Abstract
The sympathetic nervous system (SNS) innervation of white adipose tissue (WAT) is the principal initiator of lipolysis. Using pseudorabies virus, a transneuronal viral tract tracer, brain sites involved in the SNS outflow to WAT have been identified previously by us. One of these sites, the hypothalamic paraventricular nucleus (PVH) that shows predominantly unilateral sympathetic outflow from each half of the nucleus to ipsilaterally located WAT depots, was tested for laterality in lipid accumulation/mobilization in Siberian hamsters. First we tested whether unilateral PVH electrolytic lesions (PVHx) would increase lipid accumulation in WAT pads ipsilateral to the side of the PVHx. PVHx significantly increased body and WAT pad masses compared with sham PVHx; however, there was no laterality effect. In addition, bilateral PVHx increased body and WAT pad masses, as well as food intake, to a greater extent than did unilateral PVHx. We next tested for possible laterality effects on WAT lipid mobilization using food deprivation as the lipolytic stimulus in hamsters bearing unilateral or bilateral PVHx. Lipid mobilization was not prevented, as indicated indirectly by WAT mass and thus laterality of lipid mobilization could not be tested. We then tested whether removal of adrenal catecholamines via adrenal demedullation (ADMEDx) alone, or combined with bilateral PVHx, would block food deprivation–induced lipid mobilization, but neither did so. These results suggest that an intact PVH is not necessary for food deprivation–induced lipid mobilization and support the primacy of the SNS innervation of WAT, rather than adrenal medullary catecholamines, for lipid mobilization from WAT.
INTRODUCTION
Obesity is a growing epidemic in the United States with over half of the population being overweight (1). Consequently, it is not surprising that the vast majority of basic obesity research focuses on the development of increases in adiposity. By contrast, we have been studying the reversal of a naturally occurring seasonal obesity in Siberian hamsters (Phodopus sungorus; for review see refs. 2,3). Through this work we found that the reversal of this obesity occurs almost exclusively via the sympathetic nervous system (SNS) innervation of white adipose tissue (WAT; for review see refs. 4,5), rather than by circulating factors (for review see ref. 2). Indeed, the importance of the SNS innervation of WAT for lipolysis has been demonstrated by the blockade of lipid mobilization following WAT sympathetic denervation, but not by adrenal demedullation (ADMEDx) which removes circulating catecholamines thought previously to initiate lipolysis (for review see refs. 4,5).
The ventromedial hypothalamus (VMH) was thought to be a key controller of body fat and food intake, largely based on the ability of VMH lesions (VMHx) to increase body and lipid mass, and food intake (for review see ref. 6). These effects of VMHx, however, appear to be due to ancillary destruction of the descending projections from the hypothalamic paraventricular nucleus (PVH) coursing lateral to the VMH (7) on their way to the nucleus of the solitary tract, dorsal motor nucleus of the vagus (8), and the intermediolateral horn of the spinal cord (9). Thus, laboratory rats and Siberian hamsters with conventional PVH lesions (PVHx; i.e., electrolytic lesions) have significant increases in food intake and body and lipid mass (10,11). PVHx triggers anabolic responses that may be due to involvement of the PVH as part of the general autonomic circuitry projecting to the periphery that participates in energy balance (for review see ref. 4). In fact, using a viral transneuronal retrograde tract tracer, pseudorabies virus (PRV; (12)), we labeled the origins of the SNS outflow from brain to WAT for the first time in any species. Relevant to the present study, PRV injections into inguinal WAT (IWAT) of Siberian hamsters resulted in predominantly unilateral labeling in the half of the PVH ipsilateral to the injected IWAT pad (12,13). Therefore, we tested the role of the PVH in WAT lipid accumulation/mobilization. Specifically, first we tested whether unilateral PVHx would cause preferential ipsilateral adipose mass increases under ad libitum feeding conditions. We also tested whether unilateral PVHx would block lipid mobilization from WAT pads ipsilateral to the lesion using food deprivation as the lipolytic stimulus. Finally, we tested the role of the adrenal medulla in lipid mobilization with or without bilateral PVHx in food-deprived hamsters.
METHODS AND PROCEDURES
Animals and housing
For all experiments, adult (3–4 months of age) male Siberian hamsters (P. sungorus) were obtained from our breeding colony at Georgia State University. Hamsters were single housed in polypropylene cages (23 × 26 × 20 cm3) and maintained under a long day photoperiod (16-h light:8-h dark, lights-on at 0300 hours Eastern Standard Time). Room temperature was kept constant at 20 °C and relative humidity was maintained at 50 ± 5%. Food (PMI #5001; Purina, St Louis, MO) and tap water were available ad libitum throughout the experiment unless noted. Hamsters were divided into groups balanced for mean body mass and the associated standard error of the means across the first 2 weeks of single housing. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with Public Health Service and US Department of Agriculture guidelines.
Food intake and body mass measurements
Food intake and body mass were measured daily to the nearest 0.01 g for experiments 3–4, but only weekly for experiment 2 and always corrected for spillage.
