Abstract
Aim:
To explore the pathogenic role of Th17 cells and interleukin-17A (IL-17A)-associated signaling pathways in spontaneous pulmonary emphysema induced by a Toll-like receptor 4 mutant (TLR4mut).
Methods:
Lungs were obtained from wild-type (WT) or TLR4mut mice that were treated with or without recombinant mouse IL-17A (1 μg·kg−1·d−1, ip) from the age of 3 weeks to 3 months. Pulmonary emphysema was determined using histology, immunochemistry, and biochemical analysis. T cell polarization was determined with flow cytometry, the levels of cytokines were measured using ELISA, and the levels of IL-17A-associated signaling molecules were detected using Western blot.
Results:
Compared to WT mice, 3 month-old TLR4mut mice were characterized by significantly reduced infiltration of Th17 cells into lungs (2.49%±1.13 % νs 5.26%±1.39%), and significantly reduced expression levels of IL-17A (3.66±0.99 pg/μg νs 10.67±1.65 pg/μg), IL-23 (12.43±1.28 pg/μg νs 28.71±2.57 pg/μg) and IL-6 (51.82±5.45 pg/μg νs 92.73±10.91 pg/μg) in bronchoalveolar lavage fluid. In addition, p38 MAPK phosphorylation and AP-1 expression were decreased to 27%±9% and 51%±8%, respectively, of that in WT mice. Treatment of TLR4mut mice with IL-17A increased the infiltration of Th17 cells into lungs and expression levels of IL-17A, IL-6, and IL-23 in bronchoalveolar lavage fluid, attenuated MDA and apoptosis, and improved emphysema accompanied with increased phosphorylation of p38 MAPK and expression of AP-1.
Conclusion:
Th17 cells, in particular the cytokine IL-17A, play a crucial role in the pathogenesis of TLR4mut-induced spontaneous pulmonary emphysema. Both of them are potential targets for therapeutic strategies for pulmonary emphysema.
Keywords: emphysema, IL-17A, Th17 cells, Toll-like receptor 4
Introduction
Chronic obstructive pulmonary disease (COPD), which is defined as airflow obstruction that is not fully reversible, was ranked fifth among causes of death globally in 2002; however, it is expected to be the third most common cause of death in 2020. The main pathological changes associated with COPD include chronic bronchitis, emphysema and small-airway disease1. The symptoms of emphysema partially overlap with COPD, and it is one manifestation of a group of chronic, obstructive, and frequently progressive destructive lung diseases2. Emphysema is characterized by destruction of the alveolar walls and permanent enlargement of the air spaces distal to the terminal bronchioles, which contribute to a reduction in the forced expiratory volume in 1 second (FEV1)3. Inflammation, cellular apoptosis, oxidative stress, and protease/antiprotease imbalance are involved in the pathogenesis of emphysema4, 5. Chemokines and other chemoattractants, such as interleukin-8 (IL-8), monocyte chemotactic protein-1 (MCP-1), and LTB4, promote the accumulation of inflammatory cells, including macrophages and neutrophils, in the lungs. Lymph follicles containing dendritic cells (DCs) and Th1 cells are present in the parenchyma, which suggests a potential role of the immune response in emphysematous destruction. Also, macrophages and neutrophils can be activated by various insults, such as cigarette smoke, and can subsequently produce reactive oxygen species (ROS) and many inflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-1β, and IL-6. In return, inflammation-activated macrophages and neutrophils release proteinases, including elastases and matrix metalloproteinase-9. These proteinases contribute to the degradation of extracellular matrix components and eventually to the development of emphysema. However, anti-inflammatory agents or antioxidants only reduce the symptoms, and they do not affect the decline in FEV16. Thus, further investigation is needed to understand the mechanisms underlying the pathogenesis of emphysema.
There have been many studies that have indicated that Toll-like receptors (TLRs) and TLR-mediated autoimmunity play critical roles in the development and resolution of emphysema7, 8. TLRs, as a critical family of pattern recognition receptors, can initiate and orchestrate innate and adaptive immune responses; in addition, they function as sensors of damage-associated molecular pattern and are involved in multiple noninfectious inflammatory diseases, such as acute respiratory distress syndrome, asthma, and COPD. TLR4 and TLR2 play a prominent role in gene-environment interactions that are relevant to COPD-related phenotypes because the interaction between the microbial load and the immune system is a critical determinant of both immune function and asthma/emphysema susceptibility9. TLR ligands can activate DCs and subsequently determine the direction of T cell polarization10. TLR4 mutation results in pulmonary emphysema through upregulation of NADPH oxidase (Nox) 3 in the lungs and endothelial cells, which enhance oxidant generation and elastolytic activity7. Importantly, reduction/oxidation (redox) is a novel factor that has been shown to regulate T cell polarization11. Furthermore, the accumulation of ROS is critical for the differentiation of naïve Th cells into Th1 or Th17 cells12. A recent study has indicated that IL-17 promotes the growth of airway epithelial cells and that Th17 cells play an important role in inflammation and autoimmune diseases, including emphysema13.
Therefore, we wondered whether T cell polarization, and in particular differentiation into the Th17 phenotype, was involved in the pathogenesis of TLR4mut-induced spontaneous pulmonary emphysema. We found that TLR4 mut-induced spontaneous pulmonary emphysema is associated with reductions in the amount of Th17 cell infiltration into the lungs and in the production of Th17-associated cytokines. Administration of recombinant mouse IL-17A reversed TLR4mut-induced pulmonary emphysema by enhancing the phosphorylation of p38 MAP kinase and the expression of AP-1, suggesting that Th17 cells and IL-17A associated signaling pathways play a crucial role in the pathogenesis of pulmonary emphysema. Furthermore, our results indicate that the IL-17A signaling pathway is a potential target for the development of therapeutics against pulmonary emphysema.
