Abstract
Lupus is a systemic autoimmune disease characterized by anti-nuclear antibodies in humans and genetically susceptible NZB/W mice that can cause immune complex glomerulonephritis. T cells contribute to lupus pathogenesis by secreting pro-inflammatory cytokines such as IL-17, and by interacting with B cells and secreting helper factors such as IL-21 that promote production of IgG autoantibodies. In the current study, we determined whether purified NKT cells or far more numerous conventional non-NKT cells in the spleen of NZB/W female mice secrete IL-17 and/or IL-21 after TCR activation in vitro, and provide help for spontaneous IgG autoantibody production by purified splenic CD19+ B cells. Whereas invariant NKT cells secreted large amounts of IL-17 and IL-21, and helped B cells, non-NKT cells did not. The subset of IL-17 secreting NZB/W NKT cells expressed the Ly108loCD4−NK1.1− phenotype, whereas the IL-21 secreting subset expressed the Ly108hiCD4+NK1.1− phenotype and helped B cells secrete a variety of IgG anti-nuclear antibodies. α-galactocylceramide enhanced the helper activity of NZB/W and B6.Sle1b NKT cells for IgG autoantibodiy secretion by syngeneic B cells. In conclusion, different subsets of iNKT cells from mice with genetic susceptibility to lupus can contribute to pathogenesis by secreting pro-inflammatory cytokines and helping autoantibody production.
Keywords: Natural Killer T cells, Systemic lupus erythematosus, autoantibodies, IL-17, IL-21
1. Introduction
Systemic lupus erythematosus (SLE) is a multi-organ autoimmune disease that is characterized by autoantibody formation, and immune complex deposition in the skin and kidneys [1–3]. A hallmark of this disease in both NZB/WF1 (BW) hybrid female mice and humans is the development of anti-nuclear antibodies especially IgG anti-dsDNA antibodies that are present in the serum, and spontaneously secreted by spleen and blood mononuclear cells respectively [1, 4–8]. These autoantibodies contribute to immune complex glomerulonephritis that can result in renal failure [9, 10]. A variety of lupus susceptibility genes have been identified in mice. In the NZM2410 strain derived from interbred BW mice, some of these genes are included in chromosomal segments Sle1a, Sle1b, Sle2, and Sle3 that have homologies in humans with lupus [11, 12]. The Sle1b segment includes the SLAM family genes (SLAMF1-6) that form two alternative haplotypes that regulate CD4+ T cell activation; type 1 that is present in C57BL/6 (B6) mice and type 2 that is present in NZB and NZW mice [11, 12]. When the Sle1b segment from NZM2410 mice is bred on to B6 mice, the resultant B6.Sle1b (Sle1b) mice show upregulation of SLAMF6 (Ly108) expression in B cells, serum anti-chromatin antibodies, and decrease in the sensitivity of immature B cells to signaling through the BCR as judged by a decreased level and duration of calcium flux after stimulation [11, 13]. The presence of the Sle1b segment in autoimmune mice results in the preferential selection of the Ly108-1 isoform that impairs B cell anergy, and receptor revision and deletion [13].
It is of interest that inactivation of both of the genes that encode SLAMF1 and SLAMF6 results in the failure of development of invariant NKT (iNKT) cells [14]. The failure is explained by the inability of NKT cell precursors to undergo positive selection that requires homotypic interactions between the SLAM molecules expressed on these precursors and on double negative thymocytes [14, 15]. SLAM molecules are also required to optimize interactions between T and B cells that result in T cell help for immunoglobulin secretion, and germinal center formation [16, 17]. The expression of SLAM surface molecules on NKT cells in lupus prone BW mice has not been studied previously, and it is not clear whether there is differential expression on NKT cells in lupus prone versus non-autoimmune mice.