PRV transneuronal tract tracing methodology
In experiment 1, PRV injections were performed as previously described (12). In brief, hamsters (n = 4) were anesthetized with pentobarbital sodium (50 mg/kg) and an incision was made to expose the entire dorsal surface of the IWAT pad for PRV injections (Bartha’s K strain; generous gift from Lynn Enquist, Princeton University; 1 × 108 plaque-forming units/ml; 150 nl/injection; 5 injections/fat pad; total of 7.5 × 104 plaque-forming units). Based on our previous data (12), hamsters were killed 6 days later—a postinoculation survival time that allows the virus to reach the rostral forebrain. The animals were then perfused, and their brains removed for subsequent PRV immunohistochemistry exactly as previously described (14). Thoracic and lumbar spinal cords were scanned for immunohistochemical evidence of bilateral infections, a condition that indicates nonspecific infections suggestive of leakage of the virus from the inoculation sites.
PVHx
For all experiments, electrolytic lesions of the PVH were performed under isoflurane anesthesia using a skull-level coordinate system (stereotaxic coordinates: 0.15 mm anterior to bregma, 0.35 mm lateral to the midsagittal sinus, and 5.55 mm below the dura) as described previously (15). PVHx were made using a tungsten electrode (0.008 internal diameter; A and M Systems, Everett, WA) insulated with Epoxylite except for the cross-sectional tip by passing ~1.75 mA direct current through the electrode to the target site for 15 s. Sham lesions included all steps except that the current was not passed through the electrode. Unilateral lesions of the PVH were alternated between the left and right sides to control for the possibility of left side predominance of the innervation of WAT, as has been reported for the adrenal and ovary (16).
56-h Food deprivation
For experiments 3 and 4, body mass and food intake were measured beginning 2 days after surgeries. Destruction of the PVH causes immediate and progressive increases in food intake and body fat (11). Thus, in order to minimize excessive lipid accumulation and consequent changes in metabolism, food deprivation was initiated at the minimum Institutional Animal Care and Use Committee–approved postlesion interval of 7 days after the surgery. At that time, approximately half the animals receiving sham or PVHx began 56 h of food deprivation (Institutional Animal Care and Use Committee approved) with water available 2 h before lights-on. The remaining animals continued ad libitum feeding. At the end of that time, all hamsters were killed for histological measures and tissue harvesting (see below).
ADMEDx
In experiment 4, half of each group received bilateral ADMEDx with the remainder receiving sham ADMEDx performed exactly as we previously described (17) immediately after half the hamsters received bilateral PVHx or sham bilateral PVHx. It should be noted that ADMEDx does not compromise adrenal cortical function in laboratory rats (18) or Siberian hamsters (T.J. Bartness, M.F. Dallman, and B.D. Goldman, unpublished data). As in experiment 3, 1 week after recovery from these surgeries, half the animals in each group were food deprived for 56 h. At the end of this period, the same terminal measures as in experiment 3 were made and, in addition, complete ADMEDx was verified by analyzing the remaining adrenal for epinephrine/norepinephrine content by high-pressure liquid chromatography (17). Data from ADMEDx hamsters with detectable adrenal epinephrine/norepinephrine were excluded from analyses.
Tissue harvesting
For all experiments, IWAT, epididymal WAT (EWAT), and retroperitoneal WAT (RWAT) pads were harvested and weighed to the nearest 0.001 g.
Lesion verification
After harvesting tissues, the brains were processed for histological analysis of the lesion location exactly as we described previously (14). Briefly, brains were sliced at 40 μm with a cryostat. Sections were mounted on slides and stained with cresyl violet. Location and size of each lesion were verified by light microscopy and localized with a standard mouse brain atlas (19) because no Siberian hamster brain atlas exists. The mouse atlas also was used because although a Syrian atlas exists (20), the size and shape of most Siberian hamster nuclei/fiber tracts resemble mouse brain more than Syrian hamster brain. The resulting lesions were circular in shape with the center originating from the point of the 0.008 in electrode tip (Figure 2). Because these were electrolytic lesions, all tissue including neurons and fibers of passage within the diameter of ~0.45 mm, posterior, anterior, and lateral from the electrode tip, was destroyed. Only data from hamsters with lesions centered within the caudal PVH (largely parvocellular also including anterior parvicellular, medial parvicellular, lateral magnocellular, dorsal cap, ventral and posterior parvicellular) were considered positive lesions (“hits”) and only data from these animals were analyzed (Figure 2).
Figure 2.
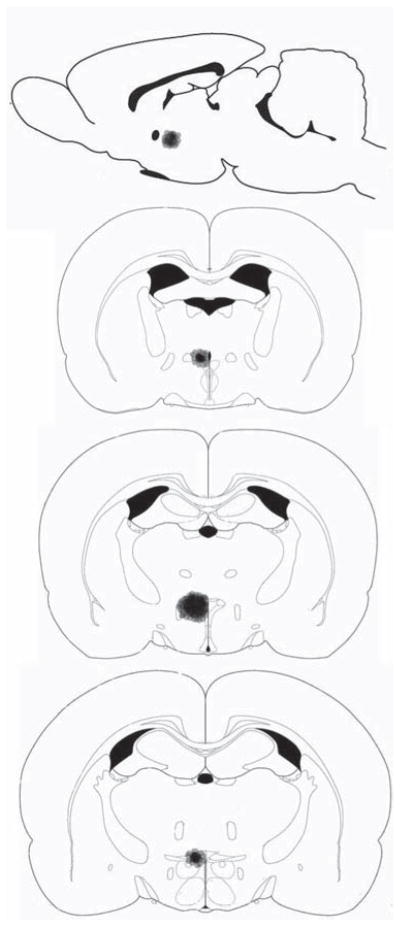
Experiments 2, 3, and 4—Representative reconstruction of typical paraventricular hypothalamus lesions. The lesion is only shown unilateral for comparison reasons. Top: sagittal view of lesion. Bottom three: progressive coronal view of lesion, rostral to caudal.
