Abstract
Aim:
To investigate the anti-proliferative effect of iptakalim (Ipt), a newly selective KATP channel opener, in endothelin-1 (ET-1)-induced human pulmonary arterial smooth muscle cells (PASMCs) using proteomic analysis.
Methods:
Human PASMCs were incubated with ET-1 (10−8 mol/L) and ET-1 (10−8 mol/L) plus iptaklim (10−5 mol/L) for 24 h. Analysis via 2-DE gel electrophoresis and MALDI-TOF-MS was employed to display the different protein profiles of whole-cell protein from cultures of control, ET-1 treatment alone, and treatment with ET-1 and iptaklim combined. Real time RT-PCR and Western blot analysis were used to confirm the proteomic analysis.
Results:
When iptakalim inhibited the proliferative effect of ET-1 in human PASMCs by opening the KATP channels, the expression of different groups of cellular proteins was changed, including cytoskeleton-associated proteins, plasma membrane proteins and receptors, chaperone proteins, ion transport–associated proteins, and glycolytic and metabolism-associated proteins. We found that iptakalim could inhibit the proliferation of human PASMCs partly by affecting the expression of Hsp60, vimentin, nucleoporin P54 (NUP54) and Bcl-XL by opening the KATP channel.
Conclusion:
The data suggest that a wide range of signaling pathways may be involved in abolishing ET-1-induced proliferation of human PASMCs following iptakalim treatment.
Keywords: pulmonary arterial smooth muscle cells, KATP channel opener, iptakalim, proteomics, proliferation
Introduction
Proliferation of pulmonary arterial smooth muscle cells (PASMCs) is a key feature during the development of hypoxia pulmonary hypertension (PH) 1, 2. Endothelin-1 (ET-1) has been implicated in the development of the pulmonary hypertension through the remodeling of pulmonary vessels 3, 4. In pulmonary hypertension, the amount of circulating ET-1 is increased, with the greatest increase seen in the small resistance arteries. The vascular remodeling associated with chronic hypoxia is likely mediated by the mitogenic effects of ET-1 on vascular smooth muscle cells (SMCs), resulting in their growth 5.
Dysfunction of the potassium channels in the plasma membrane plays a pivotal role in PASMC proliferation 6. Changes in K+ channel function may represent a universal mechanism by which Ca2+ signals are targeted toward the activation of gene expression and cell growth 7. Furthermore, activation of K+ channels can induce apoptosis in vascular SMCs 8. Recently, KATP channels have received intense scrutiny because of their biophysical properties, regulation and pharmacology 9. Vasoactive agonists such as ET-1 can alter the activity of KATP channels in vascular SMCs, which contributes to the regulation of arterial diameter 10. This observation has prompted a widespread interest in KATP channels as potential targets to regulate proliferative vascular disorders in disease conditions such as PH 9.
Iptakalim (Ipt), a lipophilic para-amino compound with a low molecular weight, has been demonstrated via pharmacological, electrophysiological, and biochemical studies and a receptor binding test to be a new selective KATP channel opener 11, 12. Our previous study revealed that iptakalim could alleviate pulmonary artery remodeling and had the potential to treat pulmonary arterial disorders in PH 13. Moreover, iptakalim could inhibit the release and synthesis of ET-1 in cultured endothelial cells and suppress the proliferation of rabbit/human ET-1-induced PASMCs in a concentration-dependent manner in vitro, with 10−5 mol/L iptakalim abolishing the effect of 10−8 mol/L ET-1 14, 15. However, the molecular mechanisms of its anti-proliferative effect remain poorly understood. In this study, a proteomic approach is used to investigate the anti-proliferative effect of iptakalim in ET-1-induced human PASMCs.
Materials and methods
Isolation and culture of human PASMCs
Approval for the use of human specimens in this study was obtained from the Jiangsu Province Hospital Ethical Review Board (the First Affiliated Hospital of Nanjing Medical University, Nanjing, China). Human PASMCs were cultured from stage 3–4 of pulmonary artery of a male lung cancer donor (specimens were obtained from Jiangsu Province Hospital). Briefly, the arteries were separated from their adventin and endothelium, minced into 1-mm squares with sterile scalpel blades and placed in a culture bottle containing Dulbecco's modified Eagle's medium (DMEM) with 20% FBS. The culture bottle was placed in a moist tissue culture incubator at 37 °C in an atmosphere of 5% CO2. The purity of human PASMCs in the primary cultures was confirmed by positive staining with a smooth muscle α-actin antibody compared with a known positive control of SMCs as previously described 16.
Experimental design for 2-DE
Experiments were performed in the third to fifth passages of the cells. Human PASMCs were exposed to the normal (control) condition, the ET-1 (10−8 mol/L) condition, or the ET-1 (10−8 mol/L) + iptakalim (10−5 mol/L) condition in parallel culture. Human PASMCs were plated in 75 mL culture bottles and cultured for 72 h before the medium was changed to serum-free DMEM containing 100 units/mL (1%) penicillin/streptomycin. The human PASMCs were cultured in serum-free medium for 24 h prior to treatment with either ET-1 (10−8 mol/L) or ET-1 (10−8 mol/L)+iptakalim (10−5 mol/L). The control was left untreated for the same period of time. The control, ET-1-treated, and ET-1+iptakalim-treated cultures from one experiment were simultaneously submitted to all the steps of 2-DE, including protein extraction, isoelectric focusing, SDS-PAGE, silver staining and quantitative analysis, to minimize the variability between samples.