The role of iNKT cells in the pathogenesis of lupus is controversial with some studies showing a disease promoting role whereas others show a disease suppressing role [18–27]. The different roles may be due to the different models used to study lupus in mice, since there are two general types; one in which lupus is induced in genetically normal (no spontaneous autoimmune manifestations) strains of mice by injecting substances such as the oil, pristane, or apoptotic cells [1, 3, 26], and a second in which lupus occurs spontaneously in genetically susceptible mice such as the BW strain [2, 11]. It is not surprising that NKT cells suppress autoimmune manifestations of lupus induced in genetically normal mice, since NKT cells in these strains have been shown to promote tolerance to allografts [28], suppress experimental autoimmune encephalitis [29], and graft versus host disease [30]. Even in the BW strain, NKT cells have been reported to suppress immunity because lupus disease activity was reported to be increased in BW mice with an inactivated CD1d gene [24]. However, the latter study was difficult to interpret because the CD1d−/− BW mice failed to show the expected elimination of NK1.1+ TCR+ T cells that is observed in C57BL/6 CD1d−/− mice [24].
On the other hand, NKT cells in BW mice have been reported to be abnormal in some studies, and promote rather than suppress autoimmunity and to infiltrate organs such as the kidneys [18–22, 31]. In particular, blocking of NKT cell function with anti-CD1d monoclonal antibodies in vivo resulted in marked improvement in lupus in BW mice, and NKT activation with α-galactocylceramide (α-GalCer) resulted in worsened lupus [18].
NKT cells rather than conventional T cells (non-NKT; Tcon) have been shown to be the predominant source of IL-21 after T cell activation via the TCR [32]. IL-21 is a pleiotropic cytokine [33] and has been shown to play a required role in promoting the development of lupus in the genetically susceptible MRL/lpr, BXSB-yaa, and sanroque strains of mice [34–36]. Although autoantibodies are the proximate cause of many of the disease manifestation of lupus, especially immune complex glomerulonephritis, T cells in lupus prone mice not only help B cells to secrete autoantibodies, but also secrete proinflammatory cytokines such as IL-17. IL-17 can contribute to inflammation and neutrophil infiltration in the diseased kidneys, and spontaneous development of germinal centers in lupus prone mice [37–42]. Two types of IL-17 producing T cells have been described; Th17 cells derived from naive Tcon cells, and invariant NKT17 cells that are a subset of iNKT cells [43, 44]. The NKT17 subset does not express the NK1.1 or CD4 receptors, but does express the neuropillin-1 (Nrp-1) receptor [44]. The relative contributions of Th17 and NKT17 cells to the production of pathogenic IL-17 in lupus prone mice are not clear.
The secretion of pathogenic high affinity anti-dsDNA autoantibodies is thought to involve the maturation of B cells in germinal centers that develop spontaneously in both mice and humans with lupus [1–3]. Differentiation of follicular B cells into germinal center B cells requires help from follicular helper T cells. The latter T cell subset is distinguished by secreting the potent B cell help factor, IL-21, and by the transcription factor Bcl-6 [45]. Two types of IL-21 producing follicular helper T cells have been described; Tfh that are derived from naive Tcon cells, and invariant NKTfh that are a subset of iNKT cells [45, 46].
A major goal of the current work was to compare the secretion of IL-17 and IL-21 by purified iNKT cells and Tcon cells from non-autoimmune B6 mice to that of lupus prone BW mice and Sle1b mice after TCR stimulation to determine whether the T cells of lupus prone mice had abnormally high cytokine secretion. A second goal was to use surface expression of Ly108 to distinguish subsets of T cells that differentially secrete IL-17 and 21 after T cell activation, and to determine whether the subset that secreted IL-21 also helped BW B cells to spontaneously secrete IgG antibody and IgG autoantibodies. We found that iNKT cells were the predominant source of the high IL-17 and IL-21 secretion after T cell activation in vitro in BW mice, and BW mice have elevated levels of IL-21 in the serum. The IL-17 secreting iNKT cells were confined to a small subset that had the Ly108loCD4−Nrp-1+NK1.1− phenotype, and had no helper activity. In contrast, the IL-21 secreting iNKT subset had the Ly108hiCD4+NK1.1− phenotype, and helped autoantibody secretion. The IL-17 iNKT secreting subset was found among T cells infiltrating the diseased kidneys.