Statistical analysis
In experiment 2, body mass data were analyzed by ANOVA for repeated measures with a group × week (3 × 11) design (SPSS for Windows, release 11.5.0; SPSS, Chicago, IL). Post hoc between-group comparisons were made with Fisher’s least significant difference pairwise multiple comparison tests. Cumulative food intake, individual fat pad masses, and total fat pad mass were analyzed using one-way between-subjects ANOVA. In experiments 3 and 4, percent cumulative body mass change was analyzed by repeated-measure ANOVA with a group × day (4 × 10) or (2 × 10) design and cumulative food intake measures and fat pad masses (IWAT, EWAT, and RWAT) were analyzed by using one-way between-subjects ANOVA. Differences among groups were considered statistically significant if P < 0.05. Exact probabilities and test values have been omitted for simplification and clarity of the presentation of the results.
RESULTS
Experiment 1—Is the SNS outflow from the PVH to WAT unilateral?
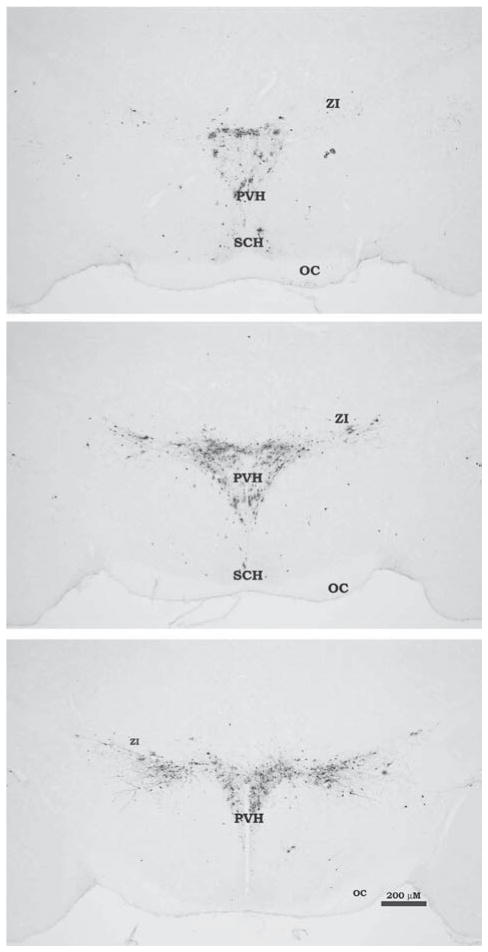
Six days postinjection there was no bilateral infection in spinal cord sections indicating no leakage of the virus from the inoculation site to adjacent or remote tissues. We previously quantified the pattern of PRV-infected neurons across the neural axis in great detail after identical IWAT injections of the virus (13,14); therefore, we present only representative microphotographs of the PVH PRV infection pattern that illustrate the largely unilateral infection of the PVH ipsilateral to the side of the inoculated IWAT pad without quantification of the infected cells (Figure 1).
Figure 1.
Experiment 1—Photomicrograph illustrating pseudorabies virus (PRV) labeling in the paraventricular hypothalamus (PVH) at three different levels, rostral to caudal after injection into inguinal white adipose tissue. Infected cells (black) were detected using standard immunohistochemistry against PRV with diaminobenzidine peroxidase–generated chromogen. Unilateral PRV injections were made into the hamster’s left inguinal white adipose tissue pad corresponding to the greater ipsilateral infection seen in the right half of each brain section for the PVH. OC, optic chiasm; SCH, suprachiasmatic hypothalamus; ZI, zona incerta.
Experiment 2—Do PVHx increase lipid accumulation and if so, is it predominately ipsilateral to the lesion?
Here we tested whether PVHx would promote increased lipid accumulation (decreased lipid mobilization) in WAT pads ipsilateral to the unilateral PVHx due to inhibition of basal lipolysis of the distally denervated WAT depots. This was accomplished by making unilateral, bilateral, or sham PVHx and measuring food intake and body mass weekly for 11 weeks. Terminally, decreases in IWAT, EWAT, and RWAT masses were considered an integrative measure of lipid mobilization.
Lesion verification
Hamsters with either bilateral or unilateral PVHx lesions centered within the caudal PVH were considered positive “hits” if at least 70% of the anterior, medial, and/or posterior PVH were destroyed on one or both sides (depending if the lesion was designed to be unilateral or bilateral, respectively) compared with their appropriate sham lesion controls (Figure 2). In addition, data from animals with lesions of a diameter greater than ~0.50 mm were discarded because of likely destruction of surrounding non-PVH tissue. Final animal numbers after discarding “misses” were: bilateral PVHx (n = 5) and unilateral PVHx (n = 8).
