Abstract
Background
The natural history of pancreatic neuroendocrine neoplasms (PNENs) in patients with Von Hippel-Lindau (VHL) disease is poorly defined. Management of patients with PNENs is challenging because there are no reliable preoperative criteria to detect malignant lesions and the majority of resected tumors are found to be benign. The aim of this study was to determine whether 18-Fluorodeoxyglucose-Positron Emission Tomography (18FDG-PET) uptake predicts growth and detects malignant VHL-associated PNENs.
Study Design
Prospective study of 197 patients with VHL-associated pancreatic lesions. Clinical and imaging characteristics were analyzed to study the associations between FDG-PET uptake, tumor growth, and the development of metastatic disease.
Results
109 of 197 patients had solid pancreatic lesions and underwent both CT and 18FDG-PET scanning, which identified 165 and 144 lesions, respectively. 18FDG-PET detected metastatic disease in 3 patients not detected by CT scan and suggested non-neoplastic disease in 3 patients. Maximum SUV uptake on 18FDG-PET correlated with tumor size on CT (r=0.47, p<0.0001) and an increase in SUVmax was associated with tumor growth (r=0.36, p=0.0062). No association was seen between 18FDG-PET uptake and age, VHL genotype, or serum chromogranin A levels.
Conclusions
FDG-PET scanning identifies metastatic disease not detected by CT scan and avoids the resection of non-PNEN lesions that have no malignant potential in patients with VHL-associated PNENs. It should be considered as a valuable functional imaging modality in the clinical management of patients with VHL-associated PNENs.
Keywords: Von Hippel-Lindau, Pancreatic neuroendocrine neoplasm, 18FDG-PET, metastatic
Introduction
Von Hippel-Lindau (VHL) disease is an autosomal dominant, multisystem familial cancer syndrome resulting from a germline inactivating mutation in the VHL tumor suppressor gene.1 Patients with VHL are at risk of developing multiple benign and malignant tumors, including hemangioblastomas of the brain and spinal cord (10-70%), retinal hemangiomas (25-60%), endolymphatic sac neoplasms (10%), renal cysts and renal cell carcinomas (25-60%), pheochromocytomas (10-20%), and solid and cystic pancreatic neoplasms (35-70%).2, 3 Most pancreatic lesions are benign cysts, comprised of simple cysts or rarely serous cystadenomas.4 In contrast, solid lesions on CT scan are less common and usually indicate pancreatic neuroendocrine neoplasms (PNENs) (15% of VHL patients),3, 5 and may be malignant in up to 17% of patients.6
The primary goal in management of patients with VHL-associated pancreatic lesions is to prevent the development of metastatic disease while minimizing possible treatment related morbidity. And although there have been recent advances in medical therapies for metastatic PNENs, surgical resection remains the only curative therapy.7-9
Several imaging modalities have been used to detect PNENs. Computed tomography (CT) is the most widely used modality. The reported sensitivity of CT scan varies between 29-94%, with higher sensitivity achieved when both arterial and portal venous phases are performed.10, 11 This wide range of sensitivity is primarily due to small PNENs missed on CT. The combination of CT and MRI is able to localize >50% of PNENs larger than 3 cm, but only 5% of lesions smaller than 1 cm.12 Recent studies suggest that functional imaging with positron emission tomography (PET) may be a useful localizing modality for PNENs. Radiolabeled 18-fluorodeoxyglucose (18F-FDG) is taken up by some PNENs, especially poorly differentiated ones, and reflects increased glucose metabolism, which is linked to cellular proliferative activity and may be useful for distinguishing between benign and malignant lesions.13, 14 However, 18FDG-PET has been shown to be of limited utility for detecting well-differentiated tumors due to their low expression of glucose transporters and low proliferative activity.15, 16
A widely used criterion for recommending surgical resection of VHL-associated PNENs is tumor size (> 3cm), however such an approach may miss some malignant tumors and may result in patients undergoing resection for a histologically benign tumor or non-PNEN. Additional adjunct preoperative imaging studies, which could predict increased risk of disease progression or detect metastatic disease may refine the management of patients with VHL-associated pancreatic lesions. Thus, the aim of the present study was to determine prospectively whether the uptake status and amount of 18FDG-PET uptake could predict PNEN growth and detect malignant VHL-associated PNENs.