Materials and methods
Materials
In situ cell death detection kits were purchased from Roche Diagnostics Ltd (East Sussex, UK). Malondialdehyde (MDA) assay kits were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Anti-cleaved caspase 3 was from Cell Signaling (Danvers, MA, USA). ELISA kits for IL-17A, IL-23, IL-6, and TGF-β1 were purchased from eBioscience (San Diego, CA, USA). FITC/PE-conjugated anti-CD4, FITC-conjugated anti-IFN-γ, PE-conjugated anti-IL-13, FITC-conjugated anti-CD4, PE-conjugated anti-CD25, PE-conjugated anti-IL-17A antibodies were purchased from eBioscience. Recombinant mouse IL-17A was purchased from R&D Systems (Minneapolis, MN, USA).
Animals
TLR4mut (C3H/HeJ) mice and corresponding wild-type (WT) mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Mice were maintained under specific pathogen free conditions at the Experimental Animal Center of the Institute of Materia Medica. For therapeutic treatment, TLR4mut mice were randomized into 2 groups and administered recombinant mouse IL-17A (1 μg/kg body weight, ip) or an identical volume of vehicle once every day from 3 weeks of age to the end of the experiment. Mice were sacrificed by injection of excess pentobarbital sodium at 3 months of age. The study protocol was approved by the Institutional Committee for the Ethics of Animal Care and Treatment.
Bronchoalveolar lavage fluid (BALF)
Mice were anesthetized and the lungs were lavaged with 0.6 mL of ice-cold phosphate balance solution (PBS). BALF was centrifuged at 100×g for 15 min at 4 °C. The supernatant was decanted and stored at −80 °C for further analysis.
Measurement of lung histology and morphometry
Animals were anesthetized and the lungs were perfused with 4% neutral buffered formalin for 20 min (n=6 in each group) via the tracheal cannula at an airway pressure of 25 cm H2O14. The fixed lungs were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE) for histological analyses. The average distance between alveolar walls and the mean linear intercept (Lm) were calculated according to established methods15. Briefly, Lm was calculated as the total length of each line of the grid using a 100 μm×100 μm grid passing randomly through the lung and divided by the number of alveolar intercepts. For each pair of lungs, 6 histological fields were evaluated. Lung sections were processed for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using an in situ cell death detection kit.
Assay of lipid peroxides
MDA was measured using an MDA assay kit from Nanjing Jiancheng Bioengineering Institute according to the manufacturer's instructions16.
ELISAs for cytokines in BALF
The concentrations of IL-17A, IL-23, IL-6, and TGF-β1 in BALF were detected using ELISA kits in accordance with the manufacturer's instructions.
Preparation of single-cell lung suspensions
Single-cell suspensions were prepared from the right lung, as previously described17. Briefly, the lung vasculature of anesthetized mice was perfused with PBS until free of blood. The lung was minced; digested with 1 mL digestion medium consisting of RPMI-1640, 1 mg/mL collagenase type 2 (Roche Diagnostics; Indianapolis, IN, USA) and 0.02 mg/mL DNase I (grade II from bovine pancreas) for 60 min at 37 °C; subjected to red blood cell lysis (eBioscience); passed through a 100 μm cell strainer; and kept on ice until labeling.
Flow cytometry
The Th1/Th2 mouse T cell subpopulations in single-cell lung suspensions were labeled with FITC-conjugated anti-CD4, PE-conjugated anti-IFN-γ, and Alexa 647-conjugated anti-IL-13 antibodies. Th1 and Th2 cells were defined as CD4+ IFN-γ+ cells and CD4+ IL-13+ cells, respectively. Similarly, regulatory T cells (Tregs) were labeled with FITC-conjugated anti-CD4 and PE-conjugated anti-CD25 antibodies and were defined as CD4+ CD25+ cells; Th17 cells were labeled with FITC-conjugated anti-CD4 and PE-conjugated anti-IL17 antibodies and were defined as CD4+ IL-17+ cells.
Surface molecule expression of single-cell lung suspensions was analyzed using multicolor flow cytometry, as previously described18. Briefly, single-cell lung suspensions were suspended in cold PBS containing 3% FBS and 0.02% NaN3. The cells were then incubated with a mixture of rat and mouse IgG (1:1) to reduce nonspecific binding, followed by serial incubations with saturating concentrations of FITC-conjugated mAb and/or PE-conjugated mAb for 1 h at 4 °C. Isotype-matched mAbs were used in control samples. After incubation, 20,000 stained cells were analyzed using CellQuest software (BD Biosciences, Sparks, MD, USA). In addition, the levels of various cytokines, such as IFN-γ, IL-13, and IL-17, were determined by an intracellular staining method, as previously described19. The cells were fixed (2% paraformaldehyde), permeabilized (0.5% saponin or methanol), and stained with PE-, Alexa 647-, or PE-conjugated mAbs specific for IFN-γ, IL-13, and IL-17, respectively, or isotype-matched mAb. The fluorescence data were collected and analyzed as described above.
Western blot analysis
Cytoplasmic and nuclear proteins were extracted from mouse lungs, and Western blots were performed as previously described20. Briefly, protein extracts were resolved on 10% SDS-PAGE. Immunoblot analysis was performed with enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Chicago, IL, USA).
Statistical analyses
Data were expressed as means±SD. Statistical analyses were performed with SPSS 13.0 using paired-samples t-tests or nonparametric tests. For the therapeutic study, one-way ANOVA analysis was performed to assess data differences among various groups. Differences were considered significant at a level of P<0.05.