Body mass, food intake, and feed efficiency
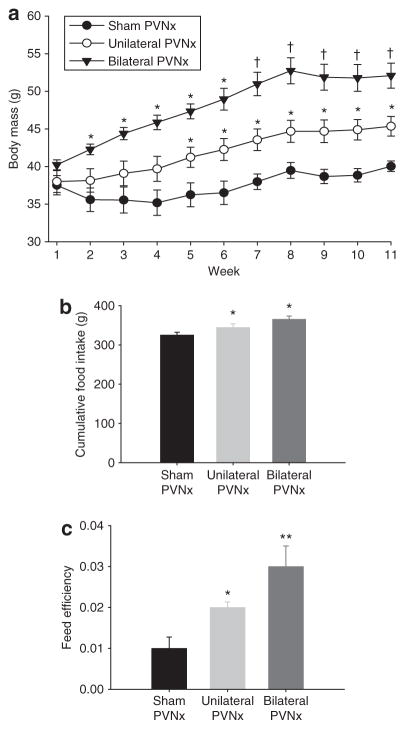
Both bilateral and unilateral PVHx significantly increased body mass compared with sham PVHx controls. Bilateral PVHx hamsters were significantly heavier than sham controls by week 3 (P < 0.05; Figure 3a) and unilateral PVHx animals by week 7 (Ps < 0.05; Figure 3a). Hamsters with unilateral PVHx were significantly heavier than controls by week 5 (P < 0.05; Figure 3a). Cumulative food intake was significantly greater than sham controls by week 2 in the bilateral PVHx group and by week 6 in the unilateral PVHx group (data not shown; P < 0.05) remaining significantly elevated in both groups until the conclusion of the experiment (Ps < 0.05; Figure 3b). Both unilateral and bilateral PVHx hamsters had significantly increased feed efficiency (body mass gained (g)/cumulative food intake (g)) compared with sham controls (Ps < 0.05; Figure 3c), with the feed efficiency of bilateral PVHx hamsters also significantly increased compared with that of the unilateral PVHx hamsters.
Figure 3.
Experiment 2—(a) Absolute body mass (g; mean ± s.e.m.) of hamsters with bilateral paraventricular hypothalamus (PVH) lesions (PVHx) (n = 5), unilateral PVHx (n = 8), sham controls (n = 8). *Ps < 0.05 vs. sham controls; †P s < 0.05 vs. sham controls and unilateral PVHx. (b) Cumulative food intake (g; mean ± s.e.m.). *P s < 0.05 vs. sham controls. (c) Feed efficiency (body mass gained (g)/cumulative food intake (g); mean ± s.e.m.). *P s < 0.05 vs. sham controls; **P s < 0.05 vs. unilateral PVHx and sham controls.
Individual and total WAT masses
Both bilateral and unilateral PVHx significantly increased all WAT pad masses compared with sham PVHx controls (Ps < 0.05; Table 1). In addition, hamsters with bilateral PVHx had EWAT masses that were significantly greater than unilateral PVHx hamsters (P < 0.05; Table 1). Contrary to our prediction, unilateral PVHx did not result in significant ipsilateral/contralateral differences in individual fat pad masses or total dissected fat (IWAT + EWAT + RWAT) relative to the side of the lesion (Table 1). Total dissected fat was, however, significantly greater in bilateral and unilateral PVHx hamsters compared with sham PVHx controls (Ps < 0.05; Table 1). Moreover, total dissected fat pad mass was significantly greater for bilateral PVHx hamsters than unilateral PVHx hamsters (P < 0.05; Table 1).
Table 1.
Mean ± s.e.m. (g) inguinal white adipose tissue (IWAT), epididymal white adipose tissue (EWAT), retroperitoneal white adipose tissue (RWAT), and total WAT pad masses of Siberian hamsters in experiment 2 with sham control or hypothalamic paraventricular nucleus lesions (PVHx) that were bilateral or unilateral
| Lesion type
|
||||||
|---|---|---|---|---|---|---|
| Left
|
Right
|
|||||
| Sham | Ipsi-unilateral | Bilateral | Sham | Contra-unilateral | Bilateral | |
| IWAT | 0.68 ± 0.06a | 0.92 ± 0.08b | 1.22 ± 0.18b | 0.66 ± 0.06a | 0.90 ± 0.09b | 1.22 ± 0.18b |
| EWAT | 0.30 ± 0.01a | 0.44 ± 0.02b | 0.57 ± 0.04c | 0.30 ± 0.02a | 0.47 ± 0.05b,c | 0.58 ± 0.02c |
| RWAT | 0.07 ± 0.01a | 0.12 ± 0.02b | 0.16 ± 0.02b | 0.07 ± 0.01a | 0.12 ± 0.01b | 0.16 ± 0.02b |
| Total pads | 1.05 ± 0.07a | 1.47 ± 0.01b | 1.95 ± 0.32c | 1.04 ± 0.08a | 1.50 ± 0.12b,c | 1.96 ± 0.29c |
For each individual fat pad, nonshared letters (a,b,c) indicate statistically significant differences (P < 0.05).