Methods
Patient Population
As part of a prospective clinical protocol, 197 patients with pancreatic manifestations of VHL were enrolled into a clinical protocol between June 2010 and December 2012 with serial axial imaging performed at the National Institutes of Health (NIH) Clinical Center (Clinicaltrials.gov: NCT00062166). All patients provided prior informed written consent and this study was approved by our Institutional Review Board.
Of the 197 patients followed in the study, 60 were excluded from this analysis because they presented with only pancreatic cysts. An additional 28 patients were excluded due to a lack of follow up imaging. This resulted in a study cohort of 109 patients with solid pancreatic lesions on CT scan. Clinical, demographic, laboratory, histopathologic and radiographic data were collected prospectively.
Imaging studies
As part of our clinical research protocol, patients with solid and complex lesions underwent pancreatic abdominal computed tomography (2mm slices, both arterial and portal venous phase) annually. 18F-FDG-PET scans were also performed annually for patients with solid lesions. The patients were required to fast for 4-6h before PET. Scans were performed 60 minutes after the administration of approximately 10 mCi of intravenous 18FDG for patients ≤90 kg and 15 mCi for patients >90 kg. Patients were scanned from the mid-thigh to the skull base. A non-contrast, non-diagnostic CT was used for attenuation and anatomic localization. Maximum standardized uptake values (SUVmax) were measured based on patient total body weight.
CT scans were assessed by two independent reviewers. The diagnosis of PNENs was made on the arterial phase of the CT. All solid radiographic lesions of the pancreas were catalogued by size and location. The largest diameter of each tumor was recorded by each reviewer and averaged to provide a consensus measurement. Average measurements less than 0.5 cm were excluded from the analysis.
Surgical management and histology
Patients who met the operative criteria at our institution underwent resection of their PNENs. Tumors were resected if they were ≥2 cm in the head of the pancreas or ≥3cm in the body/tail of the pancreas. Twenty-four patients had a total of 29 lesions surgically removed. Lesions were classified by pathologists according to the 2010 World Health Organization (WHO) classification system for tumor grade, based on a combination of mitotic count and MIB1 (Ki67) labeling index as follows: Low grade (G1): <2 mitoses/10 hpf and <3% Ki67 index, Intermediate grade (G2): 2-20 mitoses/10hpf or 3%-20% Ki67 index, High grade (G3): >20 mitoses/10 hpf or >20% Ki67 index.
Statistical Analyses
Summary statistics are presented as mean ± standard deviation (SD), except where otherwise indicated. Associations between demographic, radiologic, clinicopathologic data and imaging results were evaluated using Spearman rank correlation coefficients, reported with 95% confidence intervals (CI) and exact permutation p values. Differences between groups in quantitative variables were assessed using the Mann-Whitney test. Due to within-patient correlation of SUVmax values over multiple lesions, associations with other lesion and patient characteristics were tested on a per patient basis from bootstrapped samples of one lesion per patient with median results reported to represent a typical sample. Statistical analyses were performed in GraphPad Prism, Version 5.04, 2010 (San Diego, California) and SAS/STAT software version 9.3 (copyright, SAS Institute Inc., Cary, NC).
Results
The study cohort demographics and clinical characteristics are summarized in Table 1. In 109 patients, CT detected 165 pancreatic solid lesions, and 144 of these were avid pancreatic lesions detected by 18FDG-PET. The average number of solid lesions per patient was thus 1.51 with a mean size of 1.50 cm by CT (median 1.30 cm, range 0.5-5.2 cm) and a mean SUVmax value of 7.5 (median 5.6, range 0-35.7).
Table 1.
Characteristics of Study Cohort
| Variable | Value |
|---|---|
| n | 197 |
| Pts with solid PNEN, n (%) | 137 (69.5) |
| Pts with CT and FDG-PET | 109 |
| Male : female ratio | 47 : 62 |
| Median age, y (range) | 47 (18-74) |
| No. of lesions on CT / PET | 165 / 144 |
| Average no. of PNENs per patient (range) | 165/109= 1.51 (1-4) |
| Size of PNENs on CT, cm, mean±SD (range) | 1.50 ± 0.88 (0.5-5.2) n=165 |
| SUVmax on PET, mean±SD (range) | 7.5 ± 6.2 (0-35.7) n=144 |
| Median chromogranin a, ng/mL (range) | 150 (32-3,560) n=100 |
| Patients who underwent resection, n | 24 |
PNENs, pancreatic neuroendocrine neoplasms.