2. Materials and Methods
2.1. Mice
B6 female mice and NZB/W F1 female mice (referred to subsequently as BW mice) were obtained from Jackson Laboratories. Sle1b breeding pairs were a kind gift of Dr. Edward K. Wakeland (University of Texas Southwestern Medical Center). All mice were housed and/or bred in a pathogen-free environment in the Department of Comparative Medicine, Stanford University. All experimental protocols were approved by Stanford Administrative Panel on Laboratory Animal Care (A-PLAC).
2.2. Flow cytometry
For assessment of the expression of surface molecules, isolated single cell suspensions were stained with predetermined optimal antibody concentrations according to the manufacturer’s staining protocol. Briefly, cell suspensions were pre-incubated with mouse BD Fc Block™ purified anti-mouse CD16/CD32 mAb (2.4G2) on ice for 5 minutes, and then staining was performed in PBS buffer containing 1% FCS on ice. Labeled antibodies to TCR (H57-597), CD4 (RM4-5), CD19 (1D3), NK1.1 (PK136) were from eBioscience. Labeled anti-Ly108 (330-AJ) antibody along with specific isotype-matched control antibodies were from Biolegend. Labeled polyclonal anti-mouse Neuropilin-1 (Nrp-1) and specific isotype-matched control antibodies were from R&D Systems. PE conjugated CD1d-tetramer was obtained from the NIH Tetramer Facility, Atlanta, GA. Data were acquired on an LSRII (BD Biosciences) and were analyzed with FlowJo software (Tree Star, Inc.).
2.3. Cell preparation
To prepare kidney mononuclear cells (KMC), one or two kidneys were mechanically disrupted without collagenase digestion. Cell suspensions were passed through a 70-μm cell strainer and red blood cells were then lysed and to obtain single kidney cell suspensions. Thereafter, KMC were enriched by centrifugation in a Lympholyte-M (Cedalane Labs) solution. About 2.5~5X105 KMC were obtained from each BW mouse.
Purified B cells were obtained from spleens by a mouse B Cell Isolation Kit and MACS columns (Miltenyi Biotec) according to the manufacture’s protocol. Enriched cells were incubated with anti-CD19 mAb and sorted using a BD FACSAria II cell sorter (BD Biosciences). For DC preparation, spleens were digested with Collagenase D (Roche) at 37°C for 20min, thereafter splenocytes passed through nylon mesh screens were incubated with Pan DC MicroBeads and enriched using MACS Columns (Militenyi Biotec). iNKT cells were isolated as described before [47]. Briefly three to five mice were pooled and splenocytes were obtained and stained with PE-conjugated CD1d tetramer and α-GalCer loaded CD1d dimer-mouse IgG fusion protein (BD Biosciences). iNKT cells were enriched subsequently by incubating with anti-PE microbeads (Milteney Biotech) and passing through MACS LS columns (Milteney Biotech). Enriched cells were further stained with anti-TCRβ-FITC (BD pharmingen), or iNKT cell subset mABs, and sorted into iNKT (TCRβ+ CD1dtet+) cells or into different iNKT subsets by BD FACSAria II cell sorter (BD Biosciences). The purity of sorted cells was more than 90%.
2.4. Cell culture
Cells were cultured in RPMI 1640 supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml), L-glutamine (2 mM), and 1×10−5M β-mercaptoethanol at 37°C with 5% CO2 (complete medium). For iNKT cell functional assays, 100~200ul sorted subsets of iNKT cells (5 × 105 cells/ml) were cultured in complete medium in 96 well flat bottom plate coated with functional grade purified 10 μg/ml anti-mouse CD3 (145-2C11) and 10 μg/ml CD28 (37.51) antibodies (eBioscience). After three days’ culture, supernatants were collected stored at −80°C for later quantification of cytokines. For KMC functional assays, KMC (1X105) were incubated with DCs (5X103) and 100 ng/mL of α-GalCer for 4 days. Culture supernatants were collected and stored until analysis. For B cell help assays, 2X105 whole splenocytes or combinations of 1X105 sorted CD19+ B cells with 5X104 sorted iNKT cell subsets were cultured with or without 100 ng/mL of α-GalCer in complete RPMI medium in 96 well round bottom plate at 37°C in 5% CO2 for ten days. Cell culture supernatants were then collected for analysis of IgM, IgG and autoantigen production.
