Abstract
Aim:
Tetrandrine (Tet) is a Ca2+ channel blocker and has antiarrhythmic effects. Less information exists with regard to the mechanisms underlying its antiarrhythmic action other than blocking Ca2+ channels. In this study, the effects of Tet on the Na+ current (INa) in the atrial myocardium of patients in atrial fibrillation (AF) and sinus rhythm (SR) were investigated, and the characteristics of the Na+ current were synchronously compared between the AF and SR patients.
Methods:
Na+ currents were recorded using the whole-cell patch clamp technique in single atrial myocyte of the AF and the normal SR groups. The effects of Tet (40–120 μmol/L) on the Na+ current in the two groups were then observed.
Results:
Tet (60–120 μmol/L) decreased INa density in a concentration-dependent manner and made the voltage-dependent activation curve shift to more positive voltages in the SR and AF groups. After exposure to Tet, the voltage-dependent inactivation curve of INa was shifted to more negative voltages in the two groups. Tet delayed the time-dependent recovery of INa in a concentration dependent manner in both AF and SR cells; however, there were no differences in the effects of Tet on INa density and properties in the two groups. The INa density of AF patients did not differ from that of the SR patients.
Conclusion:
Tet can block sodium channels with slow recovery kinetics, which may explain the mechanisms underlying the antiarrhythmic action of Tet. The decreased conduction velocity (CV) in AF patients is not caused by the Na+ current.
Keywords: tetrandrine, electrophysiology, arrhythmia, sodium channels, heart
Introduction
Atrial fibrillation is the most common arrhythmia in clinical practice and can produce electrical remodeling that contributes to the self-perpetuation of AF. Electrical remodeling of AF is characterized by a marked reduction in the atrial effective refractory period, a decrease or reversal of its physiologic adaptation to heart rate, and a decrease in atrial conduction velocity (CV)1, 2, 3. Sodium current is one of the critical parameters affecting CV. In the canine rapid pacing model of AF, Gaspo et al have observed a decreased INa during AF that has been correlated with the slowing of atrial conduction4. Only small amounts of data have been reported with regard to the differences in the characteristics of INa between AF and SR patients.
Tet, an alkaloid extracted from the Chinese medicinal herb Radix stephania tetrandrae, possesses a wide spectrum of pharmacological activities. Tet is a nonspecific Ca2+ channel blocker and has been clinically used to treat patients with angina and hypertension in China5. Tet has played an antiarrhythmic role in several experimental arrhythmic models6, 7, 8, 9. Tet reduces the incidence of arrhythmias induced by cesium chloride in rabbits7. Tet has also been reported to produce equally potent ameliorating effects on arrhythmia and infarcts induced by ischemia and reperfusion without further inhibiting ischemia-reduced heart rate and coronary artery flow; the results indicate Tet may be a better choice for the treatment of arrhythmia than the classical Ca2+ channel blocker8, 9. Clinical reports have shown that Tet can terminate acute episodes of paroxysmal supraventricular tachycardia10. Previous studies have also demonstrated that Tet can block Ca2+ channels in ventricular cells11, vascular smooth muscle cells12 and hepatocytes13. Despite the antiarrhythmic action of Tet, little is known about its mechanism of action, other than that it includes blocking of Ca2+ channels; no information is available about whether the antiarrhythmic actions of Tet are related to the sodium channels of humans.
The purpose of the present study was to investigate the following: 1) the effects of Tet on Na+ current density and kinetics in human atrial myocardium in sinus rhythm; 2) whether AF can affect the action of Tet on Na+ channels in humans; and 3) the differences in sodium current density and characteristics between AF and SR patients.
Materials and methods
Characteristics of patients
Right atrial appendages were obtained during the course of cardiac surgery using the following procedures approved by the Hospital Ethics Committee. Twenty-one patients were assigned to the SR group and 12 to the chronic AF group, according to the underlying cardiac rhythm. Chronic AF was defined as permanent AF of at least 7days duration. Patient characteristics are listed in Table 1.
Table 1. Characteristics of patients with SR and AF. Mean±SD.
| SR | AF | |
|---|---|---|
| n (man/feminine) | 21 (12/9) | 12 (8/4) |
| Age (year) | 43±9 | 48±7 |
| Underlying heart disease | ||
| Rheumatic heart disease | 9 | 8 |
| Coronary artery disease | 13 | 4 |
| Heart function (NYHA) | ||
| I | 10 | 3 |
| II | 8 | 7 |
| III | 3 | 2 |
| IV | 0 | 0 |
| Medication | ||
| Digitalis | 1 | 2 |
| β-blockers | 2 | 5 |
| Ca2+-channel blockers | 1 | 3 |
| ACE-Inhibitors | 5 | 6 |
| Sotalol | 0 | 2 |
SR: sinus rhythm. AF: atrial fibrillation.
Isolation of single myocytes
Single myocytes were isolated by enzymatic dissociation using the chunk method14. The specimens were transported to the laboratory in a Ca2+-free cardioplegic solution (100% O2, room temperature) containing (in mmol/L) 50 KH2PO4, 8 MgSO4, 10 HEPES, 5 adenosine, 140 glucose, 100 mannitol, and 10 taurine, with pH adjusted to 7.4 using KOH. Chunks were washed in 25 mL EGTA solution containing (in mmol/L) 137 NaCl, 5 KH2PO4, 1 MgSO4, 10 glucose, 5 HEPES, 10 taurine, and 0.1 EGTA, with pH adjusted to 7.4 using NaOH. After blood and calcium were washed out, the chunks were incubated for 35 min in 5 mL Ca2+-free Enzyme Incubation Medium (EIM) with the addition of 150 U/mL collagenase (TypeV), 12.6 U/mL protease (TypeXXIV) and 1 mg/mL albumin (100% O2, 35 °C). The EIM solution contained the same composition of EGTA solution but without EGTA. This period of digestion was followed by a 1 min wash in EIM solution without any enzymes. Subsequently, the supernatant was removed and replaced by a fresh enzyme medium with the same composition but without protease and incubated until rod-shape cells were present. Cells were kept in a KB storage solution containing (in mmol/L) 30 KCl, 10 KH2PO4, 25 glucose, 5 mannitol, 0.1% albumin, 75 L-glutamic acid, 10 β-hydroxybutyric acid, 10 taurine, and 10 EGTA, with pH adjusted to 7.2 using KOH.
