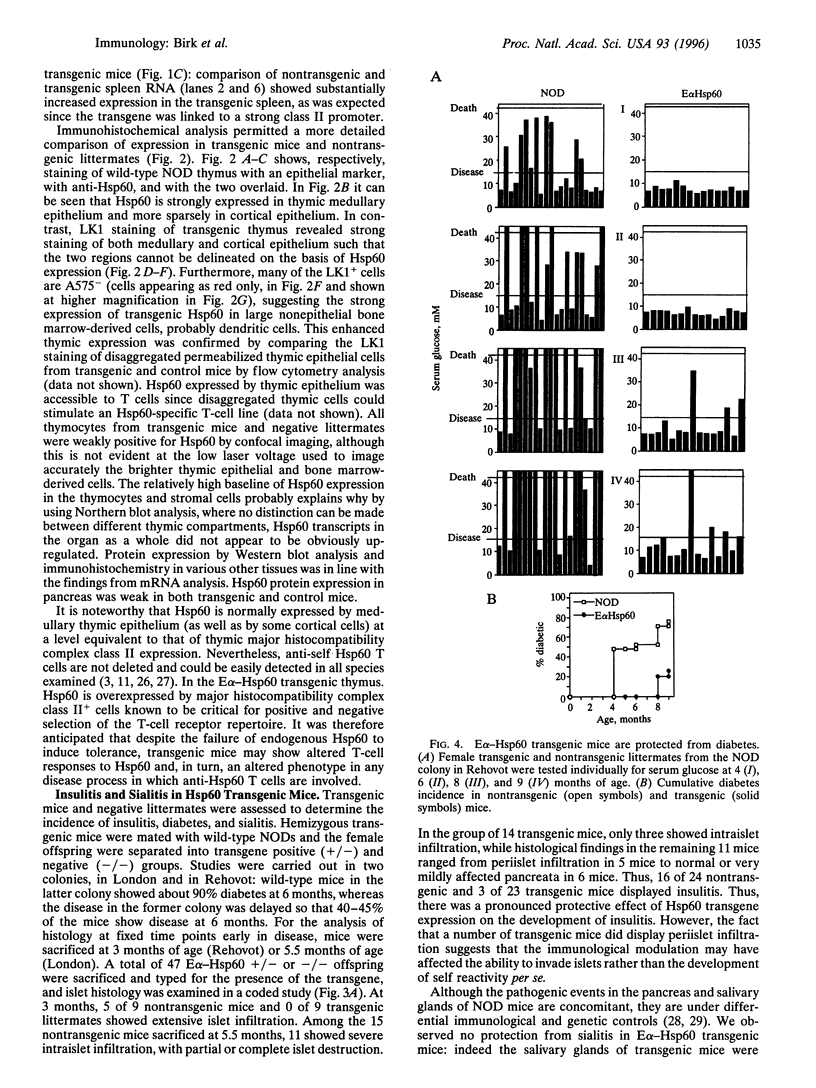
Abstract
A pathogenic role for self-reactive cells against the stress protein Hsp60 has been proposed as one of the events leading to autoimmune destruction of pancreatic beta cells in the diabetes of nonobese diabetic (NOD) mice. To examine this hypothesis, we generated transgenic NOD mice carrying a murine Hsp60 transgene driven by the H-2E alpha class II promoter. This would be expected to direct expression of the transgene to antigen-presenting cells including those in the thymus and so induce immunological tolerance by deletion. Detailed analysis of Hsp60 expression revealed that the endogenous gene is itself expressed strongly in thymic medullary epithelium (and weakly in cortex) yet fails to induce tolerance. Transgenic mice with retargeted Hsp60 showed overexpression of the gene in thymic cortical epithelium and in bone marrow-derived cells. Analysis of spontaneous T-cell responses to a panel of self and heterologous Hsp60 antigens showed that tolerance to the protein had not been induced, although responses to an immunodominant 437-460 epitope implicated in disease were suppressed, probably indicating an epitope shift. This correlated with changes in disease susceptibility: insulitis in transgenic mice was substantially reduced so that pathology rarely progressed beyond periislet infiltration. This was reflected in a substantial reduction in hyperglycemia and disease. These data indicate that T cells specific for some epitopes of murine Hsp60 are likely to be involved in the islet-cell destruction that occurs in NOD mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderuccio F., Toh B. H., Tan S. S., Gleeson P. A., van Driel I. R. An autoimmune disease with multiple molecular targets abrogated by the transgenic expression of a single autoantigen in the thymus. J Exp Med. 1993 Aug 1;178(2):419–426. doi: 10.1084/jem.178.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton S. M., van der Zee R., Goodacre J. A. Inflammation activates self hsp60-specific T cells. Eur J Immunol. 1993 Jan;23(1):33–38. doi: 10.1002/eji.1830230107. [DOI] [PubMed] [Google Scholar]
- Anderton S. M., van der Zee R., Prakken B., Noordzij A., van Eden W. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J Exp Med. 1995 Mar 1;181(3):943–952. doi: 10.1084/jem.181.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman B., Haskins K. Islet-specific T-cell clones from the NOD mouse respond to beta-granule antigen. Diabetes. 1994 Feb;43(2):197–203. doi: 10.2337/diab.43.2.197. [DOI] [PubMed] [Google Scholar]
- Boog C. J., de Graeff-Meeder E. R., Lucassen M. A., van der Zee R., Voorhorst-Ogink M. M., van Kooten P. J., Geuze H. J., van Eden W. Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med. 1992 Jun 1;175(6):1805–1810. doi: 10.1084/jem.175.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I. R. Induction of heat shock (stress) genes in the mammalian brain by hyperthermia and other traumatic events: a current perspective. J Neurosci Res. 1990 Nov;27(3):247–255. doi: 10.1002/jnr.490270302. [DOI] [PubMed] [Google Scholar]
- Elias D., Cohen I. R. Peptide therapy for diabetes in NOD mice. Lancet. 1994 Mar 19;343(8899):704–706. doi: 10.1016/s0140-6736(94)91582-2. [DOI] [PubMed] [Google Scholar]
- Elias D., Marcus H., Reshef T., Ablamunits V., Cohen I. R. Induction of diabetes in standard mice by immunization with the p277 peptide of a 60-kDa heat shock protein. Eur J Immunol. 1995 Oct;25(10):2851–2857. doi: 10.1002/eji.1830251021. [DOI] [PubMed] [Google Scholar]
- Elias D., Markovits D., Reshef T., van der Zee R., Cohen I. R. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D., Reshef T., Birk O. S., van der Zee R., Walker M. D., Cohen I. R. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3088–3091. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. F., Qin H. Y., Bhatti S., Smith D. K., Singh R. K., Dillon T., Lauzon J., Singh B. Immunization with the larger isoform of mouse glutamic acid decarboxylase (GAD67) prevents autoimmune diabetes in NOD mice. Diabetes. 1994 Dec;43(12):1494–1499. doi: 10.2337/diab.43.12.1494. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Altmann D. M. Dual T cell receptor alpha chain T cells in autoimmunity. J Exp Med. 1995 Oct 1;182(4):953–959. doi: 10.1084/jem.182.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. S., Buu N. N., Ruijs T. C., Williams K., Antel J. P. Differential expression of heat shock proteins by human glial cells. J Neuroimmunol. 1992 Dec;41(2):231–238. doi: 10.1016/0165-5728(92)90074-u. [DOI] [PubMed] [Google Scholar]
