Abstract
Aim:
Both endothelin ETA receptor antagonists and angiotensin AT1 receptor antagonists lower blood pressure in hypertensive patients. A dual AT1 and ETA receptor antagonist may be more efficacious antihypertensive drug. In this study we identified the mode and mechanism of binding of imidazole series of compounds as dual AT1 and ETA receptor antagonists.
Methods:
Molecular modeling approach combining quantum-polarized ligand docking (QPLD), MM/GBSA free-energy calculation and 3D-QSAR analysis was used to evaluate 24 compounds as dual AT1 and ETA receptor antagonists and to reveal their binding modes and structural basis of the inhibitory activity. Pharmacophore-based virtual screening and docking studies were performed to identify more potent dual antagonists.
Results:
3D-QSAR models of the imidazole compounds were developed from the conformer generated by QPLD, and the resulting models showed a good correlation between the predicted and experimental activity. The visualization of the 3D-QSAR model in the context of the compounds under study revealed the details of the structure-activity relationship: substitution of methoxymethyl and cyclooctanone might increase the activity against AT1 receptor, while substitution of cyclohexone and trimethylpyrrolidinone was important for the activity against ETA receptor; addition of a trimethylpyrrolidinone to compound 9 significantly reduced its activity against AT1 receptor but significantly increased its activity against ETA receptor, which was likely due to the larger size and higher intensities of the H-bond donor and acceptor regions in the active site of ETA receptor. Pharmacophore-based virtual screening followed by subsequent Glide SP, XP, QPLD and MM/GBSA calculation identified 5 potential lead compounds that might act as dual AT1 and ETA receptor antagonists.
Conclusion:
This study may provide some insights into the development of novel potent dual ETA and AT1 receptor antagonists. As a result, five compounds are found to be the best dual antagonists against AT1R and ETA receptors.
Keywords: imidazole, AT1 receptor, ETA receptor, quantum-polarized ligand docking (QPLD), MM/GBSA free-energy calculation, 3D-QSAR, molecular dynamics simulation, antihypertensive drug
Introduction
Hypertension is a multi-factorial trait that results from a combination of genetic and environmental factors1. Several candidate proteins that are linked to hypertension have been identified; among these, the angiotensin II type-1 receptor (AT1R) gene and endothelin receptor type A (ETA) are important targets for the design of antihypertensive drugs2,3,4,5,6.
Angiotensin II (Ang II) activates the AT1R on arterial smooth muscle cells; this activation causes vasoconstriction and increases blood pressure7, and AT1R antagonists reduce blood pressure in patients with essential hypertension8. Endothelin I (ET-1) activates the ETA and ETB receptors; the activation of these receptors induces vascular smooth muscle cell contraction and increases blood pressure9, and ETA/ETB receptor antagonists are also given to patients with essential hypertension to reduce diastolic blood pressure10.
There is a significant medical need for an antihypertensive drug that is effective as a monotherapy in a wide variety of patients11. The endogenous Ang II and ET-1 proteins are powerful vasoconstrictors and mitogens, and the pathogenesis of hypertension and cardiovascular disease is associated with both of these factors11. Elevated levels of Ang II promote the synthesis and vasoconstrictive action of ET-1, and elevated levels of ET-1 increase the synthesis and vasoconstrictive action of Ang II; therefore, these factors constitute a positive dual-feedback mechanism and an excellent target for hypertension management11. Because Ang II and ET-1 cause increases in blood pressure and promote each other's actions, it is plausible that the concomitant blockade of both the angiotensin and endothelin endocrine/paracrine pathways may lead to enhanced reductions in blood pressure12,13,14. The dual inhibition of both AT1R and ETA has more physiological benefit than the individual inhibition of either AT1R or ETA alone, as demonstrated in hypertensive transgenic rats that overexpress the human renin gene12.
In this study, combined homology modeling, molecular dynamics simulation, MM/GBSA free-energy calculations and atom-based 3D-QSAR analysis were performed to identify the mode and mechanism of binding of the imidazole series of compounds15 as dual inhibitors of AT1R and ETA. In this paper, we describe a robust combined ligand- and structure-based development of 3D-QSAR and utilize this approach to examine the mechanism of dual inhibition of AT1R and ETA. The contours generated from QSAR studies highlight the structural features required for dual inhibition, and it will be useful for further design of more potent inhibitors.
Materials and methods
Homology modeling of ETA
The sequence of the human ETA (427 residues) was retrieved from the UniProt database (accession number: P25101). The crystal structures of human β2 Adrenergic Receptor (3KJ6) and Adenosine A2A Receptor (3PWH) were retrieved from the Protein Data Bank (PDB) and selected as templates for homology modeling according to the result of a BLAST search. The templates were selected based on sequence identity and the coverage of the sequence. The sequences of ETA and the templates (3PWH and 3KJ6) were aligned using ClustalW (http://www.ichec.ie/infrastructure/software/GlustalW)16. A BLOSSUM scoring matrix was used for the ClustalW alignment17. Because the sequence identity of the target and template sequence is <30%, we chose a multiple template approach; this increased the coverage of the sequence alignment. Many studies have reported that using multiple templates improves the quality of homology modeling18,19,20. Fanelli and Benedetti reported that the selection of the proper template by careful sequence comparisons is a crucial step in comparative modeling21. The ETA tertiary structure was modeled using modeller 9.10 software (Accelrys Inc, San Diego, CA, USA)22,23,24,25.
Refinement and validation of the model
To obtain a reliable model, structural refinement was performed using stepwise constrained optimization26. First, the initial models were optimized and energy was minimized to correct disallowed torsion angles and to eliminate unfavorable atom-atom contacts using Prime 2.027 software (Schrodinger, LLC, New York, NY, USA)28. The validation of the modeled structures was performed by inspecting the psi/phi Ramachandran plot obtained from PROCHECK analysis29.
Molecular dynamics simulation
Molecular dynamics (MD) simulations were carried out for the modeled ETA protein and the theoretical AT1R protein (PDB ID: 1ZV0)30 from the Protein Data Bank (www.rcsb.org) using Desmond MD code and the OPLS-AA 2005 (Optimized Potentials for Liquid Simulations-All Atom) force field31,32 for minimization of the system. Using the Desmond system builder, a 10 Å buffered orthorhombic system with periodic boundary conditions was constructed using a DPPC lipid membrane33,34,35 and a TIP4P explicit water solvent36. The overall charge was neutralized by 0.15 mol/L NaCl. The simulations were performed in the NPT ensemble. The temperature of 325 °K and pressure of 1.013 bar were kept constant by coupling the system to a Berendsen thermostat and barostat. An integration step of 2.0 was used, Coulombic interactions were calculated using a cutoff radius of 9.0 Å, and long-range electrostatic interactions were calculated using the smooth particle mesh Ewald method37. Before each MD simulation, a default Desmond membrane protein relaxation protocol was applied38.
Preparation of protein
The starting coordinates of the AT1R and modeled ETA protein were further modified for Glide docking calculations and subjected to protein preparation using the protein preparation wizard of Glide software (Schrodinger, LLC, New York, NY, USA)39. For these calculations, the proteins were minimized using the Protein Preparation Wizard by applying an OPLS-2005 force field. Progressively weaker restraints were applied to the non-hydrogen atoms. This refinement procedure was carried out based on the recommendations of Schrodinger LLC (New York, NY, USA). Because Glide uses the full OPLS-2005 force field at an intermediate docking stage and claims to be more sensitive to geometric details than other docking tools. The most likely positions of hydroxyl and thiol hydrogen atoms, protonation states and tautomers of His residues, and Chi 'flip' assignments for Asn, Gln and His residues were selected. Progressive minimizations were performed until the average root mean square deviation of the non-hydrogen atoms reached 0.3 Å.
Active site predictions
The active sites of the modeled ETA protein and AT1R were investigated using the SiteMap program (Schrodinger, LLC, New York, NY, USA)40. This software generates information on the binding site's characteristics using novel search and analytical facilities; a SiteMap calculation begins with an initial search step that identifies or characterizes one or more regions on the protein surface that may be suitable for binding ligands to the receptor using grid points.
Ligand preparation
Twenty-four compounds that have been reported to be dual inhibitors of AT1R and ETA15 were drawn in Maestro (Schrodinger, LLC, New York, NY, USA). Each structure was assigned an appropriate bond order using the LigPrep package from Schrodinger, LLC, New York, NY, USA41. The inhibitors were converted to mae format (Maestro, Schrodinger, LLC, New York, NY, USA) and optimized by means of the Optimized Potentials for Liquid Simulations (OPLS 2005) force field using the default setting.
Glide extra precision docking (XP) and QM-polarizes ligand docking (QPLD)
All the compounds were subjected to Glide XP docking. In addition, the popular docking program Glide and QM/MM software Q-site were combined to perform a QM-Polarized Ligand Docking (QPLD) analysis42,43. For QM/MM calculations, this approach uses the QSITE program42, which is coupled with JAGUAR44 for the QM region and the IMPACT molecular modeling code for the MM region45. When there are covalent connections between the QM and MM regions, it uses frozen localized molecular orbitals along the covalent bonds to construct an interface between the two regions. The QM/MM energy is calculated as the coulomb–van der Waals force of the complex that is calculated from the electrostatic potential energy of the ligand; this is in turn generated from a single point calculation using density functional theory for the QM region with the 6-31G*/LACVP* basis set, B3LYP density functional, and “Ultrafine” SCF accuracy level (iacc=1, iacscf=2). Initially, a regular standard precision (SP) Glide docking followed by extra precision (XP) refinement was carried out, generating 5 poses per docked molecule; these were then submitted to QM-ESP charge calculation at the B3LYP/3-21G* level within the protein environment defined by the OPLS-2005 force field. Finally, the resulting poses were re-docked for another Glide run using the ESP atomic charges and XP scoring modes46.
Computational mutagenesis studies
Computational mutagenesis and alanine scanning studies were carried out using the Mutate Residues script from Schrodinger, LLC, New York, NY, USA. Computational mutagenesis is a technique used to determine the contribution of specific residues to the function of a protein by mutating the residues to alanine47,48 to understand the structural and energetic characteristics of the hotspots. The mutated systems were not minimized, and it was assumed that no local rearrangements occur with the mutation. The residues that interacted with BMS-248360 were mutated to alanine, and we subsequently analyzed the binding free energy.
