Abstract
Toll Like Receptor (TLR) activation on dendritic cells (DCs) induces DC maturation and secretion of pro-inflammatory cytokines, both of which are important for activation and differentiation of CD4 T cells. The importance of TLR activation on DCs for CD8 T cell responses is less clear. Here, we tested the ability of different TLRs to regulate CD8 T cell responses to pathogens. We found that although all TLRs are able to induce CD8 T cell activation in vitro, there are profound differences in their ability to activate CD8 T cells in vivo. The nucleic acid recognizing endosomal TLRs, TLR3 and TLR9, had a potent ability to induce CD8 T cell activation. However, the surface TLRs, TLR2 and TLR4, that recognize bacterial ligands, were not only incapable of inducing CD8 T cell priming, but had a dominant effect of inhibiting CD8 T cell expansion induced by activation of endosomal TLRs. We found that TLR2 and TLR4, acting in a MyD88-dependent manner, influenced CD8 T cell priming by altering the composition of DCs in the draining lymph nodes. Our results have important implications for combined bacterial and viral infections and suggest that bacterial infections could constrain the ability of the host to mount effective anti-viral CD8 T cell immunity.
Introduction
The presentation of pathogen derived peptides by MHC Class II or Class I molecules, either by classical or cross-presentation pathways, all achieve the activation of the adaptive immune response. The primary sensing of pathogens by DCs is however accomplished by pattern recognition receptors (PRRs) (1) that not only induce activation of DCs, but also regulate the trafficking of cargo to maximize peptide generation (2). Many different PRRs, such as Toll like receptors (TLRs) (3), retinoic acid-inducible gene I (RIG-I) like receptors (4) nucleotide-binding oligomerization domain-containing protein (NOD)-like receptors (NLRs) (5) and C-type lectin receptors (6), have all been implicated in inducing DC maturation and regulating adaptive immunity. TLRs recognize conserved microbial products from a diverse class of pathogens and initiate signaling to induce inflammatory responses. The outcome of signaling is determined by specificity of the adapter usage by each TLR (7). TLRs use MyD88 and TRIF to activate NF-κB, MAP kinases and IRFs (8) and use BCAP to activate PI3 Kinase (9, 10). The capacity of TLRs to activate adaptive immune responses is also determined by the nature of signaling events induced in DCs by different TLRs. For example, the TRIF-dependent pathway of TLR4 signaling is sufficient to induce DC maturation but is not sufficient to induce pro-inflammatory cytokine production and thus fails to activate measurable CD4 T cell responses (11, 12). All known TLRs except TLR3 signal through myeloid differentiation primary response gene 88 (MyD88) and induce up-regulation of both MHC Class I and MHC Class II molecules. While CD4 T cell activation is a direct outcome of antigen presentation by mature DCs and the ability of TLR-activated DCs to polarize and prime naive CD4 T cells has been well documented (3, 12–14), the role of different TLRs in the regulation of CD8 T cell responses is less well characterized. TLR9 and TLR3 are found in the endosomal compartment of DCs and can thus encounter intracellular pathogens such as viruses (8). As a result, viral nucleic acids activate these TLRs allowing for the generation of CD8 T cell responses. TLR9 and TLR3 ligands are also known to induce CD8 T cell responses to soluble protein antigens by enhancing APC cross-presentation (15–19). Immunostimulatory CpG DNA motifs can be found in viral and bacterial genomes and synthetic CpG DNA has been widely used as an adjuvant to enhance CD8 T cell responses in different experimental models (18, 19). Recent reports have shown that TLR7 ligands increase CD8 T cell responses by enabling the cross-priming ability of different DC subsets via Type I interferon production (20).
The role of plasma membrane TLRs, TLR2 and TLR4, in inducing CD8 T cell responses is not entirely clear. It has been proposed that LPS enhances cross-presentation due to the ability of TLR4 to recruit TAP to the ER (21). Other studies have also implicated both TLR2 and TLR4 in promoting CD8 T cell priming (22, 23). In spite of this, it has been reported that pre-treatment of animals with TLR ligands reduces the ability of mice to mount CD8 T cell responses in vivo (24). For example, a recent study has suggested that peptidoglycan contamination of LPS activated NOD receptors, which suppressed cross-presentation of antigens in vitro (25). Another study has implicated LPS in induction of IL-10 producing CD4 T cells that can suppress CD8 T cell responses in vivo (26). The precise mechanisms and general rules of how different TLRs regulate cross-presentation and CD8 T cell priming remain unresolved.
In this study, we examined how surface and endosomal TLRs differentially regulate CD8 T cell responses. While all TLR ligands induced and enhanced cross-presentation and priming of CD8 T cells in vitro, our results clearly show that only activation of endosomal TLRs enhanced CD8 T cell responses in vivo. In contrast, activation of surface TLRs led to the suppression of CD8 responses induced by endosomal TLR agonists and intracellular pathogens. This study brings to the fore the differential role of surface and endosomal TLRs in antigen cross-presentation by DCs, and provides critical insights into the importance of engaging the correct TLR to induce productive CD8 T cell responses in vivo.
Materials and Methods
Mice
All B6 mice used in experiments were 6–12 weeks of age and purchased from the UT southwestern Medical Center (UTSW) Mouse Breeding Core. OT-I RAG−/− mice were purchased from Taconic. GFP B6 mice were purchased from Jackson Laboratories and bred with OT-I RAG−/− mice to generate GFP OT-I RAG−/− mice. Unless otherwise indicated, all transfer experiments were done using CD8 T cells from OT-I-GFP Tg mice. TRIF KO, MyD88 KO, IRF3 KO, and CD11c-MyD88 Tg mice (on a MyD88 deficient background) were bred and maintained at the UTSW animal facility. All mouse experiments were done as per protocols approved by the University of Texas Southwestern (UTSW) Medical Center’s Institutional Animal Care and Use Committee (IACUC)
Antibodies
Antibodies were obtained from the following sources: BD Biosciences: CD62L-APC, CD25-PECy7, CD4-PECy5, CD25-bio, Ly6G-FITC, B220-PerCP. Biolegend: CD16/32 purified, CD8α-APC/Cy7, CD11c-PECy7, CD11c-APC, CD45.2-Pacific Blue, B220-bio, NK1.1-bio, CD11b-bio, CD11c-bio, CD4-bio, CD16/32-bio, Ly6C-bio, SA-PE. E Bioscience: CD4-AF750, CD11b-AF 700, MHC II-Pacific Blue, Ly6C-APC, F4/80-AF750. Covance: CD4 (GK1.5). Life Technologies: Q dot 605 streptavidin conjugate. Baylor Tetramer Facility: Kb SIINFEKL tetramer-PE. Bio X-cell: Anti-Ly6G (clone 1A8) antibody.
Emulsion Preparations
All emulsions were prepared with 50% of the volume being incomplete freund’s adjuvant (Sigma) and the remaining 50% of volume being reagents to be tested diluted in 1X PBS. Ovalbumin (Sigma): 10ug/footpad, CpG (W.M. Keck Oligonucleotide Synthesis Facility, Yale University): 10 μg/footpad, LPS from E. coli 055:B5 (Sigma): 5 μg/footpad, BLP (Pam3CSK4; Invivogen): 25 μg/footpad, Poly IC (Fisher): 20 μg/footpad and OVA-Alexa Fluor 647 conjugate (Life Technologies): 20ug/footpad.
Virus and Bacteria Preparations
Listeria monocytogenes 10403 serotype 1, expressing full-length ovalbumin protein (LM-OVA), was provided by Dr. Hao Shen (University of Pennsylvania School of Medicine, Philadelphia, PA. and used at 2 × 104 CFU/footpad. Salmonella typhimurium SL1344 ΔSpi-1 mutant was provided by Dr. Denise Monack (Stanford University) and used at 500 CFU/footpad. Heat-killed Salmonella typhimurium SL1344 ΔSpi-1 mutant was used at 2.5–5×107 particles/footpad. Vesicular stomatitis virus (VSV)-expressing full-length ovalbumin protein (VSV-OVA) was provided by Dr. Leo Lefrancois (University of Connecticut Health Center, Farmington, CT) and used at 106 PFU/footpad.