Sample preparation for 2-DE
The medium was removed from the culture bottles and the human PASMCs were detached from the culture bottle using trypsin digestion. The PASMCs were harvested and washed three times in ice cold 1×PBS before the addition of lysis buffer (500 μL) containing 7 mol/L urea, 2 mol/L thiourea, 4% (w/v) CHAPS, 1% (w/v) DTT, 1% (v/v) protease inhibitor cocktail, and 2% (v/v) IPG buffer (pH 3−10) on ice. Samples were sonicated (Five 1-s bursts) on ice and then centrifuged at 40 000×g for 60 min at 4°C. The supernatant was removed, assayed for protein concentration (using the Bradford method), and stored at −85°C for the next step in the analysis.
2-DE gel electrophoresis
Isoelectric focusing (IEF) was performed on an Ettan IPGphorII (Amersham Bioscience, Uppsala) with 24 cm immobilized pH gradient strips (pH 3–10; Amersham Bioscience, Uppsala). The IPG strips were rehydrated with samples containing 120 μg of protein and each IPG strip was focused simultaneously, as described previously 17. After isoelectric focusing, the IPG strips were equilibrated at room temperature and then run in an Ettan Daltsix electrophoresis system (Amersham Bioscience, Uppsala), as described previously. Gels were silver stained according to published procedures 18.
Quantitative analysis of differential protein expression
Gels were scanned using an Attrix Scan 1010 plus (Microtek, Taiwan), and the resulting images were analyzed with the ImageMasterTM 2D platinum software (Amersham Bioscience, Uppsala) for spot detection quantification and comparative and statistical analyses. In brief, the amount of each protein spot was expressed as its volume, which was calculated as the volume above the spot border situated at 75% of the spot height (measured from the peak of the spot). To reflect the quantitative variations in the protein spot volumes, the spot volumes were normalized as a percentage of the total volume of all the spots present in a gel. The experiments were repeated three times with three independent samples, and the protein expression profiles were compared. We assessed the statistical significance between each 2-DE gel (control, ET-1-treated and ET-1+iptakalim-treated) using ImageMasterTM 2D Platinum Software. In brief, each protein spot from the control, ET-1-treated and ET-1+iptakalim-treated gels was manually matched and assigned a number. The protein spots that varied with the same trends of intensity between the 2-DE gels (control, ET-1-treated and ET-1 +iptakalim-treated) in three independent experiments were selected for protein identification.
Protein identification
In-gel Trypsin Digestion: protein spots were manually excised from the gels stained with silver (each piece approximately 1 mm square) using a tip, and digested with trypsin according to the previously described protocol 17.
Protein identification by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS, Bruker Daltonics, Lepzig): dried peptides were dissolved in 2 μL of 0.5% (v/v) TFA. The matrix material was dissolved until saturated in TA (CAN:0.1% TFA:acetone=3:6:1). The matrix and the analytic solution were mixed at a ratio of 1:1 and 1 μL of the mixture was deposited onto the stainless steel sample target. The solvent was allowed to evaporate at ambient temperature. MALDI-TOF analysis was performed using a Bruker Biflex IV MALDI-TOF-MS system coupled with an N2 laser (337 nm, 3-ns pulse length) in positive ion mode with an accelerating voltage of 19 kV. Peptide data were collected in the reflectron mode. External calibration for peptide analysis was performed with peptide calibration standards. Analyses of MS data were performed by searching against the SWISS-PROT database of the National Center for Biotechnology Information (NCBI) non-redundant database using the MASCOT search program (Matrixscience, London, UK; www.matrixscience.com) to identify proteins of interest. Searches were performed using peptide mass accuracy tolerances of 0.3 Da or 200 ppm for external calibration. Peptides occurring in the blank controls were excluded. One missed cleavage per peptide was allowed, and a molecular weight range of 10% of the relative molecular weight was allowed for the matching of peptide mass values. The criteria for positive identification of proteins were set as follows: (1) at least four matching peptide masses and (2) scores greater than 50 were considered significant (P<0.05).
Expression analysis via real time quantitative RT-PCR
Total cellular RNA from control, ET-1 (10−8 mol/L)- treated and ET-1 (10−8 mol/L)+iptakalim (10−5 mol/L)- treated human PASMCs was isolated using the TRIzol reagent (Invitrogen, Jefferson). Then, 2 μg of total RNA of each sample was reverse transcribed into cDNA using the Moloney murine leukemia virus reverse transcriptase (Invitrogen, Jefferson). To amplify the selected protein transcripts, the following gene primers were used:
Hsp60 5′-CAGATGCCCTTAATGCTACAAG-3′ (forward)
5′-GCATCATAACCAACTTCTGAGG-3′ (reverse)
Vimentin 5′-CAGATGCCCTTAATGCTACAAG-3′ (forward)
5′-GCATCATAACCAACTTCTGAGG-3′ (reverse)
NUP54 5′-CAAAGGAAGAGTGGTTATGCCATT-3′ (forward)
5′-CGGCCCTTGAACTGAGTAGGT-3′ (reverse)
Bcl-XL was also amplified; the primers were 5′-AAGGAGATGCAGGTATTGGTGAGT-3′ (forward) and 5′-TCTCGGCTGCTGCATTGTT-3′ (reverse). GAPDH was used as control; the primers were 5′-GGAGCCAAACGGGTCATCATCTC-3′ (forward) and 5′-GAGGGGCCATCCACAGTCTTCT-3′ (reverse). The amplified fragment was about 200−250 bp. PCR conditions were as follows: 95 °C at 10 min for 1 cycle, 95 °C for 10 s and 60 °C for 1 min for 40 cycles in the 7300 System (Applied Biosystems Company, Foster City, CA).