Patch clamp technique
Only quiescent, rod-shape cells with clear cross-striations were studied. These cells were transferred to the tissue chamber mounted on the stage of an inverted microscope. Myocytes were superfused (2–3 mL/min) with a solution containing (in mmol/L) the following: 132.5 CsCl, 5 NaCl, 1 MgCl2, 1 CaCl2, 20 HEPES, 11 glucose, and 2 CoCl2, with pH adjusted to 7.2 using CsOH. The resistances of glass electrodes (outer diameter 1.5 mm) equaled 1–2 MΩ when filled with the following solution (in mmol/L): 135 CsF, 5 NaCl, 5 HEPES, 10 EGTA, 5 Mg-ATP, with pH adjusted to 7.35 using CsOH. K currents were suppressed with the use of this K-free solution, and ICa was blocked with 2 mmol/L Co2+. Na+ currents were recorded using the whole cell configuration of the voltage clamp technique, which was performed with an Axopatch 200B clamp amplifier (Axon Instruments). The membrane capacitance of each cell was measured using a 5 mV hyperpolarizing pulse from a holding potential of −120 mV, and these areas were divided by voltage step. The membrane capacitance was identical in the two groups: 69.17.2 pF (n=26) in SR and 73.68.8 pF (n=19) in AF; P=NS vs SR. To account for the differences in cell size, all mean current data are expressed as current densities. INa was recorded at room temperature.
Drugs
The main drugs were purchased from Sigma (St Louis, MO, USA), including adenosine, mannitol, taurine, collagenase, protease, albumin, β-hydroxybutyric acid, Mg-ATP, CsOH and CsF. Tet was obtained from the Research Institute of Drug and Biologic Product, China (lot number: 000201).
Statistics
Group data are expressed as means±SEM. Comparisons between SR and AF groups were made using a Student's non-paired t-test. A P value <0.05 was considered statistically significant. Statistical comparisons of multiple group means were obtained by ANOVA. A nonlinear, least-square, curve-fitting program (CLAMPFIT in pCLAMP 6.0) was used to perform curve-fitting procedures.
Results
In order to avoid any contaminating effects of time-dependent changes in the INa I–V relationship, all studies began 20 min after membrane rupture, with protocols performed in the same sequence in all cells, beginning with the INa density-voltage relationship, followed immediately by analysis of voltage-dependent inactivation, and finally time-dependent recovery from inactivation.
Voltage- and concentration-dependent blockade of Na+ current by Tet in SR cells
The data were obtained with a holding potential of −120 mV and a series of 5 mV depolarizing steps in voltage from −90 to 0 mV. Tet can decrease INa density in a concentration-dependent manner in the SR cells (Table 2). The maximal INa density was reduced by 28.1%, 46.5%, 67.6%, and 87.5% by Tet 60, 80, 100, and 120 μmol/L, respectively. This inhibitory effect was readily reversed on the washout of Tet. Typical original current and I–V curves are shown in Figure 1A–1D. Tet did not change the threshold potential (around −70 to −60 mV), but it did change the shape of I–V curves of INa. The potential of maximum peak was shifted to a more positive potential, indicating that the block was voltage-dependent.
Table 2. The effects of Tet on INa density in SR and AF atrial myocytes. Mean±SEM. cP <0.01 vs control.
| Maximal INa density (pA/pF) | |||||||
|---|---|---|---|---|---|---|---|
| CTet | 0 μmol/L | 40 μmol/L | 60 μmol/L | 80 μmol/L | 100 μmol/L | 120 μmol/L | Wash out |
| SR | −58.6±7.8 | −53.3±8.5 | −42.1±6.9c | −31.3±6.2c | −18.9±5.7c | −7.3±2.6c | −49.6±10.8 |
| (n=26) | (n=10) | (n=12) | (n=10) | (n=8) | (n=8) | (n=5) | |
| AF | −61.8±8.8 | 52.7±9.5 | −43.4±7.1c | −34.4±6.0c | −13.7±4.3c | −5.2±2.2c | −53.7±11.8 |
| (n=19) | (n=8) | (n=10) | (n=8) | (n=7) | (n=7) | (n=4) | |
SR: sinus rhythm. AF: atrial fibrillation. CTet: concentration of tetrandrine.
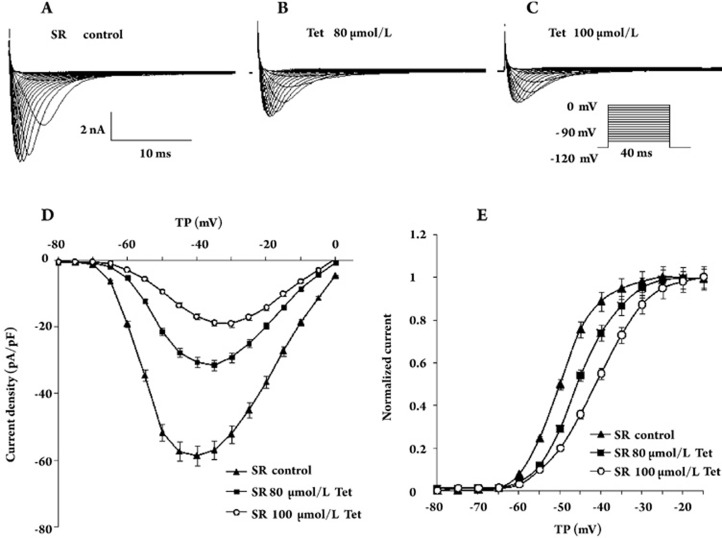
Figure 1.