In vivo priming using OVA or pathogens +/− TLR ligands
Cells were isolated and pooled from the spleen and multiple LNs of GFP OT-I Rag−/− mice and CD8 T cells were purified using FACS sorting. A cell suspension was prepared and diluted to a concentration of 104 cells/mL. Mice received 100uL (~1000 cells) via lateral tail vein injection. The next day, antigen emulsions were prepared and injected subcutaneously in the foot pads. Inguinal and popliteal LNs were harvested after 7 days to test for a primary response. If pathogens were used, spleen cells were also collected.
In vivo cell depletion of Tregs, CD4 T cells and Ly6G+ cells
In vivo depletion of Treg cells was achieved by injecting 100 μg of purified anti-CD25 monoclonal antibody in the lateral tail veins of mice 3 days prior to immunization. Total CD4 T cell depletion resulted from 3 intraperitoneal (i.p.). injections of 200 μg purified anti-GK1.5 monoclonal antibody on days −4, −1, and day 3 of immunization. To deplete Ly6G+ cells, one injection of 500μg 1A8 monoclonal antibody was given i.p. one day prior to immunization, and another injection given i.p. on day 4 post immunization. Treg and CD4 T cell depletion was confirmed by staining PBMCs for CD4 and CD25. Ly6G+ cell depletion was confirmed by sacrificing a cohort of mice and staining lymph node cells for Ly6G and Ly6C populations.
Flow cytometry
All samples were run on a LSR II (BD Biosciences). All data were analyzed using FlowJo software (TreeStar).
Preparation of dendritic cells
After 18–24 hours post-immunization, draining LNs were harvested, pooled together, and processed. When OVA-647 was used as the antigen, OVA-647+ cells were sorted and collected using a MoFlo sorter (Cytomation). In some experiments, splenic DCs from Flt3-treated B6 mice were used as antigen presenting cells (27).
In vitro activation of OT-I CD8 T cells
LNs and spleens were collected from 1–3 OT-I RAG−/− mice and processed together and sorted to obtain pure, naive CD8 T cells (>99% purity). The sorted OT-I cells were CFSE labeled and cultured with CD11c+ splenic DCs at a ratio of 10:1. Ovalbumin was added to the culture at a concentration of 20 μg/mL +/− Pam3CSK4 (200 ng/mL), or CpG (1μM), or LPS (100 ng/mL), or Poly IC (1 μg/mL). Cells were harvested 3 days later, stained, and samples run on the LSR II. Experiments using CFSE-labeled OT-I cells and ex vivo sorted APCs were co-cultured at a (5:1) ratio and after 3 days cells were analyzed by FACS. In some experiments, OT-I cells were co-cultured with ex vivo sorted APCs in a 96-well U-bottom dish. The OT-I cells were held constant at 5×104 per well and the APCs were serially diluted. After 3 days the culture was pulsed with 3H-thymidine for 12–16 hours and then harvested and subjected to scintillation counting using a Micro Beta Trilux counter (Perkin Elmer).
Quantification of LM bacterial burden
After day 3 and day 7 of infection, draining lymph nodes and/or spleen, and livers were harvested. Samples were homogenized in equal volume of water and 50 uL of sample was spread onto BHI + streptomycin plates. Plates were incubated at 37°C and colonies were counted next day. Total CFU was calculated by multiplying the number of colonies per plate with the dilution factor.
Statistical Analysis
Data are presented as mean ± SE. Statistical analyses were performed using Prism (GraphPad Software) and p values were obtained by using two-tailed unpaired Student t-tests.
Results
Differential regulation of CD8 T cell responses by Toll like receptors
In the present study we examined the role of surface versus cytosolic TLRs in regulation of CD8 T cell responses both in vitro and in vivo. We used ovalbumin (OVA) as a model antigen and measured expansion of OT-I CD8 T cells, transgenic CD8 T cells that express a TCR specific to the OVA derived peptide (SIINFEKL), as a read-out for cross-presentation. To understand the ability of different TLRs to induced cross-priming, we purified splenic DCs from B6 mice and co-cultured them with OT-I cells in the presence of titrating doses of OVA, with or without different TLR ligands. Soluble OVA by itself induced moderate CD8 T cell proliferation as reported before (28–30). However, LPS, BLP (Pam3CSK4), CpG and poly I:C all enhanced the ability of splenic DCs to induce expansion of OT-I CD8 T cells (Supplemental Fig. 1A). These data suggested that there is no inherent difference in the ability between different TLRs to activate CD8 T cells. It is understandable for an endosomal TLR such as TLR9 that recognizes viral-derived DNA to induce CD8 T cell priming, however, the benefit of priming CD8 T cells following extracellular bacterial (LPS) recognition by TLR4 is not apparent. We were therefore very interested in pursuing this question further to understand if both surface and endosomal TLRs have an equivalent ability to induce CD8 T cell priming in vivo.
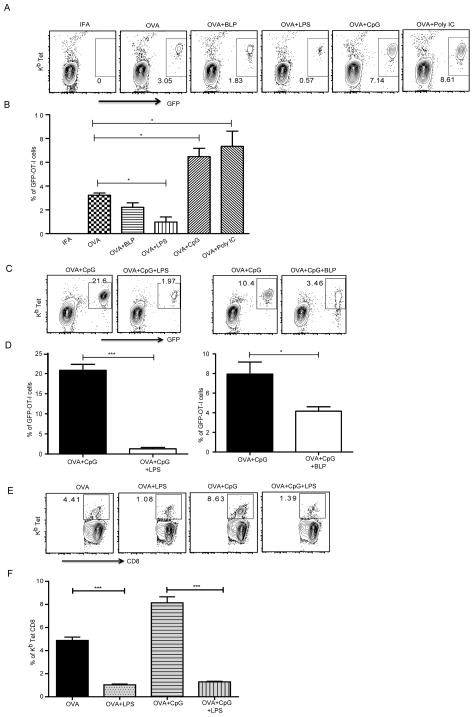
B6 mice received GFP-OT-I cells and 24 hours later were immunized subcutaneously (sc) with OVA in the presence or absence of TLR ligands, emulsified in incomplete Freund’s adjuvant (IFA). Immunization with OVA alone induced measurable expansion of OT-I cells in the draining lymph nodes (LNs). Immunization with OVA together with endosomal TLR ligands poly I:C (TLR3 ligand) or CpG (TLR9 ligand) induced significantly enhanced CD8 T cell responses (Fig. 1A, 1B). This is important since both these TLRs recognize viral ligands and there is need for the immune system to activate CD8 T cells in such a scenario. However, in complete contrast to the in vitro data, immunization of mice with OVA together with surface TLR ligands, BLP (Pam3CSK4, TLR1/2 ligand) or LPS (TLR4 ligand) led to suppression of OT-I CD8 T cell responses. This was in contrast to the ability of LPS to enhance CD4 T cell activation in vivo (Supplemental Fig. 1B, 1C). Since CpG enhanced CD8 T cell responses significantly (Fig. 1A, 1B), we tested if LPS and BLP would suppress CpG induced CD8 T cell priming. The suppression of CD8 T cell expansion was evident even when BLP or LPS were combined with OVA + cytosolic TLR ligands (Fig. 1C, 1D) suggesting that suppression of CD8 responses by surface TLRs is dominant over the ability of endosomal TLRs to prime CD8 T cells. This suppression was also evident when we measured endogenous CD8 T cell responses, without any OT-I T cell transfer, using the Kb-SIINFEKL tetramer (Fig. 1E, 1F). It is possible that activation of TLR2 or TLR4 induces early activation of CD8 T cells that are then exhausted at later time points. To test this possibility we conducted a temporal investigation of CD8 T cell expansion at days 5, 7 and 9 following immunization and found that LPS inhibited CD8 T cell responses during all time points (Supplemental Fig. 1D). CD8 T cell expansion was not measurable at time points earlier than day 5 in any of the groups. These data suggest that unlike in vitro priming, in vivo activation of CD8 T cells is a complex process and surface and endosomal TLRs have diametrically opposite effects on the outcome of CD8 T cell priming. These results prompted us to further dissect the role of different TLR signaling components in regulating priming and expansion of CD8 T cells in vivo.