- Gerling I. C., Serreze D. V., Christianson S. W., Leiter E. H. Intrathymic islet cell transplantation reduces beta-cell autoimmunity and prevents diabetes in NOD/Lt mice. Diabetes. 1992 Dec;41(12):1672–1676. doi: 10.2337/diab.41.12.1672. [DOI] [PubMed] [Google Scholar]
- Heath W. R., Allison J., Hoffmann M. W., Schönrich G., Hämmerling G., Arnold B., Miller J. F. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature. 1992 Oct 8;359(6395):547–549. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- Honeyman M. C., Cram D. S., Harrison L. C. Glutamic acid decarboxylase 67-reactive T cells: a marker of insulin-dependent diabetes. J Exp Med. 1993 Feb 1;177(2):535–540. doi: 10.1084/jem.177.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Schönrich G., Momburg F., Auphan N., Malissen M., Malissen B., Schmitt-Verhulst A. M., Arnold B. Non-deletional mechanisms of peripheral and central tolerance: studies with transgenic mice with tissue-specific expression of a foreign MHC class I antigen. Immunol Rev. 1991 Aug;122:47–67. doi: 10.1111/j.1600-065x.1991.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur C., Hanahan D., Smith K. M. T-cell tolerance toward a transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6707–6711. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. D., Wang B., Haskins K., Benoist C., Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993 Sep 24;74(6):1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- Kouskoff V., Fehling H. J., Lemeur M., Benoist C., Mathis D. A vector driving the expression of foreign cDNAs in the MHC class II-positive cells of transgenic mice. J Immunol Methods. 1993 Dec 3;166(2):287–291. doi: 10.1016/0022-1759(93)90370-m. [DOI] [PubMed] [Google Scholar]
- Li X., Golden J., Faustman D. L. Faulty major histocompatibility complex class II I-E expression is associated with autoimmunity in diverse strains of mice. Autoantibodies, insulitis, and sialadenitis. Diabetes. 1993 Aug;42(8):1166–1172. doi: 10.2337/diab.42.8.1166. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lund T., O'Reilly L., Hutchings P., Kanagawa O., Simpson E., Gravely R., Chandler P., Dyson J., Picard J. K., Edwards A. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-A beta-chain or normal I-E alpha-chain. Nature. 1990 Jun 21;345(6277):727–729. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- Mathis D. J., Benoist C. O., Williams V. E., 2nd, Kanter M. R., McDevitt H. O. The murine E alpha immune response gene. Cell. 1983 Mar;32(3):745–754. doi: 10.1016/0092-8674(83)90060-0. [DOI] [PubMed] [Google Scholar]
- Munk M. E., Schoel B., Modrow S., Karr R. W., Young R. A., Kaufmann S. H. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J Immunol. 1989 Nov 1;143(9):2844–2849. [PubMed] [Google Scholar]
- Parish N. M., Chandler P., Quartey-Papafio R., Simpson E., Cooke A. The effect of bone marrow and thymus chimerism between non-obese diabetic (NOD) and NOD-E transgenic mice, on the expression and prevention of diabetes. Eur J Immunol. 1993 Oct;23(10):2667–2675. doi: 10.1002/eji.1830231042. [DOI] [PubMed] [Google Scholar]
- Reiner S. L., Zheng S., Corry D. B., Locksley R. M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993 Sep 27;165(1):37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tisch R., Yang X. D., Singer S. M., Liblau R. S., Fugger L., McDevitt H. O. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993 Nov 4;366(6450):72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van Ewijk W., Ron Y., Monaco J., Kappler J., Marrack P., Le Meur M., Gerlinger P., Durand B., Benoist C., Mathis D. Compartmentalization of MHC class II gene expression in transgenic mice. Cell. 1988 May 6;53(3):357–370. doi: 10.1016/0092-8674(88)90156-0. [DOI] [PubMed] [Google Scholar]