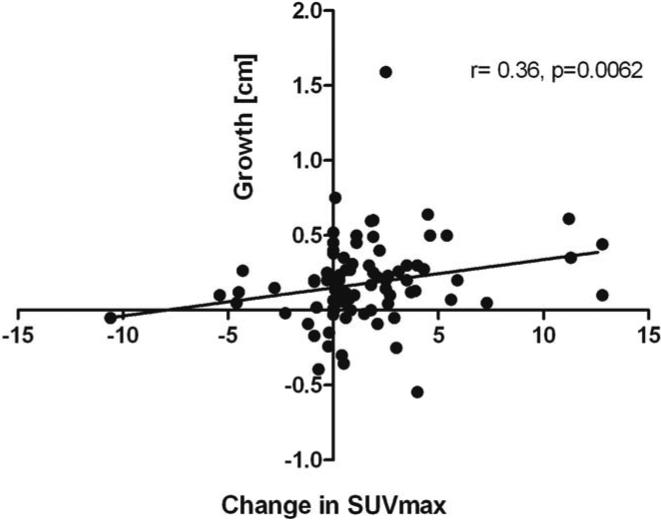
The SUVmax significantly correlated with tumor size of the pancreatic lesion as measured by CT (r=0.47, 95% CI 0.41-0.54, p<0.0001, Figure 1). Of the 21 lesions that were not PET avid (SUVmax = 0), three had no contemporaneous CT size, and the average size of the other 18 was 1.22 ± 0.99 cm. The largest non-avid lesion measured 4.95 cm by CT and pathology revealed a 1.3 cm well-differentiated neuroendocrine tumor in association with a larger microcystic serous cystadenoma (MCA). Fifty-seven patients had 83 lesions with two or more 18FDG-PET scans and with tumor growth over the same time interval, in intervals ranging from 0.5 to 2.1 years (median 1.0) the change in SUVmax between these scans significantly correlated with the tumor growth (r=0.36, 95% CI 0.24-0.48, p=0.0062, Figure 2). However, the SUVmax at the initial 18FDG-PET scan was not found to be significantly associated with future tumor growth rate.
Figure 1.
Association between 18FDG-PET uptake and the size of the pancreatic lesion on CT, measured at same time point, N=156. Spearman correlation r= 0.47, 95% CI 0.41-0.54, p<0.0001. SUVmax= Maximum standardized uptake value.
Figure 2.
Association between the change in 18FDG-PET uptake and growth of solid pancreatic lesion in cm over the same time period, N=83. Spearman correlation r=0.36, 95% CI 0.24-0.48, p=0.0062. SUVmax= Maximum standardized uptake value.
SUVmax was slightly higher in females than in males, with mean SUVmax values of 8.4 ± 6.6 for 62 females and 6.1 ± 5.4 for 47 males (p=0.07). Tumor size by CT was not significantly different by gender (1.46 cm in females versus 1.53 cm in males, median 1.30 in each). There was no association seen between SUVmax and age, genetic mutation (specific exon(s) involved) or serum chromogranin A levels (in 100 patients).
