histopathological lung injury score, interalveolar septal thickness and the number of neutrophils in alveolar spaces in mice at 24 h during LPS-induced lung injury.
| Measure | Untreated control | LPS and PBS | LPS and CXCR4 antagonist |
|---|---|---|---|
| Lung injury score | 1.1±0.1 | 3.3±0.1 | 2.5±0.2* |
| Septal thickness (µm) | 1.6±0.1 | 2.9±0.1 | 2.5±0.1* |
| No. of neutrophils in 100 alveoli | 1.2±0.3 | 68.3±4.6 | 48.5±2.9* |
Abbreviations: LPS, lipopolysaccharide; PBS, phosphate-buffered saline.
Data are represented as mean±s.e.m. n=6 per group.
*P<0.01 versus LPS and PBS group.
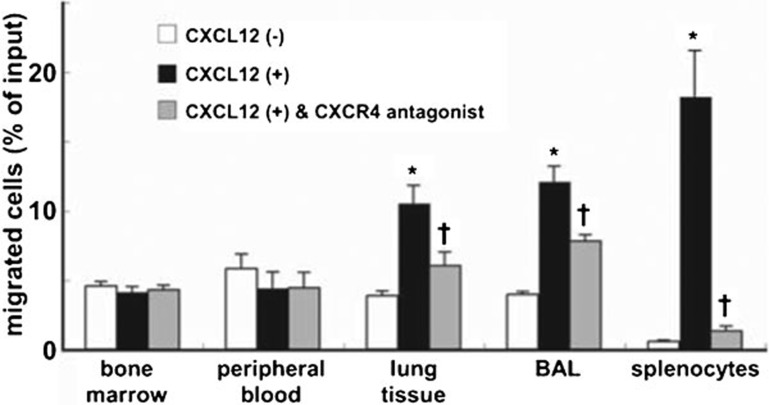
Neutrophils isolated from injured lungs exhibited migratory activity toward CXCL12
To elucidate the role of CXCR4 in the accumulation of neutrophils in the injured lung, we examined whether lung neutrophils responded to and showed the migratory activity toward CXCL12. Isolated neutrophils from the LPS-instilled mice were placed in modified Boyden chambers, and CXCL12 was added to the chambers as a chemoattractant. Splenocytes from untreated control mice were used as a positive control because splenocytes expressed high levels of surface CXCR4 (Figure 1e). Neutrophils isolated from the bone marrow or peripheral blood exhibited no migratory response toward CXCL12 (Figure 3). In contrast, neutrophils isolated from lung digests and BAL fluids exhibited migratory responses to CXCL12 (Figure 3). Furthermore, this migratory response to CXCL12 was blocked by a specific CXCR4 antagonist. These ex vivo findings demonstrated that the accumulated lung neutrophils expressing high surface levels of CXCR4 exhibited migratory activity toward CXCL12, whereas bone marrow and peripheral blood neutrophils, which express very low surface levels of CXCR4, did not respond to CXCL12.
Figure 3.
Neutrophils that accumulated in the mouse lungs during LPS-induced lung injury showed migratory responses to CXCL12. Neutrophils were isolated from the bone marrow, blood, lung tissue and BAL fluid at 24 h during LPS-induced lung injury. Cell migration assays assessing the migration of neutrophils toward CXCL12 were performed in vitro using chemotaxis chambers. Splenocytes from untreated control mice were used as a positive control because splenocytes expressed high levels of surface CXCR4 (Figure 1e). Levels of neutrophil or splenocyte migration in the absence of CXCL12, the presence of CXCL12, or after pretreatment with a CXCR4 antagonist in the presence of CXCL12, are depicted by the white, black and gray bars, respectively. The data shown represent the percentage of migration. The results were obtained from five mice in each group. Values represent mean±s.e.m. *P<0.01 versus CXCL12 (−) control group and †P<0.01 versus CXCL12 (+) group using ANOVA with Scheffé's post hoc test. BAL, bronchoalveolar lavage.