Binding free energy calculation
Prime/MM-GBSA was used to predict the free energy of binding between the receptor and the set of ligands. The binding free energy (ΔGbind) was calculated using the default parameters of Prime software, Schrodinger, LLC, New York, NY, USA27,49,50.
3D-QSAR model generation
PHASE 3.3, implemented in the Maestro 9.2 software package (Schrodinger, LLC, New York, NY, USA)51,52, was used to generate the 3D-QSAR models of the imidazole series as dual inhibitors of AT1R and ETA. A set of 24 imidazole compounds (Table 1) was selected from the available literature10 and used in the present study. The alignment was achieved using the top-ranked docking pose from Glide XP and QPLD for each inhibitor. QPLD showed a better correlation with the experimentally determined activity; we therefore used QPLD-generated conformations to generate the 3D-QSAR models using the default parameters. In the partial least-squares (PLS) regression analysis, a leave-n-out (LNO) validation was performed. Then, non-cross validated analysis was performed to obtain the final QSAR model. PHASE provides a standard set of six pharmacophore features: hydrogen bond acceptors (A), hydrogen bond donors (D), hydrophobic groups (H), negatively ionizable (N), positively ionizable (P), and aromatic ring (R)51. In this study, we performed 3D pharmacophore screening using the two top-ranked hypothetical pharmacophores, AARRR and AARRH, representing the chemical features of dual AT1R and ETA inhibitors. Using these pharmacophores, we searched chemical databases and retrieved molecules with novel and desired chemical features from the ChemBridge database (www.ChemBridge.com. The compounds retrieved from the database were subjected to structure-based screening. Glide provides three different levels of docking precision: HTVS (high throughput virtual screening), SP (standard precision) and XP (extra precision). We carried out our calculations using HTVS first, and then used the SP and XP modes to further refine the good ligand poses. Then, finally we docked the screened compounds using the more precise docking protocol, QPLD.
Table 1. Compounds selected (imidazole series) for 3D-QSAR study and their measured biological activity and predicted activity.
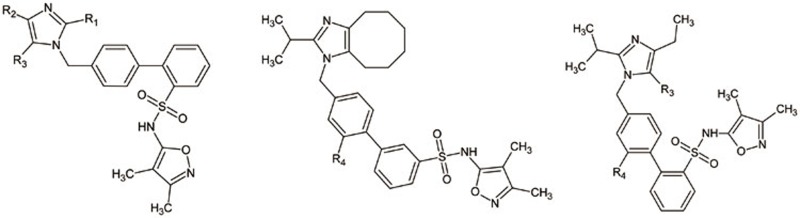
| Compound No | R1 | R2 | R3 | R4 | AT1R | ETA | ||
|---|---|---|---|---|---|---|---|---|
| Actual activity | Predicted activity | Actual activity | Predicted activity | |||||
| 1 | Bu | Cl | CONH2 | – | 7.62 | 7.992 | 6.381 | 7.094 |
| 2 | Pr | Cl | CONH2 | – | 7.678 | 8.031 | 7.398 | 7.308 |
| 3 | Pr | Et | CONH2 | – | 8.155 | 8.12 | 7.26 | 7.290 |
| 4 | Pr | Et | CONMe2 | – | 7.292 | 7.382 | 7.26 | 7.298 |
| 5 | Pr | Et | CONHMe | – | 7.886 | 8.151 | 7.796 | 7.228 |
| 6 | Pr | Cl | CO2Me | – | 7.638 | 8.287 | 6.337 | 6.975 |
| 7 | Pr | Cl | COMe | – | 7.398 | 7.608 | 7.398 | 7.083 |
| 8 | Pr | CH2CH2CH2CH2C(=O) | H | 8.097 | 8.12 | 6.979 | 7.260 | |
| 9 | – | – | – | 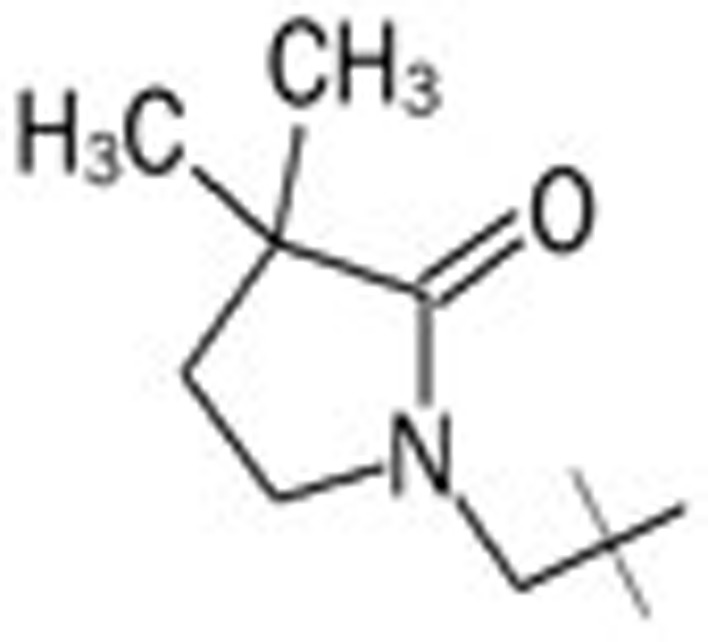 |
6.481 | 9 | 8.635 | |
| 10 | – | – | – | Methyl | 8.698 | 8.35 | 7.886 | 8.044 |
| 11 | – | – | – | Fluoro | 7.796 | 7.551 | 7.699 | 7.968 |
| 12 | – | – | – | Cyanomethyl | 7.796 | 7.833 | 7.824 | 8.349 |
| 13 | – | – | – | Hydroxymethyl | 7.523 | 7.637 | 7.854 | 8.016 |
| 14 | – | – | – | Ethoxymethyl | 8.301 | 8.157 | 7.699 | 7.587 |
| 15 | – | – | – | Methoxymethyl | 8.398 | 7.727 | 7.69 | 7.675 |
| 16 | – | – | CONH2 | Methoxymethyl | 9.222 | 8.879 | 8.398 | 7.860 |
| 17 | – | – | CONH2 | Methyl | 8.699 | 8.037 | 8.301 | 7.308 |
| 18 | – | – | CONH2 | Ethoxymethyl | 8.523 | 8.192 | 7.699 | 7.702 |
| 19 | – | – | CONH2 | Chloro | 7.456 | 7.742 | 6.155 | 6.949 |
| 20 | – | – | CONH2 | Ethyl | 8.886 | 8.419 | 8.155 | 8.176 |
| 21 | – | – | CONH2 | 2-Fluoroethoxymethyl | 8.699 | 8.56 | 8.097 | 7.550 |
| 22 | – | – | CONHMe | H | 8.097 | 8.528 | 8.155 | 8.151 |
| 23 | – | – | CONHMe | Methyl | 8.398 | 8.528 | 8.301 | 7.500 |
| 24 | – | – | CONHMe | Ethoxymethyl | 7.854 | 7.541 | 7.656 | 8.168 |
Enrichment studies
The Schrodinger decoy set was used to validate the pharmacophore hypotheses, virtual screening (VS) and QPLD. This decoy set was downloaded as a 3D SD file from the Schrodinger Website and consists of 1000 drug-like compounds (with an average molecular weight of 400 Daltons) and 27 active compounds from the published literature15. For our validation, 100 drug-like compounds were randomly selected from the Schrodinger decoy set and mixed with 27 active molecules. QPLD calculation is a time-consuming process, so we reduced our decoy set to 127. We used this method to evaluate the ability of our hypothetical pharmacophores and docking study to discriminate and separate the active compounds from the inactive compounds in our larger set of molecules.
Results and discussion
ETA sequence alignment and model generation
A sequence similarity search was performed using NCBI BLASTp, a conventional alignment tool for protein sequences. Sequence similarity is the main criterion for selecting a template for homology modeling. The sequence similarity between the ETA protein and other known GPCR proteins is very low (<30%), but we selected the templates from the GPCR superfamily that had high resolution and maximum sequence coverage with the target sequence. Therefore, in addition to sequence similarity, we used 7-TM architecture as another criterion. The amino acid sequence of ETA (427 amino acids) shows a significant percentage of identity with the thermostabilized adenosine A2A receptor [3PWH: 70/306 (23%)] and the β2-adrenergic receptor [3KJ6: 84/384 (22%)]. Even though the target-template sequence similarity falls in the twilight region (<30%), it is still possible to obtain a probable model of ETA based on these templates because all these proteins belong to the class A GPCR superfamily with a topology of seven trans-membrane helices. Sequence-structure alignment has been reported to be superior to sequence-sequence alignment for the purpose of homology modeling; therefore, the former was used for our model building. The multiple sequence alignment of the ETA sequence with 3KJ6 and 3PWH is shown in Figure 1, and conserved residues in the binding site region are shown in Supplementary Figure 1.
Figure 1.
Multiple structure sequence alignment between template crystal structures (3PWH and 3KJ6) and the ETA sequence. In the sequences, an asterisk (*) indicates an identical or conserved residue, a colon (:) indicates conserved substitutions, and a period (.) indicates semi-conserved substitutions.
Several studies have reported that homology modeling based on multiple templates improves the quality of the modeled proteins, including GPCR18,19,20,21. Therefore, in this study we used multiple templates to generate the ETA model. Ten models were generated based on the human adenosine A2A Receptor (3PWH) and the β2-Adrenergic Receptor (3KJ6) using the multiple templates protocol of modeller 9.10 software (Accelrys Inc, San Diego, CA, USA). Loop refinements were carried out using Prime software (Schrodinger, LLC, New York, NY, USA). The modeled proteins were energy minimized using the OPLS 2005 force-field.