Western blot analysis
Proteins (50 μg each) from whole-cell lysates were separated by SDS-PAGE in 12% SDS gels and then eletrotransferred onto nitrocellulose membranes (BIO-RAD, Hercules). Membranes were blocked overnight in blocking milk solution containing 5% (w/v) nonfat milk at 4 °C, followed by incubation with primary antibodies (anti-Hsp60, 1:1000; anti-vimentin, 1:100; anti-nucleoporin p54 (NUP54), 1:1000; anti-Bcl-XL, 1:1000) overnight at 4 °C. The membranes were then rinsed with 1×PBS, applied in 3×15 min washes, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:5000) at room temperature for 2 h. After a second set of the washes as described above, the reactive proteins were visualized using chemiluminescent substrates (Pierce, Rockford, IL). GAPDH was used as the control.
Statistical analysis
All values were expressed as mean±SEM of three independent experiments. The significance of the difference between the control and samples treated with the various drugs was determined by one-way ANOVA, followed by the post-hoc least significant difference (LSD) test. Differences were considered significant at P<0.05.
Results
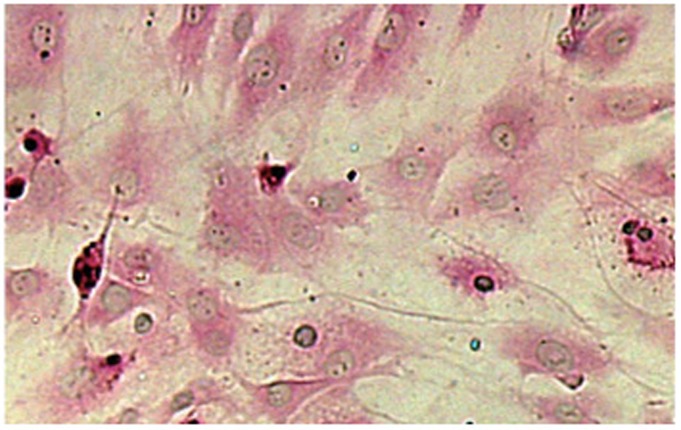
The present results showed that human PASMCs exhibited the typical “hill and valley” growth morphology. The purity of the primary culture cells was about 99%, which was determined by immunocytochemistry for α-smooth muscle actin, as shown in Figure 1.
Figure 1.
Immunocytochemical staining for α-smooth muscle actin in cells from human PASMC primary cultures (×100). Immunostaining showed that primary cultures were positive for α-smooth muscle actin.
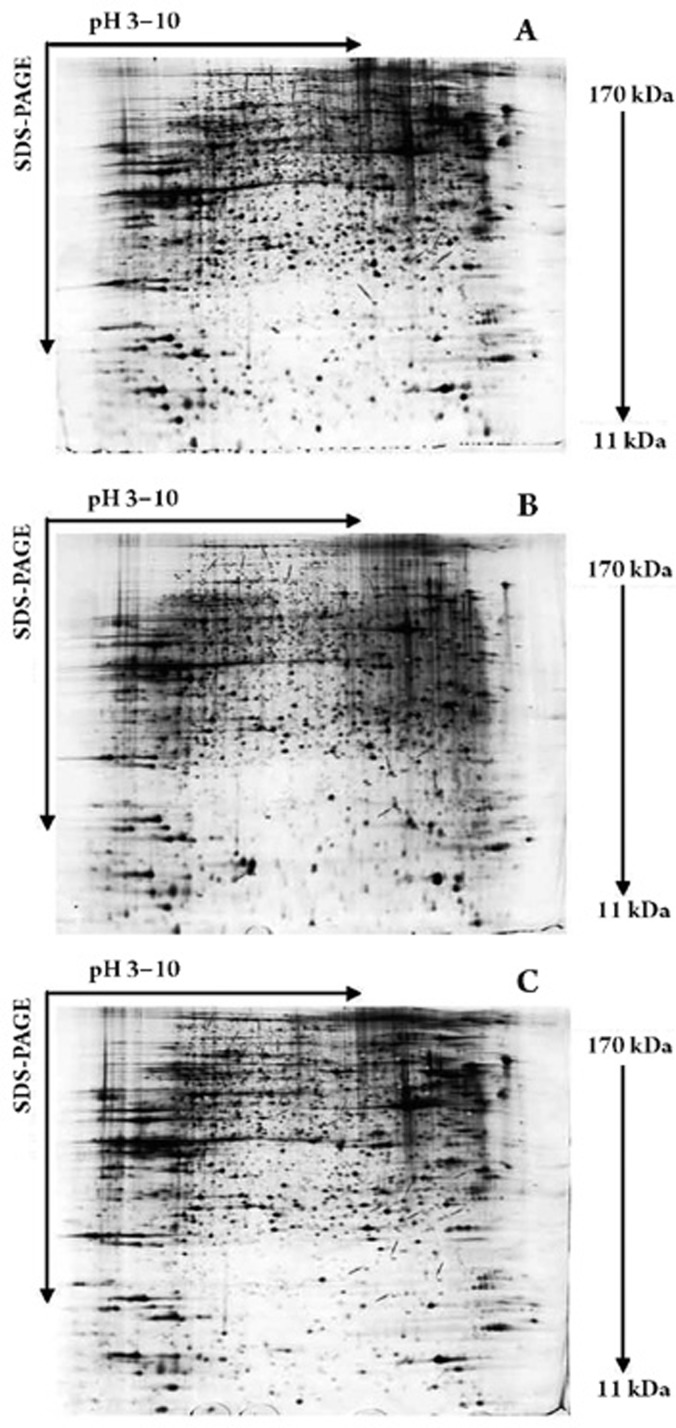
The differential protein expression in human PASMCs was examined using 2-DE. The human PASMCs were treated with ET-1 (10−8 mol/L), ET-1 (10−8 mol/L)+iptakalim (10−5 mol/L), or left untreated. Figure 2 presents the results from one of three independent experiments. More than 3000 protein spots were separated in each gel image, with an apparent range of molecular masses from 11 to 170 kDa and a range of pH values from 3 to 10. Gels were analyzed with the ImageMasterTM 2D platinum software. Proteins that had at least a 5-fold difference in intensity among the gels were excised for identification by MALDI-TOF mass spectrometry using the MASCOT search. We positively identified 27 proteins whose expressions were induced or suppressed in ET-1-treated cells compared with the control group, and were suppressed or induced in ET-1+iptakalim-treated cells compared with the ET-1-treated group (Table 1). Different functional groups of protein were regulated by iptakalim in ET-1-induced human PASMCs, including cytoskeleton-associated proteins (actin, vimentin, lamin A/C, tubulin), plasma membrane protein and receptors (annexin A5, G protein subunit beta 1, nucleoporin p54, transmembrane channel-like protein 5), chaperone proteins (Hsp60, GRP 78, T-complex protein), an ion transport-associated protein (Lasp-1), and metabolism-associated proteins (purine nucleoside phosphorylase, PGAM1 protein, Pgk1, and others). One interesting finding was that iptakalim could down-regulate the ET-1-induced expression level of the contractile protein actin in human PASMCs. We currently have no explanation for this phenomenon; the reasons for this change remain to be defined in future studies.
Figure 2.
Two-dimensional gel electrophoresis maps of proteins in human PASMCs. Proteins (120 μg) from control (A), ET-1 (10−8 mol/L)-treated (B), and ET-1 (10−8 mol/L)+iptakalim (10−5 mol/L)-treated (C) human PASMCs were run on 2-DE gels and then stained with silver solution. The protein spots that were changed more than 5-fold by ET-1 and inhibited by iptakalim were marked with arrows.
Table 1. Twenty-seven differential proteins identified in this study.
| Spot No | Accession No | Protein identified | Peptides matched | Mascot score | Molecular mass (Da) | PI | Functional class |
|---|---|---|---|---|---|---|---|
| Up-regulation in ET-1 treated group and down-regulation in ET-1+Ipt treated group | |||||||
| 1305 | Q7Z3B4 | Nucleoporin P54 | 17 | 93 | 55 515 | 6.53 | signal transduction |
| 757 | P10809 | 60 kDa heat shock protein | 35 | 230 | 61 187 | 5.7 | chaperone |
| 2433 | P08758 | Annexin A5 | 14 | 97 | 35 840 | 4.94 | apoptosis |
| 2478 | Q5T8M8 | Actin, alpha 1 | 9 | 81 | 32 370 | 5.26 | cytoskeletal |
| 1390 | Q53GK6 | Beta actin variant | 26 | 198 | 42 038 | 5.29 | cytoskeletal |
| 2788 | P00491 | Purine nucleoside phosphorylase | 25 | 193 | 32 325 | 6.45 | metabolism |
| 3164 | Q6FHk8 | PGAM1 protein | 14 | 81 | 28 931 | 6.67 | metabolism |
| 2165 | P11021 | 78 kDa glucose-regulated protein precursor | 18 | 95 | 72 402 | 5.07 | chaperone |
| 2881 | Q14847 | LIM and SH3 domain protein 1 (LASP-1) | 16 | 122 | 30 097 | 6.61 | ion transport |
| 1314 | P08670 | Vimentin | 26 | 166 | 53 545 | 5.06 | cytoskeletal |
| 2326 | P00558 | Phosphoglycerate kinase 1 | 12 | 67 | 44 854 | 8.3 | metabolism |
| 2994 | Q9H069 | Leucine rich repeat containing 48 | 9 | 63 | 61 559 | 4.66 | signal transduction |
| 2461 | P09651 | Heterogeneous nuclear ribonucleoprotein A1 | 7 | 61 | 32 532 | 8.97 | signal transduction |
| 2281 | P60709 | ACTB actin, cytoplasmic 1 | 13 | 77 | 42 052 | 5.29 | cytoskeletal |
| 2828 | Q6UXY8 | Transmembrane channel-like protein 5 | 10 | 77 | 88 648 | 9.06 | ion transport |