Effects of Tet on INa density in the SR myocytes of humans. (A) Current traces were obtained by a holding potential of −120 mV and a series of 5 mV depolarizing steps to voltages from −90 to 0 mV in the absence of Tet. (B) Original sodium current in the presence of 80 μmol/L Tet. (C) Original sodium current in the presence of 100 μmol/L Tet. (D) I–V relation curves of INa densities before and after the application of 80 μmol/L (n=10, P<0.01) and 100 μmol/L (n=8, P<0.01) Tet. (F) The effects of Tet on voltage-dependent activation curves of sodium current in SR cells; the data were fitted with a Boltzmann equation. The V1/2 was as follows: control, −50.1±0.5 mV, 80 μmol/L Tet, −45.9±0.2 mV (n=10, P<0.05 vs control), 100 μmol/L Tet, −41.3±0.3 mV (n=8, P<0.01 vs control). TP: test potential.
We studied the voltage-dependent activation properties of the current (Figure 1E). The data were well fitted by a Boltzmann equation, with a half-maximum activation voltage averaging from −50.1±0.5 mV in the control (n=26) to −45.9±0.2 mV (n=10, P<0.05 vs control) and −41.3±0.3 mV (n=8, P<0.01 vs control) by Tet 80 and 100 μmol/L, respectively. The slope factors were not changed by Tet (Table 3). These results indicate that changes of INa density by Tet in SR cells are associated with changes in the voltage dependence of INa activation.
Table 3. The effects of Tet on properties of INa in SR and AF atrial myocytes. Mean±SEM. bP<0.05, cP<0.01 vs control. eP<0.05, fP<0.01, AF vs SR.
| SR | AF | |||||
|---|---|---|---|---|---|---|
| CTet | 0 μmol/L | 80 μmol/L | 100 μmol/L | 0 μmol/L | 80 μmol/L | 100 μmol/L |
| Voltage-dependent activation | ||||||
| n | 26 | 10 | 8 | 19 | 8 | 7 |
| V1/2 act (mV) | −50.1±0.5 | −45.9±0.2b | −41.3±0.3c | −51.5±0.4 | −47.7±0.4b | −42.5±0.3c |
| Kact | 6.0±0.2 | 5.9±0.2 | 6.4±0.3 | 6. 2±0.2 | 6.2±0.3 | 6.4±0.2 |
| Voltage-dependent inactivation | ||||||
| n | 15 | 9 | 12 | 8 | ||
| V1/2 inact (mV) | −93.4±3.3 | −98.7±2.4b | −83.0±2.3f | −90.5±4.1b | ||
| Kinact | 6.2±0.2 | 6.3±0.2 | 4.6±0.2e | 4.6±0.1 | ||
| Recovery from inactivation | ||||||
| n | 10 | 8 | 7 | 8 | 6 | 5 |
| Time constant (ms) | 8.62±0.5 | 15.59±2.8c | 32.56±2.5c | 8.55±1.8 | 14.91±2.2c | 34.90±1.9c |
CTet: concentration of tetrandrine. V1/2 act: half-maximum activation voltage. Kact: slope factor for activation.
V1/2 inact: half-maximum inactivation voltage. Kinact: slope factor for inactivation.
Voltage- and concentration-dependent blockade of Na+ current by Tet in AF cells
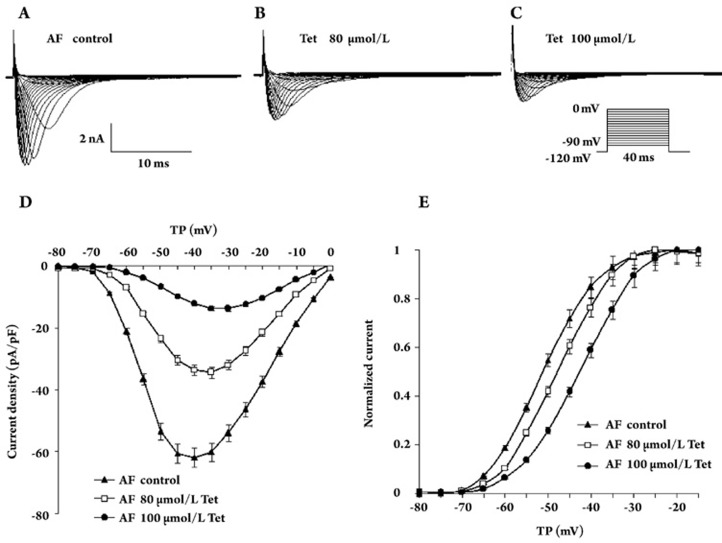
The maximal INa density of AF was −61.8±8.8 pA/pF (n=19) compared with SR cells; there was no difference between the two types of cells (Table 2). Tet also decreased INa density in a concentration-dependent manner in AF cells (Table 2). The maximal INa density was reduced by 29.7%, 44.4%, 70.9%, and 88.1% in AF cells after exposure to Tet 60, 80, 100, and 120 μmol/L, respectively. This inhibitory effect was reversed on the washout of Tet. Figure 2 shows a typical original current and I–V curves. Tet caused the potential of maximum peak to shift to a more positive potential as well. Figure 2E shows the voltage-dependent activation properties of the current. The half-maximum activation voltage averaged from −51.5±0.4 mV in the control (n=19) to −47.7±0.4 mV (n=8) and −42.5±0.3 mV (n=7) in the group exposed to Tet 80 and 100 μmol/L, respectively, P<0.05 vs control). The slope factor was not changed by Tet (Table 3). The voltage-dependent activation curves of INa in SR and AF cells were all shifted to more positive voltages after exposure to Tet, which indicated that Tet could inhibit the activation of INa; there was no difference in the inhibitory effects of Tet on SR and AF cells.