Validation of the predicted structure
The overall stereo-chemical qualities of the ten generated models were assessed by PROCHECK (Hinxton, Cambridge, United Kindgom). One model was selected based on the best Ramachandran plot characteristics. The Ramachandran plot of the best model showed 81.6% of the residues in the most favorable region, 15.1% in the additional allowed region, 2.0% in the generously allowed region and 1.3% in the disallowed region. These results revealed that the majority of the amino acids are in a phi/psi distribution that is consistent with a right-handed α-helix and suggested that the model is reliable and of good quality. The G-factors, indicating the quality of the covalent, dihedral and overall bond angles, were −0.92, −1.96, and −1.28, respectively. The overall main-chain and side-chain parameters, as evaluated by PROCHECK, were all very favorable. The Ramachandran plot characteristics confirmed the quality of the predicted model. The model selected was again subjected to MD simulation for further refinement of the structure.
Molecular dynamics simulations
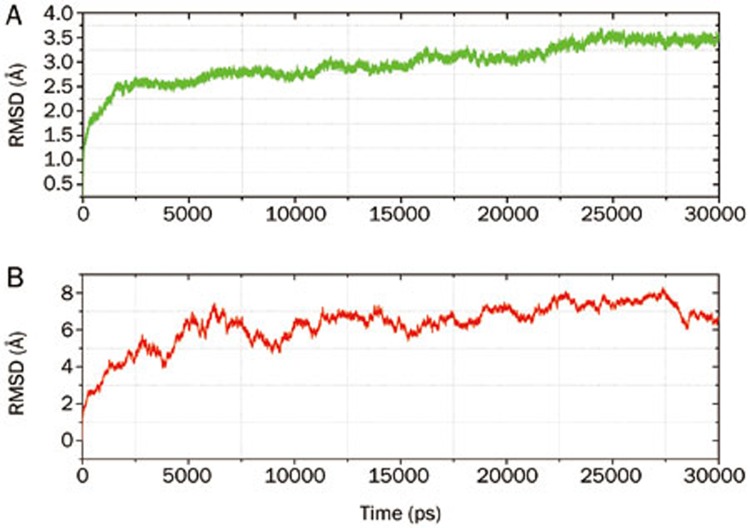
Molecular dynamics can be used to explain protein structure-function problems, such as folding, conformational flexibility and structural stability. In the simulations, we monitored the backbone atoms and the Cα-helix of the modeled protein. The RMSD values of the modeled structure (ETA) and 1ZV0 (AT1R) protein backbone atoms were plotted as a time-dependent function of the MD simulation. The results support our modeled structure; they show constant RMSD after 25 ns of the simulation process for ETA, and there is less deviation in the RMSD for AT1R after 6 ns. The time dependence of the RMSD (Å) of the backbone atoms of the modeled protein (ETA) and the 1ZV0 (AT1R) protein over the course of 30 ns of simulation is shown in Figures 2A and 2B. The graph clearly indicates that there is a change in the RMSD from 1.0 Å to 3.0 Å in the ETA homology model during the first 20 ns, but after 20 ns, the RMSD reaches a plateau. The RMSD values of the backbone atoms in the system tend to converge after 25 ns, showing fluctuations of approximately 0.5 Å. The low RMSD and the simulation time indicate that, as expected, the 3D structural model of ETA represents a stable folding conformation. The RMSD of the AT1R protein was also analyzed; there was a constant deviation of the RMSD within 2 Å after 4 ns. This result confirms that the theoretical 3D structure of the AT1R protein (Pdb id: 1ZV0) is good enough for further study.
Figure 2.
The RMSD of backbone atoms of the ETA (A) and AT1R proteins (B) from a 30 ns MD run.
Prediction and comparison of the active sites of AT1R and ETA
Taking into account the structural flexibility of the GPCR protein, we first evaluated the active sites of the modeled proteins in terms of shape, sterics, and electronic properties. From the contact scores (0.956 for AT1R and 0.923 for ETA), the strength of van der Waals contacts with the receptors also emerged; this is an important feature of each active site. The exposure and enclosure values are measures of the degree of opening of an active site to the solvent; these values are almost the same in both proteins (Table 2). However, the value of don/acc is much less in AT1R compared to that in ETA. This finding reveals that the intensities of hydrogen bond donor and acceptor regions are lower in AT1R. The overall scores (site score) are almost the same in both of the proteins. This result provides further information regarding the active sites of the AT1R and ETA proteins and will facilitate the design of potent dual inhibitors.
Table 2. Comparison of active site of AT1R and ETA protein using SiteMap (Schrodinger, LLC).
| Protein | Site score | Size | Volume | Exposure | Enclosure | Contact | Phobic | Philic | Don/acc |
|---|---|---|---|---|---|---|---|---|---|
| AT1R | 1.063 | 310 | 1117.837 | 0.597 | 0.753 | 0.956 | 1.081 | 0.904 | 0.654 |
| ETA | 1.114 | 322 | 1061.242 | 0.580 | 0.756 | 0.923 | 1.347 | 0.652 | 1.087 |
Exposure/enclosure, properties measuring the degree of opening of active site to the solvent; contact, measure of site point interaction via vdW contacts; don/acc, property related to the sizes and intensities of H-bond and acceptor regions; SiteScore: an overall score based on previous properties.
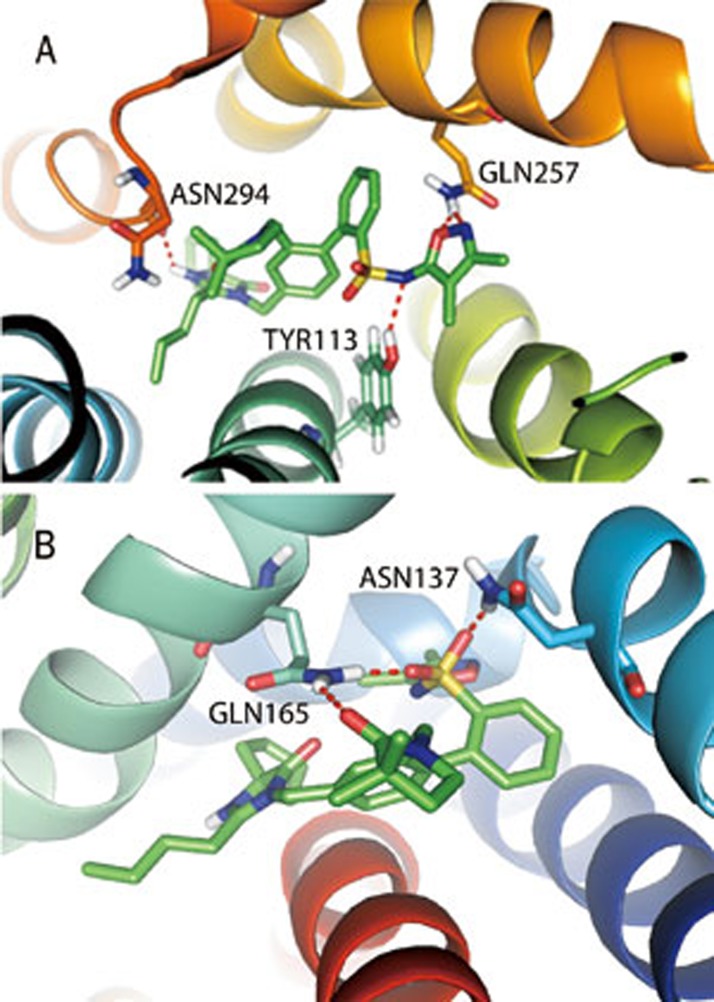
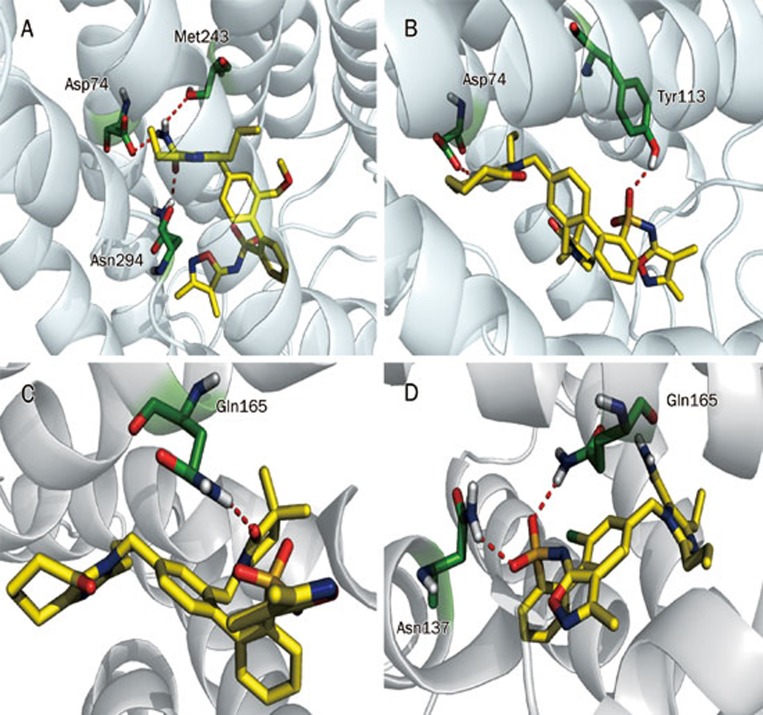
QM/MM docking of AT1R and ETA with the dual inhibitor BMS-248360
A QM/MM docking study was performed with BMS-248360, a dual AT1R and ETA inhibitor, to further optimize the protein structures (Figures 3A and 3B). N1 and O2 from oxazole form a hydrogen bond with the −NH2 group of Gln257 of AT1R, and N4 near the oxosulfane oxide forms a hydrogen bond with the −OH group of Tyr113. Two benzene rings of BMS-248360 form Π–Π stacking with Phe204, Phe249, Tyr253, and His256, respectively. In ETA, O1, and O3 from the oxosulfane oxide form hydrogen bonds with the −NH2 groups of Gln165 and −NH2 group of Asn137, respectively. O5 from 3,3-dimethylpyrrolidin-2-one forms a hydrogen bond with the −NH2 region of Gln165.
Figure 3.
Binding modes of AT1R (A) and ETA (B) with the dual inhibitor BMS-248360.