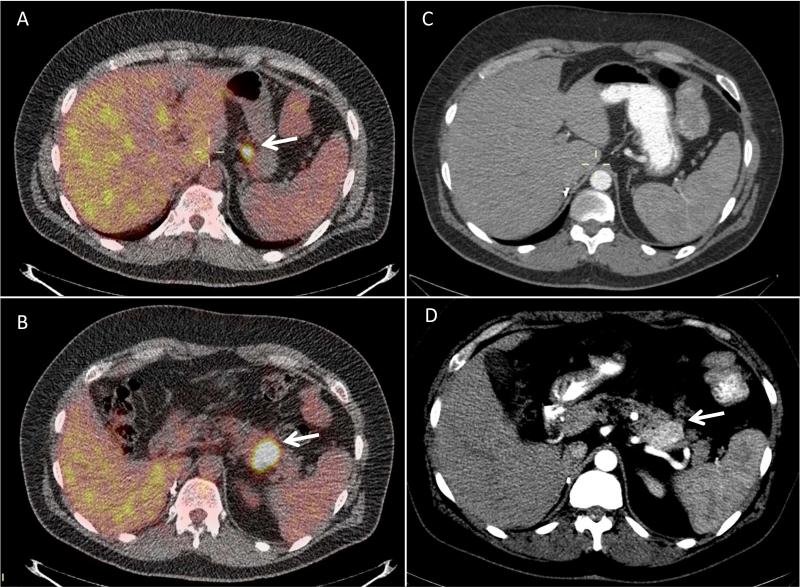
Twenty-four patients underwent resection of their PNENs during follow up. The operative intervention, pathology, and radiologic findings are summarized in Table 2. Average follow-up time after surgery was 36 months (median 37, range 19-51 months). Of the 24 patients, 22 had an 18FDG-PET scan prior to operative intervention, and CT scan demonstrated a total of 29 lesions. The majority of lesions (27/29) were avid on 18FDG-PET, with a mean SUVmax of 10.3 ± 6.2. There was no difference in SUV uptake seen by the histologic tumor grade (G1 versus G2) or nodal status (N0 versus N1). There was no difference in SUVmax seen in non metastatic versus metastatic PNENs (10.3 ±6.7 and 10.5 ±5.5). However, 18FDG-PET detected metastatic disease in 3 patients, which was not detected on CT scan. One patient had local invasion into the gastrohepatic ligament. The second patient was found to have retropancreatic lymph node involvement, and the third patient had a suspicious PET-avid peripancreatic lymph node that was metastatic PNEN on histologic examination. Representative images for the first patient are shown in Figure 3.
Table 2.
Histologic and Radiologic Features of Resected pancreatic Neuroendocrine Neoplasms in 24 Patients with Von Hippel-Lindau
| All patients | Patients with MCAs+concurrent PNENs (n=5¶) | PNENs only (n=19) | |
|---|---|---|---|
| Patients with resection for solid tumors; n (% of resected) | 24 (100) | 5 (20.8) | 19 (79.2) |
| Age at operation, y, median (range) | 47 (24-71) | 48 (33-56) | 47 (24-71) |
| Resection performed, n* | 25 | 6 | 19 |
| Total pancreatectomy | 3 | 1 | 2 |
| Pancreaticoduodenectomy | 7 | 1 | 6 |
| Distal pancreatectomy | 8 | 3 | 5 |
| Enucleation | 7 | 1 | 6 |
| FDG-PET uptake; mean ±SD (range) | 10.3 ±6.2 (0-25) | 4.5 ±4.0 (0-10.4) | 12.2 ±5.7 (3.5-25)† |
| Size of tumor lesion, cm, mean ±SD (range)‡ | 2.30 ±1.51 (0-7) | 1.72 ±2.22 (0-6) | 2.45 ±1.29 (1.3-7) |
| PNEN staging; n/no. evaluable (% of group)§ | |||
| T1, ≤2 cm | 3/4 (75) | 7/19 (37) | |
| T2, limited to pancreas >2 cm | 1/4 (25) | 10/19 (53 | |
| T3, beyond pancreas, no involvement of SMA and celiac | 0 | 2/19 (11%) | |
| T4, beyond pancreas with involvement of SMA and celiac | |||
| N1 | 1/5(20) | 6/19(32) | |
| M1; n (%) | 0 | 2/19(11) | |
| Grade, n (%) | |||
| 1 | 2/4 (50) | 11/19 (58) | |
| 2 | 2/4 (50) | 8/19 (42) | |
One patient had more than one procedure performed, thus the number of resections is greater than the number of patients.
n=17.
Largest PNEN size on final pathology.
TNM stage according to the American Joint Committee on Cancer 28, 29 and the World Health Organization histologic classification for PNEN (1 low grade NEN; 2 intermediate NEN; 3 high grade NEC).30
Overall 5 patients with MCAs, 1 patient MCA only, 4 concurrent MCA+PNENs FDG, 18-Fluorodeoxyglucose; PNENs, pancreatic neuroendocrine neoplasms; SMA, superior mesenteric artery; VHL, Von Hippel-Lindau.
Figure 3.
Representative case of a 44 year-old man with metastatic PNEN. The patient had pancreatic extension into the gastrohepatic ligament seen on 18FDG-PET and was found to have liver metastases. Images A and B show the 18FDG-PET with the body mass of SUVmax 15.6 (B, arrow) and its suspicious extension into the gastrohepatic ligament (A, arrow). Images C demonstrates absence of the lesion shown in image A and D show the CT scan of the pancreatic body mass of 4cm (D, arrow).