2.5. Cytokines, IgM, IgG and autoantibody analysis
IFNγ, IL-4 and IL-17 secretion from cell culture supernatants of iNKT cell and KMC functional assays were assessed by either a MilliplexMAP Kit (Millipore) on a Bio-Plex 200 system (Bio-Rad) or Ready-Set-Go! ELISA kit (eBioscience). IL-21 secretions were analyzed by a MilliplexMAP Kit (Millipore) on a Bio-Plex 200 system (Bio-Rad). Measurements of IgM and IgG in culture supernatants of B cell help assays were performed using an ELISA kit (SouthernBiotech) as described previously [19]. Anti-dsDNA IgG in the supernatants of whole splenocyte cultures and BW Ly108hi/lo iNKT and B cells co-cultures were assayed by mouse anti-dsDNA IgG-specific ELISA kits (Alpha Diagnostic Intl. Inc.). Anti-dsDNA IgG and other IgG autoantibodies from co-culture supernatants were assayed using nucleic acid/proteomic microarray plates described below.
2.6. Microarray assay for autoantibodies
Glass slides were treated to create a layer of plasmonic gold on the printing surface as previously described [48, 49]. The plasmonic gold film was further modified with mercaptohexadecanoic acid (Sigma-Aldrich), resulting in a monolayer of mecaptohexadecanoic acid which was further conjugated to branched poly(ethylene glycol) (PEG)-amine (SunBio) through an amide bond. The free amine group on the PEG chain was later converted into carboxylic groups with succinic anhydride (Sigma-Aldrich) and was activated by NHS ester. Whole-protein antigens (purchased from Diarect, Immunovision, Roche Applied Science or Jackson Immunoresearch) were printed on plasmonic gold slides using a VersArray ChipWriter Compact robotic microarrayer and ChipWriter Pro software (BioRad) in replicates of three in PBS plus 1% glycerol (Sigma Aldrich). The arrays were blocked with 3% FBS in PBS for 1 h at room temperature with light rocking agitation. After rinsing arrays 3 times with PBST solution (PBS plus 0.05% tween-20), the mouse cell culture supernatant was applied for 2 h at RT with light rocking agitation. the arrays were washed in PBST before applying secondary goat anti-mouse IgG (Jackson ImmunoResearch) conjugated to IRDye800 (LI-COR), diluted to 0.2 μg/ml in PBS with 20% FBS. Following incubation of detection antibody for 30 min at RT with light rocking agitation, arrays were washed and dried in microscope slide racks centrifuged at 300 g for 5 min at RT. Processed arrays were immediately scanned with an LI-COR Odyssey digital scanner and analyzed scanned images with Genepix Pro 6.1 software (Molecular Devices).
2.7. Statistical analysis
Data are presented as mean±SEM. Statistical comparison of the data was performed by paired two-tailed Student’s t test using Prism 5.0 software (GraphPad). Group difference with P<0.05 was considered statistically significant.