The binding free energy was also calculated using the MM/GBSA approach for the proteins in complex with BMS-248360; the free energy was −121.44 kcal/mol for AT1R and −120.21 kcal/mol for ETA. To explore the role of important amino acid residues in the ligand-protein interaction, we applied a computational mutagenesis (alanine scanning) to replace these residues with alanines. The results from our energetic analysis of the mutant complexes are shown in Table 3. In the ETA/BMS-248360 complex, the N137A and Q165A mutations were not favorable for ΔGbind, increased this parameter from −120.21 kcal/mol to −104.95 kcal/mol and −99.20 kcal/mol, respectively. The mutation increased the ΔGBind Covalent, the ΔGBind Lipo and the ΔGBind SolvGB by 10.38 kcal/mol, 4.97 kcal/mol and 17.409 kcal/mol, respectively. However, the ΔGBind Coulomb, the ΔGBind Hbond, and the ΔGBindvdW were lower in the N137A mutant by 12.37 kcal/mol, 0.05 kcal/mol and 4.996 kcal/mol, respectively. For the Q165A mutant, the ΔGBind Coulomb, the ΔGBind Covalent, the ΔGBind Hbond and the ΔGBind Lipo were increased, but the ΔGBind SolvGB and the ΔGBindvdW were slightly decreased. This finding shows that N137 and Q165 are important residues for ligand binding. In the AT1R/BMS-248360 complex, the Y113A, Q257A, and N294A mutations are not favorable for the ΔGbind, the ΔGBind Coulomb, the ΔGBind Covalent, the ΔGBind Hbond, the ΔGBind Lipo, the ΔGBind SolvGB, and the ΔGBindvdW, based on our energetic analysis, but the N294A mutant slightly reduced the ΔGBind Coulomb and the ΔGBind SolvGB. This result clearly shows that Y113, Q257, and N294 are important residues for ligand binding.
Table 3. Relative binding free energies (kcal/mol) between wild and mutant ETA/BMS-248360 and AT1R/BMS-248360 complex.
| ETA/BMS-248360 complex | |||||||
|---|---|---|---|---|---|---|---|
| ΔGBinda | ΔGBind Coulombb | ΔGBind Covalentc | ΔGBind Hbondd | ΔGBind Lipoe | ΔGBind SolvGBf | ΔGBindvdWg | |
| WT | −120.21 | 10.18 | 4.34 | −0.97 | −71.07 | 5.77 | −66.08 |
| N137A | −104.95 | −2.20 | 14.72 | −1.02 | −66.10 | 23.18 | −71.08 |
| Q165A | −99.20 | 17.50 | 13.54 | −0.64 | −63.25 | 2.01 | −66.18 |
| AT1R/BMS-248360 complex | |||||||
| WT | −121.44 | 32.18 | 14.14 | −1.19 | −63.37 | −16.00 | −80.91 |
| Y113A | −113.42 | 35.53 | 13.89 | −0.78 | −65.84 | −17.76 | −75.69 |
| Q257A | −115.70 | 37.07 | 13.82 | −0.85 | −66.60 | −18.05 | −79.54 |
| N294A | −120.89 | 28.40 | 13.77 | −1.17 | −67.39 | −14.14 | −78.04 |
WT, Wild Type.
aMMGBSA free energy of binding
bContribution to the MMGBSA free energy of binding from the Coulomb energy
cContribution to the MMGBSA free energy of binding from covalent binding
dContribution to the MMGBSA free energy of binding from hydrogen bonding
eContribution to the MMGBSA free energy of binding from lipophillic binding
fContribution to the MMGBSA free energy of binding from the van der Waals energy
eContribution to the MMGBSA free energy of binding from the generalized Born electrostatic solvation energy.
Glide extra-precision docking (XP), quantum polarized ligand docking and molecular mechanics-generalized born surface area (MM/GBSA) rescoring
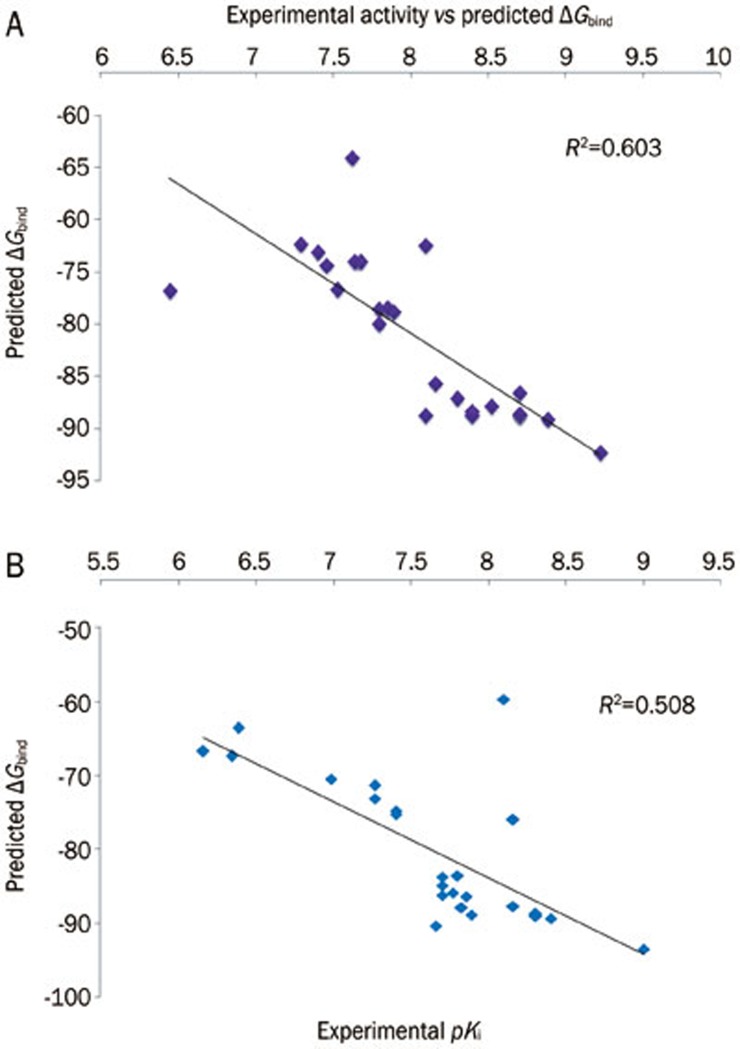
To study the molecular basis of interactions between the imidazole compound series and AT1R and ETA and to analyze the affinity of this binding, all the ligands were docked into the active site using Glide XP (Table 4 and Table 5). The correlation between experimentally determined biological activity and the binding free energy was 0.291 for AT1R (Supplementary Figure 2) and 0.232 for ETA (Supplementary Figure 3). Because XP docking predicts a very low correlation between experimental biological activity and the predicted binding free energy, we performed further study using QM-polarized docking. We assumed that this docking method would give a more accurate prediction of the electrostatic interactions46, which help to increase the docking accuracy. All 24 imidazole series compounds were docked inside the active sites of AT1R and ETA using QPLD. The binding mode of inhibitors predicted using Glide XP docking was almost identical to that predicted by QPLD. The top five poses per molecule were submitted for rescoring using the MM/GBSA process. The correlations between the MM/GBSA result and experimental activity (0.603 and 0.508 for AT1R and ETA (Figure 4), respectively) were better than the correlations between the docking scores and the experimental activity. The MM/GBSA rescoring of QPLD showed better correlation with the biological experimental activity than the MM/GBSA scoring from Glide XP docking calculation. Therefore, this docking method gives more accurate treatment of electrostatic interactions, which helps to improve the docking accuracy. We used the QPLD docked ligand conformation to generate the 3D-QSAR model.
Table 4. Docking and MM/GBSA free energy calculation result of imidazole series against AT1R.
| Compound No | Docking score | Glide XP Prime MM/GBSA ΔGbind (kcal/mol) | Docking score | QPLD Prime MM/GBSA ΔGbind (kcal/mol) | QM/MM Energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 | −5.26 | −75.56 | −6.89 | −64.13 | −2440.50 |
| 2 | −6.34 | −76.61 | −7.05 | −74.00 | −2401.45 |
| 3 | −6.29 | −81.32 | −6.77 | −85.62 | −2022.18 |
| 4 | −5.96 | −73.55 | −6.03 | −72.35 | −2100.38 |
| 5 | −7.05 | −77.10 | −6.74 | −78.74 | −2061.32 |
| 6 | −6.44 | −75.23 | −5.83 | −73.97 | −2459.14 |
| 7 | −6.09 | −74.83 | −6.17 | −73.06 | −2384.35 |
| 8 | −5.32 | −79.96 | −6.23 | −72.43 | −2043.00 |
| 9 | −7.38 | −67.69 | −7.07 | −76.72 | −2443.96 |
| 10 | −5.99 | −77.08 | −5.68 | −86.48 | −2082.07 |
| 11 | −6.32 | −77.41 | −5.64 | −78.47 | −2141.73 |
| 12 | −5.77 | −80.75 | −6.56 | −79.96 | −2173.85 |
| 13 | −5.09 | −75.18 | −5.67 | −76.67 | −2156.88 |
| 14 | −6.08 | −58.90 | −6.46 | −86.97 | −2235.04 |
| 15 | −6.46 | −87.60 | −6.67 | −88.34 | −2195.94 |
| 16 | −6.25 | −91.41 | −7.20 | −92.19 | −2174.10 |
| 17 | −6.34 | −67.28 | −6.56 | −88.70 | −2060.23 |
| 18 | −6.62 | −89.29 | −7.09 | −87.73 | −2213.40 |
| 19 | −6.23 | −50.66 | −5.84 | −74.39 | −2478.51 |
| 20 | −6.06 | −90.77 | −7.11 | −89.02 | −2099.26 |
| 21 | −6.04 | −86.25 | −6.68 | −88.56 | −2311.77 |
| 22 | −6.15 | −82.19 | −5.99 | −88.61 | −2213.07 |
| 23 | −5.72 | −84.53 | −6.20 | −88.61 | −2099.25 |
| 24 | −6.08 | −72.01 | −6.34 | −78.40 | −2252.21 |
Table 5. Docking and MM/GBSA free energy calculation result of imidazole series against ETA.