There was no 18FDG-PET uptake in two of 29 (6.9%) pancreatic lesions found on pathology, and both were small foci of PNEN (3 mm and 1.3 cm) with the large dominant lesion being MCA. A total of four patients presented with MCAs in addition to PNENs, and one had MCA only on final pathology. Of those, three had either no PNEN or concurrent small PNENs (3 mm to 1.3 cm). The comparison in SUV uptake between only PNENs and PNENs concurrent with MCA is shown in Figure 4. The SUVmax uptake was significantly lower in the group with MCA and concurrent PNENs (p=0.0014). Two lesions with high 18FDG-PET uptake in the combined PNEN and MCA group were PNEN of 6 cm of size on histologic examination. MCAs had a significantly lower 18FDG-PET SUVmax with none being above 4.2. For a total of 29 lesions with a histologic diagnosis, using a SUVmax cutoff of <4.0 versus >4.0 had a sensitivity of 92% (23/25), a specificity of 75% (3/4) and a positive and negative predictive value of 95.8% (23/24) and 60% (3/5), respectively, for detecting PNEN.
Figure 4.
Comparison of preoperative SUVmax according to final pathology (per lesion analysis). Mann-Whitney test, **p=0.0014. MCA, microcystic adenoma; PNEN, pancreatic neuroendocrine neoplasms; PreOP SUVmax, preoperative maximum standardized uptake value.
Discussion
To our knowledge this is the first prospective study evaluating the utility of 18FDG-PET in VHL-associated PNENs. Overall, 18FDG-PET localized 87% (144/165) of the solid pancreatic lesions (≥0.5cm) found on CT scan. The SUVmax by 18FDG-PET significantly correlated with lesion size by CT, but did not predict subsequent growth. However, we found increased SUVmax for those lesions that were larger on follow up imaging. Our results suggest a higher mean SUVmax in females compared to males, but we did not detect an association between 18FDG-PET uptake and age, genetic mutation status, or chromogranin A levels. 18FDG-PET identified 93% (27/29) of the tumors requiring surgical excision and helped identify metastatic lesions in 3 patients that were not seen by CT scan. MCAs had a significantly lower 18FDG-PET SUVmax with none being above 4.2, and overall the positive predictive value for 18FDG-PET in detecting PNEN was 95.8%.
In patients with PNENs, preoperative evaluation to determine the risk of malignancy or presence of metastatic disease is critical for determining the optimal treatment approach. CT scan has been traditionally used for this purpose, and studies have shown a correlation between tumor size and malignancy for sporadic nonfunctioning PNENs with a cutoff of >2cm recommended for resection because of the higher risk of malignancy.17 Blansfield et al5 showed a similar size cutoff for VHL-associated PNENs. Because PNENs generally have an indolent course,18 we tested the idea of 18FDG-PET scanning possibly providing adjunct data to anatomic imaging studies that could optimize the management of patients with VHL-associated pancreatic lesions. Indeed, we found 18FDG-PET scanning was helpful for distinguishing between PNENs and MCAs based on a SUVmax cutoff of 4.2. Because VHL-associated MCAs do not have a malignant potential they do not require operative intervention thus avoiding the potential morbidity of surgical treatment. We have implemented this finding in our protocol and only recommend surgical resection of complex or atypical enhancing pancreatic lesions on CT scan when the SUVmax is above 4.0. Our results also suggest that 18FDG-PET is useful in identifying metastatic disease missed by CT scan in patients with primary tumors which would have not fulfilled our size criteria for recommending surgical resection. Thus, 18FDG-PET allows for detection of metastatic disease and non-neoplastic pancreatic lesions in approximately 5% of patients with VHL, which has significant ramifications on their surgical management.