Results
TLR4 mutation enhances apoptosis and oxidative stress in the lungs
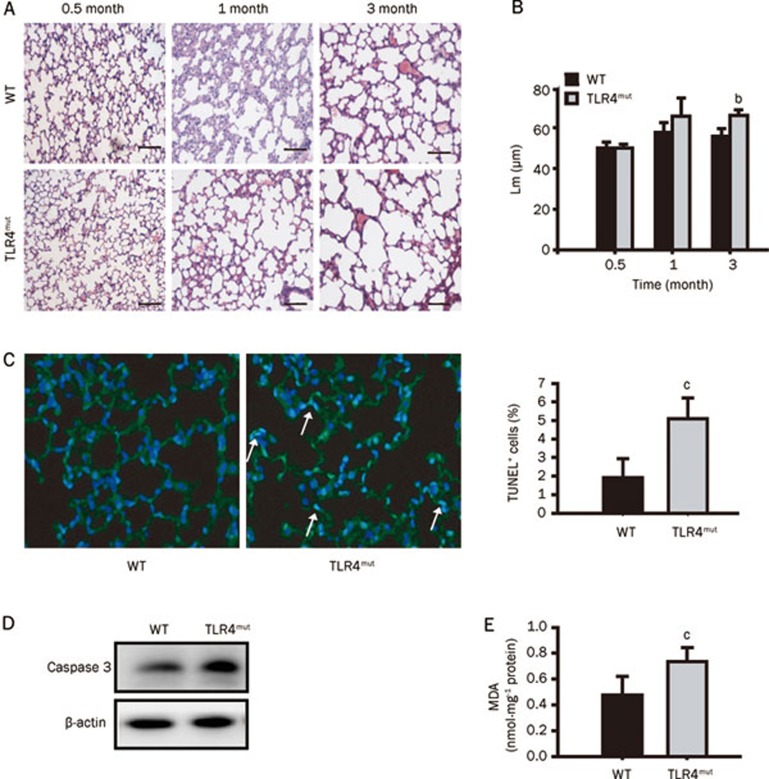
Different strains of TLR4-mutant mice (C3H/HeJ, C57BL/10ScNJ, and C57BL/6J backgrounds) all have been shown to exhibit increased lung volumes at 3 months of age, which indicates that the observed phenotypes were independent of strain7. In this study, C3H/HeJ TLR4mut mice were used to investigate the mechanisms underlying the development of TLR4mut-induced spontaneous emphysema. The lungs of TLR4mut mice showed destruction of the alveolar architecture and enlargement of the air spaces at 3 months of age but not at 0.5 or 1 month of age (Figure 1A). Also, the morphometric quantitation of airspace enlargement revealed that Lm was larger in TLR4mut mice than in WT mice at 3 months of age (66.12±2.61 μm νs 55.81±3.93 μm, P<0.05), but there were no differences between the lungs of WT and those of TLR4mut mice at either 0.5 or 1 month of age (Figure 1B). In addition, compared with WT mice, TLR4 mutation increased the number of TUNEL+ (blue-green) cells and the levels of MDA in the lungs at 3 months of age (5.09%±1.11% νs 1.92%±1.01%, P<0.01, and 0.74±0.11 nmol·mg-1 protein νs 0.47±0.15 nmol·mg−1 protein, P<0.01, respectively) (Figures 1C and 1E). Also, the expression of cleaved caspase 3, which contributes to cellular apoptosis, was enhanced in the lungs of TLR4mut mice (Figure 1D). These results indicate that TLR4 mutation causes pulmonary emphysema in mice at 3 months of age, which is consistent with the report from Zhang et al7. Therefore, three-month-old TLR4mut mice were used as our model of spontaneous emphysema for further studies.
Figure 1.
TLR4 mutation caused spontaneous pulmonary emphysema in mouse. (A) Representative histological images of lung sections from the indicated ages of WT or TLR4mut mice (HE staining) (n=6). Original magnification, ×100. Scale bar, 100 μm. (B) TLR4 mutation enhanced the mean linear intercept (Lm) of mice. Lm was the total length of each line of the grid which was divided by the number of alveolar intercepts. (C) TLR4 mutation increased the TUNEL+ apoptotic cells in lung tissues. The left panel showed the representative merged images of lung sections stained with TUNEL (green) and DAPI (blue). Arrows indicate representative TUNEL+ (blue-green) cells. Original magnification, ×400. The right panel showed the summary of TUNEL+ apoptotic cells (n=6). (D) TLR4 mutation resulted in an enhanced expression of cleaved caspase-3 detected by Western blot. Data are representative immunoblots of three independent experiments (n=4/group/experiment). (E) TLR4 mutation enhanced the levels of MDA in the lungs of 3-month-old mice (n=6). Data are means±SD. bP<0.05, cP<0.01 νs WT mice.