| Compound No | Docking score | Glide XP Prime MM/GBSA ΔGbind (kcal/mol) | Docking score | QPLD Prime MM/GBSA ΔGbind (kcal/mol) | QM/MM Energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 | −9.91 | −69.01 | −7.15 | −63.59 | −2403.67 |
| 2 | −10.64 | −79.62 | −7.15 | −74.74 | −2403.67 |
| 3 | −10.81 | −78.41 | −6.13 | −71.39 | −2024.44 |
| 4 | −9.87 | −65.88 | −7.01 | −73.10 | −2102.61 |
| 5 | −9.96 | −82.48 | −6.35 | −83.52 | −2063.51 |
| 6 | −9.92 | −70.35 | −6.87 | −67.40 | −2461.39 |
| 7 | −9.01 | −79.18 | −6.22 | −75.28 | −2386.58 |
| 8 | −8.89 | −72.03 | −6.18 | −70.50 | −2045.24 |
| 9 | −6.95 | −95.25 | −7.99 | −93.49 | −2446.21 |
| 10 | −8.31 | −88.90 | −6.37 | −88.83 | −2084.34 |
| 11 | −6.96 | −79.07 | −7.56 | −86.17 | −2143.92 |
| 12 | −7.41 | −84.99 | −7.45 | −87.80 | −2176.02 |
| 13 | −8.02 | −81.05 | −8.04 | −86.36 | −2159.09 |
| 14 | −8.43 | −78.41 | −6.60 | −84.83 | −2237.26 |
| 15 | −7.53 | −76.52 | −6.47 | −85.79 | −2198.15 |
| 16 | −9.80 | −88.43 | −6.97 | −89.33 | −2176.25 |
| 17 | −9.03 | −88.99 | −6.56 | −88.72 | −2062.40 |
| 18 | −8.14 | −78.33 | −7.39 | −83.73 | −2215.31 |
| 19 | −7.21 | −85.58 | −7.00 | −66.75 | −2480.72 |
| 20 | −7.50 | −86.65 | −7.01 | −75.99 | −2101.73 |
| 21 | −8.10 | −55.09 | −7.21 | −59.67 | −2314.04 |
| 22 | −7.89 | −85.64 | −7.43 | −87.66 | −2215.26 |
| 23 | −7.75 | −88.68 | −6.55 | −88.97 | −2101.50 |
| 24 | −8.10 | −80.69 | −6.81 | −90.30 | −2254.40 |
Figure 4.
Experimental activity vs predicted ΔGbind of AT1R (A) and ETA (B) from the QPLD pose.
Analysis of 3D-QSAR validation
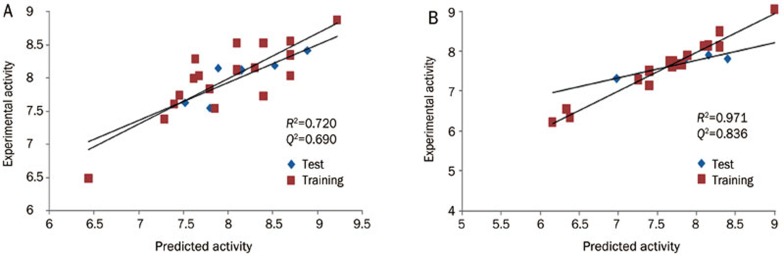
Reliable predictions can only come from statistically valid QSAR models. Several statistical parameters can be used to evaluate the robustness of a QSAR model, including leave-n-out cross validation of the training set (R2), leave-n-out cross validation of the test set (Q2), the standard deviation (SD), the Root Mean Square Error (RMSE), and the variance ratio (F). High R2 is an important condition for a predictive QSAR model. The best QSAR model will be chosen based on its predictive ability, so in addition to high R2, the best model should also have high Q2. For AT1R, we obtained a good R2 value for the training set (0.72), excellent predictive power with a Q2 of 0.69, an RMSE of 0.254, a standard deviation (SD) of 0.38, an F variance ratio of 35.7, a Pearson R correlation (between the predicted and observed activity for the test set) of 0.85, and an r2pred of 0.66. Plots of the predicted vs actual pKi for the training and test sets are shown in Figure 5A. For ETA, we obtained an R2 value for the training set of 0.971, excellent predictive power with a Q2 of 0.84, an RMSE of 0.11, a SD of 0.04, an F variance ratio of 159.1, a Pearson R correlation (predicted and observed activity for the test set) of 0.92, and an r2pred of 0.70. Plots of the predicted vs actual pKi for the training and test sets are shown in Figure 5B.
Figure 5.
Graph of actual versus predicted pKi of the training set and the test set using the atom-based QSAR model from PHASE for AT1R (A) and ETA (B).
Analysis of 3D-QSAR model
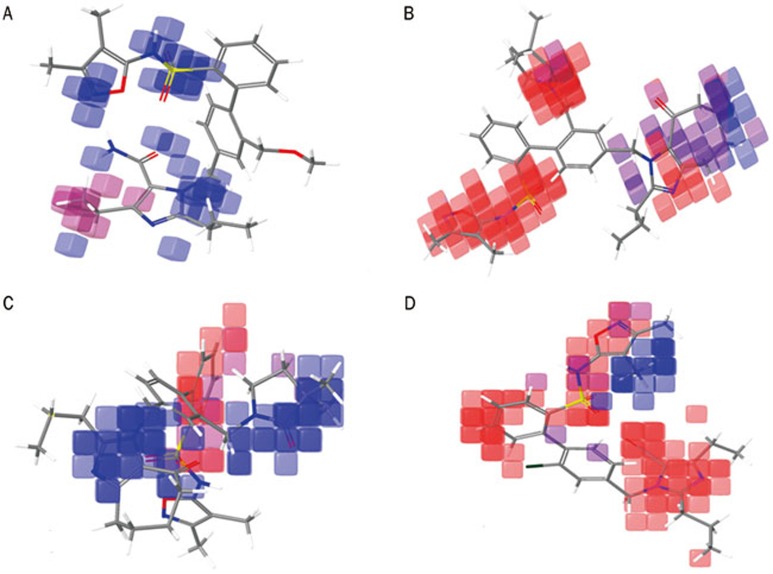
Additional insight into the inhibitory activity can be gained by visualizing the QSAR model in the context of the most and least active compounds. The contribution maps obtained from our result shows how 3D-QSAR methods can identify features that are important for the interaction between each ligand and its target protein. Such maps allow the identification of those positions that require a particular physicochemical property to enhance the bioactivity of a ligand. A pictorial representation of the contours generated for AT1R is shown in Figures 6A–6B; the contours generated for ETA are shown in Figures 6C–6D. In these representations, the blue cubes indicate favorable regions, while red cubes indicate unfavorable regions for activity.
Figure 6.
Pictorial representation of the cubes generated using the QSAR model. Blue areas indicate favorable regions, while red areas indicate unfavorable regions for the activity. The QSAR model visualized in the context most active compound, 16 (A), and the least active compound, 9 (B), against AT1R. The QSAR model visualized in the context of the most active compound, 9 (C), and the least active compound 19 (D), against AT1R.
Figures 6A and 6B (AT1R) compares the most significant favorable and unfavorable features that arise when the QSAR model is applied to the most active compound (16) and the least active compound (9) for AT1R inhibition. In the context of compound 16, the blue cubes are observed near the (2,3-dimethylcyclopenta-1,3-dien-1-yl)sulfuramidous acid and the N17 region. However, a red cube is still observed near the ethyl group of the H-imidazole region. In the context of the least active compound (9), red cubes are observed near N-(3,4-dimethyl-1,2-oxazol-5-yl)benzenesulfonamide, N17 of tetrahydro-H-benzimidazole and the trimethylpyrrolidinone region. However, blue cubes are still observed near the tetrahydro-H-benzimidazole region, which may be a result of the addition of cyclooctanone to the H-imidazole region. The activity of the molecule is greatly influenced by the substitution of methoxymethyl group in C4 of the benzene ring. The further addition of cyclooctanone to the H-imidazole region may increase the activity in the most active compound.
Figures 6C and 6D (ETA) compare the most significant favorable and unfavorable features that arise when the QSAR model is applied to the most active compound (9) and the least active compound (19) for ETA inhibition. In the context of compound 9, blue cubes are observed in the region of the pyrazole and the trimethylpyrrolidinone. However, red cubes are observed near the benzene ring. In the context of compound 19, red cubes are observed near the 5-[(aminooxy)methyl]-4-ethyl-2-propyl-1H-imidazole benzene ring and in the (3,4-dimethyl-1,2-oxazol-5-yl)sulfuramidous acid region. However, blue cubes are also observed in the two methyl groups of N-(3,4-dimethyl-1,2-oxazol-5-yl)benzenesulfonamide. This finding indicates that the substitution of cyclooctanone in the H-imidazole and trimethylpyrrolidinone increased the activity of the molecule.
From our 3D-QSAR study, we observed that the substitution of cyclooctanone and trimethylpyrrolidinone is important for the activity of the compound against the ETA protein. However, in the case of AT1R, the substitutions of trimethylpyrrolidinone reduce the activity of the compounds, and the substitution of the methoxymethyl group on R4 influences the activity of the molecule. The addition of aminooxy methane and the ethyl group on H-imidazole also increases the activity. The sizes and intensities of the H-bond donor and acceptor region are comparatively small in AT1R compared to those in the ETA protein (Table 2). This difference may explain why the addition of trimethylpyrrolidinone reduces the activity against AT1R protein but increases the activity against ETA.
Binding mode analysis of the most active compound (compound 16) and the least active compound (compound 9) against AT1R
Molecular docking studies were performed to understand the binding mode and mechanism of binding between the most active compound (16) (Figure 7A) and the least active compound (9) and AT1R (Figure 7B). To obtain information for further structure optimization, we used Quantum Polarized Ligand Docking. The −C=O from −CONH2 (R3) of compound 16 forms a hydrogen bond with N294, and the −NH2 group from −CONH2 also forms two hydrogen bonds with S115 and D74, respectively. Two benzene rings from the major scaffold have Π-Π stacking with H256 and F249, respectively. The O1 from the oxosulfane oxide group of compound 9 forms a hydrogen bond with the −OH group of Y113 of AT1R, and N1 from the imidazole near cycloheptanone interacts with the −COOH group of D74 of AT1R. Dimethyl-dihydro-oxazole and the benzene ring near oxosulfane oxide have Π–Π stacking with H256 and F204, respectively. Molecular docking also reveals that the substitution of −CONH2 forms a greater number of hydrogen bonds, which may enhance the activity of the molecule, and the substitution of trimethylpyrrolidinone may reduce the activity of the molecule. This result further confirms that the substituted bulky group (trimethylpyrrolidinone) in R4 cannot fit inside the active site of AT1R, and therefore, the activity of the molecule decreases.