3. Results
3.1. Subsets of iNKT cells identified by Ly108 expression
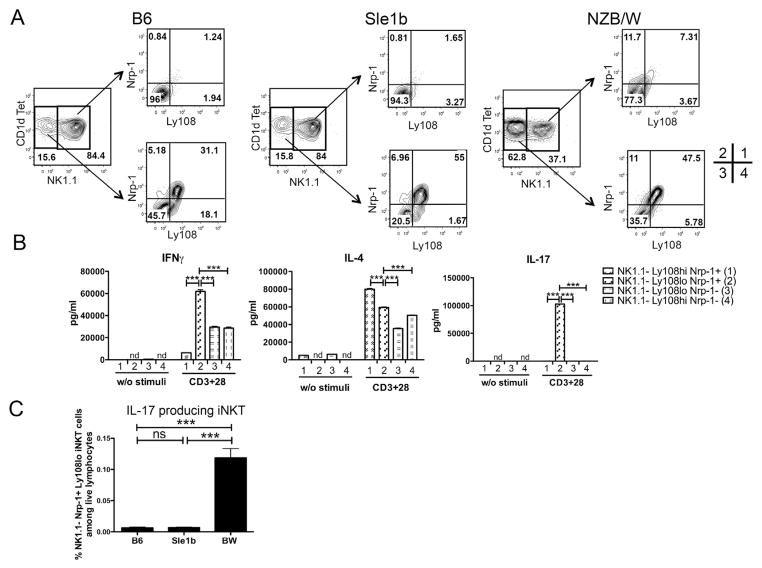
We compared the staining patterns of spleen cells from B6, Sle1b, and BW female mice that were 2~4 months (range 8 to 16 weeks, mean 14 weeks) old for the content of iNKT cells and Tcon cells, and for the surface expression of Ly108 and NK1.1 markers. Representative flow cytometry in Figure 1A using CD1d tetramers to identify iNKT cells show that about 1–2% of gated lymphocytes were CD1dtet+TCR+ iNKT cells, and CD1dtet−TCR+ Tcon cells represented about 33–49%. The mean percentage of iNKT cells among lymphocytes was significantly increased (~2.5%) in the BW spleen as compared to the B6 (~1%) and Sle1b (~0.7%) spleen. A similar pattern was observed for the absolute number of iNKT cells in B6, Sle1b and BW mice (Supplemental Figure 1A). The mean percentage of Tcon cells in the BW spleen (~43%) was significantly increased as compared to B6 (~30%) or Sle1b (~35%). Ly108 was expressed on all splenic iNKT and Tcon cells of B6, Sle1b, and BW mice (Supplemental Figure 1B). iNKT cells could be clearly separated into two populations with Ly108hi and Ly108lo staining. Ly108hi cells were most frequent among iNKT cells in the BW spleen (mean of ~25%), and lowest in the B6 spleen (~8%). The order was reversed among Tcon cells (Figure 1B). A similar pattern was found for the absolute number of Ly108hi iNKT and Ly108hi Tcon populations in B6, Sle1b, and BW mice (Supplemental Figure 1A). A similar pattern was found in old (about 6 months old) mice (data not shown).
Figure 1.
Flow cytometric analyses of Ly108 expression on iNKT and Tcon cells from B6, Sle1b and BW splenocytes. Single cell suspensions were obtained from young mice that range 8 to 16 weeks old (mean 14 weeks) and analyzed by flow cytometry. (A) iNKT (TCR+CD1dtet+) and Tcon (TCR+CD1dtet−) populations of B6, Sle1b and BW mice are enclosed in boxes, and percentage within each box is shown; mean frequencies (percentage among gated lymphocytes) of iNKT and Tcon cells are shown on the right. (B) Percentage of Ly108hi cells among iNKT and Tcon cells from B6, Sle1b and BW mice are enclosed in boxes; mean percentages for Ly108hi population in iNKT and Tcon gates are shown on the right. (C) NK1.1 staining on iNKT cells from B6, Sle1b and BW mice, and boxes show NK1.1+ and NK1.1− cells; mean percentages for NK1.1− population in iNKT cells are shown on the right. (D) Ly108hi cells among NK1.1+ and NK1.1− iNKT populations from BW mice shown on the left; mean percentages for Ly108hi population in NK1.1+ (closed square) and NK1.1− (open circle) iNKT cells from B6, Sle1b and BW mice are shown on the right. Each symbol in graphs represents an individual mouse. Data are presented as the mean ± s.e.m. Group differences with P>0.05 were not considered statistically significant (ns). *, P<0.05; **, P<0.01; ***, P<0.001 (two-tailed Student’s t-test).