| 1023 | P68363 | Tubulin alpha-ubiquitous chain | 14 | 96 | 46 797 | 4.96 | cytoskeletal |
| 2041 | P60709 | ACTB actin, cytoplasmic 1s | 15 | 89 | 42 052 | 5.29 | cytoskeletal |
| 1293 | P14618 | PKM2 isoform M2 of pyruvate kinase isozymes M1/M2 | 20 | 114 | 58 470 | 7.96 | metabolism |
| Down-regulation in ET-1 treated group and up-regulation in ET-1+Ipt treated group | |||||||
| 273 | Q9UPY3 | Endoribonuclease Dicer | 17 | 62 | 220 227 | 5.45 | signal transduction |
| 655 | Q14195 | Dihydropyrimidinase-related protein 3 | 14 | 77 | 62 323 | 6.04 | metabolism |
| 799 | Q16555 | Dihydropyrimidinase-related protein 2 | 15 | 87 | 62 711 | 5.95 | metabolism |
| 547 | P49368 | T-complex protein 1 subunit gamma | 25 | 136 | 61 066 | 6.1 | chaperone |
| 441 | P43304 | Glycerol-3-phosphate dehydrogenase, mitochondrial precursor | 36 | 240 | 81 296 | 7.23 | metabolism |
| 950 | P14618 | Pyruvate kinase isozymes M1/M2 | 20 | 143 | 58 339 | 7.95 | metabolism |
| 783 | P02545 | Lamin-A/C | 21 | 91 | 74 380 | 6.57 | cytoskeletal |
| 310 | P02545 | Lamin-A/C | 21 | 91 | 74 380 | 6.57 | cytoskeletal |
| 1959 | P62873 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta 1 | 10 | 54 | 38 020 | 5.6 | signal transduction |
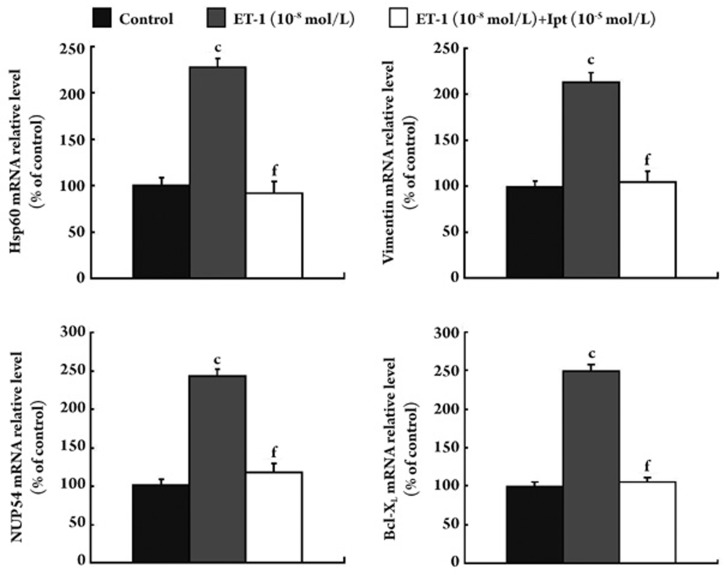
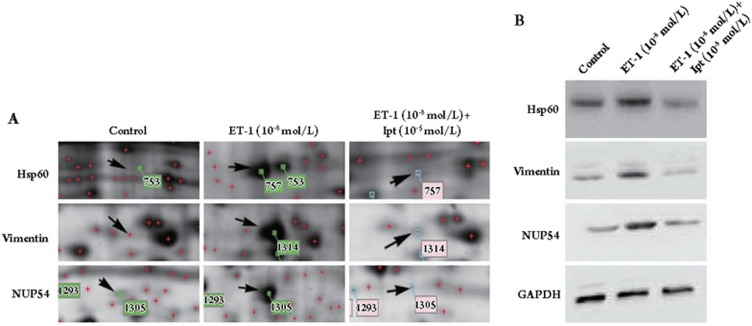
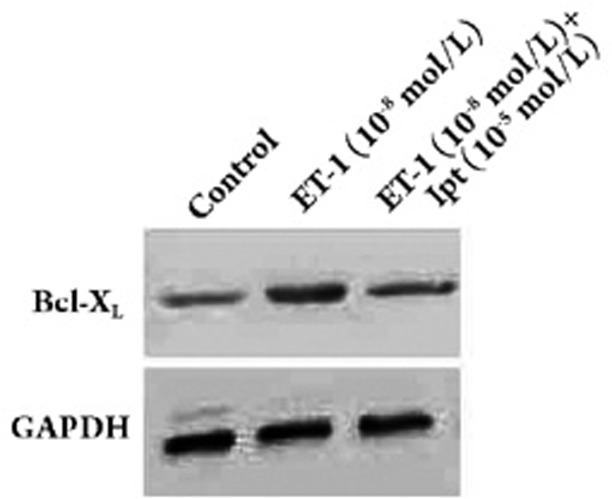
Among the 27 proteins, Hsp60, vimentin and NUP54 were selected for further analysis by real-time PCR and western blot analysis because their expressions were dramatically changed on the 2-DE gels. The results of both real-time PCR and Western blot analysis confirmed the data from the 2-DE analysis. As shown in Figures 3 and 4, the mRNA levels and protein levels of Hsp60, vimentin, and NUP54 were up-regulated by ET-1, however, this up-regulation was abolished by iptakalim in human PASMCs. In light of the anti-apoptotic effect of Bcl-XL and its possible interaction with Hsp60, the expression of Bcl-XL was also examined. Interestingly, the expression of Bcl-XL was increased in ET-1-treated cells and showed little change in ET-1+iptakalim-treated cells as compared to controls (Figures 3 and 5).
Figure 3.
Real-time quantitative RT-PCR analysis of mRNA expression of Hsp60, vimentin, NUP54, and Bcl-XL in human PASMCs. Human PASMCs were incubated with ET-1 (10−8 mol/L) or ET-1 (10−8 mol/L)+iptaklim (10−5 mol/L) or left untreated for 24 h. The mRNA was extracted and amplified by real-time quantitative RT-PCR. Values are represented as means±SEM from three independent experiments. The results showed that iptakalim could decrease the mRNA expression of Hsp60, vimentin, NUP54 and Bcl-XL induced by ET-1 in human PASMCs. n=3 experiments. Mean±SEM. cP<0.01 vs control. fP<0.01 vs ET-1 10−8 mol/L.
Figure 4.