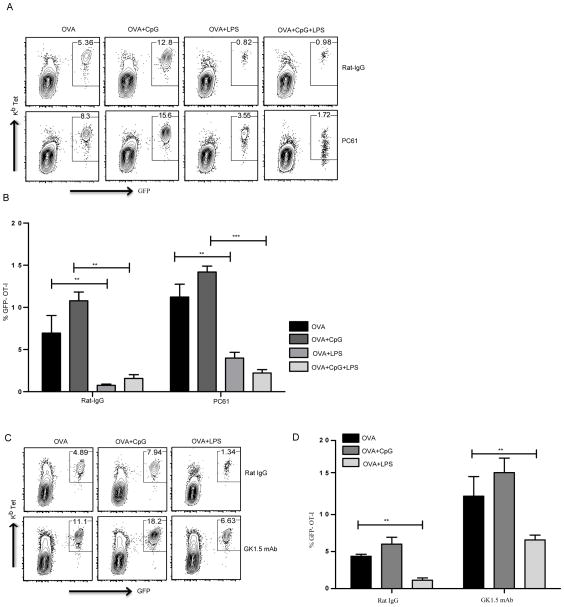
Figure 1. Differential regulation of in vivo CD8 T cell responses by endosomal and plasma membrane TLRs.
Mice that received GFP-OT-I T cells were immunized as indicated and cells from the draining lymph node were stained using Kb-SIINFEKL tetramer. (A) CD8+ T cells that express GFP and stain for the Class I tetramer are shown. (B) Mean ± SEM of quantification of GFP+ OT-I T cells as a percentage of total CD8 T cells from 3 independent mice immunized with OVA and different TLR ligands. (C) Representative plots of OT-I T cell expansion in draining lymph nodes, 7 days after immunization. (D) Mean ± SEM from 3 independent mice. (E) Mice without any OT-I T cell transfer were immunized as indicated and cells from draining lymph nodes were stained on day 7 for CD8 and Kb tetramer to reveal SIINFEKL-specific CD8 T cell expansion. (F) Mean ± SEM of tetramer positive, CD8 T cells from 5 independent mice. The data of all above experiments are representative of at least three independent experiments with 3 mice per group. *, P < 0.05; ***, P < 0.005.
LPS mediated CD8 T cell suppression is dependent on TLR4 and is induced by the MyD88 dependent signaling pathway in DCs
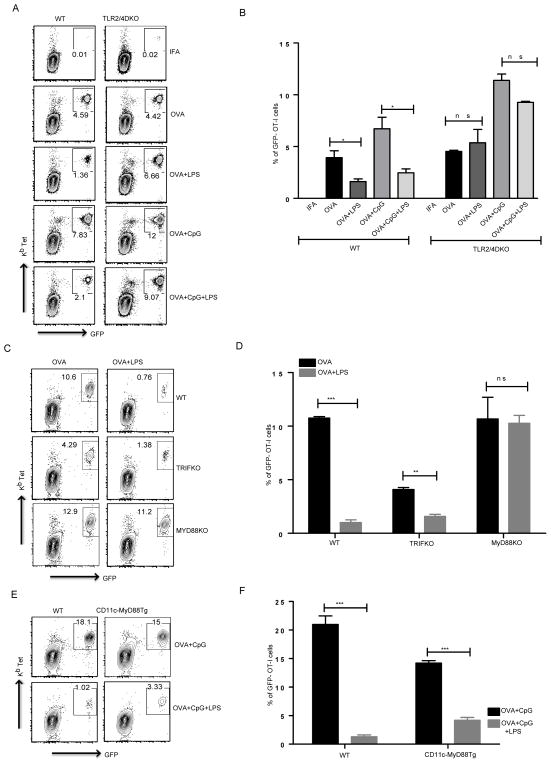
LPS is a cell wall component of gram-negative bacteria and it is possible that even highly purified commercial grade LPS preparations can contain other contaminants such as NOD ligands. It has been recently reported that suppression of CD8 T cell activation in vitro is mediated by a contaminant present in LPS, which signals through a cytosolic NOD like receptor (25). It is not clear whether LPS acts on DCs or CD8 T cells directly to suppress their expansion. We designed our next set of experiments to address both of these issues. We transferred WT OT-I CD8 T cells into TLR2/4 double KO mice and immunized them as described before, either using OVA or OVA mixed with LPS in IFA. The LPS mediated CD8 T cell suppression was completely abrogated in TLR2/4 double knockout mice (Fig. 2A, 2B) suggesting that LPS acts directly via TLR4 and that the in vivo suppression of CD8 T cell responses is not due to contaminating NOD ligands. Furthermore, the data also establish that LPS does not act directly on CD8 T cells and that TLR4 expression in non T cell compartments, potentially in DCs, could play a major role in LPS mediated suppression of CD8 T cell responses.
Figure 2. LPS mediated suppression of CD8 T cell responses depends on the TLR4-MyD88 signaling axis in CD11c+ cells.
(A) WT and TLR2/4 DKO mice received WT OT-I T cells and were immunized as indicated. Representative plots show CD8 T cells positive for GFP and Kb tetramer. (B) Mean ± SEM of GFP-OT-I T cells as a percentage of total CD8 T cells from 3 independent mice per group. (C) WT OT-I T cells were transferred into WT, TRIF KO and MyD88 KO mice and immunized with OVA or OVA mixed with LPS. Representative plots show CD8 T cells positive for GFP and Kb tetramer. (D) Mean ± SEM of GFP-OT-I T cells as a percentage of total CD8 T cells from 3 independent mice per group. (E) After OT-I CD8 T cell transfer, WT and CD11c MyD88 Tg mice were immunized as indicated and cells were stained for CD8 and Kb tetramer and representative plots show percentage of CD8 T cells that expressed GFP and stained positive for the tetramer. (F) Mean ± SEM of GFP-OT-I T cells as a percentage of total CD8 T cells from 3 independent mice per group. All the data above are representative of two independent experiments with 3 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
TLRs use several signaling adapters and specificity of signaling is determined by differential usage of adapter proteins. For example, TLR9 uses MyD88 as its only signaling adapter and all signaling downstream of TLR9 is abrogated in the absence of MyD88. Not surprisingly, the TLR9 ligand CpG induced enhancement of cross-priming of CD8 T cells is dependent on MyD88 (data not shown). TLR4 signaling is more complex as it can signal in response to LPS in the absence of MyD88. The MyD88 dependent signaling pathway of TLR4 activates NF-κB and MAP kinases while the MyD88 independent pathway uses TRIF as an adapter protein and in addition to NF-κB and MAP kinases, activates IRF3 (31). We were interested in determining the role of MyD88 and TRIF signaling pathways downstream of TLR4 in influencing CD8 T cell priming in vivo. To do so, we immunized wild type, MyD88 KO and TRIF KO mice with OVA in the presence or absence of LPS. Consistent with previous experiments, OVA+LPS immunized wild type mice had a reduced CD8 T cell response when compared to OVA alone, while OVA+LPS immunized MyD88 KO mice showed no impairment/reduction in CD8 T cell responses (Fig. 2C, 2D). TRIF KO mice however exhibited a reduced CD8 T cell response to OVA alone compared to wild type mice (Fig. 2C, 2D) and this might be a result of compromised basal type I interferon production and signaling in these mice as type I IFNs are known to play a critical role in priming of CD8 T cells (32, 33). This observation is further supported by experiments in IRF3 KO mice where CD8 T cell priming by OVA alone was reduced compared to WT mice (Supplemental Fig. 2A). The reduced CD8 T cell responses to OVA in TRIF KO mice are still however suppressed by LPS (Fig. 2C) suggesting that LPS mediated CD8 T cell suppression is induced by signaling pathways downstream of MyD88.