Figure 2.
Effects of Tet on INa density in human AF myocytes. (A–C) Representative sodium currents recorded from AF cells before and after incubation with 80 μmol/L and 100 μmol/L Tet. Currents were recorded at test potentials between −90 mV and 0 mV with 5 mV depolarizing steps. (D) I–V relation curves of INa densities in control, 80 μmol/L (n=8, P<0.01) and 100 μmol/L (n=7, P<0.01) Tet. (F) The voltage-dependent activation curves are shifted to more positive voltages by Tet in AF cells. The V1/2 was as follows: control, −51.5±0.4 mV, 80 μmol/L Tet, −47.7±0.4 mV (n=8, P<0.05 vs control), 100 μmol/L Tet, −42.5±0.3 mV (n=8, P<0.01 vs control). TP: test potential.
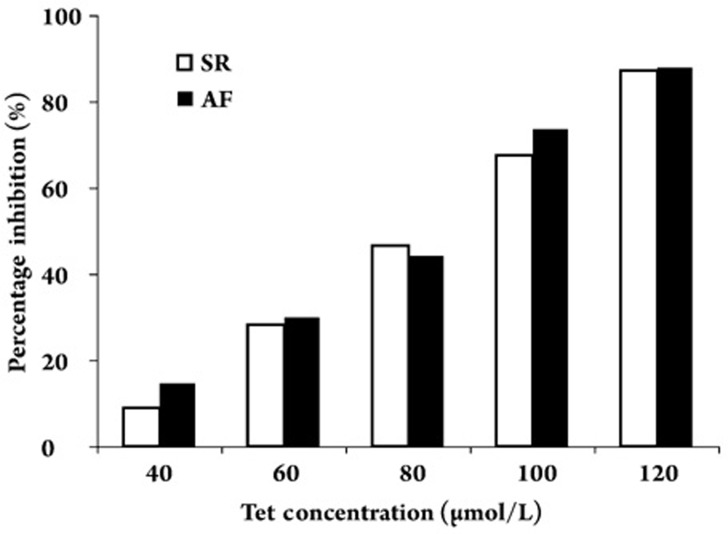
Figure 3 shows the relationships between the concentration of Tet and the percentage inhibition of Na+ current density in the SR and AF cells. The half-maximal concentration required for the inhibitory effect of Tet on the Na+ current was 76.9±4.1 μmol/L and 74.4± 3.6 μmol/L in the SR and AF cells, respectively (n=7, P=NS). There were no differences between the effects of Tet on INa in AF and SR cells. That is, Tet identically inhibited INa in AF and SR cells.
Figure 3.
Relationship between the concentration of Tet and the percentage inhibition of Na+ current density in SR and AF cells. There were no differences between SR and AF with respect to the inhibition rate of Tet on INa.
Effects of Tet on the voltage dependence of INa inactivation
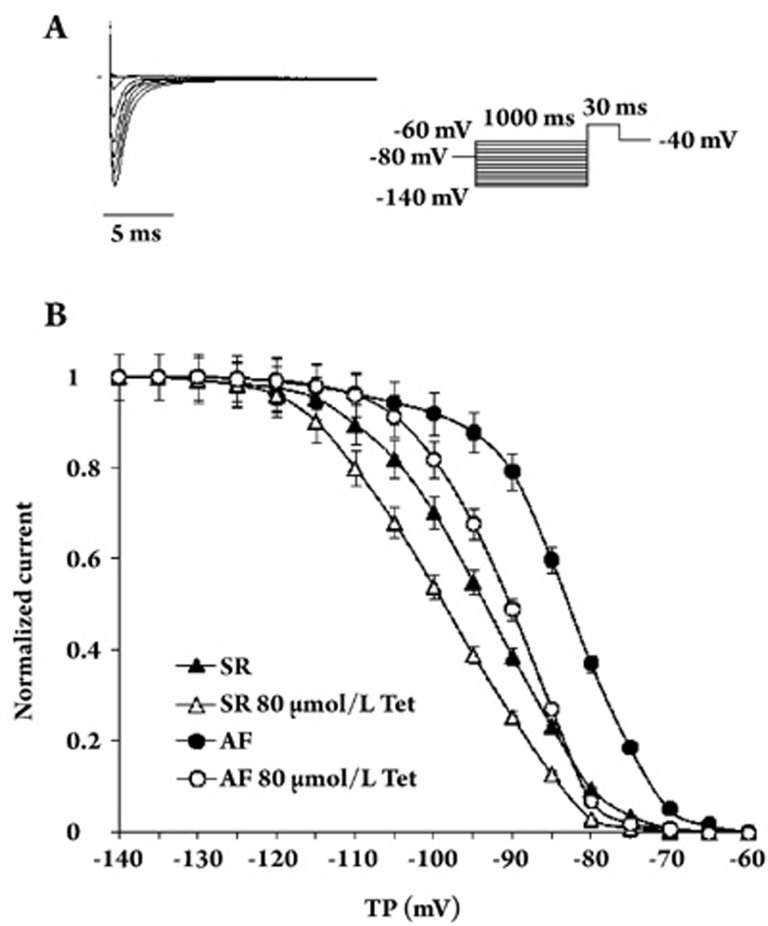
To further characterize the inhibitory effects of Tet on INa, we examined whether Tet had an effect on voltage-dependent inactivation of INa. A double-pulse protocol was used to assess the voltage dependence of INa inactivation. A 1-s conditioning pulse of voltages between −140 and −60 mV was followed by a 30 ms test pulse of −40 mV. Figure 4A shows a representative original recording of the steady-state inactivation of the sodium channel. The peak current elicited by the test pulse was normalized to a current without a prepulse. The results were well fitted by a Boltzmann relation. The voltage-dependent inactivation curve of INa (Figure 4B) was shifted to a more positive voltage in AF cells. The mean values for V1/2 changed from −93.4±3.3 mV (n=15) in SR to −83.0±2.3 mV (n=12) in AF (P<0.01 vs SR). Exposure to Tet shifted the voltage-dependent inactivation curves of INa to more negative voltages in both AF and SR cells; V1/2 was shifted from −93.4±3.3 mV (n=15) to −98.7±2.4 mV (n=9) (P<0.05 vs control) with application of 80 μmol/L Tet in SR, from −83.0±2.3 mV (n=12) to −90.5±4.1 mV (n=8) (P<0.05 vs control) with application of 80 μmol/L Tet in AF. The slope factor (K) was not altered by Tet (Table 3). These results indicate that Tet can inhibit the amplitude of INa in a voltage-dependent manner in AF and SR cells.