Protein expression of Hsp60, vimentin, and NUP54 in human PASMCs. Human PASMCs were incubated with ET-1 (10−8 mol/L) or ET-1 (10−5 mol/L)+iptakalim (10−5 mol/L) or left untreated for 24 h. Proteins (50 μg each) from whole-cell lysates were run on 2-DE gels (A) or separated by SDS-PAGE and immunoblotted with antibodies against Hsp60, vimentin, NUP54, or GAPDH (B). We confirmed that the Western blot and proteomic results were identical.
Figure 5.
Protein expression of Bcl-XL in human PASMCs. Human PASMCs were incubated with ET-1 (10−8 mol/L) or ET-1 (10−8 mol/L)+iptakalim (10−5 mol/L) or left untreated for 24 h. Proteins (50 μg each) from whole-cell lysates were separated by SDS-PAGE and immunoblotted with antibodies against Bcl-XL or GAPDH.
Discussion
It has been demonstrated that iptakalim could selectively open the KATP channels and increase the KATP current 11, 12, 15. Our previous work has shown that iptakalim has an anti-proliferative effect on PASMCs and has potential for the treatment of pulmonary hypertension 13, 14, 15. To further investigate the mechanism of iptakalim's anti-proliferative effect, we took a proteomic approach to examine the protein expression in human PASMCs. ET-1 was able to close the KATP channels and reduce the KATP current in a concentration-dependent manner in PASMCs 19. It was selected as the agonist in our experimental model because it has been implicated in the development of pulmonary hypertension 3, 4.
One of the findings of this work was that the expression of Hsp60 in ET-1-induced human PASMCs was decreased by iptakalim. The chaperone molecular heat shock proteins are primarily anti-apoptotic in cells 20, 21. Hsp60 can form a complex with Bax, Bak, and Bcl-XL; when Hsp60 decreases, both Bax and Bak increase, while Bcl-2 decreases 22, 23. It has been reported that acute ablation of Hsp60 could activate caspase-dependent apoptosis by the disruption of an Hsp60-p53 complex, which results in p53 stabilization, increasing expression of pro-apoptotic Bax, and Bax-dependent apoptosis 22. In contrast, overexpression of Hsp60 could lead to the increased expression of Bcl-XL and Bcl-2 and decreased expression of Bax 23. Therefore, the down-regulation of Hsp60 or interference with the binding of Hsp60 to Bax, Bak, or Bcl-XL could potentially induce apoptosis 24, 25. In this regard, iptakalim could decrease the ET-1-induced Hsp60 expression in human PASMCs, suggesting that iptakalim might have a pro-apoptotic effect in ET-1-induced PASMCs.
Structural protein expression changes are usually involved in cell proliferation. In the present work, we also identified the protein vimentin, which has intermediate filaments (IFs) and may play an important role in cell integrity, mobility, and differentiation 26, 27, as well as in maintaining cellular function 28, 29, 30. Various cellular processes, including signal transduction, cytoskeletal stability, and cell apoptosis, have been associated with alterations of IFs 29. Vimentin contains several phosphorylation sites 31 and is a substrate for many protein kinases, such as p34 Cdc2 kinase 32, PKA, PKC 33, Ca2+-calmodulin-dependent protein kinase II 34, p21-activated kinase 35 and MAPK-activated protein kinase-2 36. The proteolytic degradation of vimentin is a component of the executive process of cell apoptosis 37. However, whether the decreased expression of vimentin in ET-1-induced PASMCs results from proteolytic degradation has yet to be determined.
The nuclear pore complexes (NPCs), consisting of nucleoporins p62, p54, and p58/p45, may be the only known gateway between the cytoplasm and nucleus that allows for the exchange of macromolecules in eukaryotic cells and is involved in signal import and export 38, 39. Functional endothelin receptors (ETAR and ETBR) are present on the plasma membrane and nuclei, and protein-protein interactions between the receptors and p62 subcomplex have been demonstrated 40. Stimulation of the plasma membrane and nuclear ETRs by ET-1 can increase the [Ca2+]cyt and nuclear cisterna or nucleoplasm 41. Nucleoplasmic Ca2+ can regulate many key nuclear functions, including gene transcription, apoptosis, and gene repair 42, 43. Moreover, the Ca2+ level can alter the conformational state of the nuclear pore complex and control the transport of molecules between the cytosol and nucleoplasm. It has been confirmed that a transient rise in [Ca2+]cyt induced by ET-1 could be blocked by iptakalim in human PASMCs. Furthermore, the present study showed that iptakalim could abolish the effect of ET-1 on the induction of NUP54 expression in human PASMCs. The data suggest that iptakalim regulates signaling between the cytoplasm and nucleus, not only through the concentration of Ca2+ but also through the expression of NUP54 in ET-1-induced PASMCs.
Because of technical issues, there are limits to the ability of the 2-DE MS approach to analyze proteins of medium to low abundance. In this regard, we plan to investigate the mechanism of the anti-proliferative effect of iptakalim using the modified 2-DE method in association with another approach, such as microarray analysis.
Conclusion
The present work investigated the mechanism of the anti-proliferative effect of the newly selective KATP channel opener iptakalim in ET-1-induced human PASMCs. Our data suggested that iptakalim might achieve its anti-proliferative effect at least in part through the regulation of Hsp60, Bcl-XL, vimentin and NUP54 expression and modulate a wide range of signaling pathways in ET-1-induced human PASMCs.
