Abstract
Aim:
Proteasome inhibitors have been found to suppress glioma cell proliferation and induce apoptosis, but the mechanisms are not fully elucidated. In this study we investigated the mechanisms underlying the apoptosis induced by the proteasome inhibitor MG-132 in glioma cells.
Methods:
C6 glioma cells were used. MTT assay was used to analyze cell proliferation. Proteasome activity was assayed using Succinyl-LLVY-AMC, and intracellular ROS level was evaluated with the redox-sensitive dye DCFH-DA. Apoptosis was detected using fluorescence and transmission electron microscopy as well as flow cytometry. The expression of apoptosis-related proteins was investigated using Western blot analysis.
Results:
MG-132 inhibited C6 glioma cell proliferation in a time- and dose-dependent manner (the IC50 value at 24 h was 18.5 μmol/L). MG-132 (18.5 μmol/L) suppressed the proteasome activity by about 70% at 3 h. It induced apoptosis via down-regulation of antiapoptotic proteins Bcl-2 and XIAP, up-regulation of pro-apoptotic protein Bax and caspase-3, and production of cleaved C-terminal 85 kDa PARP). It also caused a more than 5-fold increase of reactive oxygen species. Tiron (1 mmol/L) effectively blocked oxidative stress induced by MG-132 (18.5 μmol/L), attenuated proliferation inhibition and apoptosis in C6 glioma cells, and reversed the expression pattern of apoptosis-related proteins.
Conclusion:
MG-132 induced apoptosis of C6 glioma cells via the oxidative stress.
Keywords: C6 glioma cells, apoptosis, proteasome inhibitor, MG-132, tiron, oxidative stress, Bcl-2, Bax, caspase-3, PARP, X chromosome-linked inhibitor of apoptosis (XIAP)
Introduction
The proteasome is an evolutionarily conserved protease complex with multiple catalytic activities and is mainly responsible for selective degradation of abnormal intracellular proteins and many cellular regulatory proteins1. It is also involved in cellular differentiation, antigen presentation and cell cycle modulation2. Therefore, inhibition of proteasome activity has emerged as a new chemotherapy strategy for malignant tumors. Although studies have shown that inhibition of proteasome activity could inhibit cellular proliferation in several tumor cell lines including melanoma, prostate cancer and glioma cells3, 4, 5, 6, its underlying mechanism is not fully understood. The proteasome inhibitor bortezomib has been authorized by the US Food and Drug Administration to enter clinical trials, but it is used mainly in the leukemia field. Thus, more studies are needed to investigate the effects of proteasome inhibition on solid cancers. MG-132 is an aldehyde peptide compound that has shown potent inhibitory effects on proteasome chymotrypsin-like activity and is chemically distinct from bortezomib (a boronic acid dipeptide)7. Although our previous studies in vitro showed that it could induce apoptosis in human glioma cells8, its upstream events are largely unknown.
Oxidative stress is a complex and dynamic situation characterized by overproduction of reactive oxygen species (ROS) that cannot be cleared from cells9. Oxidative stress can damage macromolecules such as lipids, nucleic acids and proteins and trigger cell death through the effects of ROS on signal transduction pathways10. Therefore, oxidative stress could cause cell death via apoptotic pathways. Several studies have shown that oxidative stress is closely associated with the proteasome, as the proteasome is involved in regulating anti-oxidants, including catalase, heme oxygenase-1 (HO-1), copper/zinc-superoxide dismutase and γ-GCS11, 12, 13, 14. However, the relationship between proteasome dysfunction and oxidative stress is still a matter of dispute. In the central nervous system, proteasome dysfunction serves as an important switch for the induction of oxidative stress15. On the other hand, proteasome inhibition has protective effects on vascular cells undergoing oxidative stress14. Even for tumor cells, proteasome inhibition also has varied effects on the production of ROS in different thyroid cancer cell lines16.
Therefore, in this study, we examined the effects of proteasome inhibition on rat C6 glioma cells and investigated the relationship between apoptosis and oxidative stress generated by inhibition of proteasome activity. Thus, tiron (4,5-dihydroxy-1,3-benzene disulfonic acid), a well-known and widely used antioxidant17, was used in this study to attenuate the oxidative stress that proteasome inhibition might induce.
Materials and methods
Reagents
MG-132, obtained from Calbiochem (San Diego, CA, USA), was dissolved in PBS to a storage concentration of 50 μmol/L. DMEM medium was from Gibco (Rockville, MD, USA). Fetal bovine serum (FBS) was from Life Technologies (Grand Island, NY, USA). Antibodies against caspase-3, Bax, and Bcl-2 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-XIAP (X chromosome-linked inhibitor of apoptosis) was from R&D systems (Minneapolis, MN, USA). Anti-poly (ADPribose) polymerase (PARP) and PARP/85 were from BD Biosciences (San Jose, CA, USA). Protein concentration assay kits were from Bio-Rad (Hercules, CA, USA). ECL Western blotting detection reagents were from Amersham (Piscataway, NJ, USA). PVDF membranes were from Millipore Company (Billerica, MA, USA). Other reagents were from Sigma (St Louis, MO, USA).