Figure 7.
The binding mode of AT1R and its inhibitors. (A) The most active compound, 16, and (B) the least active compound, 9. The binding mode of ETA and its inhibitors (C) the least active compound, 19, and (D) the most active compound, 19.
Binding mode analysis of the most active compound (compound 9) and the least active compound (compound 19) against ETA
In the most active compound against ETA (9) (Figure 7C), the O5 from 3,3-dimethylpyrrolidin-2-one (R4) forms a hydrogen bond with the NH2 of Q165 in ETA. Imidazole participates in Π–Π stacking with W240. In the case of the least active compound against ETA (19) (Figure 7D), O1 and O2 of the oxosulfane oxide group form hydrogen bonds with the NH2 of Gln165 and the NH2 of Asn137, respectively. From this docking result, we also observed that there was Π–Π stacking for the most active compound and that this may play an important role in the activity of the molecule. We also observed that the substitution of trimethylpyrrolidinone does not affect the activity of the molecule, and this substituted compound is able to fit inside the active site of ETA. This docking study also revealed that the substitution of trimethylpyrrolidinone (R4) may influence the activity of the molecule.
Pharmacophore- and structure-based screening
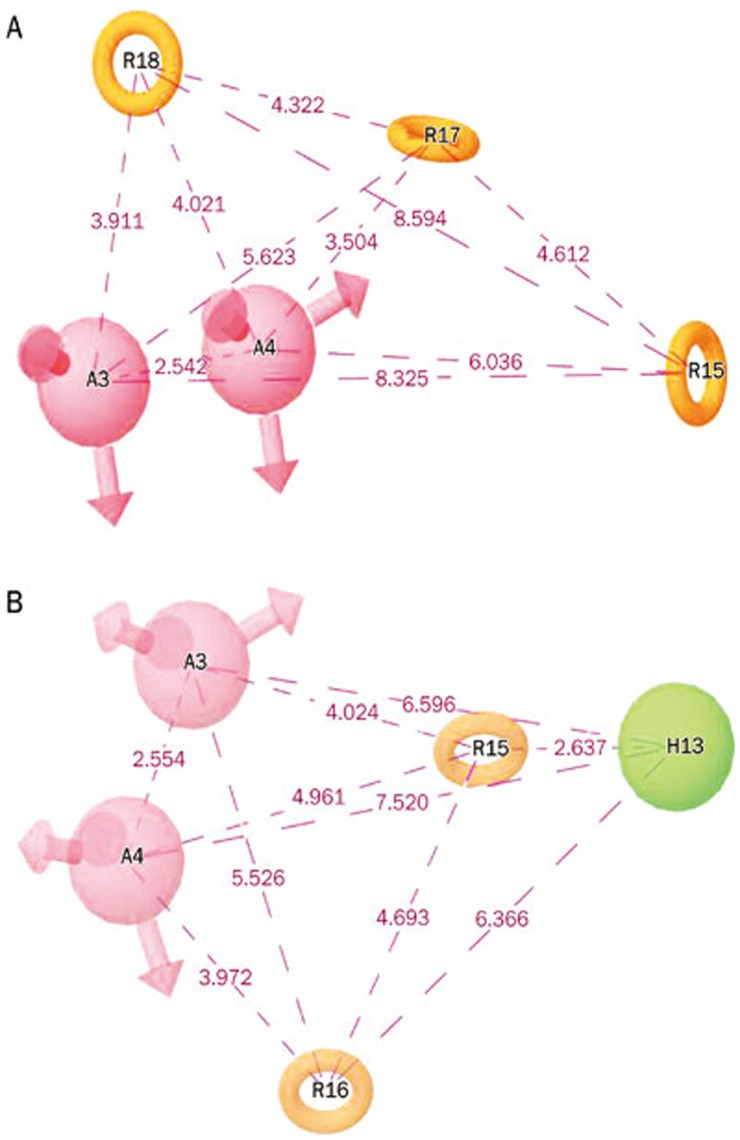
Pharmacophore models containing five sites were generated using the selected molecules for this study (24 imidazole series compounds)15, which were reported as dual inhibitors of both AT1R and ETA. The common hypothetical pharmacophores with three and four features were rejected for the study based on the molecule occupancy of the pharmacophoric features. The best two hypothetical pharmacophores, AARRR and AARRH, were selected based on the scoring function for further pharmacophore-based screening (Figure 8). The selected hypothetical pharmacophore consists of two hydrogen bond acceptors (A) and three aromatic rings (R) for the first hypothesis, and the second hypothetical pharmacophore consists of two hydrogen bond acceptors (A), two aromatic rings (R) and one hydrophobic group (H).
Figure 8.
The two top-ranked pharmacophore models with the pharmacophore distance (Å).
A database search retrieved all the positive hits and filtered out the inactive compounds. The interpretation of how the pharmacophore maps onto the positive hits may provide insight into the structural requirements for dual inhibition of AT1R and ETA and can act as a guide for the further refinement of the molecules to design a better molecule with good absorption, distribution, metabolism, and excretion (ADME) properties. The generated pharmacophore model screened against the ChemBridge chemical databases of 511,324 compounds. The compounds that possessed fitness scores higher than 1.500 (2873 compounds) were subjected to high-throughput virtual screening (HTVS) against AT1R and ETA separately. After HTVS, SP docking and XP docking, which are considered to be more precise modes of docking simulation, were performed. We identified 46 compounds targeting AT1R and 33 compounds targeting ETA that were able to effectively bind to their active site through this docking procedure. Further, we re-docked (using XP flexible docking) these 46 compounds targeting ETA and 33 compounds targeting AT1R; this approach identified 17 compounds that are able to effectively bind to the active site of both proteins. The selection of compounds was based on the glide score, glide energy, emodel, binding free energy and conformation of binding, which are similar to those of the known compounds. It has been reported that ensemble rescoring53,54,55 increases the percentage of correctly docked poses. Therefore, we used ensemble rescoring to identify the potential dual inhibitors. Henriksen et al also applied ensemble rescoring in identifying lead molecules using virtual screening56. They found that 19 out of 41 predicted compounds were active in an enzyme assay and that 14 of these compounds were active in subsequent whole cell assays.
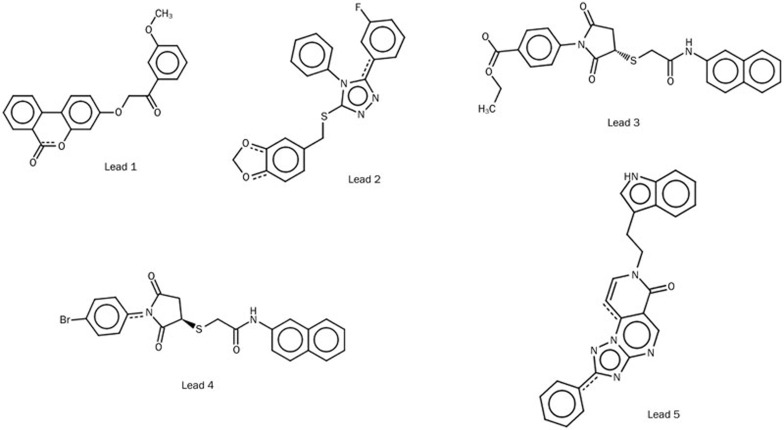
Next, we subjected these 17 dual-inhibitory compounds to quantum polarized ligand docking, which we found to be more accurate for this study, and we identified 5 compounds that bind very effectively and close to the binding mode of the most active known compounds with good docking score and good binding free energy. The 2D structures of the five identified compounds are shown in Figure 9. The binding mode and docking scores are shown in Table 6 and Table 7. The binding modes of the five lead compounds inside the binding pocket of AT1R and ETA are shown in Supplementary Figures 4 and 5, respectively.
Figure 9.
The chemical structure of five leading molecules that may be potential dual inhibitors of AT1R and ETA.
Table 6. Docking score and predicted activity of ETA protein. Predicted activity was calculated using the equation Y=−9.470x−5.020, where Y is the activity and x is the ΔGbind. The equation was derived from the correlation equation of experimentally predicted activity and ΔGbind of the compounds studied for QSAR analysis.
| Compound name | Docking score | QM/MM Energy (kcal/mol) | MM/GBSA (kcal/mol) | Predicted activity |
|---|---|---|---|---|
| Lead 1 | −7.52 | −1216.96 | −78.18 | 7.45 |
| Lead 2 | −9.31 | −1651.27 | −76.15 | 7.25 |
| Lead 3 | −7.51 | −1839.31 | −88.57 | 8.47 |
| Lead 4 | −7.96 | −4134.87 | −84.92 | 8.11 |
| Lead 5 | −7.42 | −1321.34 | −71.75 | 6.83 |
Table 7. Docking score and predicted activity of AT1R protein. Predicted activity was calculated using the equation Y=−10.26x−1.717, where Y is the activity and x is the ΔGbind. The equation was derived from the correlation equation of experimentally predicted activity and ΔGbind of the compounds studied for QSAR analysis.
| Compound name | Docking score | QM/MM Energy (kcal/mol) | MM/GBSA (kcal/mol) | Predicted activity |
|---|---|---|---|---|
| Lead 1 | −8.00 | −1216.95 | −60.37 | 5.85 |
| Lead 2 | −5.98 | −1651.27 | −64.82 | 6.31 |
| Lead 3 | −7.85 | −1839.33 | −85.01 | 8.45 |
| Lead 4 | −7.32 | −4138.87 | −88.23 | 8.79 |
| Lead 5 | −7.30 | −1321.34 | −74.50 | 7.34 |
Enrichment of the virtual screening protocol
In a virtual screening process, the best hypothesis or docking calculation should distinguish the active compounds from the inactive ones. A decoy set, composed of 27 known dual inhibitors of AT1R and ETA and 100 decoy molecules from Schrodinger, was used to validate whether the use of the hypothetical pharmacophores (AARRH and AARRR), VS, and QPLD could discriminate the active compounds from the inactive compounds. The result was analyzed using a set of parameters such as the hit list (Ht), number of active percent of yields (%Y), percent ratio of actives in the hit list (%A), enrichment factor (E), false negatives, false positives, and goodness of hit score (GH) (Supplementary Table 1 and 2)57,58. We observed that all the GH values were between 0.6 and 0.8, which indicates that the quality of the pharmacophore models and VS are acceptable. Based on our overall validations, we can assume that both the pharmacophore hypothesis and the docking calculation were able to predict most of the compounds in the same order of magnitude and that it can discriminate the active inhibitors from the inactive or low-activity compounds. Because QPLD has better GH values, we assumed that this docking protocol would be able to discriminate the active inhibitors from the inactive ones. Therefore, we propose that our virtual screening protocol can effectively discriminate the most suitable dual inhibitors of AT1R and ETA.