Experiment 3—Does unilateral PVHx block or diminish food deprivation–induced WAT lipid mobilization ipsilateral to the lesion?
Here we tested whether PVHx would block or diminish food deprivation–induced lipid mobilization from WAT pads ipsilateral to the lesion. This was accomplished by comparing WAT pad masses from ad libitum–fed or 56-h food-deprived hamsters bearing unilateral, bilateral, or sham PVHx.
Lesion verification
Lesion verification yielded the following group sizes: bilateral PVHx ad libitum–fed (n = 5), bilateral PVHx food-deprived (n = 8), unilateral PVHx ad libitum–fed (n = 6), unilateral PVHx food-deprived (n = 6), sham ad libitum–fed (n = 11), and sham food-deprived animals (n = 11).
Body mass and food intake
By 7 days after PVHx, all bilateral PVHx groups had significantly greater body masses compared with the sham controls (Ps < 0.05; Table 2). Food deprivation caused a significant decrease in the percent body mass change for all groups compared with their ad libitum–fed counterparts (Ps < 0.05). At the end of the experiment, the cumulative body mass change of the bilateral PVHx ad libitum–fed group was significantly greater than that of sham and unilateral PVHx ad libitum–fed controls (P < 0.05; Table 2). The food-deprived, bilateral PVHx group lost significantly less body mass compared with their food-deprived sham PVHx counterparts, but their body mass was not different than unilateral PVHx hamsters within the same group (P < 0.05; Table 2). Six-day cumulative food intake was compared among the groups with the intake of the nonfood-deprived groups not considered for the days encompassing the 56-h food deprivation period to equate the total number of days when food was eaten for all animals. In as few as 6 days, bilateral PVHx increased food intake, but was only significant in the group assigned to be food deprived (Table 2).
Table 2.
Mean ± s.e.m. (g) percent body mass change of hamsters with sham, unilateral, or bilateral paraventricular nucleus lesions (PVHx) before or after food deprivation, as well as 6-day cumulative prefood deprivation–induced food intake in experiment 3
|
Ad libitum fed
|
Food deprived
|
|||||
|---|---|---|---|---|---|---|
| Sham | Unilateral | Bilateral | Sham | Unilateral | Bilateral | |
| % Body mass Δ food deprivation | 1.72 ± 0.88a | 3.23 ± 2.23a | 10.86 ± 1.98b | 3.31 ± 0.91a | 4.36 ± 0.86a,b | 7.46 ± 2.10b |
| % Body mass Δ end | 3.87 ± 0.98a | 6.22 ± 3.42a | 17.55 ± 2.53b | −12.66 ± 1.29a,* | −10.44 ± 0.68a,b,* | −7.17 ± 2.18b,* |
| 6-Day food intake | 34.81 ± 1.52 | 34.51 ± 3.33 | 38.04 ± 2.51 | 31.85 ± 0.88a | 35.38 ± 0.86a | 37.53 ± 2.89b |
Nonshared letters (a,b,c) indicate statistically significant differences (P < 0.05). Asterisk denotes statistical significance (P < 0.05) between nonfood and food-deprived representative counterparts.
WAT pad masses
All groups had significant food deprivation–induced decreases in all WAT pad masses compared with their ad libitum–fed counterparts (Ps < 0.05; absolute data not shown). There was no difference in the food deprivation– induced decreases in WAT pad mass among any of the groups; thus, no laterality effect of lipid mobilization was seen (data not shown).
Experiment 4—Are both bilateral PVHx and ADMEDx required to block food deprivation–induced lipid mobilization?
Here we tested whether eliminating adrenal medullary catecholamines via bilateral ADMEDx alone, or in combination with PVHx, would block WAT lipid mobilization. This was accomplished by making either bilateral or sham PVHx with half the animals in each group receiving ADMEDx or sham ADMEDx. As before half of each subgroup was food deprived or ad libitum fed.
Lesion verification
Lesion verification yielded the following eight group sizes: PVHx + sham ADMEDx ad libitum fed (n = 5), PVHx + sham ADMEDx food deprived (n = 5), PVHx + ADMEDx ad libitum fed (n = 5), PVHx + ADMEDx food deprived (n = 6), sham PVHx + ADMEDx ad libitum fed (n = 5), sham PVHx + ADMEDx food deprived (n = 5), sham PVHx + sham ADMEDx ad libitum fed (n = 7), and sham PVHx + sham ADMEDx food deprived (n = 7).
Body and WAT pad masses
At day 7 postsurgery, before food deprivation was instated, all PVHx groups, with or without ADMEDx, had significantly increased body mass compared with respective sham controls (Table 3; Ps < 0.05). ADMEDx without PVHx did not affect the percent body mass change at this time (Table 3). The ad libitum–fed PVHx hamsters continued to increase body mass from this point through the conclusion experiment, remaining significantly heavier than their sham counterparts (Table 3; P < 0.05). PVHx and/or ADMEDx did not significantly alter food deprivation–induced decreased body mass compared with their respective ad libitum controls (Ps < 0.05; Table 3). Lipid mobilization, as assessed by the integrative measure of WAT pad mass, also was not affected by bilateral ADMEDx alone or in combination with PVHx (Table 4).