We found that PET avidity of VHL-associated PNENs was slightly higher in women. In non-small cell lung cancer, 18FDG-PET uptake has been shown to correlate negatively with survival in men when compared to women, with body composition not being a factor in the sex difference (lean body mass).19 These data suggest there may be a difference in tumor biology between sexes. In our study, the difference in 18FDG-PET SUVmax by sex could be confounded by body mass differences between sexes as we could not adjust for lean body mass. However, other investigators have shown body mass index (BMI) and gender influence mediastinal and liver 18FDG uptake, with lower uptake in men.20
Several studies have shown 18FDG-PET to be promising as an alternative to tissue sampling for determining aggressiveness of tumors 21 and to be of prognostic value in several other cancers including lung and head and neck cancers.22, 23 In patients with neuroendocrine neoplasms (NENs), investigators have shown that 18FDG-PET may be associated with tumors with high Ki67 indices.24 Binderup et al 25 showed 18FDG-PET to be a predictor of progression-free survival in NENs when the SUVmax was >3.0. However, our data did not show any association between PET avidity and WHO tumor grade, but the number of patients who required an operation was relatively small. We did not observe any association between 18FDG-PET avidity and exon 3 mutation or chromogranin A levels, both of which may be associated with metastatic or persistent/recurrent PNENs.5, 26, 27
There are several limitations to the current study. First, CT was used as the “gold-standard” for the detection of PNENs in patients without pathologic confirmation in all cases. The combination of different modalities is known to provide the greatest accuracy in detection of PNENs,10, 11 and thus our results may overestimate the accuracy of 18FDG-PET in detecting PNENs. Our study was performed in patients with VHL-associated pancreatic lesions so may not be generalizable to other familial cancer syndromes such as MEN1 in which PNENs occur or in cases of sporadic PNENs. Our study also did not adjust for lean body mass when calculating SUVmax, which could have led to the finding of differential uptake by sex but this is not the default in clinical practice and thus our data is more applicable to the clinical setting most physicians obtain interpretations of 18FDG-PET scans. We do not routinely use endoscopic ultrasound with or without biopsy in patients with VHL-associated pancreatic lesions as most neuroendocrine tumors have typical features with arterial enhancement on CT scan and if the lesion is not a neuroendocrine tumor it is consistent with a cystadenoma which have no malignant potential in VHL. Moreover, the purpose of imaging studies in patients with VHL-associated pancreatic lesions is for follow up to detect disease progression and or metastatic disease warranting an operation. We do perform endoscopic ultrasound with or without biopsy in special cases where patients have severe chronic kidney insufficiency as a result of multiple nephrectomies for renal cell carcinoma to avoid the risk of renal failure from intravenous contrast. Lastly, the 28 patients excluded from the cohort analyzed, for the lack of follow up imaging, had on average smaller tumors at enrollment. This may influence the observed association of SUVmax uptake and tumor size.
In conclusion, our data indicate that 18FDG-PET scanning should be considered a valuable adjunct test in the management of patients with VHL-associated PNENs. Semiquantitative SUVmax evaluation might give clinically valuable information as it accurately detects many of these lesions and identifies metastatic disease not seen by CT scan. More accurate identification of patient disease burden and better differentiation between nonmetastatic and metastatic lesions will improve treatment decision-making and avoid unnecessary treatment related morbidity.
Precis.
18-Fluorodeoxyglucose-PET improves detection of metastatic disease and non-neoplastic disease in patients with Von Hippel-Lindau -associated pancreatic lesions.
Acknowledgments
Support: This research was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Abbreviations
- CT
Computed Tomography
- 18FDG-PET
18-Fluorodeoxyglucose-Positron Emission Tomography
- HPF
High power field
- MCA
microcystic adenoma
- MRI
Magnetic Resonance Imaging
- NIH
National Institutes of Health
- NPV
Negative Predictive Value
- PNENs
Pancreatic Neuroendocrine Neoplasms
- PPV
Positive Predictive Value
- VHL
Von Hippel-Lindau
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
References
- 1.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 2.Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 3.Charlesworth M, Verbeke CS, Falk GA, et al. Pancreatic lesions in von Hippel-Lindau disease? A systematic review and meta-synthesis of the literature. J Gastrointest Surg. 2012;16:1422–1428. doi: 10.1007/s11605-012-1847-0. [DOI] [PubMed] [Google Scholar]