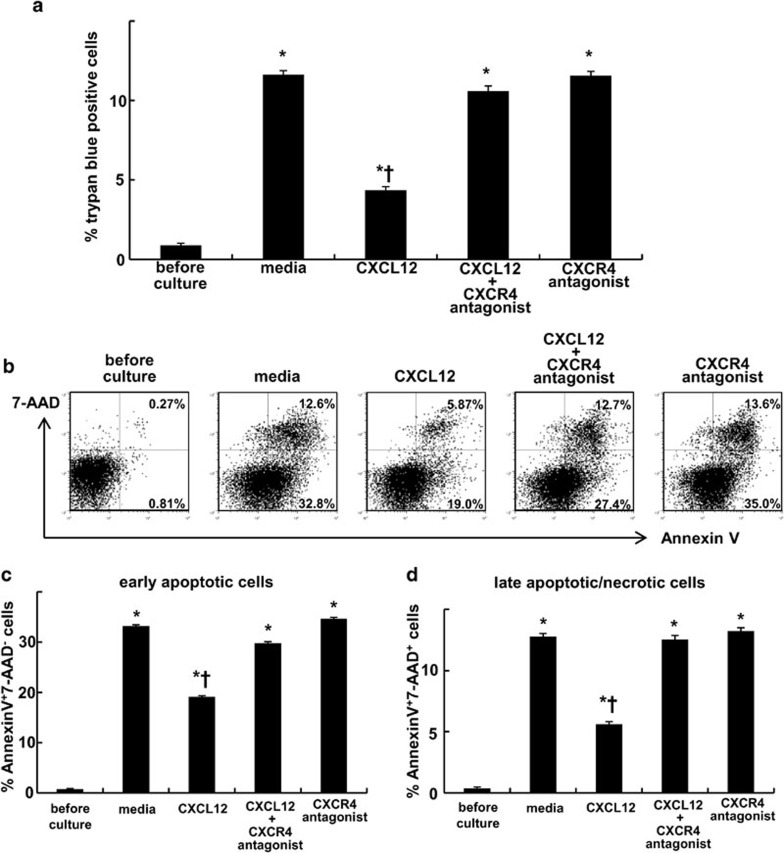
Activation of CXCR4 by CXCL12 attenuated cell death of isolated neutrophils from the injured lungs
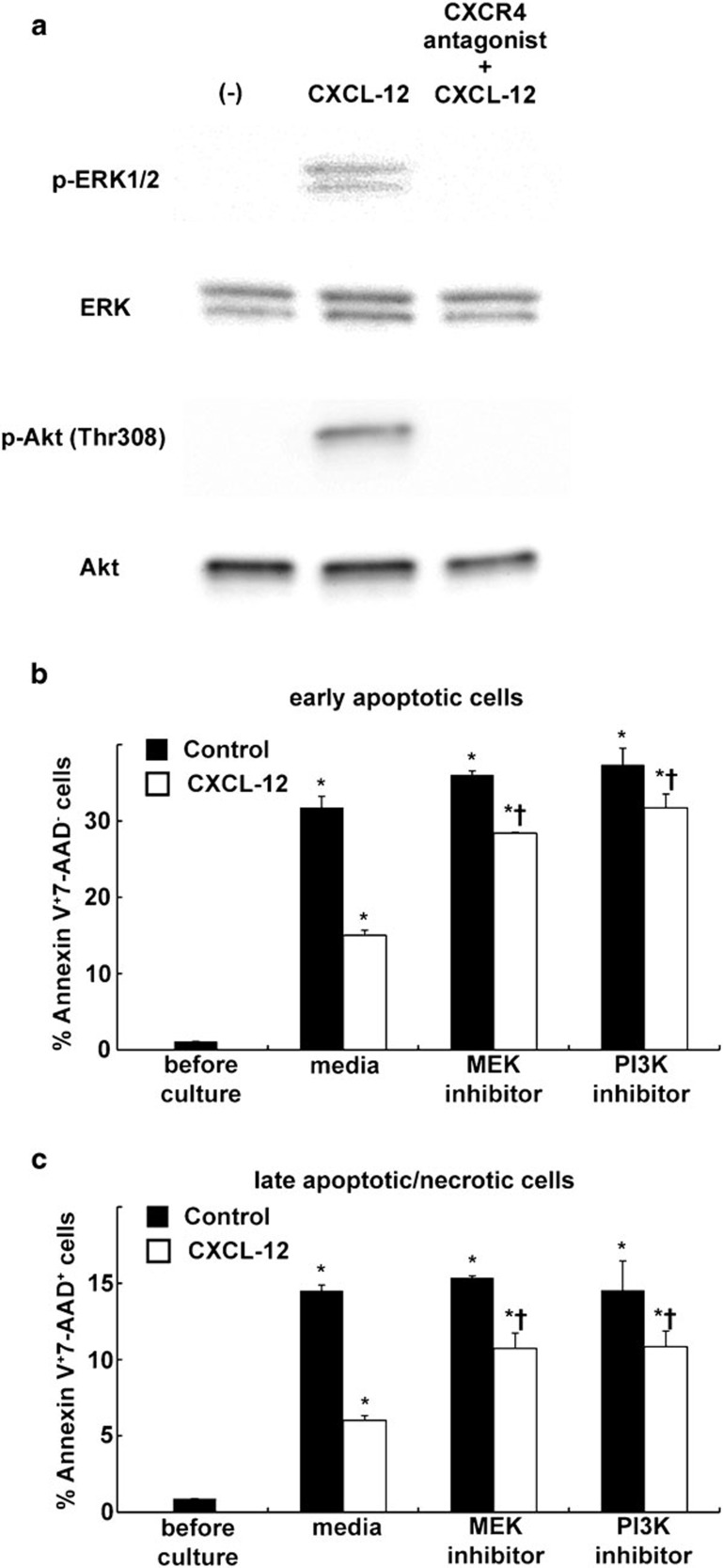
The observation that blocking the CXCL12/CXCR4 pathway decreased the number of neutrophils in the air space suggests the involvement in neutrophil recruitment during LPS-induced lung injury. However, in contrast to the enhanced migratory activity toward CXCL12 observed in the accumulated lung neutrophils, the blood neutrophils, which expressed very low levels of CXCR4 (Figure 1), did not exhibit a migratory response to CXCL12 (Figure 3). This suggested that the CXCL12/CXCR4 pathway was not critically involved in the recruitment of neutrophils, at least from circulating blood into the injured lungs. It has been reported that CXCR4 activation by CXCL12 suppressed cell death of CD34+ hematopoietic cells32 and CD4+ T cells.33 To investigate whether the CXCL12/CXCR4 pathway contributes to the accumulation of neutrophils in the injured lung by suppressing the neutrophil death, we examined the role of CXCL12 in suppressing cell death of the lung neutrophils. We performed trypan blue staining to assess cell death after ex vivo culture either with or without CXCL12. The administration of CXCL12 decreased cell death levels of the neutrophils (Figure 4a). This protective