The experiments using TLR2/4 DKO suggest that a non-T cell compartment was responsible for TLR4 induced suppression of CD8 responses. Since many myeloid and stromal cells express TLR4, we wanted to narrow down the cell type responsible for suppression of CD8 T cell priming. We have previously generated a chimeric mouse that expresses MyD88 under a CD11c promoter (CD11c-MyD88 Tg) and only CD11c expressing cells (DCs and macrophages) get activated in response to TLR ligands (34). Since the data above suggest that MyD88 signaling downstream of TLRs is responsible for impairment of CD8 T cell responses, we asked if MyD88 in DCs is directly responsible for this effect. We immunized WT and CD11c-MyD88 Tg mice with OVA+CpG or OVA+CpG+LPS and measured OT-I expansion as described before. CpG induced expansion of OT-I T cells in both B6 and CD11c-MyD88 Tg mice while LPS compromised the ability of CpG to induce expansion of CD8 T cells (Fig. 2E). In the CD11c-MyD88 Tg mice, OVA induced expansion of CD8 T cells was also inhibited when OVA was co-injected along with LPS (Supplemental Fig 2B). These data suggest that MyD88, downstream of TLR4 in CD11c expressing cells is responsible for inhibition of CD8 T cell activation and expansion in vivo.
TLR2 and TLR4 activation suppresses pathogen CD8 T cell responses in vivo
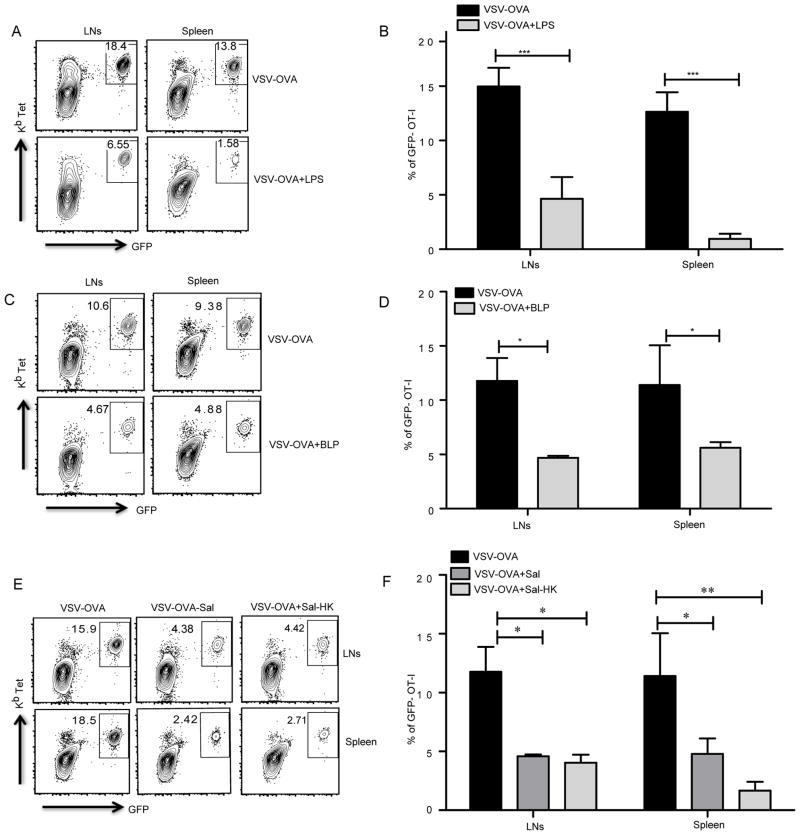
Viral and bacterial co-infections are common occurrences and can lead to severe morbidity and mortality due to dysregulation of various immune responses. Several reports have shown that polymicrobial sepsis leads to impairment in APC functions and subsequent T cell responses. To examine whether TLR2 and TLR4 activation regulate the outcome of CD8 T cell responses to viral infection, we used vesicular stomatitis virus expressing OVA (VSV-OVA) to induce CD8 T cell priming in vivo and investigated the ability of LPS and BLP to influence these responses. Mice were infected with live VSV-OVA in the presence or absence of LPS or BLP. VSV-OVA alone elicited strong CD8 T cell responses, both in the draining lymph nodes and the spleen (Fig. 3A). Consistent with our results from immunization using OVA, mice infected with VSV-OVA together with LPS or BLP, respectively, showed a marked reduction in CD8 T cell responses (Fig. 3A–D, Supplemental Fig. 3A). We observed that co-infections of mice with VSV-OVA and live Salmonella typhimurium also lead to similar suppression of CD8 T cell responses (Fig. 3E, 3F). Heat-killed Salmonella typhimurium when co-injected with VSV-OVA behaved similar to LPS and led to significant dampening of CD8 T cell responses induced by the virus. These results support the notion that TLRs play a critical role in regulation of CD8 T cell responses in the event of viral infections in that activation of TLR2 or TLR4 by bacterial ligands can suppress anti-viral CD8 immunity.
Figure 3. Anti-viral CD8 responses are compromised by activation of plasma membrane TLRs.
(A) Mice that received OT-I CD8 T cells were infected using VSV-OVA or VSV-OVA mixed with different TLR ligands or live/heat killed Salmonella typhimurium (Sal/Sal-HK) and draining lymph nodes and the spleen were harvested on day 7 after infection to measure OT-I CD8 T cell expansion. (A, C, E). Representative plots show CD8 T cells that express GFP and stain positive for Kb-SIINFEKL tetramer. (B, D, F). Mean ± SEM of GFP-OT-I T cells as a percentage of total CD8 T cells from 5 independent mice per group. The data are representative of three (A, B, C and D) or two independent experiments (E and F). *, P < 0.05; **, P < 0.01; ***, P < 0.005.