Analysis of identified compounds using QSAR model generated against AT1R
We superimposed our QSAR model generated on the most active compound with the five identified compounds (Supplementary Figure 6). In lead 1, blue cubes were observed on the 6H-benzo[c]chromen-6-one regions of the compound, and the −C=O from 6H-benzo[c]chromen-6-one formed a hydrogen bond with Val179 of AT1R. Therefore, this functional group may be important for the activity against AT1R. In lead 2, blue cubes were observed near the triazole and the benzene ring, but unfortunately, there were no interactions in this region. In lead 3, blue cubes were observed on ethyl formate, and the benzene ring attached to it had Π–Π stacking with Trp253 of AT1R. Ethyl formate has a high electron-withdrawing capacity and may influence the activity of the molecule. In lead 4, the blue cubes were observed near the pyrrolidine-2,5-dione region, and O11 formed H-bonds with Tyr113 of AT1R. The bromobenzene near pyrrolidine-2,5-dione also formed Π–Π stacking with Trp253. In lead 5, blue cubes were observed on the indole, and the −NH from the indole formed a H-bond and Π–Π stacking with Tyr292. Therefore, the indole of lead 5 may be an important functional group for the activity of the molecule.
Analysis of identified compounds using QSAR model generated against ETA
In the context of lead 1, blue cubes were observed on methoxybenzene, and this benzene formed Π–Π stacking with Tyr50 (Supplementary Figure 7). A hybrid of red cubes and blue cubes were observed near the 6H-benzo[c]chromen-6-one. The O from cyclohexane formed an H-bond with Gln86 of ETA, and the benzene ring formed Π–Π stacking with Tyr186. Because 6H-benzo[c]chromen-6-one influenced the activity of the leads against both AT1R and ETA, this may be a particularly important substitution for the dual inhibitory activity. In lead 2, blue cubes were observed on the benzodioxole group, and red cubes were observed on the fluorobenzene group. Therefore, the substitution of benzodioxole may be an important substitution for the activity against ETA. In lead 3, blue cubes were observed on ethyl formate, but unfortunately, there was no interaction with this substitution. However, ethyl formate is an important substitution for the activity of this lead compound against AT1R; the substitution of ethyl formate may be an important substitution for dual inhibitory activity. In lead 4, blue cubes were observed on naphthalene; this could be an important substitution for the activity against ETA. In the context of lead 5, blue cubes were observed on the ethyl group, and a mix of red cubes and blue cubes was observed near the indole substitution. The −NH from the indole group formed an H-bond with Gln86 of ETA. The indole group is an important substitution for both the proteins; thus, this substitution could be important for dual inhibitory activity.
Conclusion
In summary, a structurally reasonable and energetically reliable model of ETA was built using the multi-template homology modeling technique and a subsequent stepwise refinement procedure by molecular dynamics simulation; the known inhibitors were then docked into the active site of the initial model. With this refined ETA model and the structure of AT1R, retrieved from PDB, we performed QPLD docking with the known dual inhibitor BMS-248360 to further optimize the model structure. We found that Tyr113, Gln257, and Asn294 from AT1R formed hydrogen bonds with BMS-248360 and that Asn137 and Gln165 from ETA formed hydrogen bonds with BMS-248360. Further, we subjected these structures to Glide XP docking and QPLD with dataset compounds. The QPLD docking result showed better correlation with the experimental activity for both the proteins. We generated a 3D-QSAR model from this conformation, with good correlations of R2=0.72 and Q2=0.69 for AT1R and R2=0.97 and Q2=0.84 for ETA. Further, the visualization of the 3D-QSAR model in the context of the molecules under study provided details of the relationship between structure and activity; our model therefore provides explicit indications for the design of better analogues. The substitution of methoxymethyl and cyclooctanone may increase the activity of the molecule against the AT1R protein. In the case of the ETA protein, the substitution of cyclohexone and trimethylpyrrolidinone is important for the activity of the compound. From this study, we also observed that the addition of trimethylpyrrolidinone to compound 9 significantly reduced its activity against AT1R but significantly increased its activity against ETA due to the larger size and greater intensities of the H-bond donor and acceptor regions. Pharmacophore-based screening, followed by subsequent Glide SP, XP, and QPLD calculation, identified five potential leading molecules that could be potential dual inhibitors for both AT1R and ETA. The results from this study may provide some insights into the development of novel potent dual ETA and AT1R antagonists.
Author contribution
Karthikeyan MUTHUSAMY designed research; Khuraijam Dhanachandra SINGH performed research; Karthikeyan MUTHUSAMY and Khuraijam Dhanachandra SINGH contributed new analytical tools and reagents; Karthikeyan MUTHUSAMY and Khuraijam Dhanachandra SINGH analyzed data; Karthikeyan MUTHUSAMY and Khuraijam Dhanachandra SINGH wrote the paper.
Acknowledgments
This work was supported by the University Grant Commission (UGC), Government of India [Grant No 37-12/2009 (SR)]. KDS gratefully acknowledges CSIR for providing the Senior Research Fellowship (SRF). The authors thank Dr M RAVIKUMAR (Application Scientist from Schrödinger, Bangalore) for the detailed discussion and suggestions. The suggestions from the anonymous reviewers of this manuscript are cordially appreciated.
Footnotes
Supplementary information is available at the Acta Pharmacologica Sinica website.
Supplementary Information
The sequence alignments of ETA protein sequence with the sequence of templates (PDB id: 3PWH and 3KJ6) generated by Clustal W. In the sequences, an asterisk (*) indicates an identical or conserved residue; a colon (:) indicates conserved substitutions; a stop (.) indicates semi-conserved substitutions.
Experimental activity vs predicted ΔGBind from Glide XP docking pose of (AT1R).
Experimental activity vs predicted ΔGBind from Glide XP docking pose of ETA.
Binding mode of 5 lead molecules in the active site of AT1R.
Binding mode of 5 lead molecules in the active site of ETA.
Aligning of 3D-QSAR model of AT1R with the most active compound and 5 lead molecules identified from virtual screening.
Aligning of 3D-QSAR model of ETA with the most active compound and 5 lead molecules identified from virtual screening.
Statistical parameters of GH score validation for two top ranked hypotheses AARRH and AARRR.
Statistical parameters of GH score validation for VSW and QPLD.
References
- Ceolotto G, Papparella I, Bortoluzzi A, Strapazzon G, Ragazzo F, Bratti P, et al. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am J Hypertens. 2011;24:241–6. doi: 10.1038/ajh.2010.211. [DOI] [PubMed] [Google Scholar]
- Papadopoulos DP, Papademetriou V. Targeting angiotensin II type I receptors to reduce the risk of stroke in patients with hypertension. Expert Opin Ther Targets. 2006;10:231–7. doi: 10.1517/14728222.10.2.231. [DOI] [PubMed] [Google Scholar]
- Gradman AH. Role of angiotensin II type 1 receptor antagonists in the treatment of hypertension in patients aged >or=65 years. Drugs Aging. 2009;26:751–67. doi: 10.2165/11316790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B. New drug targets for pulmonary hypertension: Rho GTPases in pulmonary vascular remodelling. Postgraduate Med J. 2008;84:348–53. doi: 10.1136/pgmj.2007.066555. [DOI] [PubMed] [Google Scholar]
- Kingman M, Ruggiero R, Torres F Ambrisentan. An endothelin receptor type A — selective endothelin receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2009;10:1847–58. doi: 10.1517/14656560903061275. [DOI] [PubMed] [Google Scholar]
- Barst RJ. A review of pulmonary arterial hypertension: role of ambrisentan. Vasc Health Risk Manag. 2007;3:11–22. [PMC free article] [PubMed] [Google Scholar]
- van den Meiracker AH, Admiraal PJ, Janssen JA, Kroodsma JM, de Ronde WA, Boomsma F, et al. Hemodynamic and biochemical effects of the AT1 receptor antagonist irbesartan in hypertension. Hypertension. 1995;25:22–9. doi: 10.1161/01.hyp.25.1.22. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Brunner HR. Clinical experience with angiotensin II receptor antagonists. J Hum Hypertens. 1993;7:S33–S36. [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature (Lond) 1988;332:411–5. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlton V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. N Engl J Med. 1998;338:784–90. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- Murugesan N, Gu Z, Fadnis L, Tellew JE, Baska RA, Yang Y, et al. Dual angiotensin II and endothelin A receptor antagonists: synthesis of 2′-substituted N-3-isoxazolyl biphenylsulfonamides with improved potency and pharmacokinetics. J Med Chem. 2005;48:171–9. doi: 10.1021/jm049548x. [DOI] [PubMed] [Google Scholar]
- Kowala MC, Murugesan N, Tellew J, Carlson K, Monshizadegan H, Ryan C, et al. Novel dual action AT1 and ETA receptor antagonists reduce blood pressure in experimental hypertension. J Pharmacol Exp Ther. 2004;309:275–84. doi: 10.1124/jpet.103.055855. [DOI] [PubMed] [Google Scholar]
- Imai T, Hirata Y, Emori T, Yanagisawa M, Masaki T, Marumo F. Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension. 1992;19:753–7. doi: 10.1161/01.hyp.19.6.753. [DOI] [PubMed] [Google Scholar]
- Chua BH, Chua CC, Diglio CA, Siu BB. Regulation of endothelin-1 mRNA by angiotensin II in rat heart endothelial cells. Biochim Biophys Acta. 1993;1178:201–6. doi: 10.1016/0167-4889(93)90010-m. [DOI] [PubMed] [Google Scholar]
- Tellew JE, Baska RAF, Beyer SM, Carlson KE, Cornelius LA, Fadnis L, et al. Discovery of 4′-[(Imidazol-1-yl)methyl]biphenyl-2-sulfonamides as dual endothelin/angiotensin II receptor antagonists. Bioorg Med Chem Lett. 2003;13:1093–6. doi: 10.1016/s0960-894x(03)00018-0. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Henikoff S, Henikoff JG. Amino-acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A. 1992;89:10915–9. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitzky T, Levit A, Niv MY. Homology modeling of G-protein-coupled receptors with X ray structures on the rise. Curr Opin Drug Discov Devel. 2010;13:317–25. [PubMed] [Google Scholar]
- Larsson P, Wallner B, Lindahl E, Elofsson A. Using multiple templates to improve quality of homology models in automated homology modeling. Protein Sci. 2008;17:990–1002. doi: 10.1110/ps.073344908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokkar P, Mohandass S, Ramachandran M.Multiple templates-based homology modeling enhances structure quality of AT1 receptor: validation by molecular dynamics and antagonist docking J Mol Model201; 171565–77. [DOI] [PubMed] [Google Scholar]
- Fanelli F, De Benedetti PG. Update 1 of computational modeling approaches to structure-function analysis of G protein-coupled receptors. Chem Rev. 2011;111:PR438–535. doi: 10.1021/cr100437t. [DOI] [PubMed] [Google Scholar]
- Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, et al. Comparative protein structure modeling using modeller. Curr Protoc Bioinformatics. Hoboken (New Jersey): John Wiley & Sons, Inc; 2002 [DOI] [PMC free article] [PubMed]
- Mart-Renom MA, Stuart AC, Fiser AS, Sanchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Fiser A, Do RKG, Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9:1753–73. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Thornton JM. Validation of protein models derived from experiment. Curr Opin Struct Biol. 1998;8:631–9. doi: 10.1016/s0959-440x(98)80156-5. [DOI] [PubMed] [Google Scholar]
- Prime, version 3.0, Schrödinger, LLC, New York, NY, 2011.