Figure 4.
Effects of Tet on INa voltage-dependent inactivation in SR and AF cells. (A) Representative original recording of the steady-state inactivation of sodium channels. After a 1-s conditional pulse at voltages between −140 and −60 mV, the membrane potential was held to a test pulse (−40 mV, 30 ms). (B) Voltage-dependent inactivation curves of INa in both SR and AF cells. Peak current elicited by the test pulse was normalized to a current without a prepulse. The results were well fitted by a Boltzmann relation. Compared with the SR cells, the voltage-dependent inactivation of INa was shifted to a more positive voltage in AF cells. However, the voltage-dependent inactivation of INa by Tet was shifted to more negative voltages in both AF and SR cells. TP: test potential.
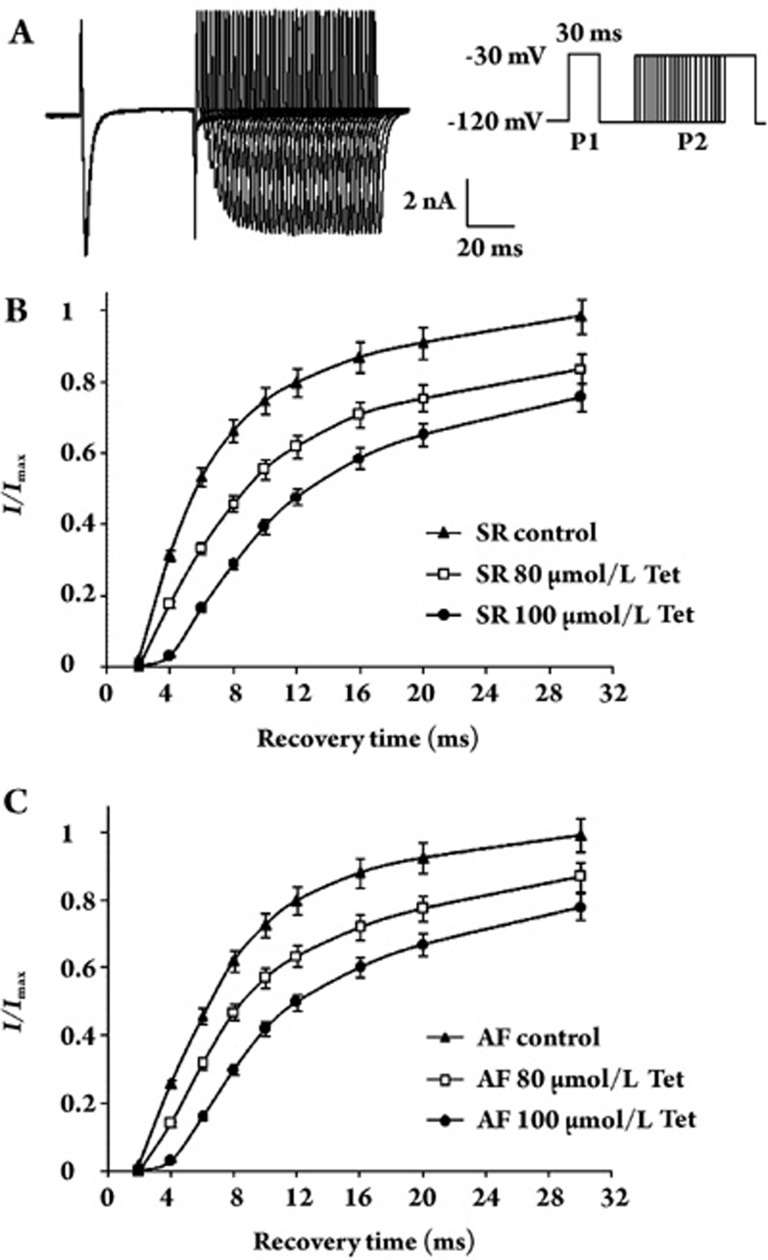
Effects of Tet on the time-dependent recovery of INa
The time-dependent recovery of INa was studied with the use of a double pulse protocol with two identical 30-ms pulses (P1 and P2) at an increasing P1–P2 interval. Figure 5A shows the original recording of time-dependent recovery of sodium channels. The P2 current amplitude was normalized to the P1 amplitude and then plotted with the P1–P2 interval. Tet delayed the time-dependent recovery of INa in a concentration-dependent manner in both AF and SR cells (Figure 5B, 5C). Recovery kinetics was best fitted with a monoexponential function. The time constant of SR changed from 8.62±0.5 ms (n=10) to 15.59±2.8 (n=8, P<0.01 vs control) and 32.56±2.5 ms (n=7, P<0.01 vs control) by Tet (80 and 100 μmol/L, respectively). The time constant of AF changed from 8.55±1.8 ms (n=10) to 14.91±2.2 (n=8, P<0.01 vs control) and 34.90±1.9 ms (n=7, P<0.01 vs control), respectively, with exposure to Tet (80 and 100 μmol/L, respectively). There were no differences between the effects of Tet on INa recovery kinetics in AF and SR cells.