Author contributions
Hong WANG, Chang-liang ZHU, Wei-ping XIE, and Ming-xia YANG designed the research; Ming-xia YANG, Zheng-xia LIU, Shi-jiang ZHANG, and Lei MA performed the research; Ming-xia YANG, Yu JING, and Shi-jiang ZHANG analyzed the data; and Ming-xia YANG, Zheng-xia LIU, and Shu ZHANG wrote and revised the paper.
Acknowledgments
We thank Dr H WANG (Institute of Pharmacology and Toxicology, Academy of Military Medical Sciences, Beijing, China) and Dr Gang HU (Department of Pharmacology, Nanjing Medical University, Nanjing, China) for their technical assistance in the study. This work was supported by the Jiangsu Commission of Science and Technology (Hong WANG) under contract No BJ200608, the National Natural Science Foundation of China (Hong WANG) under contract No 30871139 and by a research fund from the Natural Science Foundation of Jiangsu Province (Wei-ping XIE) under contract No BK2006246.
References
- Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta. 2007;1772:895–906. doi: 10.1016/j.bbadis.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, et al. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med. 2003;348:500–9. doi: 10.1056/NEJMoa021650. [DOI] [PubMed] [Google Scholar]
- Zhang YM, Wang KQ, Zhou GM, Zuo J, Ge JB. Endothelin-1 promoted proliferation of vascular smooth muscle cell through pathway of extracellular signal-regulated kinase and cyclin D1. Acta Pharmacol Sin. 2003;24:563–8. [PubMed] [Google Scholar]
- Nakanishi K, Tajima F, Nakata Y, Osada H, Tachibana S, Kawai T, et al. Expression of endothelin-1 in rats developing hypobaric hypoxia-induced pulmonary hypertension. Lab Invest. 1999;79:1347–57. [PubMed] [Google Scholar]
- Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir Res. 2001;2:90–101. doi: 10.1186/rr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Wang H, Ding J, Wang H, Hu G. Anti-proliferating effect of iptakalim, a novel KATP channel opener, in cultured rabbit pulmonary arterial smooth muscle cells. Eur J Pharmacol. 2005;511:81–7. doi: 10.1016/j.ejphar.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Neylon CB. Potassium channels and vascular proliferation. Vascul Pharmacol. 2002;38:35–41. doi: 10.1016/s1537-1891(02)00124-6. [DOI] [PubMed] [Google Scholar]
- Brevnova EE, Platoshyn O, Zhang S, Yuan JX. Overexpression of human KCNA5 increases IKV and enhances apoptosis. Am J Physiol Cell Physiol. 2004;287:C715–22. doi: 10.1152/ajpcell.00050.2004. [DOI] [PubMed] [Google Scholar]
- Cole WC, Clement-Chomienne O. ATP-sensitive K+ channels of vascular smooth muscle cells. J Cardiovasc Electrophysiol. 2003;14:94–103. doi: 10.1046/j.1540-8167.2003.02376.x. [DOI] [PubMed] [Google Scholar]
- Cui Y, Tran S, Tinker A, Clapp LH. The molecular composition of KATP channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am J Respir Cell Mol Biol. 2002;26:135–43. doi: 10.1165/ajrcmb.26.1.4622. [DOI] [PubMed] [Google Scholar]
- Hu LF, Shi XR, Yao HH, Sun YH, Ding JH, Liu SY, et al. ATP-sensitive potassium channel opener iptakalim protected against the cytotoxicity of MPP+ on SH-SY5Y cells by decreasing extracellular glutamate level. J Neurochem. 2005;94:1570–9. doi: 10.1111/j.1471-4159.2005.03306.x. [DOI] [PubMed] [Google Scholar]
- Misaki N, Mao X, Lin YF, Suga S, Li GH, Liu Q, et al. Iptakalim, a vascular ATP-sensitive potassium (KATP) channel opener, closes rat pancreatic beta-cell KATP channels and increases insulin release. J Pharmacol Exp Ther. 2007;322:871–8. doi: 10.1124/jpet.107.121129. [DOI] [PubMed] [Google Scholar]
- Xie W, Wang H, Wang H, Hu G. Effects of iptakalim hydrochloride, a novel KATP channel opener, on pulmonary vascular remodeling in hypoxic rats. Life Sci. 2004;75:2065–76. doi: 10.1016/j.lfs.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang S, Xie W, Li Q, Zhou Y, Wang H. Iptakalim inhibited endothelin-1-induced proliferation of human pulmonary arterial smooth muscle cells through the activation of KATP channel. Vascul Pharmacol. 2008;48:92–9. doi: 10.1016/j.vph.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Wang H, Long C, Duan Z, Shi C, Jia G, Zhang Y. A new ATP-sensitive potassium channel opener protects endothelial function in cultured aortic endothelial cells. Cardiovasc Res. 2007;73:497–503. doi: 10.1016/j.cardiores.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Zhu P, Huang L, Ge X, Yan F, Wu R, Ao Q. Transdifferentiation of pulmonary arteriolar endothelial cells into smooth muscle-like cells regulated by myocardin involved in hypoxia-induced pulmonary vascular remodelling. Int J Exp Pathol. 2006;87:463–74. doi: 10.1111/j.1365-2613.2006.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu YF, Guo XJ, Huo R, Ma X, Lin M, et al. A two-dimensional electrophoresis reference map of human ovary. J Mol Med. 2005;83:812–21. doi: 10.1007/s00109-005-0676-y. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–8. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Park WS, Ko EA, Han J, Kim N, Earm YE. Endothelin-1 acts via protein kinase C to block KATP channels in rabbit coronary and pulmonary arterial smooth muscle cells. J Cardiovasc Pharmacol. 2005;45:99–108. doi: 10.1097/01.fjc.0000150442.49051.f7. [DOI] [PubMed] [Google Scholar]