Cell line and culture
Rat C6 glioma cells were obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM supplemented with 10% fetal bovine serum, 2 mmol/L glutamine (Gibco, Grand Island, NY, USA), penicillin (100 U/mL) and streptomycin (100 μg/mL) and maintained at 37 °C and 5% CO2 in a humid environment. Cells in the mid-log phase were used for experiments.
Cell viability assay
C6 glioma cells were seeded onto 96-well microplates (3×104 cells/well) and cultured for 24 h. The cells were treated with PBS or MG-132 final concentrations of 10, 20, 30, and 40 μmol/L, respectively. Cell viability was assessed using an MTT assay at 3, 6, 12, and 24 h after MG-132 treatment. The absorbance value (A) at 570 nm was read using an automatic multi-well spectrophotometer (Bio-Rad, Richmond, CA, USA).
Hoechst 33342 staining
C6 glioma cells (3×105 cells/well) were allowed to grow on coverslips in 6-well culture plates (Nunc, Denmark) for 24 h. The cells were then treated with either PBS (control) or 18.5 μmol/L MG-132 at 37 °C for 24 h. Cells growing on glass coverslips were fixed in methanol for 5 min at room temperature. The fixed cells were washed twice with PBS and then incubated with Hoechst 33342 for 5 min at room temperature and observed under a fluorescence microscope. Fragmented or condensed nuclei were scored as apoptotic.
Transmission electron microscopy
C6 glioma cells were cultured and treated as described above and harvested using 0.25% trypsin and washed with PBS. Then the cells were collected by centrifugation at 800×g for 10 min and treated as described by Watkins and Cullen18. Briefly, the cells were fixed in ice-cold 2.5% glutaraldehyde in PBS (pH 7.3), rinsed with PBS and post-fixed in 1% osmium tetroxide with 0.1% potassium ferricyanide, dehydrated through a graded series of ethanol (30%–90%) and embedded in Epon (Energy Beam Sciences, Agawam, MA, USA). Semithin (300 nm) sections were cut using a Reichart Ultracut, stained with 0.5% toluidine blue and examined under a light microscope. Ultrathin sections (65 nm) were stained with 1% uranyl acetate and 0.1% lead citrate and examined on a JEM2000EX transmission electron microscope (JEOL, Pleasanton, CA, USA).
Proteasome activity assay
After growing on six-well plates (3×105 cells/well) for 24 h, C6 glioma cells were treated with either PBS (control) or 18.5 μmol/L MG-132 for 3, 6, 12, or 24 h at 37 °C. Cells were thoroughly scraped from the culture dishes with a cell scraper and washed with cold PBS. After centrifugation for 10 min at 800×g, the cell pellets were suspended in ice-cold buffer (50 mmol/L Tris-HCl, pH 7.5, 20 μmol/L ATP, 5 mmol/L MgCl2, 1 mmol/L dithiothreitol, and 20% glycerol) and homogenized with a Pyrex glass microhomogenizer (20 strokes). The homogenate was centrifuged at 15 000×g for 10 min at 4 °C to obtain supernatant. Protein concentration was determined using protein assay kits (Bio-Rad Laboratories). A total of 10 μL (1 μg/μL) of each freshly made supernatant was incubated in a 96-well plate at 37 °C for 30 min with 10 μL of 300 μmol/L of Succinyl-LLVY-AMC (Calbiochem, San Diego, CA, USA) and 85 μL of assay buffer (20 mmol/L Tris-HCl, pH 7.5, and 20% glycerol). Release of fluorescent AMC was measured with a spectrofluorometer (Perkin-Elmer Life and Analytical Sciences, Inc, Wellesley, MA, USA) at 440 nm with an excitation wavelength of 380 nm.
Measurement of intracellular ROS levels
The average level of intracellular ROS in C6 glioma cells was evaluated in cells loaded with the redox-sensitive dye DCFH-DA (Molecular Probes, OR, USA). Cells treated with 18.5 μmol/L MG-132 alone were examined at 3, 6, 12, or 24 h; cells pretreated with 1.0 mmol/L tiron before incubation with 18.5 μmol/L MG-132 were examined at 24 h. The cells treated with PBS were used as control. All experimental cells were washed twice in phosphate-buffered saline (PBS) and stained in the dark for 30 min with 20 μmol/L DCFH-DA and harvested. Cells were dissolved with 1% Triton X-100, and fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength 530 nm using a fluorescence spectrometer (HTS 7000, Perkin Elmer, Boston, MA, USA). ROS levels were expressed as arbitrary units/mg protein, then as a percentage of controls.