Figure 5.
The effects of Tet on INa time-dependent recovery. (A) Representative original recording of the time-dependent recovery of sodium channels. A double pulse protocol with two identical 30 ms, −30 mV pulses (P1 and P2) at an increasing P1–P2 interval was used to record the current. (B) The effect of Tet on the time-dependent recovery curve of INain SR cells. The P1–P2 interval is shown on the X-axis; the P2 current amplitude was normalized to the P1, which is shown on the Y-axis. The recovery curve is best fitted with a monoexponential function; the time constant of SR was prolonged by Tet. (C) The effect of Tet on the time-dependent recovery curve of INain an AF cell. Tet also prolonged the recovery process of inactive sodium channels in AF cells.
Discussion
Conduction velocity and sodium current of AF
AF can lead to electrical remodeling that contributes to the self-perpetuation of AF. Some data obtained from the animal model of AF by rapid pacing have shown that the slowing of CV is related to the decrease in INa4, 15. Our results found that neither current density nor time-dependent recovery of INa was altered by AF in humans; however, voltage-dependent inactivation of INa was shifted to more positive voltages in AF cells, which would have increased sodium channel availability at the resting potential and thus increased CV. This result is similar to research on human myocardium by Bosch14. The reasons for these contrasting results might include differences in the type of AF (experimental vs clinical AF), the influence of the underlying cardiac disease, or antiarrhythmic drugs. Other mechanisms must be involved in the slowing of atrial conduction in AF. Atrial gap junctions are another major determinant in myocardial conduction. Several studies found that sustained AF led to downregulation of connexin4016, 17 and abnormal phosphorylation of connexin4016 in humans, which decreased the atrial CV and resulted in the initiation and/or perpetuation of AF.
Mechanisms of atrial fibrillation
Moe and coworkers postulated that AF was maintained by multiple wavelets circulating randomly through the myocardium18. This hypothesis was confirmed by Allessie and colleagues, who observed atrial activation patterns during AF in isolated canine hearts and estimated that the critical number of wavelets for sustained fibrillation was between 4 and 619. The number of wavelets in the atrium is strongly affected by the wavelength, defined as the distance traveled by the activation wave during functional refractoriness, which is determined by the local effective refractory period and the local CV. Therefore, modification of these parameters is thought to affect the vulnerability and stability of AF. Other evidence shows that multiple-wavelet fibrillation is not the only mechanism of fibrillation. Mother rotor fibrillation, which is fibrillation driven by a stable high-frequency source, may also be involved20, 21.
Sodium channel blockers characteristically slow the CV. According to the wavelength theory, there is a possibility that slowing CV could decrease the wavelength and increase the number of wavelets, thus compromising the antifibrillatory effect of sodium channel blockers. Yet sodium channel blockers can terminate atrial fibrillation effectively, as shown in clinical and experimental studies22, 23, 24. Nattel23 and Kneller25 used computer simulations to show that Na+ channel blockade destabilized the mother rotor, favoring AF termination.
Interaction between Tet and the Na+ channel
According to the modulated receptor hypothesis26, the affinity of the Na+ channel blocker for the binding site is determined by the electrophysiological state of the Na+ channel (ie, resting, activated and inactivated states). Our results show that Tet not only reduced the maximal conductance of INa, but also produced a negative shift in the steady-state inactivation curve, indicating that the effect of Tet on INa is voltage-dependent and that the extent of current inhibition caused by Tet can be altered by the membrane potential. This negative-shift of the inactivation curve suggests that Tet has a preferential affinity for the inactivated states of Na+ channels. Reactivation of INa is slowed down in the presence of Tet in our results, which indicates that the speed of the dissociation of Tet from inactivated Na+ channels decreases.
Mechanisms of antiarrhythmic action of Tet
Our results indicate that Tet can delay the time-dependent recovery of INa from inactivation in a concentration-dependent manner. Tet is a type of sodium channel blocker with slow recovery kinetics in atrial myocardium. Previous reports have indicated that the efficacy of sodium channel blocking agents with slow recovery kinetics for terminating atrial fibrillation and maintaining sinus rhythm is relatively high27. The reason for this is that, at any given time during the repolarization process, fewer sodium channels have recovered from inactivation in the presence of this kind of drug than in the drug-free state. It then follows that the degree of sodium channel recovery that will produce an action potential occurs at a more hyperpolarized potential, thereby prolonging refractoriness28. Sodium channel blockers with slow recovery kinetics can prevent progressive encroachment by prolonging refractoriness near or beyond repolarization, an effect that has been called postrepolarization refractoriness (PRR)27, 29. PRR can oppose premature excitations and induction of reentrant arrhythmias and therefore protect the myocardium, which is the most important mechanism for the treatment of AF by sodium channel blockers27, 29, 30. Moreover, sodium channel blockers with slow recovery kinetics are use-dependent, which can maintain the ability of PRR to terminate AF even in the case of high frequent stimulation27.