- Chandra D, Choy G, Tang DG. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J Biol Chem. 2007;282:31289–301. doi: 10.1074/jbc.M702777200. [DOI] [PubMed] [Google Scholar]
- Arrigo A. In search of the molecular mechanism by which small stress proteins counteract apoptosis during cellular differentiation. J Cell Biochem. 2005;94:241–6. doi: 10.1002/jcb.20349. [DOI] [PubMed] [Google Scholar]
- Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. J Biol Chem. 2008;283:5188–94. doi: 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang H. Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J Mol Cell Cardiol. 2003;35:1135–43. doi: 10.1016/s0022-2828(03)00229-3. [DOI] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. HSP60, Bax, apoptosis and the heart. J Cell Mol Med. 2005;9:51–8. doi: 10.1111/j.1582-4934.2005.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. Cytosolic heat shock protein 60, hypoxia, and apoptosis. Circulation. 2002;106:2727–33. doi: 10.1161/01.cir.0000038112.64503.6e. [DOI] [PubMed] [Google Scholar]
- Salvador-Silva M, Ricard CS, Agapova OA, Yang P. Expression of small heat shock proteins and intermediate filaments in the human optic nerve head astrocytes exposed to elevated hydrostatic pressure in vitro. J Neurosci Res. 2001;66:59–73. doi: 10.1002/jnr.1197. [DOI] [PubMed] [Google Scholar]
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Ermakova S, Choi BY, Choi HS, Kang BS, Bode AM, Dong Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J Biol Chem. 2005;280:16882–90. doi: 10.1074/jbc.M414185200. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Goto H, Matsui S, Manser E, Lim L, Nagata Ki, et al. Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene. 2001;20:2868–76. doi: 10.1038/sj.onc.1204407. [DOI] [PubMed] [Google Scholar]
- Tsujimura K, Ogawara M, Takeuchi Y, Imajoh-Ohmi S, Ha MH, Inagaki M. Visualization and function of vimentin phosphorylation by cdc2 kinase during mitosis. J Biol Chem. 1994;269:31097–106. [PubMed] [Google Scholar]
- Strelkov SV, Herrmann H, Geisler N, Lustig A, Ivaninskii S, Zimbelmann R, et al. Divide-and-conquer crystallographic approach towards an atomic structure of intermediate filaments. J Mol Biol. 2001;306:773–81. doi: 10.1006/jmbi.2001.4442. [DOI] [PubMed] [Google Scholar]
- Chou YH, Bischoff JR, Beach D, Goldman RD. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990;62:1063–71. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Ando S, Tanabe K, Gonda Y, Sato C, Inagaki M. Domain- and sequence-specific phosphorylation of vimentin induces disassembly of the filament structure. Biochemistry. 1989;28:2974–9. doi: 10.1021/bi00433a035. [DOI] [PubMed] [Google Scholar]
- Tokui T, Yamauchi T, Yano T, Nishi Y, Kusagawa M, Yatani R. Ca2+-calmodulin-dependent protein kinase II phosphorylates various types of non-epithelial intermediate filament proteins. Biochem Biophys Res Commun. 1990;169:896–904. doi: 10.1016/0006-291x(90)91977-z. [DOI] [PubMed] [Google Scholar]
- Goto H, Tanabe K, Manser E, Lim L, Yasui Y, Inagaki M. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK) Genes Cells. 2002;7:91–7. doi: 10.1046/j.1356-9597.2001.00504.x. [DOI] [PubMed] [Google Scholar]
- Chen A, Zhang L. The antioxidant (-)-epigallocatechin-3-gallate inhibits rat hepatic stellate cell proliferation in vitro by blocking the tyrosine phosphorylation and reducing the gene expression of platelet-derived growth factor-beta receptor. J Biol Chem. 2003;278:23381–9. doi: 10.1074/jbc.M212042200. [DOI] [PubMed] [Google Scholar]
- Fujita J, Bandoh S, Yang Y, Wu F, Ohtsuki Y, Yoshinouchi T. High molecular weight vimentin complex is formed after proteolytic digestion of vimentin by caspase-3: detection by sera of patients with interstitial pneumonia. Microbiol Immunol. 2003;47:447–51. doi: 10.1111/j.1348-0421.2003.tb03369.x. [DOI] [PubMed] [Google Scholar]
- Paulillo SM, Phillips EM, Koser J, Sauder U, Ullman KS, Powers MA, et al. Nucleoporin domain topology is linked to the transport status of the nuclear pore complex. J Mol Biol. 2005;351:784–98. doi: 10.1016/j.jmb.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Schwarz-Herion K, Maco B, Sauder U, Fahrenkrog B. Domain topology of the p62 complex within the 3-D architecture of the nuclear pore complex. J Mol Biol. 2007;370:796–806. doi: 10.1016/j.jmb.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Boivin B, Chevalier D, Villeneuve LR, Rousseau E, Allen BG. Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. J Biol Chem. 2003;278:29153–63. doi: 10.1074/jbc.M301738200. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M, Peri KG, Almazan G, Ribeiro-da-Silva A, Shichi H, Durocher Y, et al. Nuclear localization of prostaglandin E2 receptors. Proc Natl Acad Sci USA. 1998;95:15792–7. doi: 10.1073/pnas.95.26.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimmeck D, Mayer U, Ungerer N, Warnken U, Schnolzer M, Frings S, et al. Calcium-signaling networks in olfactory receptor neurons. Neuroscience. 2008;151:901–12. doi: 10.1016/j.neuroscience.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]