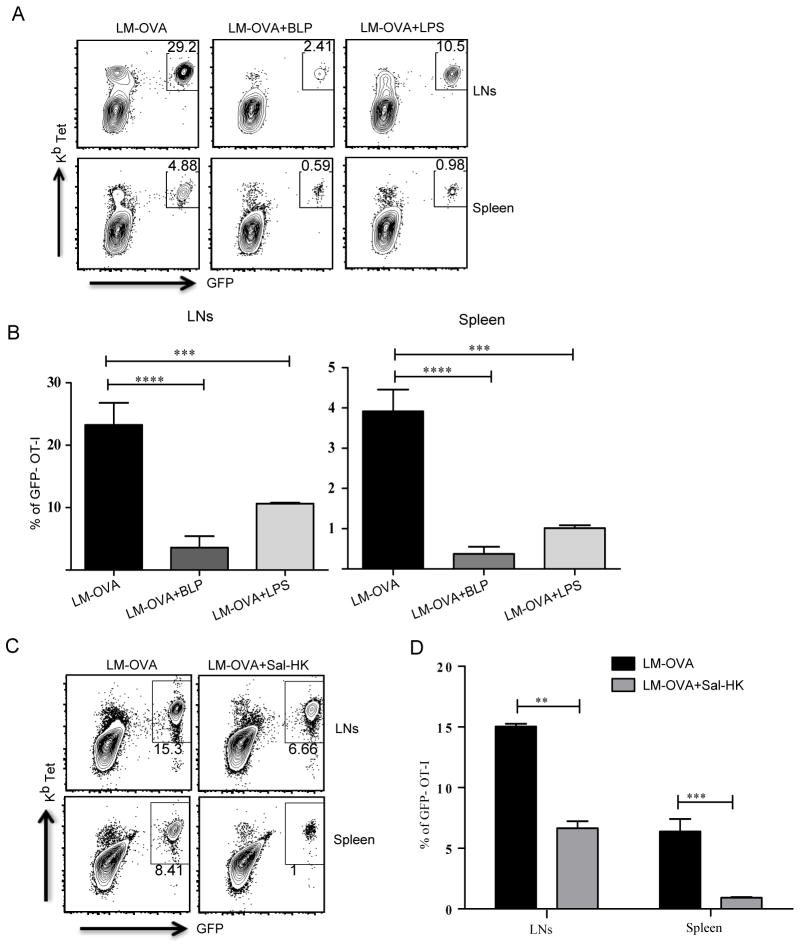
Several cytosolic and vacuolar bacterial pathogens also induce robust CD8 T cell responses and we were interested in understanding if TLR2 and TLR4 activation would dampen CD8 T cell responses induced by intracellular bacteria. We used Listeria monocytogenes expressing OVA (LM-OVA) and tested the ability of TLR2 and TLR4 ligands to suppress CD8 T cell responses. As expected, LM-OVA induced robust CD8 T cell responses both in the spleen and the lymph nodes (Fig. 4A). However, both LPS and BLP significantly dampened CD8 T cell expansion induced by LM-OVA (Fig. 4A, 4B, Supplemental Fig. 3B). Mice were also co-infected with LM-OVA and heat-killed Salmonella and consistent with the VSV-OVA experiments, heat-killed Salmonella, compromised the CD8 T cell response induced by LM-OVA (Fig. 4C, 4D). Although the VSV and Listeria experiments phenocopy the results of soluble protein immunizations, it is possible that type I IFNs (IFN-β and IFN-α4) induced by LPS alter the ability of these pathogens to replicate, lowering the pathogen load and thus affecting the magnitude of CD8 T cell responses. However, it is important to note here that BLP (Pam3CSK4), which does not induce type I IFN production (35) also reduces the magnitude of CD8 T cell responses induced by VSV. Additionally the TLR9 ligand CpG, which induced type I IFNs (36) does not inhibit CD8 T cell responses induced by VSV-OVA (Supplemental Fig 3C). To still consider this possibility that LPS induced type I IFNs could be affecting bacterial replication we measured Listeria load in the lymph nodes at day 3 following injection and saw no difference in the bacterial burden irrespective of whether the mice received LPS (Supplemental Fig. 3D). We could not detect bacteria in the draining lymph nodes at time points earlier than day 3. Furthermore, when we looked at bacterial burden at day 7 following injection, we saw that, consistent with lower CD8 T cell responses, the mice that received LPS had higher bacterial loads in the liver, lymph nodes and the spleen (Supplemental Fig. 3E). The LM-OVA results are very intriguing since Listeria has natural ligands to activate TLR2. It has been observed in an earlier study that LM infection of TLR2 KO mice led to a greater CD8 T cell response when compared to WT mice (37). It is possible that Listeria evades detection by TLR2 thus allowing the immune system to mount robust CD8 T cell responses. Our data clearly establish the dominant ability of surface TLRs to inhibit the CD8 T cell response induced by both a live virus and a cytosolic bacterium.
Figure 4. TLR2 and TLR4 activation inhibit CD8 T cell responses against Listeria monocytogenes.
After OT-I T cell transfer, mice were infected with LM-OVA or LM-OVA mixed with LPS, BLP or heat-killed Salmonella typhimurium (Sal-HK). (A and C) Representative plots show CD8 T cells from draining lymph nodes and spleen that express GFP and stain positive for Kb tetramer. (B and D) Mean ± SEM of GFP-OT-I T cells as a percentage of total CD8 T cells from three independent mice per group. The experiments are representative of two independent experiments with three mice per group. **, P < 0.01; ***, P < 0.005; ****, P < 0.001.
LPS mediated suppression is independent of Tregs and CD4 T cells
Regulatory CD4 T cells (Tregs) are known to play a critical role in regulating immune responses. Using different experimental systems, several studies have shown the ability of Tregs to suppress CD8 T cell responses (38, 39). Other studies have shown that IL10 secreted by antigen specific non-Treg CD4 T cells can also suppress CD8 T cell responses in a CD25 and FoxP3 independent manner (19, 26). Therefore, we tested if there is a potential role for Tregs in LPS-mediated CD8 T cell suppression. We depleted Tregs in vivo using an anti-CD25 antibody and immunized these mice with OVA with or without different TLR ligands. Treg depletion led to a slight basal enhancement of CD8 T cell responses in all groups but did not abrogate the ability of LPS to limit expansion of CD8 T cells (Fig. 5A, 5B). The extent of LPS mediated suppression was similar to the control group of mice that received Rat IgG. These results support the idea that Tregs have a very limited role in LPS mediated impairment of CD8 T cell priming and expansion. Next we assessed the role of total CD4 T cells in LPS mediated suppression of CD8 T cell responses in vivo. CD4 T cells were depleted using the GK1.5 monoclonal antibody and these mice were immunized with OVA in the presence or absence of TLR ligands. CD4 depletion led to an overall enhancement of CD8 T cell expansion in all groups, including the mice that received OVA+LPS. However, LPS inhibited the CD8 T cell response in a significant manner (Fig. 5C, 5D). Similar to Treg depletion, these results demonstrate that CD4 T cells have little to no influence on LPS mediated suppression of CD8 T cell responses.
Figure 5. Inhibition of CD8 T cell responses by LPS is independent of Tregs and CD4 T cells.
After OT-I CD8 T cell transfer, groups of mice either received control Rat-IgG or anti-CD25 (clone PC61) to deplete CD25+ Tregs. CD8 T cell priming was measured 7 days following immunization with OVA with or without TLR ligands, as indicated (A, B). Groups of mice were depleted of CD4 T cells as described and immunized as indicated following OT-I T cell transfer (C, D). (A, C) Representative plots show CD8 T cells from draining lymph nodes that express GFP and stain positive for Kb tetramer. (B, D) Mean ± SEM of GFP-OT-I T cells as a percentage of total CD8 T cells from three independent mice per group. Data are representative of three independent experiments. **, P < 0.01; ***, P < 0.005.
Differential recruitment of myeloid cells by TLR4 activation
Many studies have revealed a variety of ways in which different myeloid derived cells exert modulatory effects on immune responses (40). The suppressive role of myeloid suppressors has been reported in both cancer and infection models (41–43). In hopes of clarifying how TLR ligation on APCs could alter CD8 T cell responses, we began with a phenotypic analysis of APCs at early time points after immunization. We analyzed cells from draining lymph nodes at 1, 6, 12, 24 and 48 hrs to look at different population of cells recruited to the draining lymph nodes. We found that OVA+LPS immunized mice show a significantly enhanced CD11b+ Ly6Cint Ly6G+ population of cells (Supplemental Fig. 4A) compared to the OVA and OVA+CpG immunized groups. Several recent studies have reported that CD11b+Ly6G+ neutrophils influence antigen presentation to T lymphocytes (44–46). Since this was a major cell population that was different between LPS immunized and other groups of mice, we tested the possibility that this population of cells could directly suppress the CD8 T cell responses. We depleted these cells using a Ly6G monoclonal antibody (Clone: 1A8) and the mice were immunized with OVA with or without LPS. Depletion of the Ly6G population did not relieve the inhibitory effects of LPS on CD8 T cell responses (Supplemental Fig. 4B, 4C).
LPS fails to inhibit CD8 responses induced by peptide immunization
Although the enhanced recruitment of Ly6G positive cells by LPS immunization is interesting, our experiments above make it unlikely that LPS induces recruitment of any kind of specialized cells that could be directly suppressing CD8 T cell responses in trans. However, it is possible that LPS affects the ability of DCs to target antigens to the cross-presentation pathway. We wanted to understand if we could abrogate the inhibitory effects of LPS on CD8 T cell expansion by immunizing with a peptide and thus bypassing the MHC Class I presentation pathway. We immunized mice with SIINFEKL in IFA or SIINFEKL+LPS mixed in IFA and measured CD8 responses seven days after immunization. SIINFEKL mixed with IFA induced robust expansion of CD8 T cells in the draining lymph nodes. However, unlike OVA protein or VSV-OVA and LM-OVA induced CD8 responses, LPS failed to inhibit SIINFEKL induced CD8 T cell priming and expansion (Supplemental Fig. 4D, 4E). These data are a clear indication that the inhibitory effects of TLR2 and TLR4 activation are because of their affects on the ability of APCs to cross-present antigens on MHC Class-I.