Th2, Th17, and Th17-associated cytokines are attenuated in the lungs of TLR4mut mice
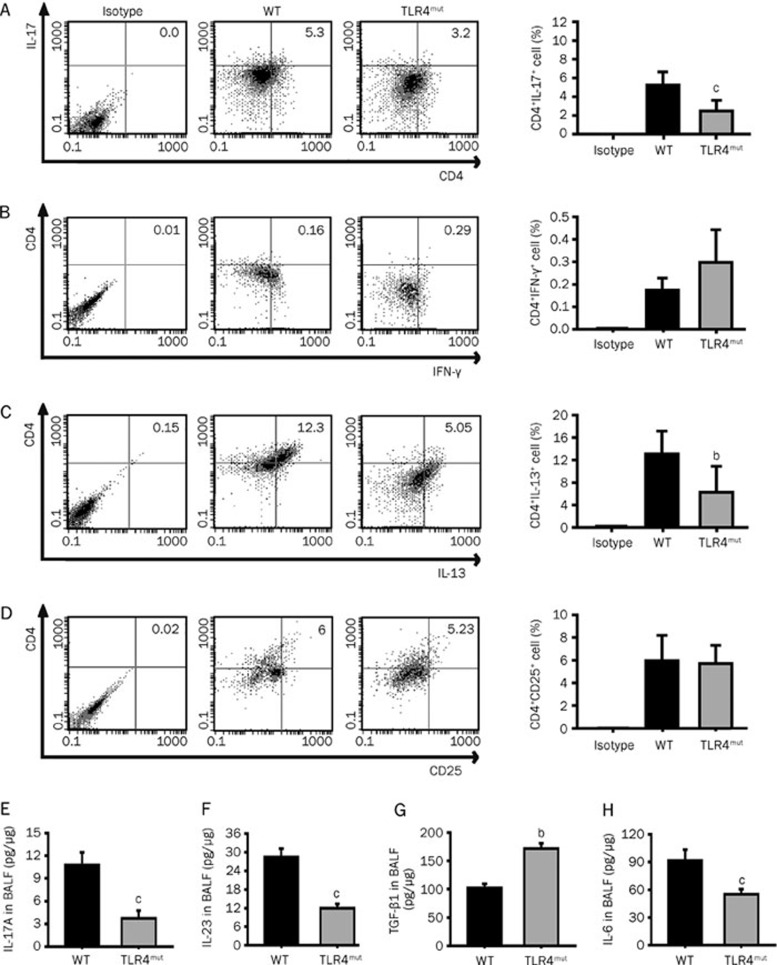
TLR activation regulates the maturity of DCs, which prime naïve T cells to determine their polarization into Th1, Th2, Treg or Th17 cells21. Th1 cells primarily secrete IL-1, IL-2, IL-12, IL-15, IL-18, IFN-γ, and TNF-α Th2 cells mainly secrete IL-4, IL-5, IL-6, IL-13; Treg cells principally secrete IL-10 and TGF-β and Th17 cells predominantly secrete IL-17, IL-6, TNF-α, and IL-2210. TLR4 mutation has been shown to reduce the activity of antioxidants and enhance the activity of Nox3 and the production of ROS7, which contribute to T cell polarization by changing the redox status11; our results indicate that the numbers of CD4+IL-17+ Th17 cells and CD4+IL-13+ Th2 cells were decreased in the lungs of 3-month-old TLR4mut mice (2.49%±1.13% νs 5.26%±1.39%, P<0.01, and 6.25%±4.62% νs 13.1%±4.07%, P<0.05, respectively; Figures 2A and 2C). However, TLR4 mutation did not change the number of CD4+CD25+Treg cells or CD4+IFN-γ+ Th1 cells (Figures 2B, D).
Figure 2.
TLR4 mutation regulated the polarization of T cells and the production of cytokines in the lung. Lung single-cell suspensions were prepared with lung tissues from the 3-month-old WT or TLR4mut mice and analyzed with flow cytometry. (A–D) TLR4 mutation reduced the counts of Th17 cells (CD4+IL-17+) (A) and Th2 cells (CD4+IL-13+) (C) but did not change the counts of Th1 cells (CD4+IFN-γ+) (B) and Treg cells (CD4+CD25+) (D). Data are means±SD (n=6). (E–H) TLR4 mutation reduced the levels of IL-17A (E), IL-23 (F), and IL-6 (H) but enhanced the levels of TGF-β1 (G) in the BALF. Data are means±SD of 3 independent experiments. bP<0.05, cP<0.01 νs WT mice.
The development and differentiation of Th17 cells are regulated by many cytokines, such as IL-6, transforming growth factor (TGF)-β1, IL-23, IL-21, and IL-1. In particular, synergistic activation of TGF-β1 and IL-6 can effectively induce the differentiation of naïve CD4+ T cells into highly pathogenic Th17 cells, while IL-23 is considered to play a vital role in the generation of Th17 cells, which can mediate the immune response22. Compared with WT mice, TLR4mut mice contained lower levels of IL-17A (Figure 2E), IL-23 (Figure 2F), and IL-6 (Figure 2H) in the BALF at 3 months of age (3.66±0.99 pg/μg νs 10.67±1.65 pg/μg, 12.43±1.28 pg/μg νs 28.71±2.57 pg/μg, and 51.82±5.45 pg/μg νs 92.73±10.91 pg/μg, respectively, P<0.01), while they displayed higher levels of TGF-β1 (168.18±9.09 pg/μg νs 102.27±6.82 pg/μg, P<0.05; Figure 2G). These results indicate that the infiltration of Th17 cells and the production of Th17-associated cytokines were attenuated in TLR4mut mice at 3 months of age.
TLR4 mutation reduces phosphorylation of p38 MAPK and the expression of AP-1
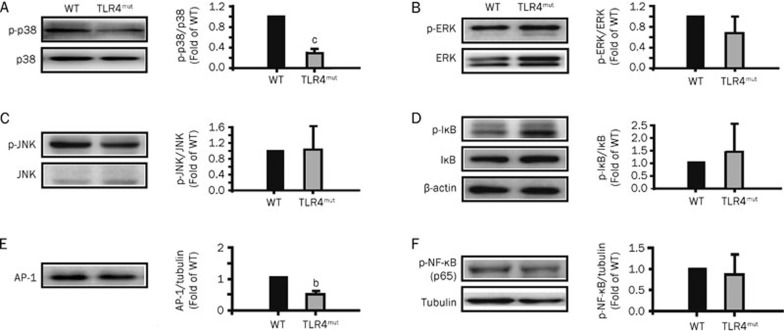
TLR4mut mice have been shown to be characterized by reduced antioxidant activity and enhanced Nox3 expression and ROS production7. Oxidative stress plays a fundamental role in the induction of inflammation through the upregulation of redox-sensitive protein kinases and transcription factors, such as MAPK, AP-1, and NF-κB, which are also the downstream signaling molecules of IL-17A23. Thus, we examined the changes in the levels of these signaling molecules in the lungs of 3-month-old TLR4mut mice. Compared with WT mice, TLR4 mutation inhibited the phosphorylation of p38 MAPK in the lungs (0.27±0.09 fold of WT levels, P<0.01; Figure 3A) but did not affect the phosphorylation of ERK and JNK kinases in the lungs (Figures 3B and 3C). In addition, TLR4 mutation decreased the expression of AP-1 in the lungs (0.51±0.08 fold of WT levels, P<0.05; Figure 3E) but did not affect the phosphorylation of IκB and NF-κB in the lungs (Figures 3D and 3F). These results indicate that TLR4 mutation results in a reduction in p38 MAPK phosphorylation and AP-1 expression.
Figure 3.