Indeed, sodium channel blockers slow CV, therefore increasing the number of wavelets. This is the main reason why sodium channel blockers are proarrhythmic. However, INa is not the only factor affecting CV; atrial gap junctions are another major determinant in myocardial conduction. Recent research has shown that Tet can inhibit the degradation of connexin 40 in an atrial rabbit model of AF31, which can increase the atrial CV of AF patients and therefore increase the wavelength and reduce the proarrhythmic action of Tet. Consequently, Tet may terminate AF effectively.
It should be noted that Tet is a non-specific calcium channel blocker. It produces a negative inotropic effect and vasodilation. Consequently, administration of Tet can reduce blood pressure. On the other hand, Tet can induce apoptosis in the hepatic cells and produce hepatotoxicity32. If patients have AF combined with cardiac insufficiency, hypotension or abnormal function of the liver, we do not suggest using Tet for treatment. However, Tet is being used for the treatment of angina and hypertension in China5. Tet can reduce the size of infarcts induced by myocardial ischemia33. In addition, Tet can reverse cardiac and vascular remodeling34 and reduce arterial blood pressure. Tet is recommended for the treatment of AF patients who have complications of hypertension or angina or a myocardial infarct.
The results of this experimental study suggest that the reason of CV decrease in AF patients is not the change in sodium current. Tet can block sodium channels with slow recovery kinetics, which can explain the mechanism underlying the antiarrhythmic action of Tet. Tet could be a promising agent for the effective treatment of AF in humans.
Author contribution
Xiao-rong ZENG, Li CHEN, and Yan YANG designed the research; Li CHEN, Zhong-wen LI, and Qi-yong LI performed the research; Qi-yong LI and Li CHEN analyzed data; Li CHEN and Qi-yong LI wrote the paper.
Acknowledgments
This work was supported by the foundation grant of Ministry of Education of the People's Republic of China (No 03109).
We thank the Department of Anesthesiology, People's Hospital of Sichuan Province, for providing specimens.
References
- Gaspo R, Bosch RF, Talajic M, Nattel S. Functional mechanisms underlying tachycardia-induced sustained atrial fibrillation in a chronic dog model. Circulation. 1997;96:4027–35. doi: 10.1161/01.cir.96.11.4027. [DOI] [PubMed] [Google Scholar]
- Shinagawa K, Shiroshita-Takeshita A, Schram G, Nattel S. Effects of antiarrhythmic drugs on fibrillation in the remodeled atrium: insights into the mechanism of the superior efficacy of amiodarone. Circulation. 2003;107:1440–6. doi: 10.1161/01.cir.0000055316.35552.74. [DOI] [PubMed] [Google Scholar]
- Ashikaga K, Kobayashi T, Kimura M, Owada S, Sasaki S, Iwasa A, et al. Effects of amiodarone on electrical and structural remodeling induced in a canine rapid pacing-induced persistent atrial fibrillation model. Eur J Pharmacol. 2006;536:148–53. doi: 10.1016/j.ejphar.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Gaspo R, Bosch RF, Elias BA, Nattel S. Tachycardia-induced changes in Na+ current in a chronic dog model of atrial fibrillation. Circ Res. 1997;81:1045–52. doi: 10.1161/01.res.81.6.1045. [DOI] [PubMed] [Google Scholar]
- Wong TM, Wu S, Yu XC, Li HY. Cardiovascular actions of Radix Stephaniae Tetrandrae: a comparison with its main component, tetrandrine. Acta Pharmacol Sin. 2000;21:1083–8. [PubMed] [Google Scholar]
- Cha L, Qian JQ, Lue FH. Antiarrhythmic action of tetrandrine and the total alkaloids of sophora flavescens. Acta Pharmacol Sin. 1981;2:26–8. [PubMed] [Google Scholar]
- Jiang JQ, Zeng QT, Cao LS.Effects of tetrandrine on early after depolarizations and arrhythmias induced by cesium chloride in rabbits J Cardiovasc Pulm Dis 200221239–41.Chinese. [Google Scholar]
- Yu XC, Wu S, Wang GY, Shan J. Cardiac effects of the extract and active components of radix stephaniae tetrandrae. II. Myocardial infarct, arrhythmias, coronary arterial flow and heart rate in the isolated perfused rat heart. Life Sci. 2001;68:2863–72. doi: 10.1016/s0024-3205(01)01067-0. [DOI] [PubMed] [Google Scholar]
- Yu XC, Wu S, Chen CF, Pang KT, Wong TM. Antihypertensive and anti-arrhythmic effects of an extract of Radix Stephaniae Tetrandrae in the rat. J Pharm Pharmacol. 2004;56:115–22. doi: 10.1211/0022357022458. [DOI] [PubMed] [Google Scholar]
- Li WH.The clinical cardiac electrophysiologic effects of tetrandrine Zhonghua Xin Xue Guan Bing Za Zhi 199321225–54.Chinese. [PubMed] [Google Scholar]
- Liu QY, Karpinski E, Pang PK. Tetrandrine inhibits both T and L calcium channel current in ventricular cells. J Cardiovasc Pharmacol. 1992;20:513–9. doi: 10.1097/00005344-199210000-00001. [DOI] [PubMed] [Google Scholar]