LPS alters the DC populations in the draining lymph nodes and directly affects the ability of DCs to present antigen to CD8 T cells
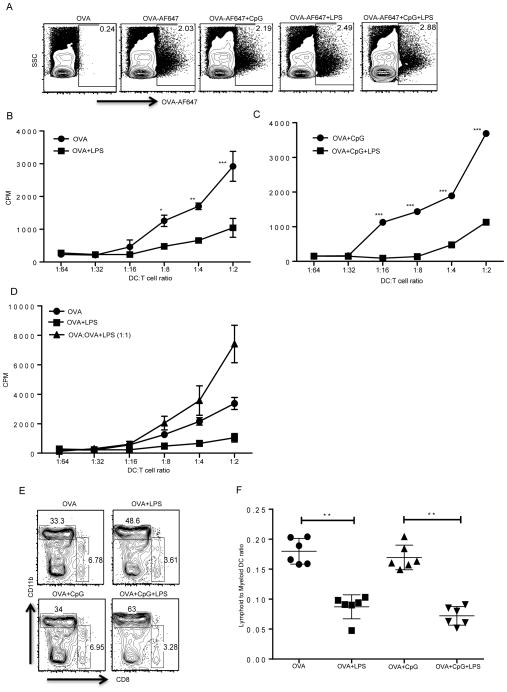
The experiments above strongly suggest the possibility that LPS could influence the directing and processing of cargo inside the cell. This could lead to compromised presentation of peptides on MHC Class-I and our data suggest that we could bypass that influence by providing a processed peptide (Supplemental Fig. 4D, 4E). In order to further address the possibility that LPS could affect cross-presentation of peptides on MHC Class I, we sorted cells that take up antigen in the draining lymph nodes following immunization. We used Alexa Flour (AF) 647 tagged OVA and immunized mice with or without different TLR ligands. We observed that the cells that took up OVA under different conditions were all CD11c+ DCs. After 18–24 hrs, OVA-AF647+ cells (Fig. 6A) were sorted and were co-cultured at titrating concentrations with sorted naive OT-I CD8 T cells. This experiment allowed us to directly investigate the ability of APCs from different groups of mice to prime OT-I CD8 T cells. We found that the sorted APCs from OVA-AF647 alone or OVA-AF647+CpG immunized group induced OT-I proliferation, whereas APCs from the OVA-AF647+LPS immunized group were defective in their ability to induce OT-I proliferation (Fig. 6B, 6C). Additionally, sorted APCs from the OVA+CpG+LPS immunized mice were also defective in priming OT-I T cells compared to APCs from the OVA+CpG immunized group, again highlighting the dominant effect of TLR4 over TLR9 in the regulation of CD8 T cell priming (Fig. 6B, 6C). There was no major difference in the levels of MHC Class I molecules expressed by APCs from any of these groups (Supplemental Fig. 4F). It is also possible that APCs from LPS immunized mice secrete soluble factors that inhibit CD8 T cell priming. The role of soluble factors secreted by APCs upon TLRs engagement have been implicated in CD8 T cell priming and specifically LPS induced IL10 production has been linked to the inhibition of CD8 T cell responses (26, 47). To address this possibility, we mixed sorted APCs from OVA-AF647+CpG and OVA-AF647+LPS in a 1:1 ratio and observed no inhibition of CD8 T cell priming when compared to the OVA-AF647+CpG group (Fig. 6D). Rather, the mixing of APCs induced greater OT-I proliferation in an additive manner.
Figure 6. TLR4 activation in vivo affects the ability of APCs to present antigen to CD8 T cells and induces differential recruitment of lymphoid and myeloid DCs.
Mice were injected with OVA or OVA-AF647 with or without different TLR ligands. (A) Cells that take up OVA can be identified in the draining lymph nodes by flow cytometry. (B, C) OVA positive cells from the draining lymph nodes of different groups of mice were sorted and incubated with OT-I CD8 T cells and proliferation was measured at the end of 72 hours by a 3H thymidine incorporation assay. (D) OVA+ APCs from draining lymph nodes of OVA primed group were mixed with the OVA+ APCs from OVA+LPS group and incubated with OT-I CD8 T cells and proliferation of CD8 T cells was measured as described above. (E). Mice were immunized with OVA-647 with or without different TLR ligands and 24hrs later cells from draining lymph nodes were analyzed for OVA-647+ cells and further analyzed for expression of lymphoid DCs (CD11c+, CD8+, CD11b−) and myeloid DC markers (CD11c+, CD11b+, CD8−). Representative plot shows staining for CD11b and CD8 on CD11c+ cells. (F) Ratio of lymphoid DCs to myeloid DCs from several independent mice following immunization and staining as described in E. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
We wanted to explore the possibility that LPS induces differential recruitment of DC subpopulations in the draining lymph nodes. For example, it is well known that lymphoid DCs are able to induce cross-presentation of exogenous antigens and induce CD8 T cell priming against extracellular antigens. (48–50). Myeloid DCs on the other hand are important for CD4 T cell priming. We performed further characterization of DCs that had taken up OVA-AF647 and observed that the mice that received LPS had a lowered representation of lymphoid DCs in the total DC pool (Fig 6E). These results suggest that LPS mediated suppression is not due to soluble factors secreted by different APCs but rather because of its effect on differential recruitment of lymphoid versus myeloid DCs.
Discussion
It is now well established that stimulation of TLRs and/or other PRRs in DCs is important for activation and differentiation of naïve CD4 T cells (13, 51). CD4 T cells play an important role in different facets of adaptive immunity. They are important to secrete effector cytokines that mobilize macrophages and neutrophils to the site of infection, induce activation and differentiation of B cells and assist in the generation and functioning of CD8 memory T cells. While it is therefore important for the immune system to induce CD4 T cell responses to all pathogens, irrespective of whether they are extracellular or intracellular, the usefulness of CD8 T cell responses for extracellular pathogens is less clear.
In the present study, we examined the effects of different TLRs on the regulation of CD8 T cell responses. Although all TLR ligands induced cross-presentation of soluble antigens in vitro, our in vivo experiments revealed an important biological understanding on how different TLRs regulate CD8 T cell priming in the lymphoid organs. In particular, we discovered that plasma membrane TLRs and endosomal TLRs have diametrically opposite effects on CD8 T cell priming and expansion in vivo. In agreement with previous findings, we observed enhanced CD8 T cell responses to TLR3 and TLR9 ligands, supporting the notion that these endosomal TLR ligands can be used as adjuvants to help induce CD8 T cell responses (16–18, 52). There are several reports on the effects of the TLR4 ligand LPS on CD8 T cell responses (15, 25, 26, 47, 52–54). Many reports indicate that signaling through TLR4 inhibits CD8 priming (15, 52, 53) while other studies indicate that LPS enhances CD8 activation (54, 55). There are also other studies that demonstrate that LPS can act as an adjuvant to enhance CD8 T cell responses induced by SIINFEKL peptide (56). We observe a very robust response induced by SIINFEKL immunization itself and a modest enhancement of that response when LPS is co-injected. There are yet other studies where mice are immunized with OVA and LPS to induce OT-I T cell activation and expansion (55). However since other TLR ligands were not used in these studies, it is hard to understand the relative magnitude of OT-I responses when compared to LPS. It is possible that the dose of antigen and LPS used and the number of OT-I T cell that were transferred prior too immunization also contribute to the observed differences. The studies that indicate that LPS augments CD8 responses in vivo (54, 55) are difficult to compare with our work since they use high doses of peptide for immunization that could bypass physiological mechanisms by which LPS induces suppression of responses. However, as demonstrated by our studies, in the context of whole protein immunizations and infection with pathogens, LPS has a detrimental effect on the outcome of CD8 T cell responses. LPS clearly has the ability to induce activation and expansion of antigen specific CD4 T cells, but our and other studies (26) demonstrate that LPS has an inhibitory effect on CD8 T cell activation and expansion. Additionally, both Mycobacterium tuberculosis (57) and Japanese encephalitis virus (58) use the TLR2 signaling pathway to inhibit cross-presentation of antigens.