Analysis of NK1.1 expression on iNKT cells was performed in the three strains (Figure 1C). Whereas NK1.1− NKT cells were the minority in the B6 and Sle1b mice (~18%), the NK1.1− NKT cells were predominant in the BW spleen (~60%). This observation is consistent with previous reports [18, 22]. The NK1.1− NKT cells had a discrete population of Ly108hi cells that accounted for about 40% of cells in BW mice, and the NK1.1+ NKT cells had a smaller percent without a discrete concentric contour staining pattern (Figure 1D). The mean percent of Ly108hi cells among the NK1.1− NKT cells was highest among the Sle1b cells (~65%) as compared to the B6 (~35%) or BW (~38%) cells. In all three strains, the mean percentage of Ly108hi cells among the NK1.1+ NKT cells was less than 5%. In summary, the Ly108hi cells formed a discrete subset of iNKT cells that was confined mainly to the NK1.1− NKT cells, and the NK1.1− subset was predominant only in the BW mice.
3.2. Ly1o8loCD4−Nrp-1+NK1.1− iNKT cells are the predominant source of IL-17 in BW mice
We compared the ability of iNKT cells and Tcon cells from 3–4 month old female BW mice to secrete the cytokines IFN-γ, IL-4, and IL-17 after in vitro stimulation with anti-CD3 and anti-CD28 mAbs. Figure 2A shows the cytokine concentrations in supernatants after 72 hours. Whereas BW Tcon cells made similar large amounts of IFN-γ (mean ~28,000pg/ml), they made lower amounts of IL-4 (mean ~1000pg/ml) and IL-17 (mean ~400pg/ml). In contrast, the iNKT cells made at least 10 fold higher levels of IL-4 (~28000pg/ml) and IL-17 (~4000pg/ml) as compared to equal numbers of Tcon cells (Figure 2A).
Figure 2.
BW iNKT cells, produce significantly higher amounts of IL-4 and IL-17 than Tcon cells and Ly108lo iNKT cells are predominant IL-17 producing iNKT cells. Cells were sorted from three pooled spleens of young mice and cultured (5X105/ml) for 3 days with or without plate bound anti-CD3 and CD28 mAbs, culture supernatants were then collected for IFNγ, IL-4 and IL-17 measurement by ELISA or Luminex. (A) IFNγ, IL-4 and IL-17 concentrations in supernatants of stimulated iNKT and Tcon cells from BW mice. Cytokines measurements are shown on the right. (B) IFNγ, IL-4 and IL-17 production by BW CD4+ Ly108hi, CD4+ Ly108lo and CD4− Ly108lo iNKT cells. Sorting gates are shown on the left, and cytokines measurements were shown on the right. (C) IFNγ, IL-4 and IL-17 production by BW NK1.1+ and NK1.1− iNKT cells. FACS profile is shown for the sorting gates on the left, and cytokines measurements are shown on the right. (D) IFNγ, IL-4 and IL-17 production by BW NK1.1−Ly108hi and NK1.1−Ly108lo iNKT cells. FACS profile is shown for the sort gates on the left, and cytokines measurements are shown on the right. Group differences with P>0.05 was not considered statistically significant (ns). *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001 (two-tailed Student’s t-test). Data (mean ± s.e.m.) are calculated from four independent experiments (A, B) or derived from triplicate cultures collected from one representative experiment of three with similar results (C, D). Cytokine concentrations without anti-CD3 and CD28 mAbs were less than 100pg/ml.
In further experiments, we determined whether a subset of iNKT cells was the predominant source of increased secretion of IL-17 in the BW mice. A previous study showed that CD4−NK1.1− iNKT cells with surface expression of Nrp-1 was the predominant source of IL-17 after selective activation of iNKT cells from B6 mice [44]. We sorted the gated iNKT cells from the spleen of BW mice into 3 subsets of CD4+Ly108hi, CD4+Ly108lo, and CD4−Ly108lo cells (Figure 2B). Almost all CD4− cells were Ly108lo, and CD4+ cells contained both Ly108hi and Ly108lo cells. After stimulation with anti-CD3 and CD28 mAbs, concentrations of IFN-γ, IL-4 and IL-17 in supernatants were determined as above. Whereas all 3 subsets secreted both IFN-γ and IL-4, only the CD4−Ly108lo subset secreted IL-17 (mean ~15,000pg/ml). (Figure 2B).