effect of CXCL12 against cell death was blocked by the administration of a specific CXCR4 antagonist (Figure 4b–d). We further performed Annexin V and 7-AAD staining to assess apoptosis levels after culture. The incidences of both early apoptotic cells and late apoptotic/necrotic cells were reduced by CXCL12. This protective effect against apoptosis was also blocked by a CXCR4 antagonist (Figure 4b–d).
Figure 4.
CXCR4 activation by CXCL12 attenuated the cell death of mouse neutrophils isolated from the injured lungs. Neutrophils were isolated from the mouse lungs at 24 h after LPS instillation. The isolated neutrophils were cultured in either media alone (RPMI 1640 supplemented with 10% FCS), media containing CXCL12, media containing CXCL12 and a specific CXCR4 antagonist or media with a specific CXCR4 antagonist alone for 24 h at 37 °C. Subsequently, cell death was assessed by trypan blue staining (a). Staining with Annexin V and 7-AAD was also performed to detect early apoptotic (Annexin V+7-AAD−; b, c) cells and late apoptotic/necrotic (Annexin V+7-AAD+; b, d) cells. Representative dot plots are shown. A total of six mice were used in each group. The values represent mean±s.e.m. *P<0.01 versus before culture group and †P<0.01 versus media only group using ANOVA with Scheffé's post hoc test. 7-AAD, 7-aminoactinomycin D; FCS, fetal calf serum.