Detection of apoptosis and cell cycle
After 12 h of starvation in serum free DMEM/F12, C6 glioma cells were treated with 18.5 μmol/L MG-132 for 24 h or pretreated with 1.0 mmol/L tiron prior to 24 h incubation with 18.5 μmol/L MG-132. Cells treated with PBS were used as controls. Then, all the cells were washed with PBS, counted and adjusted to 1×106 cells/mL. The cells were fixed in 70% ethanol at 4 °C overnight, treated with 100 mg/L RNase at 37 °C for 30 min and stained with 50 mg/L propidium iodide (Sigma) for 30 min. The cells were analyzed using flow cytometry (FAC2Scan, Becton Dickinson, San Jose, CA, USA). The rate of apoptosis and cell cycle status were analyzed using CELLquest software (Becton Dickinson). Data were acquired by collecting 20 000 cells per tube; the numbers of viable and apoptotic cells were determined for each experimental condition.
Western blotting
C6 glioma cells were cultured, harvested and washed as described above. After centrifugation for 10 min at 1000×g, the cell pellets were suspended in ice-cold buffer (15 mmol/L Tris, pH 7.6, 250 mmol/L sucrose, 1 mmol/L MgCl2, 2.5 mmol/L EDTA, 1 mmol/L EGTA (ethylene glycol-bis (b-amino ethylether) tetraacetic acid), 1 mmol/L dithiothreitol, 1.25 mg/mL pepstatin A, 10 mg/mL leupeptin, 2.5 mg/mL aprotinin, 1.0 mmol/L phenylmethylsulfonyl fluoride (PMSF), 0.1 mmol/L Na3VO4, 50 mmol/L NaF, and 2.0 mmol/L Na4P2O7) and homogenized with a Pyrex glass microhomogenizer (20 strokes). Homogenates were centrifuged at 10 000×g at 4 °C for 10 min to obtain supernatant. The protein content of the supernatant was determined using Bio-Rad protein assay kits.
Equal amounts of protein were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gels and then transferred to PVDF membranes. The membranes were blocked with 3% bovine serum albumin in TBS for 30 min and then incubated overnight at 4 °C with the following primary antibodies: caspase-3, Bax, Bcl-2, XIAP, PARP, PARP/85, and β-actin. After being incubated with horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG, the blots were washed, and immunoreactive proteins were visualized on Kodak X-omat LS film (Eastman Kodak Company, New Haven, CT, USA) with an enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ, USA). Densitometry was performed with Kodak ID image analysis software (Eastman Kodak Company).
Statistical analysis
All data represent at least 4 independent experiments and are expressed as the mean±SD. Statistical comparisons were made using Student's t-test. P values of less than 0.05 were considered significant.