Figure 2C shows the results of sorting the gated iNKT cells into NK1.1+ and NK1.1− subsets. Whereas the NK1.1+ and NK1.1− subsets secreted similar amounts of IFN-γ and IL-4, the NK1.1− subset secreted significantly higher levels of IL-17 than the NK1.1+ subset. The NK1.1− subset could be divided further into 2 distinct subsets of about equal numbers of Ly108hi and Ly108lo cells as shown in Figure 2D. Whereas the supernatants of the activated sorted subsets of latter cells had similar concentrations of IFN-γ and IL-4, the Ly108lo subset secreted about 10 fold more IL-17 than the Ly108hi subset (Figure 2D). Thus, the IL-17 secreting cells were markedly enriched in the Ly108loCD4−NK1.1− subset.
In further experiments, the B6, Sle1b, and BW spleen cells were stained for Ly108 versus Nrp-1 among the gated NK1.1− iNKT cells. In all 3 strains, most Nrp-1+ cells were Ly108hi, and most Nrp-1− cells were Ly108lo (Figure 3A). However, there was a small subset of cells that were Nrp-1+ and Ly108lo (quadrant 2) that accounted for about 5–10% of the gated NK1.1− cells. The highest levels were observed in the BW cells. The subsets of BW NK1.1− cells were sorted on the basis of Ly108 and Nrp-1 expression as shown in the quadrants of Figure 3A, and the sorted cells were assayed for the ability to secrete IFN-γ, IL-4, and IL-17 after stimulation in vitro. Figure 3B shows that cells in quadrant 2 (Ly108loNrp-1+) made high levels of IL-17 (~15,000 pg/ml), whereas cells in quadrants 1, 3 and 4 made less than 100 pg/ml IL-17. High levels of IL-4 (~40,000–80,000 pg/ml) were made by cells in all quadrants and those in quadrants 2–4 made high levels of IFN-γ (>40,000 pg/ml). In summary, the high IL-17 production of BW as compared to B6 or Sle1b NKT cells was confined to the Ly108loCD4−Nrp-1+NK1.1− NKT cell subset.
Figure 3.
NK1.1−Ly108loNrp-1+ iNKT cells are IL-17 producing iNKT cells. (A) Ly108 and Nrp-1 expression on gated NK1.1+ and NK1.1− iNKT cells from young B6, Sle1b and BW mice. (B) IFNγ, IL-4 and IL-17 production by sorted BW NK1.1−Ly108hiNrp-1+ (indicated as population 1 as shown in A), NK1.1−Ly108lo Nrp-1+ (population 2), NK1.1−Ly108loNrp-1− (population 3), NK1.1−Ly108hiNrp-1− (population 4) iNKT cells. Cells were sorted from three to five pooled spleens from young BW mice and cultured (5X105/ml) for 3 days with anti-CD3 and CD28 mAbs. (C) Percentages of IL-17 producing iNKT cells, defined as NK1.1−Ly108loNrp-1+TCR+CD1dTet+ population, among live lymphocytes of B6, Sle1b and BW mice. nd, not detected. Group differences with P>0.05 was not considered statistically significant (ns); ***, P<0.001 (two-tailed Student’s t-test). Bar graphs show mean± s.e.m. Results are representative of four independent experiments (A), or two independent experiments (B), or four independent experiments (C). Independent experiments showed similar p values for group comparisons.
We compared the mean percentages of iNKT cells in the spleen that were the Ly108loNrp-1+NK1.1− among all lymphocytes in the B6, Sle1b, and BW mice in Figure 3C. Whereas the percentage was similar in B6 and Sle1b mice (about 0.006%), the percentage in BW mice was about 20 fold higher (about 0.12%). The increase was due to a combination of higher percentages of iNKT cells among splenic lymphocytes, higher percentages of NK1.1− cells among iNKT cells, and higher percentages of Ly108loNrp-1+ cells among NK1.1− iNKT cells (Figure 1, and 3).