It has been proposed that TLR4 signaling leads to recruitment of TAP and associated proteins to the endosomes leading to direct peptide transportation into endosomes followed by their loading on MHC Class I (21). There are also additional studies that show that some LPS preparations suppress cross-presentation, due to contamination with Nod ligands or through induction of IL-10 producing CD4 T cells (25). In our experiments, both TLR2 and TLR4 ligands inhibited CD8 T cell responses to both soluble antigens and infectious agents. Strikingly, activation of these plasma membrane TLRs had a dominant effect of inhibiting enhanced CD8 T cell priming mediated by TLR3 and TLR9. Our experiments involving transfer of OT-I T cells into TLR2/TLR4 DKO mice clearly establish that the suppression by LPS is not due to possible contamination of Nod or other cytosolic ligands. In addition, although CD4 T cell and Treg cell depletion led to basal enhancement of CD8 T cell responses, these responses were still suppressed by LPS suggesting that there are additional mechanisms that lead to LPS and BLP induced suppression of CD8 T cell priming.
Our experiments using VSV and Listeria monocytogenes also establish that suppression of CD8 T cell priming by LPS and BLP is not limited to soluble antigens. In addition, heat-killed Salmonella typhimurium behaved like LPS and BLP and dramatically suppressed the CD8 T cell responses induced by Listeria and VSV. The Listeria data are especially intriguing since the pathogen naturally contains TLR2 ligands and suggests the possibility that Listeria could exploit this effect to reduce CD8 T cell responses during an active infection. An earlier study in fact observed higher CD8 T cell responses in TLR2 deficient mice following Listeria infection (37). It is also possible that TLR2 plays differential roles in induction of innate and adaptive immunity against Listeria. Different experimental systems could lead to different results in assessing the importance of TLR2 in inducing anti-Listeria immunity. It is clear that TLR2 mediated detection of Listeria is important for host protection and also other aspects of anti-Listeria immune responses such as CD4 responses are unlikely to be hampered by TLR2 signaling. Our studies only reveal previously unappreciated effects of enhanced TLR2 ligation on the outcome of anti-Listeria CD8 responses. We observe that only additional activation of TLR2 by BLP during infection with Listeria led to a dramatic reduction of CD8 T cell responses. These experiments have important implications for the outcome of adaptive immunity during combined multiple infections and suggest that anti-viral or anti-bacterial CD8, but not CD4, responses could be comprised under such circumstances. CD8 T cell responses can be highly destructive and can induce severe pathology by killing infected or peptide loaded cells and suppression of CD8 responses could also allow induction of CD4 immune responses against relevant pathogens that need CD4 T cells for clearance and protection.
It is possible that type I IFNs (IFN-β and IFN-α4) induced by LPS alter the ability of Listeria or VSV to replicate, lowering their load and thus affecting the magnitude of CD8 T cell responses. However, we show that both TLR4 and TLR2 ligands inhibit OVA (as well as LM and VSV) induced CD8 responses. Although Vaccinia Virus via TLR2 can induce type I IFNs, bacterial ligands such as Pam3CSK4 do not induce type I IFNs (35) and in agreement with these findings we do not see any type I IFN production by DCs stimulated with Pam3CSK4 (data not shown). Moreover CPG that induces type I IFNs does not inhibit CD8 responses induced by VSV. So, it is highly unlikely that type I IFN production by LPS causes reduced viral load leading to reduced CD8 T cell priming. The VSV data are in support of the data with OVA and Listeria and strengthen the overall argument that TLR2 and TLR4 signaling have a dominant effect of downregulating CD8 T cell responses generated by both soluble proteins and infectious agents. In addition, a clear common theme that emerges from our experiments is that the location of the TLR at the time of signaling is an important determinant of the outcome of CD8 T cell responses. For example, the plasma membrane TLRs, TLR2 and TLR4 do not induce CD8 T cell priming in vivo, whereas the endosomal TLRs, TLR3 and TLR9 enhance CD8 T cell priming and expansion. It is known that TLR4 can signal from both plasma membrane and endosomes in MyD88 and TRIF dependent manner respectively (59) and it is clear from our data that TLR4 mediates suppression of CD8 T cell responses is dependent on MyD88 and not the TRIF pathway of signaling. Also, although endosomal TLRs traffic through different routes before localization to the endosome (60), the final location of the endosomal TLRs at the time of signaling seems to be important to enhance CD8 T cell expansion. It is also very interesting that although TLR9, TLR4 and TLR2 all use MyD88 as a signaling adapter, the suppression mediated by TLR4 is dependent on MyD88 suggesting that the biological outcomes of MyD88 signaling downstream of different TLRs are vastly different. It is important to note that TRIF-IRF3 pathway of signaling is critical for basal cross-presentation of antigens to CD8 T cells. The defects in CD8 T cell priming in TRIF and IRF3 deficient mice were however overcome by activation of the MyD88 signaling pathway downstream of TLR9 (data not shown).
There could be several mechanisms by which TLR2 and TLR4 could be inhibiting activation of CD8 T cells. An attractive hypothesis is that plasma membrane TLRs could induce recruitment of cells that suppress priming of CD8 T cells. Interestingly, we found that LPS injection led to recruitment of a Ly6G+ population of cells but depletion experiments suggested that this population was not responsible for the inhibition of CD8 T cell priming. There are however several reports demonstrating that myeloid derived cells play a prominent role in immune suppression to chronic viral infections and different cancers (41, 42). The importance of recruitment of this population of cells for adaptive immunity needs to be further investigated. A second possibility is that activation of TLR2 and TLR4 altered the DC-T cell interaction by interfering with levels of MHC Class I. We observed no differences in the level of MHC Class I expressed on DCs in the draining lymph nodes, and when mice were immunized with peptide instead of whole protein, LPS was no longer able to inhibit CD8 T cell priming.
Our final set of experiments provide compelling evidence that activation of TLR4 and TLR2 in vivo affects the ability of DCs to prime CD8 T cells. DCs from mice that received LPS were clearly deficient in their ability to activate OT-I CD8 T cells in vitro. Given that there is no alteration in levels of MHC Class I and that peptide induced CD8 T cell expansion in not inhibited by LPS, the reduced ability of DCs from either LPS immunized or LPS+CpG immunized mice could be because of alteration in lymphoid to myeloid DC ratios in the draining lymph nodes. The lower number of lymphoid DCs in the draining lymph nodes can lead to reduced priming of CD8 T cells. Our in vitro DC mixing experiments also provide important evidence that there is no active inhibition of CD8 T cells, either by a contact dependent manner or by secretion of soluble suppressive factors by DCs from LPS injected mice. The experiment in chimeric mice that express MyD88 only in CD11c positive DCs and macrophages also supports the notion that TLR4-MyD88 signaling in a cell intrinsic fashion could regulate handling of the cargo inside the cells. The TLR9-MyD88 signaling axis on the other hand promotes cross-presentation of antigens on MHC Class- I. The exact mechanism of how different DCs are recruited to the draining lymph nodes needs further investigation. It also remains to be examined if an individual dendritic cell makes distinct decisions of whether to promote or diminish targeting of antigens to the MHC Class I pathway depending on the TLR that is activated. Given that neither TLR2 nor TLR4 activation in vitro hampers the ability of DCs to activate CD8 T cells, it is more likely that the specific composition of the DC populations at the time of CD8 T cell priming determine the outcome of CD8 T cell activation and expansion. Our work provides important insights on how plasma membrane and endosomal TLRs influence CD8 T cell priming and these results have important implications for choosing TLR adjuvants for vaccines as well as for understanding how anti-viral immunity could be hampered by bacterial co-infections.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant AI082265 to CP and AI45764 to JF.