TLR4 mutation regulated the phosphorylation of p38 MAPK and the expression of AP-1. The lung extracts were prepared with the lung tissues obtained from the 3-month-old WT or TLR4mut mice. The expression and phosphorylation of the MAPKs-NF-κB signaling molecules were detected by Western blotting with specific antibodies as indicated. Bands were quantified and presented as fold-difference compared with that of WT mice. (A) The expression and phosphorylation of p38 MAPK were reduced in the lungs from 3-month-old TLR4mut mice. (B–D) The phosphorylation of ERK kinases (B), JNK kinases (C) and IκB (D) in the lungs from 3-month-old TLR4mut mice had no significant change compared with WT mice. (E) TLR4 mutation reduced the expression of AP-1 in the lungs from 3-month-old mice. (F) TLR4 mutation did not change the phosphorylation of NF-κB p65 in the lungs. Data are representatives of three independent experiments with identical results. bP<0.05, cP<0.01 νs WT mice.
IL-17A administration ameliorates TLR4mut-induced pulmonary emphysema
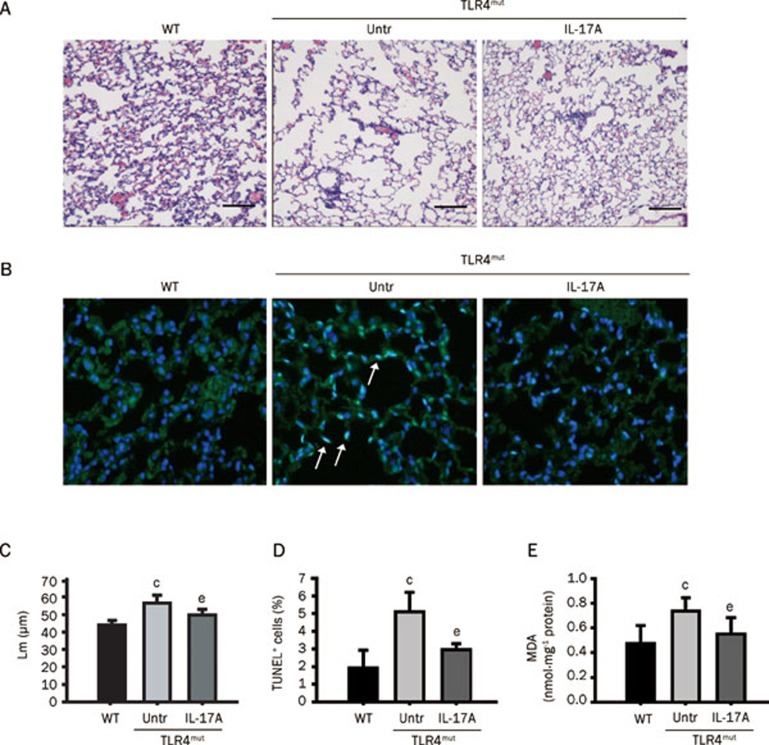
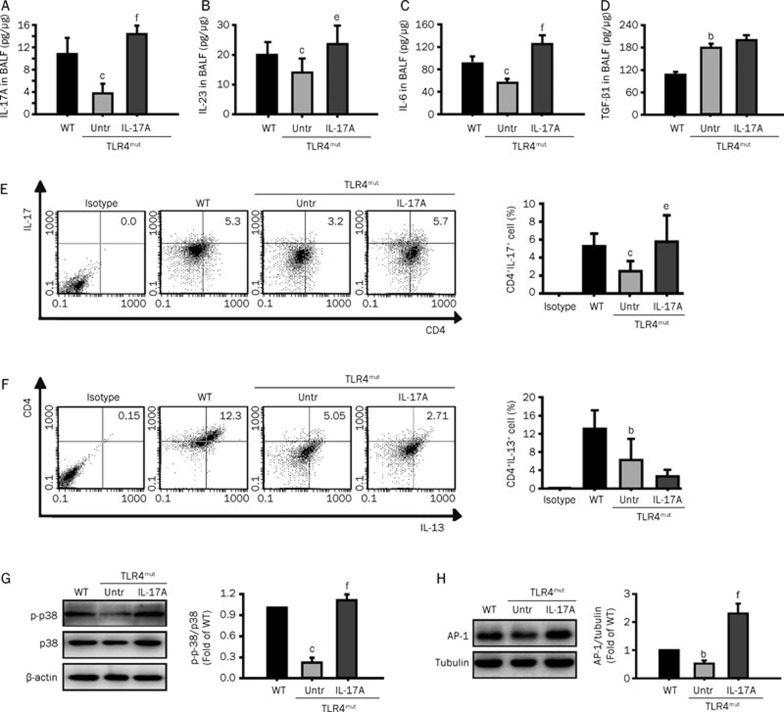
To restore the effects caused by Th17 cells, TLR4mut mice were administered mouse IL-17A. As expected, IL-17A reduced the TLR4mut-induced destruction of the normal alveolar architecture and enlargement of the air spaces distal to the terminal bronchioles, and it also decreased Lm (Figures 4A, 4C), TUNEL+ apoptotic cells (Figures 4B, 4D) and the levels of MDA (Figure 4E) in the lungs. To evaluate the effectiveness of IL-17A treatment, we examined the infiltration of Th2 cells and Th17 cells into the lungs and the levels of IL-17A, IL-23, IL-6, and TGF-β1 in the BALF of TLR4mut mice. IL-17A administration enhanced the production of IL-17A (Figure 5A), IL-23 (Figure 5B), and IL-6 (Figure 5C) (14.58±1.31 pg/μg νs 3.56±1.50 pg/μg, P<0.001; 25.30±8.22 pg/μg νs 16.33±5.58 pg/μg, P<0.05; and 129.6±10.87 pg/μg νs 57.6±3.66 pg/μg, P<0.01, respectively) but did not enhance the levels of TGF-β1 (Figure 5D). Additionally, IL-17A treatment increased Th17 cell infiltration (5.77%±2.91% νs 2.49%±1.13%, P<0.05; Figure 5E), but it did not significantly enhance Th2 cell infiltration (Figure 5F). Furthermore, IL-17A treatment reversed TLR4mut-reduced phosphorylation of p38 MAPK (Figure 5G) and expression of AP-1 (Figure 5H) in the lungs of 3-month-old TLR4mut mice (1.06±0.08 fold higher than WT νs 0.27±0.09 fold of WT levels, P<0.01 and 2.34±0.24 fold higher than WT νs 0.51±0.08 fold of WT levels. P<0.01, respectively). These results indicate that treatment of TLR4mut mice with IL-17A ameliorates pulmonary emphysema though restoration of Th17 cell infiltration and enhancement of IL-17-associated signaling.