- Maestro, version 9.2, Schrödinger, LLC, New York, NY, 2011.
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst. 1993;26:283–91. [Google Scholar]
- Clément M, Martin SS, Beaulieu ME, Chamberland C, Lavigne P, Leduc R, et al. Determining the environment of the ligand binding pocket of the human angiotensin II type I (hAT1) receptor using the methionine proximity assay. J Biol Chem. 2005;280:27121–9. doi: 10.1074/jbc.M413653200. [DOI] [PubMed] [Google Scholar]
- Jorgensen WL, Maxwell DS, Tirado-Rives J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc. 1996;118:11225–36. [Google Scholar]
- Kaminski GA, Friesner RA, Tirado-Rives J, Jorgensen WL. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J Phys Chem B. 2001;105:6474–87. [Google Scholar]
- Braganza LF, Worcester DL. Hydrostatic pressure induces hydrocarbon chain interdigitation in single-component phospholipid bilayers. Biochemistry. 1986;25:2591–6. doi: 10.1021/bi00357a047. [DOI] [PubMed] [Google Scholar]
- Faraldo-Gómez JD, Smith GR, Sansom MS. Setting up and optimization of membrane protein simulations. Eur Biophys J. 2002;31:217–27. doi: 10.1007/s00249-002-0207-5. [DOI] [PubMed] [Google Scholar]
- Schlegel B, Sippl W, Höltje HD. Molecular dynamics simulations of bovine rhodopsin: influence of protonation states and different membrane-mimicking environments. J Mol Model. 2005;12:49–64. doi: 10.1007/s00894-005-0004-z. [DOI] [PubMed] [Google Scholar]
- Jorgensen WL, Madura JD. Temperature and size dependence for Monte Carlo simulations of TIP4P water. Mol Phys. 1985;56:1381–92. [Google Scholar]
- Singh K, Kirubakaran P, Nagarajan S, Sakkiah S, Muthusamy K, Velmurgan D, et al. Homology modeling, molecular dynamics, e-pharmacophore mapping and docking study of Chikungunya virus nsP2 protease. J Mol Model. 2012;18:39–51. doi: 10.1007/s00894-011-1018-3. [DOI] [PubMed] [Google Scholar]
- Isberg V, Balle T, Sander T, Jorgensen FS, Gloriam DE. G protein- and agonist-bound serotonin 5-HT2A receptor model activated by steered molecular dynamics simulations. J Chem Inf Model. 2011;51:315–25. doi: 10.1021/ci100402f. [DOI] [PubMed] [Google Scholar]
- Glide, version 5.7, Schrödinger, LLC, New York, NY, 2011.
- SiteMap, version 2.5, Schrödinger, LLC, New York, NY, 2011.
- LigPrep, version 2.5, Schrödinger, LLC, New York, NY, 2011.
- Schrödinger Suite 2011 QM-Polarized Ligand Docking protocol; Glide version 5.7, Schrödinger, LLC, New York, NY, 2011; Jaguar version 7.8, Schrödinger, LLC, New York, NY, 2011; QSite version 5.7, Schrödinger, LLC, New York, NY, 2011.
- Cho AE, Guallar V, Berne BJ, Friesner R. Importance of accurate charges in molecular docking: Quantum mechanical/molecular mechanical (QM/MM) approach. J Comput Chem. 2005;26:915–31. doi: 10.1002/jcc.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaguar, version 7.8, Schrödinger, LLC, New York, NY, 2011.
- Impact, version 5.5, Schrödinger, LLC, New York, NY, 2005
- Yang Y, Qin J, Liu H, Yao X. Molecular dynamics simulation, free energy calculation and structure-based 3D-QSAR studies of B-RAF kinase inhibitors. J Chem Inf Model. 2012;51:680–92. doi: 10.1021/ci100427j. [DOI] [PubMed] [Google Scholar]
- Massova I, Kollman PA. Computational alanine scanning to probe protein-protein interactions: a novel approach to evaluate binding free energies. J Am Chem Soc. 1999;121:8133–43. [Google Scholar]
- Moreira IS, Fernandes PA, Ramos MJ. Computational alanine scanning mutagenesis — an improved methodological approach. J Comput Chem. 2007;28:644–54. doi: 10.1002/jcc.20566. [DOI] [PubMed] [Google Scholar]
- Afroz Alam M, Naik P. Molecular modelling evaluation of the cytotoxic activity of podophyllotoxin analogues. J Comput Aided Mol Des. 2009;23:209–25. doi: 10.1007/s10822-008-9252-1. [DOI] [PubMed] [Google Scholar]
- Dhanachandra Singh Kh, Karthikeyan M, Kirubakaran P, Nagamani S. Pharmacophore filtering and 3D-QSAR in the discovery of new JAK2 inhibitors. J Mol Graph Model. 2011;30:186–97. doi: 10.1016/j.jmgm.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Phase, version 3.3, Schrödinger, LLC, New York, NY, 2011.
- Dixon S, Smondyrev A, Knoll E, Rao S, Shaw D, Friesner R. PHASE: a new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening: 1. Methodology and preliminary results. J Comput Aided Mol Des. 2006;20:647–71. doi: 10.1007/s10822-006-9087-6. [DOI] [PubMed] [Google Scholar]
- Guimarães CR, Cardozo M. MM-GB/SA rescoring of docking poses in structure-based lead optimization. J Chem Inf Model. 2008;48:958–70. doi: 10.1021/ci800004w. [DOI] [PubMed] [Google Scholar]
- Guimarães CR. MM-GB/SA rescoring of docking poses. Methods Mol Biol. 2012;819:255–68. doi: 10.1007/978-1-61779-465-0_17. [DOI] [PubMed] [Google Scholar]
- Henriksen ST, Liu J, Estiu G, Oltvai ZN, Wiest O. Identification of novel bacterial histidine biosynthesis inhibitors using docking, ensemble rescoring, and whole-cell assays. Bioorg Med Chem. 2010;18:5148–56. doi: 10.1016/j.bmc.2010.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DC, Humblet C, Joseph-McCarthy D. Investigation of MM-PBSA rescoring of docking poses. J Chem Inf Model. 2008;48:1081–91. doi: 10.1021/ci700470c. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Choo H, Cho YS, Park WK, Pae AN. Identification of novel serotonin 2C receptor ligands by sequential virtual screening. Bioorg Med Chem. 2009;17:4559–68. doi: 10.1016/j.bmc.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Sakkiah S, Thangapandian S, John S, Lee KW. Pharmacophore based virtual screening, molecular docking studies to design potent heat shock protein 90 inhibitors. Eur J Med Chem. 2011;46:2937–47. doi: 10.1016/j.ejmech.2011.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The sequence alignments of ETA protein sequence with the sequence of templates (PDB id: 3PWH and 3KJ6) generated by Clustal W. In the sequences, an asterisk (*) indicates an identical or conserved residue; a colon (:) indicates conserved substitutions; a stop (.) indicates semi-conserved substitutions.
Experimental activity vs predicted ΔGBind from Glide XP docking pose of (AT1R).
Experimental activity vs predicted ΔGBind from Glide XP docking pose of ETA.
Binding mode of 5 lead molecules in the active site of AT1R.
Binding mode of 5 lead molecules in the active site of ETA.
Aligning of 3D-QSAR model of AT1R with the most active compound and 5 lead molecules identified from virtual screening.
Aligning of 3D-QSAR model of ETA with the most active compound and 5 lead molecules identified from virtual screening.
Statistical parameters of GH score validation for two top ranked hypotheses AARRH and AARRR.
Statistical parameters of GH score validation for VSW and QPLD.