We then investigated the mechanisms for how CXCL12/CXCR4 signaling protects neutrophils from apoptosis. We focused on both ERK and PI3K/Akt signaling pathways because it has been reported that CXCL12/CXCR4 signaling protects T cells,33 CD34+ hematopoietic cells32 and blood neutrophils of Warts, hypogammaglobulinemia, infections, and myelokathexis syndrome (an inherited immune disorder associated with CXCR4 gene mutation, causing a defect of CXCR4 internalization) patients34 via ERK1/2 and/or PI3K/Akt signaling pathway. Western blotting revealed that CXCL12 induced phosphorylation of both ERK1/2 and Akt (Figure 5a) in the neutrophils isolated from the injured lungs. Moreover, the protective effect of CXCL12 against spontaneous apoptosis on the neutrophils was suppressed by the presence of MEK1/2 inhibitor or PI3K inhibitor (Figure 5b and c). These findings suggested the protective effect of CXCL12/CXCR4 against cell death of the neutrophils accumulated in the lungs during LPS-induced lung injury through ERK and PI3K/Akt signaling pathways.
Figure 5.
CXCL12 protects lung-accumulated neutrophils of LPS-injured mice from apoptosis through MEK/ERK and PI3K/Akt pathways. (a) Neutrophils were isolated from the mouse lungs at 24 h after LPS instillation. Isolated neutrophils were untreated or pre-incubated with a CXCR4 antagonist then treated CXCL12. Western blot analysis showed that CXCL12 induced the phosphorylation of ERK1/2 and Akt. (b, c) The isolated neutrophils were cultured for 24 h at 37 °C in the presence (white bar) or absence (black bar) of CXCL12 alone or in combination with a MEK1/2 inhibitor (U0126) or a PI3K inhibitor (LY294002). Subsequently, staining with Annexin V and 7-AAD was also performed to detect early apoptotic (Annexin V+7-AAD−; b) cells and late apoptotic/necrotic (Annexin V+7-AAD+; c) cells. Note the suppression of the inhibitory effect of CXCL12 against apoptosis in the presence of MEK1/2 inhibitor or PI3K inhibitor. The values represent mean±s.e.m. (n=6). *P<0.01 versus before culture group and †P<0.01 versus media only group using ANOVA with Scheffé's post hoc test. 7-AAD, 7-aminoactinomycin D; LPS, lipopolysaccharide.
L-selectin may be involved in the increase in surface CXCR4 expression on neutrophils
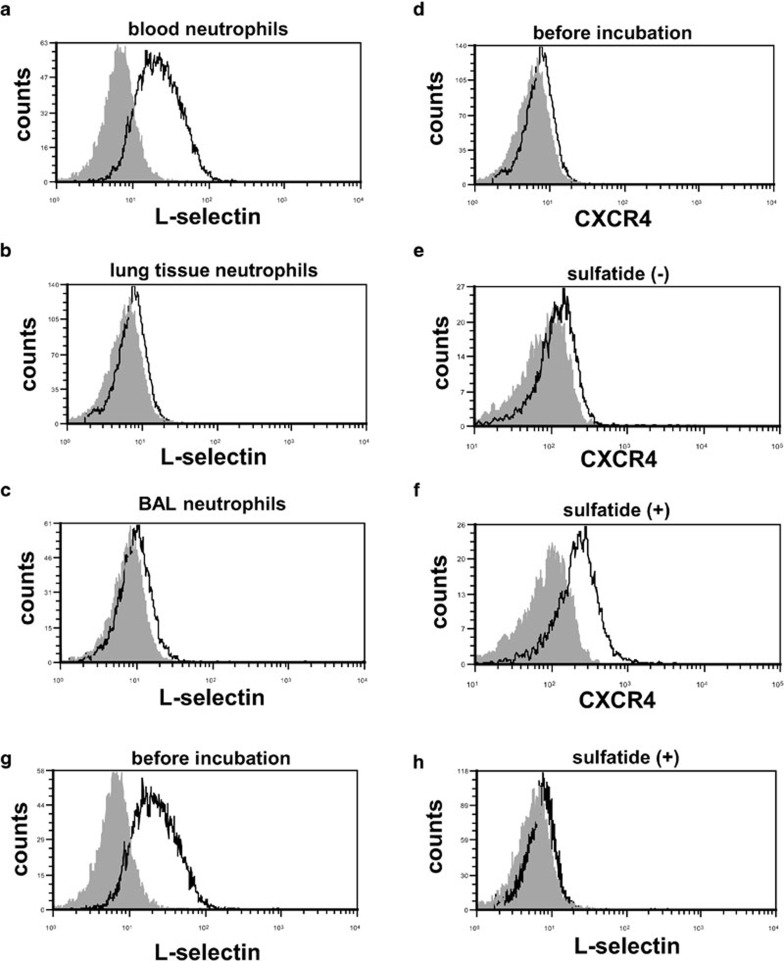
Our findings suggested that the increase in surface CXCR4 expression levels occurred during or after the extravasation of neutrophils in the lungs during LPS-induced lung injury. Circulating neutrophils expressed L-selectin on their surface (Figure 6a), whereas significant levels of surface L-selectin expression were not observed on neutrophils in lung digests and BAL fluids during LPS-induced lung injury (Figure 6b and c). These findings suggested that the shedding of L-selectin occurred in the process of extravasation of neutrophils into the injured lungs. To elucidate the participation of L-selectin in the increase in