Figure 4.
Treatment of TLR4mut mice with IL-17A ameliorated spontaneous pulmonary emphysema. (A) IL-17A ameliorated alveolar enlargement and Lm of lung tissue in TLR4mut mice. Left panel, the alveolar enlargement was detected with histological analysis lung sections by HE staining (n=6). Original magnification, ×100. Scale bar, 100 μm. (B) Treatment with IL-17A decreased the TUNEL+ apoptotic cells in the lungs. The panel showed the representative merged images of lung sections stained with TUNEL (green) and DAPI staining (blue). Arrows indicate representative TUNEL+ (blue-green) cells. Original magnification, ×200. (C) The summary of Lm. (D) The summary for the number of TUNEL+ cells in lungs. (E) IL-17A treatment reduced the levels of MDA in the lungs from 3-month-old TLR4mut mice (n=6). Data are means±SD (n=6). bP<0.05, cP<0.01 νs WT mice; eP<0.05, fP<0.01 νs TLR4mut mice.
Figure 5.
Administration with IL-17A reversed TLR4mut-reduced IL-17A, Th17, and the phosphorylation of p38 MAPK and expression of AP-1 in the lungs. The TLR4mut mice were administered with mouse IL-17A as described in the Methods section. (A–D) Treatment with IL-17A enhanced the levels of IL-17A (A), IL-23 (B), and IL-6 (C), but did not enhance the levels of TGF-β1 (D) in the BALF of TLR4mut mice (n=6). (E–F) Treatment of mice with IL-17A increased the infiltration of Th17 cells into the lungs from TLR4mut mice. Flow cytometry was used to analyze the levels of Th17 cells (CD4+IL-17+, E) and Th2 cells (CD4+IL-13+, F) in the lung single-cell suspensions prepared from the lung tissues of 3-month-old WT or TLR4mut mice (n=6). (G–H) Administration of IL-17A reversed the TLR4mut-reduced phosphorylation of p38 MAPK and expression of AP-1 in the lungs. Mouse IL-17A treatment enhanced the phosphorylation of p38 MAPK (G) and the expression of AP-1 (H) in the lungs from the 3-month-old TLR4mut mice. The tissue extracts were prepared with the lung tissues obtained from the 3-month-old WT or TLR4mut mice. The phosphorylation of p38 MAPK and expression of AP-1 were detected by Western blotting with indicated antibodies. Bands were quantified and presented as fold-difference of that of WT mice (n=3). Data are means±SD. bP<0.05, cP<0.01 νs WT mice; eP<0.05, fP<0.01 νs TLR4mut mice.
Discussion
In this study, we observed that TLR4 mutation results in age-dependent development of spontaneous pulmonary emphysema in 3-month-old mice, which is consistent with the report by Zhang et al7. The progression of emphysema in TLR4-mutant mice is akin to the progression of emphysema that develops in humans. Therefore, spontaneous emphysema in TLR4-mutant mice is an excellent model for investigating the pathogenesis of emphysema. Emphysema is considered an autoimmune disease8, while IL-17 has been demonstrated to play vital roles in autoimmune diseases as a major pro-inflammatory mediator that promotes the production of many chemokines, cytokines, and growth factors24. Thus, it is important to provide evidence to establish whether there is a relationship between Th17 cells or IL-17 cytokines and TLR4mut-induced emphysema. Our studies demonstrate that infiltration of Th17 cells and expression of IL-17 are attenuated in the lungs of 3-month-old TLR4mut mice with emphysema, and the p38 MAPK and AP-1 pathways are inhibited in the lung tissue of TLR4mut mice with emphysema. Moreover, administration of recombinant mouse IL-17A resulted in restoration of Th17 cell infiltration and enhanced IL-17A, IL-23, and IL-6 expression, which reversed the attenuated phosphorylation of p38 MAPK and expression of AP-1, decreased the levels of MDA, inhibited cellular apoptosis, and reversed TLR4mut-induced pulmonary emphysema. However, although the infiltration of Th2 cells was attenuated in the lungs of 3-month-old TLR4mut mice with emphysema, administration of recombinant mouse IL-17A did not raise the levels of infiltrating Th2 cells into the lungs. Our findings indicate that Th17 cells, and in particular the cytokine IL-17A secreted from these cells, play a crucial role in lung epithelia integration during animal development, suggesting that Th17 cells and IL-17A-associated signaling pathways are potential targets for novel pulmonary emphysema therapies.
The mechanism governing the enhanced expression of IL-17A and Th17-associated cytokines after administration of IL-17A is associated with an increase in lung-infiltrating Th17 cells. TGF-β1 and IL-6 have been shown to be required for the differentiation of naïve T cells into Th17 cells in mice22, while IL-23 has been shown to stabilize and expand the population of Th17 cells25. In TLR4mut mice, the expression levels of IL-17, IL-6 and IL-23 in the lungs are all depressed, which results in an attenuation of the lung-infiltrating Th17 cells. A previous study indicated that IL-17 is an important inducer of IL-6 expression26; our recent work showed that IL-17A directly induces the expression of TGF-β1 (Mi et al, unpublished observations). In our current study, we found that administration of IL-17A recovers the expression of IL-6 and IL-23 in the lungs of TLR4mut mice. Indeed, Mudter et al found that overexpression of IRF4 using retroviral infection induces IL-17 production and that IL-17 together with IL-6 induces RORγt expression, thereby controlling Th17-dependent colitis27.