Results
MG-132 inhibited C6 glioma cell proliferation and inhibited proteasome chymotrypsin-like activity
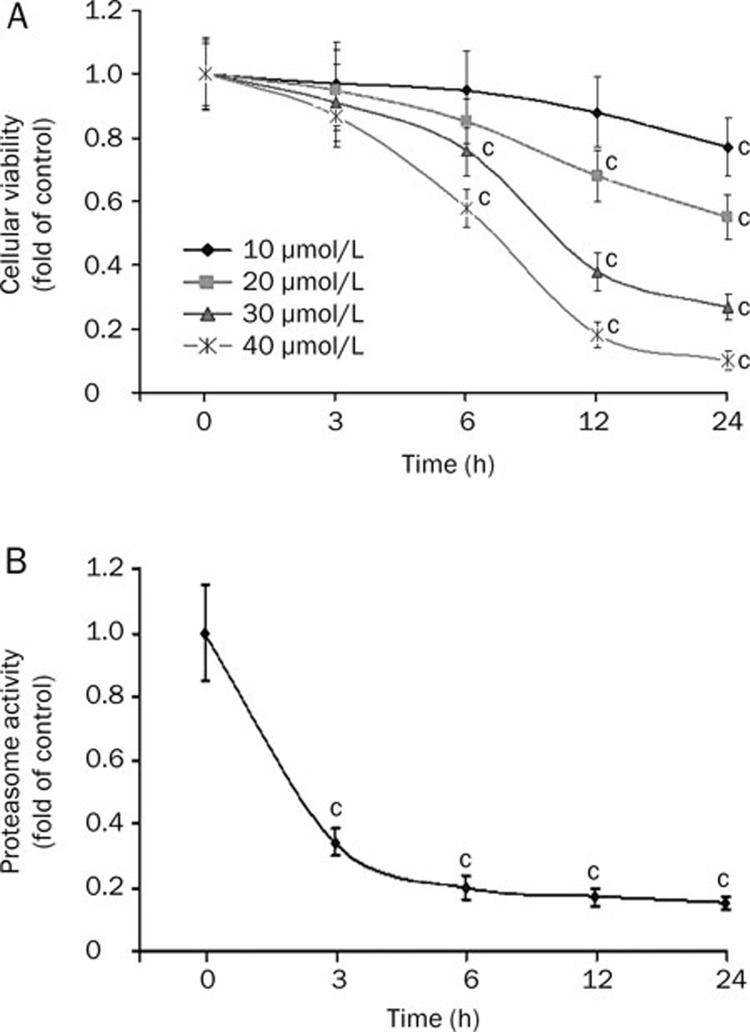
MG-132 significantly reduced the viability of C6 glioma cells beginning at 6 h in both time- and concentration-dependent manners (Figure 1A). At 24 h, the inhibitory effect reached a maximum, with inhibition rates of 23%, 45%, 73%, and 90% at concentrations of 10, 20, 30, and 40 μmol/L, respectively. The IC50 of MG-132 at 24 h was 18.5 μmol/L. Then, we assayed the inhibitory effect of 18.5 μmol/L MG-132 on proteasomal chymotrypsin-like activity using Succinyl-LLVY-AMC as a specific substrate18. MG-132 gradually inhibited enzymatic activity from 3 to 24 h (P<0.01, Figure 1B). This result suggested that 18.5 μmol/L MG-132 could effectively suppress proteasome activity and that proteasome inhibition occurred earlier (3 h) than that of viability reduction (6 h).
Figure 1.
Cellular viability assay and proteasome activity assay. (A) MTT assay of cell viability. The proliferation of C6 glioma cells was inhibited after 3 h incubation with MG-132; at 24 h, the maximal inhibitory effect was reached. (B) Proteasome activity assay. Proteasome activity in C6 glioma cells was significantly inhibited at 3 h by 18.5 μmol/L MG-132. (cP<0.01 vs control group).
Tiron blocked MG-132-induced oxidative stress
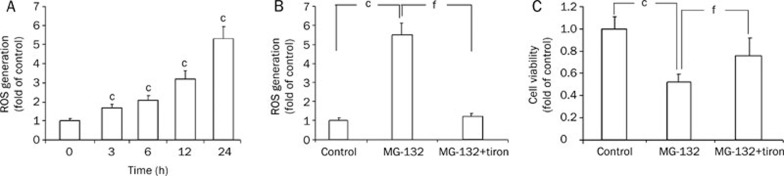
Compared with control cells, the ROS generated by MG-132 increased in a time-dependent manner in C6 glioma cells from 3 h to 24 h (P<0.01, Figure 2A). Then, tiron was used to investigate the relationship between oxidative stress and the viability reduction of C6 glioma cells after these cells were treated with 18.5 μmol/L MG-132 for 24 h. We found that 1.0 mmol/L tiron effectively blocked the generation of ROS (P<0.01 versus MG-132 group, Figure 2B), but it did not influence the viability of C6 glioma cells (data not shown). Furthermore, pretreatment with tiron significantly attenuated MG-132 (18.5 μmol/L)-induced cell viability reduction (P<0.01, Figure 2C), indicating that the effects of MG-132 in C6 glioma cells might be due partly to oxidative stress.
Figure 2.
MG-132-induced oxidative stress. (A) ROS was generated in the MG-132-treated (18.5 μmol/L) C6 glioma cells at 3 h, increasing continuously to a peak at 24 h. When tiron (1 mmol/L) was used, ROS generation was blocked (B), and the viability of C6 glioma cells also increased significantly (C). Mean±SD. n=4. cP<0.01 vs control group, fP<0.01 vs MG-132 group.