surface CXCR4 expression on neutrophils, we stimulated circulating neutrophils ex vivo with sulfatide, one of the native L-selectin ligands,35 and examined the changes in CXCR4 expression. Sulfatide induced a significant increase in surface CXCR4 expression on neutrophils (Figure 6d–f) and shedding of L-selectin (Figure 6g and h). This finding suggested that L-selectin may be involved in the increase in surface CXCR4 expression on neutrophils after the emigration of these cells into the injured lungs.
Figure 6.
L-selectin may be involved in the increase in surface CXCR4 expression in mouse neutrophils. (a–c) The surface L-selectin expression levels of neutrophils isolated from blood (a), lung tissue (b) and BAL fluid (c) at 24 h during LPS-induced lung injury were assessed by flow cytometry. The filled images show the staining using an isotype-matched control antibody. (d–f) Sulfatide induced the increase in surface CXCR4 expression in neutrophils isolated from mouse blood. The surface CXCR4 expression levels were examined before incubation (d) and after incubation for 1 h either with (f) or without (e) sulfatide. (g, h) Sulfatide induced shedding of L-selectin on neutrophils. Surface expressions of L-selectin on neutrophils were examined before incubation (g) and after incubation for 1 h with sulfatide (h). Representative histograms from one of three experiments that showed similar results are presented. BAL, bronchoalveolar lavage; LPS, lipopolysaccharide.
Discussion
In the present study, we demonstrated that cell surface CXCR4 expression levels increase on extravascular neutrophils, but not on intravascular neutrophils, in the injured lung during LPS-induced lung injury. Because CXCL12 is also upregulated in the injured lungs,17, 18, 19 these findings suggest that the increase in surface CXCR4 expression levels on extravasated neutrophils acts together with the increase of CXCL12 in the lungs to promote neutrophil migration and/or retention within the airspace.
To investigate whether the CXCL12/CXCR4 signaling system contributes to neutrophil migration to the lung or retention in the injured lung, we examined the migratory activities of neutrophils isolated from the bone marrow, blood, lung digests and BAL fluids of LPS-injured mice toward CXCL12. Our findings revealed that neutrophils isolated from lung digests and BAL fluids exhibited enhanced migratory activities toward CXCL12, whereas neutrophils isolated from bone marrow or blood did not. These findings were consistent with the low levels of surface CXCR4 expression found on neutrophils isolated from bone marrow and blood (Figure 1a and b). These data suggest that the neutrophils that were accumulated in the lung acquired the ability to migrate toward CXCL12 through the increase in CXCR4 and that the CXCL12/CXCR4 signaling pathway contributes primarily to neutrophil retention, not migration, in cases of LPS-induced lung injury.