Table 3.
Mean ± s.e.m. (g) percent body mass change before and after food deprivation of hamsters bearing bilateral paraventricular nucleus lesions (PVNx) or sham lesions that had sham adrenal demedullation (ADMEDx) or ADMEDx in experiment 4
|
Ad libitum fed
|
Food deprived
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Sham PVHx
|
PVHx
|
Sham PVHx
|
PVHx
|
|||||
| Sham
|
Sham
|
Sham
|
Sham
|
|||||
| ADMEDx | ADMEDx | ADMEDx | ADMEDx | ADMEDx | ADMEDx | ADMEDx | ADMEDx | |
| Body mass Δ before food deprivation | 3.45 ± 2.72a | 3.32 ± 1.57a | 11.81 ± 1.85b | 8.59 ± 0.45b | 0.53 ± 2.00a | 3.69 ± 1.28a | 8.34 ± 1.71b | 8.74 ± 1.57b |
| Body mass Δ after food deprivation | 2.71 ± 2.39a | 4.14 ± 1.40a | 14.88 ± 2.54b | 14.59 ± 1.84b | −18.48 ± 1.64a,* | −15.56 ± 3.80a,* | −16.52 ± 1.24a,* | −15.02 ± 2.15a,* |
Non shared letters (a,b,c) indicate statistically significant differences (P < 0.05). Asterisk denotes statistical significance (P < 0.05) between nonfood and food-deprived representative counterparts.
Table 4.
Mean ± s.e.m. percent decrease in white adipose tissue (WAT) pad masses: IWAT, EWAT, and RWAT for ad libitum–fed and food-deprived hamsters bearing sham or paraventricular nucleus lesions (PVHx), with or without adrenal demedullation (ADMEDx) as affected by food deprivation in experiment 4
| Sham PVHx
|
PVHx
|
|||
|---|---|---|---|---|
| Sham
|
Sham
|
|||
| ADMEDx | ADMEDx | ADMEDx | ADMEDx | |
| IWAT | −37.05 ± 4.97 | −41.60 ± 11.75 | −38.96 ± 12.58 | −40.39 ± 8.46 |
| EWAT | −27.27 ± 13.00 | −38.40 ± 10.15 | −33.26 ± 9.42 | −22.88 ± 9.23 |
| RWAT | −49.07 ± 3.66 | −37.21 ± 16.89 | −37.91 ± 12.37 | −39.78 ± 6.12 |
DISCUSSION
Siberian hamsters bearing PVHx had increased body and WAT masses, as seen previously in this species (11,21). More importantly, bilateral PVHx produced greater increases in individual WAT and total WAT masses, as well as in food intake, than did unilateral PVH lesions, an effect not previously reported in any species to our knowledge. As suggested previously for this study and studies were the SNS outflow from the brain to WAT was investigated using PRV (12–14), we confirmed the predominance of PVH-infected cells ipsilateral to the unilateral IWAT PRV inoculation. Because of this predominantly ipsilateral PVH infection pattern, we predicted greater increases in lipid accumulation by WAT pads ipsilateral to the unilateral PVHx than for WAT pads contralateral to the lesion because this “distal” WAT sympathetic denervation should diminish or block basal WAT lipolysis; this was not found. We then reasoned that the predominate unilaterality of PVH sympathetic premotor and higher order outflow neurons to WAT might only be revealed functionally under conditions promoting lipid mobilization, such as with food deprivation, rather than lipid accumulation. Food-deprived PVHx hamsters, however, had similarly reduced WAT pad masses regardless of whether the unilateral PVHx was ipsilateral or contralateral to the fat depots. Because of the possibility that lipolysis stimulated by adrenal medullary catecholamines could be obfuscating the role of the PVH in lipid mobilization, despite the now accepted lesser role of the adrenal medulla in lipid mobilization (for review see refs. 4,5), we combined bilateral PVHx with bilateral ADMEDx to eliminate this sole source of circulating epinephrine. We found, however, no differences in the food deprivation–induced decreases in individual or total WAT masses regardless of whether the adrenal medullas and/or the PVH was intact. The lack of effect of ADMEDx on WAT mobilization reinforces previous findings suggesting the primacy of the SNS drive to WAT initiating lipolysis (for review see refs. 4,5). Collectively, these data suggest that an intact PVH is not necessary for lipid mobilization.
One potential reason that greater unilateral lipid accumulation did not occur ipsilateral to the unilateral PVHx in experiment 2 may have been because the PVHx triggered general changes in energy metabolism such as increases in food intake resulting in global increases in WAT lipid storage, as well as in decreases in energy expenditure that often are associated with PVHx (22). In addition, bilateral PVHx and perhaps even unilateral PVHx could alter several circulating factors that affect lipid deposition also concealing potential functional laterality effects on lipid metabolism in WAT, such as the PVHx-associated increases in corticosterone (23).