Our findings have been supported by other studies as well. Inoue et al have reported that IL-17A can stimulate keratinocytes to produce vascular endothelial growth factor (VEGF), granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, IL-8, and CXCL10 and subsequently promote the growth and repair of airway epithelial cells13. Additionally, up-regulation of IL-17A has been demonstrated to induce the process of tissue fibrosis, which is opposite to the process of emphysema, in chronic periodontitis sites, heart, skin and lungs28, 29. However, there have also been some reports that are opposed to the above-mentioned results regarding the association between IL-17A and emphysema. Others have indicated that airway infiltration of CD4+CCR6+ Th17 cells are associated with chronic cigarette smoke induced airspace enlargement30, 31. Four-month exposure to smoke, a major stimulator of emphysema, can induce significant increases in IL-17 levels in the pulmonary airway32. These studies indicate that the physiological concentration of IL-17A is a critical factor for lung epithelial development and for tissue repair or regeneration after tissue injury. However, excessive expression levels of IL-17A (plus other inflammatory factors, such as TGF-β1) can result in tissue fibrosis and even organ dysfunction.
The attenuation of Th17 cells and IL-17A-associated factors is mainly due to a mutation in TLR4 signaling. Indeed, there are a number of studies that have indicated a regulatory role for TLR4 activation in the development of Th17 cells and the production of Th17-associated cytokines. Under physiological conditions, basal activity of TLR4 induced by damage-associated molecular pattern molecules, such as myeloid-related protein-8 (Mrp8) and Mrp14, is required for Th17-induced protective immunity that contributes to the defense against infection and the repair of slight tissue injury33, 34. Under infectious conditions, pathogens induce a higher level of Th17-associated factors, such as IL-17, IL-1β, pSTAT3, and RORγt, through activation of TLR435. Indeed, TLR4 activity has been shown to be required for gram-negative bacterial pneumonia-induced IL-17 production36 and for the mycobacterium tuberculosis-induced IL-17A response37. TLR4-mutant mice display lower expression levels of IL-1β, TNF-α, IFN-γ, IL-17, and IL-23 in the lungs after vaccination with pertussis vaccines38. Interestingly, TLR2-promoted Th2/Th17 responses are caused by activation of TLR4 and TLR7/8 and subsequent abrogation of the type I IFN amplification loop39. However, further investigations are needed to fully understand the mechanisms concerning how TLR4 activity regulates the development of Th17 cells and the production of Th17-associated factors.
In summary, our current study indicates that Th17 cells and IL-17A-associated signaling molecules play a crucial role in TLR4mut-induced spontaneous pulmonary emphysema. Importantly, treatment of TLR4-mutant mice with exogenous IL-17A enhanced the phosphorylation of p38 MAP kinase and the expression of AP-1 and subsequently decreased apoptosis levels and reversed TLR4mut-induced pulmonary emphysema. Our study highlights the fact that Th17 cells and IL-17A-associated signaling molecules are potential targets for the development of therapeutic strategies against pulmonary emphysema.
Author contribution
Qing-qing WANG performed research experiments, analyzed data and wrote the manuscript; Hong-zhen YANG performed part of the research experiments and helped write the manuscript; Han-zhi LIU, Su MI, Xiao-wei ZHANG, Hui-min YAN, Yong-gang MA, and Xiao-xing WANG performed part of the research experiments together with Qing-qing WANG; and Zhuo-wei HU conceived and designed the experiments, edited and modified the manuscript.
Acknowledgments
This study was supported by grants from the National Major Basic Research Program of China (2006CB503808), the Creation of Major New Drugs (2009ZX09301-003-13), and the National Natural Science Foundation of China (30672468; 30901814; 81030056). Dr Zhuo-wei HU is also supported by the Cheung-Kong Scholars Programme from the Ministry of Education and the Senior Oversea Chinese Scholar Fund from the Ministry of Personnel of People's Republic of China.
References
- Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87:1047–82. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- Taraseviciene-Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. J Clin Invest. 2008;118:394–402. doi: 10.1172/JCI31811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–27. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutten A, Goven D, Boczkowski J, Bonay M. Oxidative stress targets in pulmonary emphysema: focus on the Nrf2 pathway. Expert Opin Ther Targets. 2010;14:329–46. doi: 10.1517/14728221003629750. [DOI] [PubMed] [Google Scholar]
- Horowitz JC, Martinez FJ, Thannickal VJ. Mesenchymal cell fate and phenotypes in the pathogenesis of emphysema. COPD. 2009;6:201–10. doi: 10.1080/15412550902905953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PM, Rennard SI. What have we learned from large drug treatment trials in COPD. Lancet. 2007;370:774–85. doi: 10.1016/S0140-6736(07)61381-6. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–9. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–9. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- Vercelli D. Gene-environment interactions in asthma and allergy: the end of the beginning. Curr Opin Allergy Clin Immunol. 2010;10:145–8. doi: 10.1097/ACI.0b013e32833653d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48:1121–32. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won HY, Sohn JH, Min HJ, Lee K, Woo HA, Ho YS, et al. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid Redox Signal. 2010;13:575–87. doi: 10.1089/ars.2009.2989. [DOI] [PubMed] [Google Scholar]