We examined whether CXCR4 activation by CXCL12 prevented cell death of lung neutrophils, because the apoptosis of neutrophils and subsequent macrophage phagocytosis of apoptotic cells contribute to neutrophil clearance. We found that CXCL12 reduced the levels of cell death of the extravasated neutrophils within the injured lungs. This protective effect was inhibited when a specific CXCR4 antagonist was administered. We investigated the mechanisms for how CXCL12/CXCR4 signaling protects the lung accumulated neutrophils and then revealed that CXCL12 protects the neutrophils from apoptosis through MEK/ERK and PI3K/Akt pathways. Although we do not have clear evidence confirming that CXCR4 activation is critical for the survival of accumulated neutrophils in vivo, this idea is compatible with a recent clinical report describing increased CXCL12 concentrations and the presence of primarily non-apoptotic neutrophils with enhanced CXCR4 expression levels in the BAL fluid of lipopolysaccharide patients.36 Taken together, our ex vivo findings suggest that this protective effect of the CXCL12/CXCR4 signaling pathway against cell death contributes to neutrophil accumulation and retention in the lungs during inflammatory diseases, including cases of acute lung injury.
To investigate the stimuli that induce the increase in surface CXCR4 expression on neutrophils, we focused on L-selectin, a cell adhesion molecule that belongs to the selectin family, because it has been also reported that L-selectin is involved in the increase in surface CXCR4 expression on human peripheral blood lymphocytes and mouse lymphocytes.20, 21 We observed that the shedding of L-selectin, which is induced by L-selectin activation, occurred during the process of extravasation of neutrophils (Figure 6a–c). We also found that sulfatide, a natural ligand for L-selectin, induced the surface expression of CXCR4 on neutrophils isolated from mouse blood. These findings suggest that the activation of L-selectin may be involved in the increase in surface CXCR4 expression levels on neutrophils in the lungs.
In summary, we have shown that the surface CXCR4 expression levels on neutrophils increase after extravasation into the mouse lungs during LPS-induced lung injury. In addition, the activation of L-selectin may be a key regulator of this surface CXCR4 increase. Our findings suggest that the CXCL12/CXCR4 signaling pathway is involved in neutrophil accumulation and retention in the inflammatory site through both its chemotactic effect and its protective effect against cell death.
Acknowledgments
This work was supported by grants from the Japanese Society for the Promotion of Science (no. 17590776 to HK and no. 17790524 to MY). The authors declare no financial or commercial conflict of interest.
Footnotes
Supplementary information accompanies the paper on Cellular & Molecular Immunology's website(http://www.nature.com/cmi/).
Supplementary Information
References
- Kubo H, Graham L, Doyle NA, Quinlan WM, Hogg JC, Doerschuk CM. Complement fragment-induced release of neutrophils from bone marrow and sequestration within pulmonary capillaries in rabbits. Blood. 1998;92:283–290. [PubMed] [Google Scholar]
- Kubo H, Morgenstern D, Quinian WM, Ward PA, Dinauer MC, Doerschuk CM. Preservation of complement-induced lung injury in mice with deficiency of NADPH oxidase. J Clin Invest. 1996;97:2680–2684. doi: 10.1172/JCI118718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Kunkel SL, Bone RC. Role of tumor necrosis factor-alpha in disease states and inflammation. Crit Care Med. 1993;21:S447–S463. doi: 10.1097/00003246-199310001-00006. [DOI] [PubMed] [Google Scholar]
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- Zachariae CO. Chemotactic cytokines and inflammation. Biological properties of the lymphocyte and monocyte chemotactic factors ELCF, MCAF and IL-8. Acta Derm Venereol Suppl (Stockh) 1993;181:1–37. [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul CC, Yoshie O, et al. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci USA. 1996;93:14726–14729. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–4719. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, et al. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, et al. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148–8157. doi: 10.4049/jimmunol.178.12.8148. [DOI] [PubMed] [Google Scholar]