It was suggested that unilateral lesions of the VMH (likely the PVH) selectively disrupt the ipsilateral sympathetic out-flow to WAT, thereby demonstrating laterality in the blockade of food deprivation–induced lipolysis (24). Close inspection of these data, however, suggest that although differences in WAT mass are claimed, the values with their associated variability suggest that this was unlikely. We found a similar, albeit nonsignificant, suggestion of a unilateral diminution of food deprivation–induced lipid mobilization in RWAT mass ipsilateral to the unilateral PVHx compared with its contralateral mate, but not for other fat pads.
Bilateral knife cuts that sever PVH descending projections to the brainstem block food deprivation–induced lipid mobilization in laboratory rats (25). This effect contrasts with the inability of unilateral parasagittal PVH knife cuts to do so ipsilateral or contralateral to the cut (26), a lack of effect not due to an insufficient lipolysis-generating stimulus, as the laboratory rats were food deprived for 4 or 5 days (26). Reconciliation of these results could be explained by the demonstrated crossed descending innervation of WAT emanating from each half of the PVH, albeit an innervation that is considerably less than the descending uncrossed innervation revealed by the PRV injections here and previously for this structure (4,5). The ability of bilateral PVH knife cuts to block food deprivation–induced lipid mobilization, but not bilateral conventional PVHx used here, could represent species differences (laboratory rats vs. Siberian hamsters) and/or differences in the neural destruction by each lesion type given that our lesions destroyed both PVH cells and fibers of passage, whereas knife cuts only sever fibers of passage.
Adrenal medullary catecholamines are robust stimulators of lipolysis in vitro (27), but they do not appear to play significant roles in lipid mobilization in vivo (for review see refs. 4,5) as also shown here where ADMEDx did not block food deprivation–induced lipid mobilization.
The hypothalamus has received more than the lion’s share of attention for the regulation of body fat and feeding, perhaps due to the early report of its damage affecting food intake and body mass (28), as well as due to the discovery of leptin receptors in some of its nuclei (e.g., arcuate (29)). We took advantage of the chronic decerebrate model while the present study was underway, where the forebrain is completely disconnected from the brainstem at the meso-diencephalic juncture. Decerebrate rats have complete abolition of the bidirectional communication between the forebrain and hind-brain including all descending forebrain sympathetic outflow circuits passing to and/or through the brainstem in route to WAT. Chronic decerebration, therefore, tests whether the caudal hindbrain is capable of triggering “normal” metabolic responses to food deprivation independent of the forebrain including lipid mobilization. We found chronically decerebrated rats to be fully capable of what appeared to be normal food deprivation–induced lipid mobilization shown by their neurally intact, sham-operated controls (30). It is important to realize, however, that despite the apparent abilities of animals with this truncated neuroaxis, it would be inappropriate to conclude that the hindbrain alone is responsible for the control of lipid mobilization from WAT, but appropriate to say that the PVH is not solely responsible.
Collectively, we found no functional laterality in the PVH component of the sympathetic outflow to WAT and more generally, that an intact PVH is not necessary for food deprivation–induced lipid mobilization. Clearly, the hindbrain is sufficient to trigger lipid mobilization by food deprivation (30) and clearly the SNS is involved in food deprivation–induced WAT lipolysis, as sympathetic denervation at the level of the WAT pad blocks food deprivation–induced lipid mobilization (31). The present data, and those of others, give further testament to the apparent distributed nature of neural components involved in energy balance (32) and suggest that forebrain components may contribute to the fine-tuning of responses capable of being elicited by the hindbrain in absence of forebrain connections.
Acknowledgments
We thank Bonnie Williams, and William Festuccia, Haifei Shi, and Rob Bowers for their assistance with various aspects of these experiments. This research was supported, in part, by the National Institutes of Health grant R37 DK35254 to T.J.B.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Bartness TJ, Demas GE, Song CK. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp Biol Med (Maywood) 2002;227:363–376. doi: 10.1177/153537020222700601. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, Wade GN. Photoperiodic control of seasonal body weight cycles in hamsters. Neurosci Biobehav Rev. 1985;9:599–612. doi: 10.1016/0149-7634(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol. 1998;275:R1399–R1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res. 2007;48:1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.King BM, Frohman LA. The role of vagally-medicated hyperinsulinemia in hypothalamic obesity. Neurosci Biobehav Rev. 1982;6:205–214. doi: 10.1016/0149-7634(82)90056-2. [DOI] [PubMed] [Google Scholar]