- Inoue D, Numasaki M, Watanabe M, Kubo H, Sasaki T, Yasuda H, et al. IL-17A promotes the growth of airway epithelial cells through ERK–dependent signaling pathway. Biochem Biophys Res Commun. 2006;347:852–8. doi: 10.1016/j.bbrc.2006.06.137. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Matsuo Y, Yoshiura K, Nagase T, Yamashita N. Sequential and quantitative analysis of a murine model of elastase–induced emphysema. Biol Pharm Bull. 2008;31:1434–8. doi: 10.1248/bpb.31.1434. [DOI] [PubMed] [Google Scholar]
- D'Hulst AI, Vermaelen KY, Brusselle GG, Joos GF, Pauwels RA. Time course of cigarette smoke-induced pulmonary inflammation in mice. Eur Respir J. 2005;26:204–13. doi: 10.1183/09031936.05.00095204. [DOI] [PubMed] [Google Scholar]
- Zha RP, Xu W, Wang WY, Dong L, Wang YP. Prevention of lipopolysaccharide-induced injury by 3,5-dicaffeoylquinic acid in endothelial cells. Acta Pharmacol Sin. 2007;28:1143–8. doi: 10.1111/j.1745-7254.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Traidl-Hoffmann C, Mariani V, Hochrein H, Karg K, Wagner H, Ring J, et al. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med. 2005;201:627–36. doi: 10.1084/jem.20041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HZ, Cui B, Liu HZ, Chen ZR, Yan HM, Hua F, et al. Targeting TLR2 attenuates pulmonary inflammation and fibrosis by reversion of suppressive immune microenvironment. J Immunol. 2009;182:692–702. doi: 10.4049/jimmunol.182.1.692. [DOI] [PubMed] [Google Scholar]
- Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;161:3042–9. [PubMed] [Google Scholar]
- Cai WF, Zhang XW, Yan HM, Ma YG, Wang XX, Yan J, et al. Intracellular or extracellular heat shock protein 70 differentially regulates cardiac remodelling in pressure overload mice. Cardiovasc Res. 2010;88:140–9. doi: 10.1093/cvr/cvq182. [DOI] [PubMed] [Google Scholar]
- Amati L, Pepe M, Passeri ME, Mastronardi ML, Jirillo E, Covelli V. Toll-like receptor signaling mechanisms involved in dendritic cell activation: potential therapeutic control of T cell polarization. Curr Pharm Des. 2006;12:4247–54. doi: 10.2174/138161206778743583. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SC, Lee SH. Role of Th17 cell and autoimmunity in chronic obstructive pulmonary disease. Immune Netw. 2010;10:109–14. doi: 10.4110/in.2010.10.4.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: from basic science to medicine — 40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- Mudter J, Yu J, Zufferey C, Brüstle A, Wirtz S, Weigmann B, et al. IRF4 regulates IL-17A promoter activity and controls RORγt-dependent Th17 colitis in vivo. Inflamm Bowel Dis. 2011;17:1343–58. doi: 10.1002/ibd.21476. [DOI] [PubMed] [Google Scholar]
- Duarte PM, Santos VR, Dos Santos FA, de Lima Pereira SA, Rodrigues DB, Napimoga MH. Role of smoking and type 2 diabetes in the immunobalance of advanced chronic periodontitis. J Periodontol. 2011;82:429–38. doi: 10.1902/jop.2010.100215. [DOI] [PubMed] [Google Scholar]
- Yoshizaki A, Yanaba K, Iwata Y, Komura K, Ogawa A, Akiyama Y, et al. Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. J Immunol. 2010;185:2502–15. doi: 10.4049/jimmunol.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison OJ, Foley J, Bolognese BJ, Long E, 3rd, Podolin PL, Walsh PT. Airway infiltration of CD4+ CCR6+ Th17 type cells associated with chronic cigarette smoke induced airspace enlargement. Immunol Lett. 2008;121:13–21. doi: 10.1016/j.imlet.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Lane N, Robins RA, Corne J, Fairclough L. Regulation in chronic obstructive pulmonary disease: the role of regulatory T-cells and Th17 cells. Clin Sci (Lond) 2010;119:75–86. doi: 10.1042/CS20100033. [DOI] [PubMed] [Google Scholar]
- Melgert BN, Timens W, Kerstjens HA, Geerlings M, Luinge MA, Schouten JP, et al. Effects of 4 months of smoking in mice with ovalbumin-induced airway inflammation. Clin Exp Allergy. 2007;37:1798–808. doi: 10.1111/j.1365-2222.2007.02843.x. [DOI] [PubMed] [Google Scholar]
- Tang H, Pang S, Wang M, Xiao X, Rong Y, Wang H, et al. TLR4 activation is required for IL-17-induced multiple tissue inflammation and wasting in mice. J Immunol. 2010;185:2563–9. doi: 10.4049/jimmunol.0903664. [DOI] [PubMed] [Google Scholar]
- Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 2010;16:713–7. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- Mu HH, Hasebe A, Van Schelt A, Cole BC. Novel interactions of a microbial superantigen with TLR2 and TLR4 differentially regulate IL-17 and Th17-associated cytokines. Cell Microbiol. 2011;13:374–87. doi: 10.1111/j.1462-5822.2010.01540.x. [DOI] [PubMed] [Google Scholar]
- Bhan U, Ballinger MN, Zeng X, Newstead MJ, Cornicelli MD, Standiford TJ. Cooperative interactions between TLR4 and TLR9 regulate interleukin 23 and 17 production in a murine model of gram negative bacterial pneumonia. PLoS One. 2010;5:e9896. doi: 10.1371/journal.pone.0009896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Teirlinck AC, Kleinnijenhuis J, Kullberg BJ, van Crevel R, van der Meer JW, et al. Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukoc Biol. 2010;88:227–32. doi: 10.1189/jlb.0809550. [DOI] [PubMed] [Google Scholar]
- Banus S, Stenger RM, Gremmer ER, Dormans JA, Mooi FR, Kimman TG, et al. The role of Toll-like receptor-4 in pertussis vaccine-induced immunity. BMC Immunol. 2008;9:21. doi: 10.1186/1471-2172-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenink MH, Santegoets KC, Broen JC, van Bon L, Abdollahi–Roodsaz S, Popa C, et al. TLR2 promotes Th2/Th17 responses via TLR4 and TLR7/8 by abrogating the type I IFN amplification loop. J Immunol. 2009;183:6960–70. doi: 10.4049/jimmunol.0900713. [DOI] [PubMed] [Google Scholar]