- 4.Mohr VH, Vortmeyer AO, Zhuang Z, et al. Histopathology and molecular genetics of multiple cysts and microcystic (serous) adenomas of the pancreas in von Hippel-Lindau patients. Am J Pathol. 2000;157:1615–1621. doi: 10.1016/S0002-9440(10)64799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blansfield JA, Choyke L, Morita SY, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery. 2007;142:814–818. doi: 10.1016/j.surg.2007.09.012. discussion 818 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corcos O, Couvelard A, Giraud S, et al. Endocrine pancreatic tumors in von Hippel-Lindau disease: clinical, histological, and genetic features. Pancreas. 2008;37:85–93. doi: 10.1097/MPA.0b013e31815f394a. [DOI] [PubMed] [Google Scholar]
- 7.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 9.Ehehalt F, Saeger HD, Schmidt CM, Grutzmann R. Neuroendocrine tumors of the pancreas. Oncologist. 2009;14:456–467. doi: 10.1634/theoncologist.2008-0259. [DOI] [PubMed] [Google Scholar]
- 10.Noone TC, Hosey J, Firat Z, Semelka RC. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab. 2005;19:195–211. doi: 10.1016/j.beem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Rockall AG, Reznek RH. Imaging of neuroendocrine tumours (CT/MR/US). Best Pract Res Clin Endocrinol Metab. 2007;21:43–68. doi: 10.1016/j.beem.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Oberg K. Pancreatic endocrine tumors. Semin Oncol. 2010;37:594–618. doi: 10.1053/j.seminoncol.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Binderup T, Knigge U, Loft A, et al. Functional imaging of neuroendocrine tumors: a head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J Nucl Med. 2010;51:704–712. doi: 10.2967/jnumed.109.069765. [DOI] [PubMed] [Google Scholar]
- 14.Tatsumi M, Isohashi K, Onishi H, et al. 18F-FDG PET/MRI fusion in characterizing pancreatic tumors: comparison to PET/CT. Int J Clin Oncol. 2011;16:408–415. doi: 10.1007/s10147-011-0202-x. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson B, Orlefors H, Oberg K, et al. Developments in PET for the detection of endocrine tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:311–324. doi: 10.1016/j.beem.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Adams S, Baum R, Rink T, et al. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med. 1998;25:79–83. doi: 10.1007/s002590050197. [DOI] [PubMed] [Google Scholar]
- 17.Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150:75–82. doi: 10.1016/j.surg.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 19.Wainer Z, Daniels MG, Callahan J, et al. Sex and SUVmax: sex-dependent prognostication in early non-small cell lung cancer. J Nucl Med. 2012;53:1676–1685. doi: 10.2967/jnumed.112.105197. [DOI] [PubMed] [Google Scholar]
- 20.Malladi A, Viner M, Jackson T, et al. PET/CT mediastinal and liver FDG uptake: Effects of biological and procedural factors. J Med Imag Radiat Oncol. 2013;57:169–175. doi: 10.1111/1754-9485.12015. [DOI] [PubMed] [Google Scholar]
- 21.Vesselle H, Schmidt RA, Pugsley JM, et al. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837–3844. [PubMed] [Google Scholar]
- 22.Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–261. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- 23.Torizuka T, Tanizaki Y, Kanno T, et al. Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR. 2009;192:W156–160. doi: 10.2214/AJR.08.1429. [DOI] [PubMed] [Google Scholar]
- 24.Pasquali C, Rubello D, Sperti C, et al. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J Surg. 1998;22:588–592. doi: 10.1007/s002689900439. [DOI] [PubMed] [Google Scholar]
- 25.Binderup T, Knigge U, Loft A, et al. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978–985. doi: 10.1158/1078-0432.CCR-09-1759. [DOI] [PubMed] [Google Scholar]
- 26.Bajetta E, Ferrari L, Martinetti A, et al. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer. 1999;86:858–865. doi: 10.1002/(sici)1097-0142(19990901)86:5<858::aid-cncr23>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Nykjaer KM, Gronbaek H, Nielsen DT, et al. Description of patients with midgut carcinoid tumours: clinical database from a Danish centre. In Vivo. 2007;21:679–684. [PubMed] [Google Scholar]
- 28.Bilimoria KY, Bentrem DJ, Merkow RP, et al. Application of the pancreatic adenocarcinoma staging system to pancreatic neuroendocrine tumors. J Am Coll Surg. 2007;205:558–563. doi: 10.1016/j.jamcollsurg.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Edge S, Labow D, Carduci M. AJCC Cancer Staging Manual. 7th ed. Springer; New York, NY: 2010. [Google Scholar]
- 30.Bosman F, Carneiro F, Hruban R, Theise N. WHO Classification of Tumors of the Digestive System. 4th ed International Agency for Research on Cancer; Lyon, France: 2010. [Google Scholar]