Tiron suppressed MG-132-induced apoptosis and cell cycle arrest
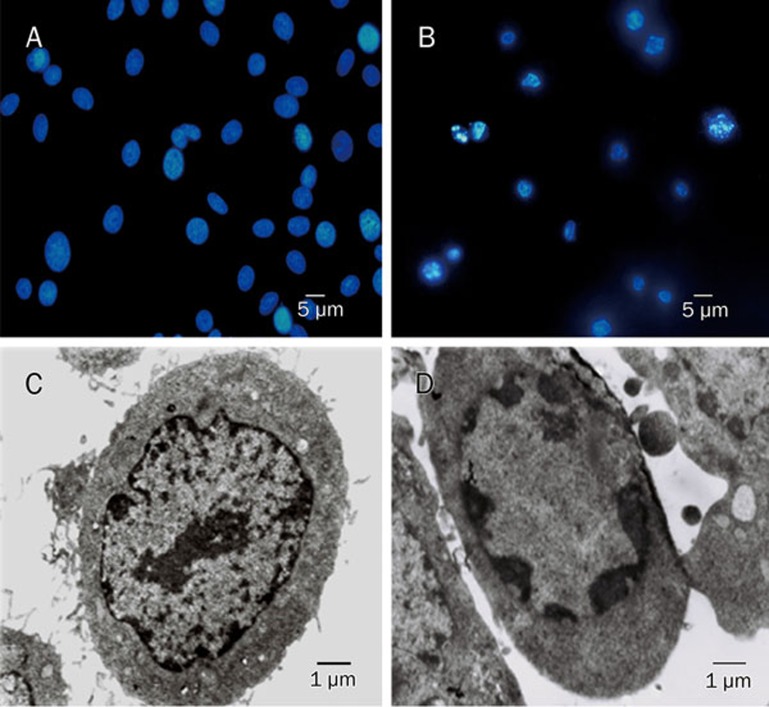
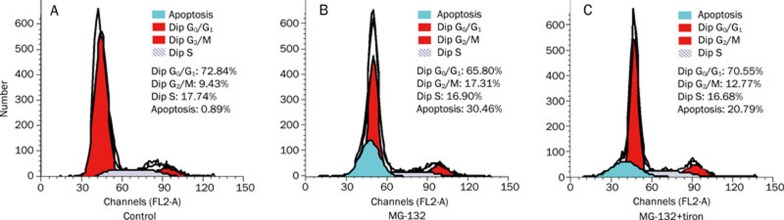
Chromatin accumulation under the nuclear membrane, nuclear condensation and DNA fragmentation were revealed by both fluorescence microscopy and transmission electron microscopy after C6 glioma cells were treated with 18.5 μmol/L MG-132 for 24 h (Figure 3). These morphological features were consistent with the characteristics of apoptosis reported previously18, indicating that apoptosis was induced by MG-132 in C6 glioma cells. Subsequent examination by flow cytometry showed that tiron suppressed MG-132-induced apoptosis in C6 glioma cells. The apoptosis rate decreased from 30.46% to 20.79%, and the percentage of cells arrested in G2/M phase was reduced from 17.31% to 12.77% (Figure 4). These results suggest that the apoptosis induced by MG-132 was closely associated with oxidative stress.
Figure 3.
C6 glioma cells displayed apoptotic morphological features after incubation with MG-132. Fluorescence microscopy (A: control group and B: MG-132 group) and transmission electron microscopy (C: control group and D: MG-132 group) showed chromatin condensation, nuclear condensation and nuclear fragmentation in the nucleus of C6 glioma cells treated with MG-132.
Figure 4.
Tiron suppressed apoptosis and cell cycle arrest induced by MG-132. Flow cytometry analysis of apoptotic rate and cell cycle. In the control group (A), the apoptotic rate was 0.89%, and the percentage of the cells in G2/M phase was 9.43%. Tiron effectively suppressed the increase of apoptosis rate (from 30.46% to 20.79%) and cell cycle arrest in G2/M phase (from 17.31% to 12.77%) induced by MG-132 (B and C).
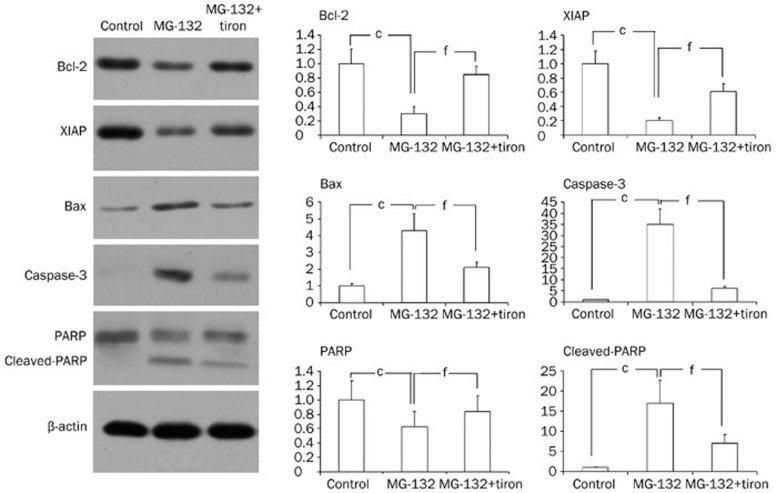
Tiron attenuated the expression of apoptosis-related proteins
Previous studies have shown that the expression of apoptotic machinery proteins such as Bcl-2, XIAP (X chromosome-linked inhibitor of apoptosis), Bax, caspase-3, and PARP is altered by oxidative stress19. In the present study, Western blot analysis revealed that the expressional level of pro-apoptotic proteins Bax and caspase-3 increased significantly, whereas the expressional level of anti-apoptotic proteins Bcl-2 and XIAP decreased markedly after C6 glioma cells were incubated with 18.5 μmol/L MG-132 for 24 h (Figure 5). However, tiron attenuated the MG-132-induced expression patterns of the above-mentioned pro-apoptotic and anti-apoptotic proteins (Figure 5). Meanwhile, tiron significantly reduced the quantity of cleaved PARP as well (Figure 5). These results indicated that tiron rescues MG-132-induced apoptosis in C6 glioma cells by influencing the expression of apoptosis-related proteins.