We wish to thank the UTSW Flow cytometry core facility. We thank Dr. H. Shen, Dr. L. Lefrancois and Dr. D. Monack for the gift of LM-OVA, VSV-OVA and Salmonella typhimurium SL1344 ΔSpi-1 mutant strain respectively. We also thank Dr. S. Rath, W. Hu and T. Troutman for helpful discussions and critical reading of the manuscript.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 4.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature immunology. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 5.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends in immunology. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nature reviews Immunology. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akira S, Yamamoto M, Takeda K. Role of adapters in Toll-like receptor signalling. Biochemical Society transactions. 2003;31:637–642. doi: 10.1042/bst0310637. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual review of immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 9.Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF, Pasare C. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:273–278. doi: 10.1073/pnas.1118579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni M, MacFarlane AWt, Toft M, Lowell CA, Campbell KS, Hamerman JA. B-cell adaptor for PI3K (BCAP) negatively regulates Toll-like receptor signaling through activation of PI3K. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:267–272. doi: 10.1073/pnas.1111957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. Endotoxin-induced maturation of MyD88-deficient dendritic cells. Journal of immunology. 2001;166:5688–5694. doi: 10.4049/jimmunol.166.9.5688. [DOI] [PubMed] [Google Scholar]
- 12.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunological reviews. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Q, Egelston C, Vivekanandhan A, Uematsu S, Akira S, Klinman DM, Belyakov IM, Berzofsky JA. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16260–16265. doi: 10.1073/pnas.0805325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta SK, Redecke V, Prilliman KR, Takabayashi K, Corr M, Tallant T, DiDonato J, Dziarski R, Akira S, Schoenberger SP, Raz E. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. Journal of immunology. 2003;170:4102–4110. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- 16.Jelinek I, Leonard JN, Price GE, Brown KN, Meyer-Manlapat A, Goldsmith PK, Wang Y, Venzon D, Epstein SL, Segal DM. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. Journal of immunology. 2011;186:2422–2429. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 18.Maurer T, Heit A, Hochrein H, Ampenberger F, O’Keeffe M, Bauer S, Lipford GB, Vabulas RM, Wagner H. CpG-DNA aided cross-presentation of soluble antigens by dendritic cells. European journal of immunology. 2002;32:2356–2364. doi: 10.1002/1521-4141(200208)32:8<2356::AID-IMMU2356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz K, Storni T, Manolova V, Didierlaurent A, Sirard JC, Rothlisberger P, Bachmann MF. Role of Toll-like receptors in costimulating cytotoxic T cell responses. European journal of immunology. 2003;33:1465–1470. doi: 10.1002/eji.200323919. [DOI] [PubMed] [Google Scholar]
- 20.Oh JZ, Kurche JS, Burchill MA, Kedl RM. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2011;118:3028–3038. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nature immunology. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 22.Pufnock JS, Cigal M, Rolczynski LS, Andersen-Nissen E, Wolfl M, McElrath MJ, Greenberg PD. Priming CD8+ T cells with dendritic cells matured using TLR4 and TLR7/8 ligands together enhances generation of CD8+ T cells retaining CD28. Blood. 2011;117:6542–6551. doi: 10.1182/blood-2010-11-317966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quigley M, Martinez J, Huang X, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009;113:2256–2264. doi: 10.1182/blood-2008-03-148809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, de Koning-Ward TF, Belz GT, Carbone FR, Crabb BS, Heath WR, Villadangos JA. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nature immunology. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 25.Wagner CS, Cresswell P. TLR and nucleotide-binding oligomerization domain-like receptor signals differentially regulate exogenous antigen presentation. Journal of immunology. 2012;188:686–693. doi: 10.4049/jimmunol.1102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.den Haan JM, Kraal G, Bevan MJ. Cutting edge: Lipopolysaccharide induces IL-10-producing regulatory CD4+ T cells that suppress the CD8+ T cell response. Journal of immunology. 2007;178:5429–5433. doi: 10.4049/jimmunol.178.9.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu W, Troutman TD, Edukulla R, Pasare C. Priming microenvironments dictate cytokine requirements for T helper 17 cell lineage commitment. Immunity. 2011;35:1010–1022. doi: 10.1016/j.immuni.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbone FR, Hosken NA, Moore MW, Bevan MJ. Class I MHC-restricted cytotoxic responses to soluble protein antigen. Cold Spring Harbor symposia on quantitative biology. 1989;54(Pt 1):551–555. doi: 10.1101/sqb.1989.054.01.065. [DOI] [PubMed] [Google Scholar]
- 29.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Access of soluble antigens to the endoplasmic reticulum can explain cross–presentation by dendritic cells. Nature immunology. 2005;6:107–113. doi: 10.1038/ni1147. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 32.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. The Journal of experimental medicine. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. Journal of immunology. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 34.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 35.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nature immunology. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 37.Kursar M, Mittrucker HW, Koch M, Kohler A, Herma M, Kaufmann SH. Protective T cell response against intracellular pathogens in the absence of Toll-like receptor signaling via myeloid differentiation factor 88. International immunology. 2004;16:415–421. doi: 10.1093/intimm/dxh047. [DOI] [PubMed] [Google Scholar]
- 38.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. Journal of immunology. 2001;167:1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 39.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. The Journal of experimental medicine. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagaraj S, Collazo M, Corzo CA, Youn JI, Ortiz M, Quiceno D, Gabrilovich DI. Regulatory myeloid suppressor cells in health and disease. Cancer research. 2009;69:7503–7506. doi: 10.1158/0008-5472.CAN-09-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of immunology. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. The Journal of experimental medicine. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. Journal of immunology. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribeiro-Gomes FL, Peters NC, Debrabant A, Sacks DL. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS pathogens. 2012;8:e1002536. doi: 10.1371/journal.ppat.1002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang CW, Strong BS, Miller MJ, Unanue ER. Neutrophils influence the level of antigen presentation during the immune response to protein antigens in adjuvants. Journal of immunology. 2010;185:2927–2934. doi: 10.4049/jimmunol.1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duffy D, Perrin H, Abadie V, Benhabiles N, Boissonnas A, Liard C, Descours B, Reboulleau D, Bonduelle O, Verrier B, Van Rooijen N, Combadiere C, Combadiere B. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity. 2012;37:917–929. doi: 10.1016/j.immuni.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Bogunovic D, Manches O, Godefroy E, Yewdall A, Gallois A, Salazar AM, Marie I, Levy DE, Bhardwaj N. TLR4 engagement during TLR3-induced proinflammatory signaling in dendritic cells promotes IL-10-mediated suppression of antitumor immunity. Cancer research. 2011;71:5467–5476. doi: 10.1158/0008-5472.CAN-10-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annual review of immunology. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 49.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annual review of immunology. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 50.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature immunology. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 52.Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 53.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 54.Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nature immunology. 2003;4:1223–1229. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- 55.Dawicki W, Watts TH. Expression and function of 4-1BB during CD4 versus CD8 T cell responses in vivo. European journal of immunology. 2004;34:743–751. doi: 10.1002/eji.200324278. [DOI] [PubMed] [Google Scholar]
- 56.Sercan O, Hammerling GJ, Arnold B, Schuler T. Innate immune cells contribute to the IFN-gamma-dependent regulation of antigen-specific CD8+ T cell homeostasis. Journal of immunology. 2006;176:735–739. doi: 10.4049/jimmunol.176.2.735. [DOI] [PubMed] [Google Scholar]
- 57.Simmons DP, Canaday DH, Liu Y, Li Q, Huang A, Boom WH, Harding CV. Mycobacterium tuberculosis and TLR2 agonists inhibit induction of type I IFN and class I MHC antigen cross processing by TLR9. Journal of immunology. 2010;185:2405–2415. doi: 10.4049/jimmunol.0904005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aleyas AG, Han YW, Patil AM, Kim SB, Kim K, Eo SK. Impaired cross-presentation of CD8alpha+ CD11c+ dendritic cells by Japanese encephalitis virus in a TLR2/MyD88 signal pathway-dependent manner. European journal of immunology. 2012;42:2655–2666. doi: 10.1002/eji.201142052. [DOI] [PubMed] [Google Scholar]
- 59.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nature immunology. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee BL, Moon JE, Shu JH, Yuan L, Newman ZR, Schekman R, Barton GM. UNC93B1 mediates differential trafficking of endosomal TLRs. eLife. 2013;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.