- Yamada M, Kubo H, Kobayashi S, Ishizawa K, Sasaki H. Stromal cell-derived factor-1 contributes to lipopolysaccharide-induced lung injury. Am J Respir Crit Care Med. 2004;169:A875. [Google Scholar]
- Yamada M, Kubo H, Kobayashi S, Ishizawa K, Sasaki H. Stromal cell-derived factor-1 contributes to lipopolysaccharide-induced neutrophil emigration within the airspace. Proc Am Thorac Soc. 2005;2:A350. [Google Scholar]
- Duchesneau P, Gallagher E, Walcheck B, Waddell TK. Up-regulation of leukocyte CXCR4 expression by sulfatide: an L-selectin-dependent pathway on CD4+ T cells. Eur J Immunol. 2007;37:2949–2960. doi: 10.1002/eji.200737118. [DOI] [PubMed] [Google Scholar]
- Ding Z, Issekutz TB, Downey GP, Waddell TK. L-selectin stimulation enhances functional expression of surface CXCR4 in lymphocytes: implications for cellular activation during adhesion and migration. Blood. 2003;101:4245–4252. doi: 10.1182/blood-2002-06-1782. [DOI] [PubMed] [Google Scholar]
- Palecanda A, Walcheck B, Bishop DK, Jutila MA. Rapid activation-independent shedding of leukocyte L-selectin induced by cross-linking of the surface antigen. Eur J Immunol. 1992;22:1279–1286. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Jutila MA, Berg EL, Kishimoto TK, Picker LJ, Bargatze RF, Bishop DK, et al. Inflammation-induced endothelial cell adhesion to lymphocytes, neutrophils, and monocytes. Role of homing receptors and other adhesion molecules. Transplantation. 1989;48:727–731. doi: 10.1097/00007890-198911000-00001. [DOI] [PubMed] [Google Scholar]
- Fujii N, Nakashima H, Tamamura H. The therapeutic potential of CXCR4 antagonists in the treatment of HIV. Expert Opin Investig Drugs. 2003;12:185–195. doi: 10.1517/13543784.12.2.185. [DOI] [PubMed] [Google Scholar]
- Tamamura H, Hiramatsu K, Mizumoto M, Ueda S, Kusano S, Terakubo S, et al. Enhancement of the T140-based pharmacophores leads to the development of more potent and bio-stable CXCR4 antagonists. Org Biomol Chem. 2003;1:3663–3669. doi: 10.1039/b306613b. [DOI] [PubMed] [Google Scholar]
- Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L807–L815. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- Tamamura H, Fujisawa M, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N, et al. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett. 2004;569:99–104. doi: 10.1016/j.febslet.2004.05.056. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo implications for acute pulmonary inflammation. Am J Pathol. 2001;158:153–161. doi: 10.1016/S0002-9440(10)63953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel K, Liles WC, Broxmeyer HE, Rodger E, Wood B, Cooper S, et al. Leukocytosis and mobilization of CD34+ hematopoietic progenitor cells by AMD3100, a CXCR4 antagonist. Support Cancer Ther. 2004;1:165–172. doi: 10.3816/SCT.2004.n.008. [DOI] [PubMed] [Google Scholar]
- Lataillade JJ, Clay D, Bourin P, Herodin F, Dupuy C, Jasmin C, et al. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G0/G1 transition in CD34+ cells: evidence for an autocrine/paracrine mechanism. Blood. 2002;99:1117–1129. doi: 10.1182/blood.v99.4.1117. [DOI] [PubMed] [Google Scholar]
- Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, Paya CV. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169:5546–5554. doi: 10.4049/jimmunol.169.10.5546. [DOI] [PubMed] [Google Scholar]
- Sanmun D, Garwicz D, Smith CI, Palmblad J, Fadeel B. Stromal-derived factor-1 abolishes constitutive apoptosis of WHIM syndrome neutrophils harbouring a truncating CXCR4 mutation. Br J Haematol. 2006;134:640–644. doi: 10.1111/j.1365-2141.2006.06240.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Toda Y, Tamatani T, Watanabe T, Suzuki T, Nakao T, et al. Sulfated glycolipids are ligands for a lymphocyte homing receptor, L-selectin (LECAM-1), binding epitope in sulfated sugar chain. Biochem Biophys Res Commun. 1993;190:426–434. doi: 10.1006/bbrc.1993.1065. [DOI] [PubMed] [Google Scholar]
- Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F, et al. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol. 2008;181:8053–8067. doi: 10.4049/jimmunol.181.11.8053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.