Figure 5.
Tiron altered the expression of apoptosis-related proteins. Western blot analysis revealed that MG-132 induced down-regulation of anti-apoptotic proteins Bcl-2 and XIAP, up-regulated expression of pro-apoptotic protein Bax and caspase-3, and produced cleaved C-terminal 85 kDa PARP. Additionally, tiron reversed these alterations. cP<0.01 vs control; fP<0.01 vs MG-132 group.
Discussion
In this study, we found that MG-132, a competitive inhibitor of proteasome chymotrypsin-like activity, induces apoptosis of C6 glioma cells via oxidative stress. We found that proteasome activity is increased in malignant cells derived from different types of cancer as compared with normal cells20, indicating that malignant cells have a need for higher proteasome activity. Therefore, proteasome inhibition would theoretically exert fatal effects on malignant tumor cells, but not on normal cells.
Although it has been reported that nontoxic proteasome inhibition protected cells from oxidative stress21, our results showed that proteasome inhibitor MG-132 at toxic concentrations could generate oxidative stress and induce apoptosis in glioma cells. Oxidative stress has been demonstrated to be closely associated with cellular death in various tumor cells9, 16, 22. Studies investigating the relationship between oxidative stress and apoptosis have indicated that oxidative stress may be a precursor to apoptosis in C6 glioma cells23, 24. In the present study, we found that selective inhibition of proteasome activity by MG-132 produced a cause-effect relationship between oxidative stress and apoptosis in rat C6 glioma cells.
Although we did not further investigate the mechanism underlying the oxidative stress produced via the inhibition of proteasome activity, previous reports showed that abnormal or unfolded intracellular proteins would aggregate if they could not be degraded via the proteasome pathway, leading to the generation of reactive oxygen species, the most prominent marker of oxidative stress25.
In addition, the present study showed that blocking oxidative stress with the anti-oxidant tiron attenuated the decreased expression of Bcl-2 and XIAP, both of which are not only anti-apoptotic factors but also function as regulators of anti-oxidants26, 27. Bcl-2 increases cellular resistance to H2O2 by increasing glutathione levels and Cu/Zn-superoxide dismutase (SOD1) activity28. XIAP induces up-regulation of at least three antioxidants residing in mitochondria, including superoxide dismutase 2, thioredoxin 2 and lysine oxoglutarate reductase29. Therefore, decreased expression of Bcl-2 and XIAP would exacerbate the severity of oxidative stress caused by MG-132, forming a malignant feedback loop.
In addition to our findings on the alterations of anti-apoptotic and pro-apoptotic proteins in the present study, previous studies have revealed some typical features of apoptosis when glioma cells were treated with different proteasome inhibitors. It has been reported that MG-132 could trigger cytochrome c release and disruption of mitochondrial membrane potential, which were concurrent with DNA breaks and loss of membrane integrity. Further studies showed that proteasome inhibitor-induced apoptosis was dependent on caspases because multiple caspases such as caspase 2, 3, 7, 8, and 9 were activated and apoptosis was inhibited when glioma cells were exposed to the broad-spectrum caspase inhibitor zVAD-fmk (benzoyl-VAD-fluoromethyl ketone). Additionally, proteasome inhibitors could reduce the transcriptional activity and expression level of anti-apoptotic nuclear NF-kappaB30. These findings indicated that inhibition of proteasome activity is an effective strategy for inducing apoptosis in glioma cells.
Proteasome inhibitors could also activate intracellular signaling pathways. However, activation of the PI3K/AKT pathways impaired the response of tumor cells to proteasome inhibitor treatment, and inhibition of the PI3K/AKT pathways increased the antitumor effects of proteasome inhibitors31. By contrast, activation of the JNK/c-Jun pathway was closely associated with growth inhibition in human glioblastoma cells5.
In addition to biochemical changes, morphological alteration is an important criterion for evaluating apoptosis. As described previously18, apoptotic changes in morphology such as chromatin accumulation, nuclear condensation and DNA fragmentation were observed in the present study. Moreover, dilated rough endoplasmic reticulum (ER), dense mitochondrial deposits and cytoplasmic vacuolization could be found when proteasome activity was inhibited30. Interestingly, these non-apoptotic alterations could still exist even if zVAD-fmk was used to block apoptosis, indicating that proteasome inhibition might lead to cellular death via another mechanism.