- Wu SN, Hwang TL, Jan CR, Tseng CJ. Ionic mechanisms of tetrandrine in cultured rat aortic smooth muscle cells. Eur J Pharmacol. 1997;327:233–8. doi: 10.1016/s0014-2999(97)89666-5. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Wang F, Cheng L, Fu LY, Zhou J, Yao WX. Effects of tetrandrine on calcium and potassium currents in isolated rat hepatocytes. World J Gastroenterol. 2003;9:134–6. doi: 10.3748/wjg.v9.i1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kühlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–31. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- Yue L, Melnyk P, Gaspo R. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Cir Res. 1999;84:776–84. doi: 10.1161/01.res.84.7.776. [DOI] [PubMed] [Google Scholar]
- Nao T, Ohkusa T, Hisamatsu Y, Inoue N, Matsumoto T, Yamada J, et al. Comparison of expression of connexin in right atrial myocardium in patients with chronic atrial fibrillation versus those in sinus rhythm. Am J Cardiol. 2003;91:678–83. doi: 10.1016/s0002-9149(02)03403-3. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Kirste W, Kuly S, Amann K, Neuhuber W, Weyand M, et al. Atrial distribution of connexin 40 and 43 in patients with intermittent, persistent, and postoperative atrial fibrillation. Heart Lung Circ. 2006;15:30–7. doi: 10.1016/j.hlc.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J. 1964;67:200–20. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- Allessie M, Lammers WJ, Bonke FI, Hollen J.Experimental evaluation of Moe's multiple wavelet hypothesis of atrial fibrillationIn: Zipes DP, Jalife J, eds. Cardiac electrophysiology and arrhythmias New York: Grune & Stratton; 1985265–75. [Google Scholar]
- Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–9. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- Nash MP, Mourad A, Clayton RH, Sutton PM, Bradley CP, Hayward M, et al. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation. 2006;114:536–42. doi: 10.1161/CIRCULATIONAHA.105.602870. [DOI] [PubMed] [Google Scholar]
- Chou CC, Zhou S, Miyauchi Y, Pak HN, Okuyama Y, Fishbein MC, et al. Effects of procainamide on electrical activity in thoracic veins and atria in canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol. 2004;286:H1936–45. doi: 10.1152/ajpheart.00754.2003. [DOI] [PubMed] [Google Scholar]
- Nattel S, Kneller J, Zou R, Leon LJ. Mechanisms of termination of atrial fibrillation by class I antiarrhythmic drugs: evidence from clinical, experimental, and mathematical modeling studies. J Cardiovasc Electrophysiol. 2003;14:S133–9. doi: 10.1046/j.1540.8167.90302.x. [DOI] [PubMed] [Google Scholar]
- Kawase A, Ikeda T, Nakazawa K, Ashihara T, Namba T, Kubota T, et al. Widening of the excitable gap and enlargement of the core of reentry during atrial fibrillation with a pure sodium channel blocker in canine atria. Circulation. 2003;107:905–10. doi: 10.1161/01.cir.0000050148.72502.3a. [DOI] [PubMed] [Google Scholar]
- Kneller J, Kalifa J, Zou R, Zaitsev AV, Warren M, Berenfeld O, et al. Mechanisms of AF termination by pure sodium channel blockade in an ionically-realistic mathematical model. Circ Res. 2005;96:e35–47. doi: 10.1161/01.RES.0000160709.49633.2b. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Katzung BG. Time-and voltage-dependent interaction of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977;472:373–98. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Kanki H, Mitamura H, Takatsuki S, Sueyoshi K, Shinagawa K, Sato T, et al. Postrepolarization refractoriness as a potential anti-atrial fibrillation mechanism of pilsicainide, a pure sodium channel blocker with slow recovery kinetics. Cardiovasc Drugs Ther. 1998;12:475–82. doi: 10.1023/a:1007758217189. [DOI] [PubMed] [Google Scholar]
- Roden DM.Treatment of cardiovascular disorders: arrhythmiasIn: Melmon KL, Morrelli HF, Hoffman BB, Nierenberg DW, editors. Clinical pharmacology: basic principles in therapeutics New York: McGraw-Hill; 1992. p151–85. [Google Scholar]
- Kirchhof PF, Fabritz CL, Franz MR. Postrepolarization refractoriness versus conduction slowing caused by class I antiarrhythmic drugs: antiarrhythmic and proarrhythmic effects. Circulation. 1998;97:2567–74. doi: 10.1161/01.cir.97.25.2567. [DOI] [PubMed] [Google Scholar]
- Koller BS, Karasik PE, Soloman AJ, Franz MR. The relationship between repolarization and refractoriness during programmed electrical stimulation in the human right ventricle: implications for ventricular tachycardia induction. Circulation. 1995;91:2378–84. doi: 10.1161/01.cir.91.9.2378. [DOI] [PubMed] [Google Scholar]
- Li DQ, Feng Y, Zhang JM, Jang JM, Zhang HQ, Hu HZ. Tetrandrine prevent connexin 40 degradation in the rabbit atria by rapid pacing. J Fourth Milit Med Univ. 2004;25:150–2. [Google Scholar]
- Cai Y, Qi XM, Gong LK, Liu LL, Chen FP, Xiao Y, et al. Tetrandrine-induced apoptosis in rat primary hepatocytes is initiated from mitochondria: caspases and endonuclease G (Endo G) pathway. Toxicology. 2006;218:1–12. doi: 10.1016/j.tox.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Shen YC, Chen CF, Sung YJ. Tetrandrine ameliorates ischemia-reperfusion injury of rat myocardium through inhibition of neutrophil priming and activation. Br J Pharmacol. 1999;128:1593–601. doi: 10.1038/sj.bjp.0702958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MR. Effects of tetrandrine on cardiac and vascular remodeling. Acta Pharmacol Sin. 2002;23:1075–85. [PubMed] [Google Scholar]