- 7.Gold RM. Hypothalamic obesity: the myth of the ventromedial nucleus. Science. 1973;182:488–490. doi: 10.1126/science.182.4111.488. [DOI] [PubMed] [Google Scholar]
- 8.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 9.Swanson LW. Immunohistochemical evidence for a neurophysin-containing autonomic pathway arising in the paraventricular nucleus of the hypothalamus. Brain Res. 1977;128:346–353. doi: 10.1016/0006-8993(77)91000-9. [DOI] [PubMed] [Google Scholar]
- 10.Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981;27:1031–1040. doi: 10.1016/0031-9384(81)90366-8. [DOI] [PubMed] [Google Scholar]
- 11.Bittman EL, Bartness TJ, Goldman BD, DeVries GJ. Suprachiasmatic and paraventricular control of photoperiodism in Siberian hamsters. Am J Physiol. 1991;260:R90–101. doi: 10.1152/ajpregu.1991.260.1.R90. [DOI] [PubMed] [Google Scholar]
- 12.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 13.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–R1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 14.Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express MEL receptors may mediate seasonal adiposity. Am J Physiol Regul Integr Comp Physiol. 2001;281:R666–R672. doi: 10.1152/ajpregu.2001.281.2.R666. [DOI] [PubMed] [Google Scholar]
- 15.Wood AD, Bartness TJ. Partial lipectomy, but not PVN lesions, increases food hoarding by Siberian hamsters. Am J Physiol. 1997;272:R783–R792. doi: 10.1152/ajpregu.1997.272.3.R783. [DOI] [PubMed] [Google Scholar]
- 16.Gerendai I, Tóth IE, Boldogkoi Z, Halász B. Recent findings on the organization of central nervous system structures involved in the innervation of endocrine glands and other organs; observations obtained by the transneuronal viral double-labeling technique. Endocrine. 2009;36:179–188. doi: 10.1007/s12020-009-9189-8. [DOI] [PubMed] [Google Scholar]
- 17.Demas GE, Bartness TJ. Direct innervation of white fat and adrenal medullary catecholamines mediate photoperiodic changes in body fat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1499–R1505. doi: 10.1152/ajpregu.2001.281.5.R1499. [DOI] [PubMed] [Google Scholar]
- 18.Aguilera G, Kiss A. Activation of magnocellular vasopressin responses to non-osmotic stress after chronic adrenal demedullation in rats. J Neuroendocrinol. 1993;5:501–507. doi: 10.1111/j.1365-2826.1993.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2007. [Google Scholar]
- 20.Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. Academic Press; New York: 2001. [Google Scholar]
- 21.Maharaj MP, Youngstrom TG, Bartness TJ. Rapid gonadal recrudescence and body and lipid mass increases with hypothalamic lesions in photoregressed Siberian hamsters. Neuroendocrinology. 1992;55:552–562. doi: 10.1159/000126169. [DOI] [PubMed] [Google Scholar]
- 22.Vander Tuig JG, Kerner J, Romsos DR. Hypothalamic obesity, brown adipose tissue, and sympathoadrenal activity in rats. Am J Physiol. 1985;248:E607–E617. doi: 10.1152/ajpendo.1985.248.5.E607. [DOI] [PubMed] [Google Scholar]
- 23.Tokunaga K, Fukushima M, Kemnitz JW, Bray GA. Comparison of ventromedial and paraventricular lesions in rats that become obese. Am J Physiol. 1986;251:R1221–R1227. doi: 10.1152/ajpregu.1986.251.6.R1221. [DOI] [PubMed] [Google Scholar]
- 24.Bray GA, Nishizawa Y. Ventromedial hypothalamus modulates fat mobilisation during fasting. Nature. 1978;274:900–902. doi: 10.1038/274900a0. [DOI] [PubMed] [Google Scholar]
- 25.Bray GA, Sclafani A, Novin D. Obesity-inducing hypothalamic knife cuts: effects on lipolysis and blood insulin levels. Am J Physiol. 1982;243:R445–R449. doi: 10.1152/ajpregu.1982.243.3.R445. [DOI] [PubMed] [Google Scholar]
- 26.Jones AP, Assimon SA, Gold RM, Sylvan A. Adipose tissue mobilization is unaffected by obesifying hypothalamic knife cuts. Physiol Behav. 1985;34:29–31. doi: 10.1016/0031-9384(85)90072-1. [DOI] [PubMed] [Google Scholar]
- 27.White JE, Engel FL. A lipolytic action of epinephrine and norepinephrine on rat adipose tissue in vitro. Proc Soc Exp Biol Med. 1958;99:375–378. doi: 10.3181/00379727-99-24355. [DOI] [PubMed] [Google Scholar]
- 28.Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat Rec. 1940;78:149–172. [Google Scholar]
- 29.Håkansson ML, Hulting AL, Meister B. Expression of leptin receptor mRNA in the hypothalamic arcuate nucleus—relationship with NPY neurones. Neuroreport. 1996;7:3087–3092. doi: 10.1097/00001756-199611250-00059. [DOI] [PubMed] [Google Scholar]
- 30.Harris RB, Kelso EW, Flatt WP, Bartness TJ, Grill HJ. Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology. 2006;147:1365–1376. doi: 10.1210/en.2005-1156. [DOI] [PubMed] [Google Scholar]
- 31.Cantu RC, Goodman HM. Effects of denervation and fasting on white adipose tissue. Am J Physiol. 1967;212:207–212. doi: 10.1152/ajplegacy.1967.212.1.207. [DOI] [PubMed] [Google Scholar]
- 32.Grill HJ, Kaplan J, Kaplan JM. Caudal brainstem participates in the distributed neural control of feeding. In: Stricker EM, editor. Neurobiology of Food and Fluid Intake. Plenum Press; New York: 1990. pp. 125–149. [Google Scholar]