In summary, we showed that inhibition of proteasome activity by MG-132 is an effective way to suppress the proliferation of C6 glioma cells via the induction of apoptosis. Tiron not only blocked the oxidative stress caused by MG-132 but also suppressed MG-132-induced apoptosis by decreasing the expression of Bax and caspase-3, rescuing the expression of Bcl-2 and XIAP and attenuating PARP cleavage. These results suggest that there is a cause and effect relationship between oxidative stress and apoptosis induced by MG-132. Although the present study demonstrated that the inhibition of proteasome activity induced apoptosis in C6 glioma cells via oxidative stress, further research is needed to evaluate this phenomenon on solid gliomas in vivo.
Author contribution
Prof Yi-nan LUO and Dr Peng-fei GE designed the research; Wen-hai FAN, Yi HOU, and Fan-kai MENG performed the research; Xiao-fei WANG analyzed data; and Peng-fei GE wrote the paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81072071 and 30973110), Fundamental Research Funds for the Central Universities (No 421030863428), and an Outstanding Youth Grant (No 20080139) from the Science and Technology Department of Jilin Province.
References
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–6. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 2005;12:1191–7. doi: 10.1038/sj.cdd.4401702. [DOI] [PubMed] [Google Scholar]
- Sorolla A, Yeramian A, Dolcet X, de Santos AMPérez, Llobet D, Schoenenberger JA, et al. Effect of proteasome inhibitors on proliferation and apoptosis of human cutaneous melanoma-derived cell lines. Br J Dermatol. 2008;158:496–504. doi: 10.1111/j.1365-2133.2007.08390.x. [DOI] [PubMed] [Google Scholar]
- Momose I, Iijima M, Kawada M, Ikeda D. A new proteasome inhibitor, TP-110, induces apoptosis in human prostate cancer PC-3 cells. Biosci Biotechnol Biochem. 2007;71:1036–43. doi: 10.1271/bbb.60697. [DOI] [PubMed] [Google Scholar]
- Yin D, Zhou H, Kumagai T, Liu G, Ong JM, Black KL, et al. Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM) Oncogene. 2005;24:344–54. doi: 10.1038/sj.onc.1208225. [DOI] [PubMed] [Google Scholar]
- Legnani FG, Pradilla G, Thai QA, Fiorindi A, Recinos PF, Tyler BM, et al. Lactacystin exhibits potent anti-tumor activity in an animal model of malignant glioma when administered via controlled-release polymers. J Neurooncol. 2006;77:225–32. doi: 10.1007/s11060-005-6937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan BZ, Chapman J, Reynolds SH. Proteasome inhibitors induce apoptosis in human lung cancer cells through a positive feedback mechanism and the subsequent Mcl-1 protein cleavage. Oncogene. 2009;28:3775–86. doi: 10.1038/onc.2009.240. [DOI] [PubMed] [Google Scholar]
- Ge PF, Zhang JZ, Wang XF, Meng FK, Li WC, Luan YX, et al. Inhibition of autophagy induced by proteasome inhibition increases cell death in human SHG-44 glioma cells. Acta Pharmacol Sin. 2009;30:1046–52. doi: 10.1038/aps.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvorovic J, Tramer F, Granzotto M, Candussio L, Decorti G, Passamonti S. Oxidative stress-based cytotoxicity of delphinidin and cyanidin in colon cancer cells. Arch Biochem Biophys. 2010;501:151–7. doi: 10.1016/j.abb.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Yu JW, Yang R, Kim YS. Differential cytoprotective effect of copper- and iron-containing chlorophyllins against oxidative stress-mediated cell death. Free Radic Res. 2010;44:655–67. doi: 10.3109/10715761003733893. [DOI] [PubMed] [Google Scholar]
- Wu WT, Chi KH, Ho FM, Tsao WC, Lin WW. Proteasome inhibitors up-regulate haem oxygenase-1 gene expression: requirement of p38 MAPK (mitogen-activated protein kinase) activation but not of NF-kappaB (nuclear factor kappaB) inhibition. Biochem J. 2004;379:587–93. doi: 10.1042/BJ20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Leng Y, Liu X, Yi Y, Li P, Kufe D. Catalase is regulated by ubiquitination and proteosomal degradation. Role of the c-Abl and Arg tyrosine kinases. Biochemistry. 2003;42:10348–53. doi: 10.1021/bi035023f. [DOI] [PubMed] [Google Scholar]
- Hoffman EK, Wilcox HM, Scott RW, Siman R. Proteasome inhibition enhances the stability of mouse Cu/Zn superoxide dismutase with mutations linked to familial amyotrophic lateral sclerosis. J Neurol Sci. 1996;139:15–20. [PubMed] [Google Scholar]
- Yamamoto N, Sawada H, Izumi Y, Kume T, Katsuki H, Shimohama S, et al. Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress: relevance to Parkinson disease. J Biol Chem. 2007;282:4364–72. doi: 10.1074/jbc.M603712200. [DOI] [PubMed] [Google Scholar]
- Ding Q, Dimayuga E, Keller JN. Proteasome regulation of oxidative stress in aging and age-related diseases of the CNS. Antioxid Redox Signal. 2006;8:163–72. doi: 10.1089/ars.2006.8.163. [DOI] [PubMed] [Google Scholar]
- Du ZX, Zhang HY, Meng X, Guan Y, Wang HQ. Role of oxidative stress and intracellular glutathione in the sensitivity to apoptosis induced by proteasome inhibitor in thyroid cancer cells. BMC Cancer. 2009;9:56. doi: 10.1186/1471-2407-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Cho EW, Chung HW, Kim IG. Effects of Tiron, 4, 5-dihydroxy-1, 3-benzene disulfonic acid, on human promyelotic HL-60 leukemia cell differentiation and death. Toxicology. 2006;223:36–45. doi: 10.1016/j.tox.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Watkins SC, Cullen MJ. A qualitative and quantitative study of the ultrastructure of regenerating muscle fibres in Duchenne muscular dystrophy and polymyositis. J Neurol Sci. 1987;82:181–92. doi: 10.1016/0022-510x(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Jungas T, Motta I, Duffieux F, Fanen P, Stoven V, Ojcius DM. Glutathione levels and BAX activation during apoptosis due to oxidative stress in cells expressing wild-type and mutant cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:27912–8. doi: 10.1074/jbc.M110288200. [DOI] [PubMed] [Google Scholar]
- Chen L, Madura K. Increased proteasome activity, ubiquitinconjugating enzymes, and eEF1A translation factor detected in breast cancer tissue. Cancer Res. 2005;65:5599–606. doi: 10.1158/0008-5472.CAN-05-0201. [DOI] [PubMed] [Google Scholar]
- Meiners S, Ludwig A, Lorenz M, Dreger H, Baumann G, Stangl V, et al. Nontoxic proteasome inhibition activates a protective antioxidant defense response in endothelial cells. Free Radic Biol Med. 2006;40:2232–41. doi: 10.1016/j.freeradbiomed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu G, et al. Pro-oxidative activities and dose-response relationship of (–)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010;31:902–10. doi: 10.1093/carcin/bgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wätjen W, Beyersmann D. Cadmium-induced apoptosis in C6 glioma cells: influence of oxidative stress. Biometals. 2004;17:65–78. doi: 10.1023/a:1024405119018. [DOI] [PubMed] [Google Scholar]
- Chen TJ, Jeng JY, Lin CW, Wu CY, Chen YC. Quercetin inhibition of ROS-dependent and -independent apoptosis in rat glioma C6 cells. Toxicology. 2006;223:113–26. doi: 10.1016/j.tox.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Tabner BJ, El-Agnaf OM, German MJ, Fullwood NJ, Allsop D. Protein aggregation, metals and oxidative stress in neurodegenerative diseases. Biochem Soc Trans. 2005;33:1082–6. doi: 10.1042/BST20051082. [DOI] [PubMed] [Google Scholar]
- Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–51. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Zhu C, Xu F, Fukuda A, Wang X, Fukuda H, Korhonen L, et al. X chromosome-linked inhibitor of apoptosis protein reduces oxidative stress after cerebral irradiation or hypoxia-ischemia through up-regulation of mitochondrial antioxidants. Eur J Neurosci. 2007;26:3402–10. doi: 10.1111/j.1460-9568.2007.05948.x. [DOI] [PubMed] [Google Scholar]
- Lee M, Hyun DH, Marshall KA, Ellerby LM, Bredesen DE, Jenner P, et al. Effect of overexpression of BCL-2 on cellular oxidative damage, nitric oxide production, antioxidant defenses, and the proteasome. Free Radic Biol Med. 2001;31:1550–9. doi: 10.1016/s0891-5849(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Resch U, Schichl YM, Sattler S, de Martin R. XIAP regulates intracellular ROS by enhancing antioxidant gene expression. Biochem Biophys Res Commun. 2008;375:156–61. doi: 10.1016/j.bbrc.2008.07.142. [DOI] [PubMed] [Google Scholar]
- Wagenknecht B, Hermisson M, Groscurth P, Liston P, Krammer PH, Weller M. Proteasome inhibitor-induced apoptosis of glioma cells involves the processing of multiple caspases and cytochrome c release. J Neurochem. 2000;75:2288–97. doi: 10.1046/j.1471-4159.2000.0752288.x. [DOI] [PubMed] [Google Scholar]
- Sloss CM, Wang F, Liu R, Xia L, Houston M, Ljungman D, et al. Proteasome inhibition activates epidermal growth factor receptor (EGFR) and EGFR-independent mitogenic kinase signaling pathways in pancreatic cancer cells. Clin Cancer Res. 2008;14:5116–23. doi: 10.1158/1078-0432.CCR-07-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]