3.3. BW or Sle1b iNKT cells can induce B cells to produce IgG autoantibodies
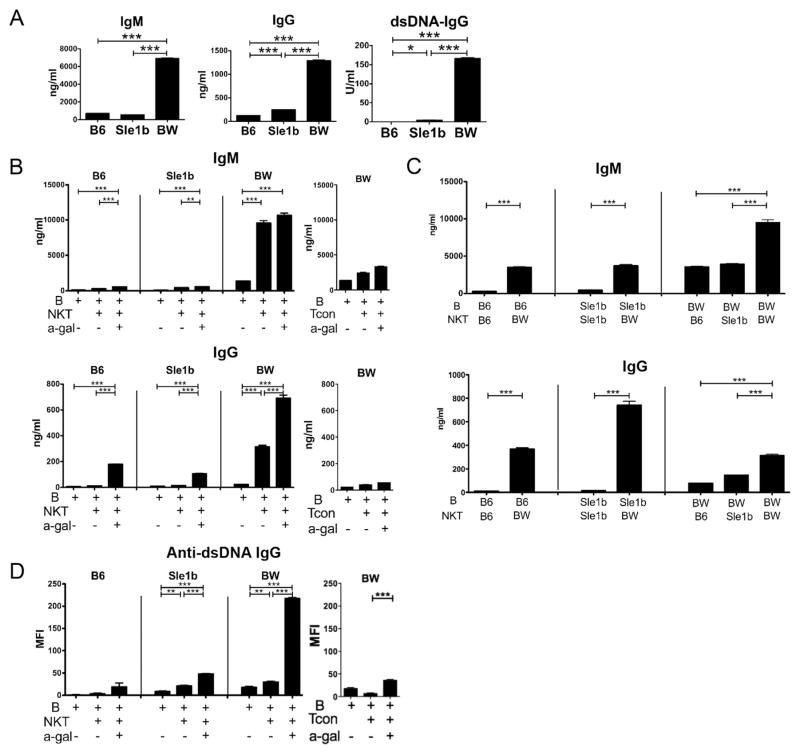
We reported previously that iNKT cells but not Tcon cells from the spleen of female BW mice that were at least 6 months old could help B cells to spontaneously secrete IgM, IgG, and IgG anti-dsDNA autoantibodies by culturing the 2 cell types together without the addition of T or B cell polyclonal activators [18–20]. Figure 4A shows that whole spleen cells of 6 month old BW mice cultured in vitro in the absence of activators spontaneously secreted dramatically higher levels of IgM, IgG, and anti-dsDNA antibodies as compared to spleen cells from age and sex matched B6 and Sle1b mice. Spontaneous secretion of autoantibodies by lymphocytes in vitro is a feature of the lupus-like disease in BW mice and in humans with severe lupus [4–8]. Although Sle1b spleen cells secreted considerably less IgG and IgG anti-dsDNA antibodies than BW cells, the Sle1b cells secreted significantly increased levels of these antibodies as compared to the B6 cells.
Figure 4.
BW iNKT cells provide help for spontaneous IgG secretion of B cells from B6, Sle1b and NZB/W mice, and α-GalCer augments production of IgG and IgG autoantibodies. Splenocytes or sorted CD19+ B cells with or without sorted iNKT cells were cultured for 10 days, and supernatants were collected for determination of IgM, IgG and IgG anti-dsDNA antibody concentrations. (A) IgM, IgG and IgG anti-dsDNA antibody concentrations from 10-day culture supernatants of 6 month old B6, Sle1b and NZB/W whole splenocytes. (B, C) IgM and IgG production from 6 month old mice, with B cells cultured with or without iNKT or Tcon cells from same strain (B) or different strains (C), in the presence of α-GalCer or not. (D) IgG anti-dsDNA antibody production from cell cultures of (B) detected by proteomic microarray. *, P<0.05; **, P<0.01; ***, P<0.001 (two-tailed Student’s t-test). Results (mean ± s.e.m.) are calculated from triplicate cultures from one representative experiment of two or three experiments with similar results.
In further experiments, purified iNKT cells and purified CD19+ B cells from the spleens of 6 month old B6, Sle1b, and BW mice were co-cultured, and supernatants were assayed for the concentrations of IgM and IgG (Figure 4B). When the co-cultured cells were obtained from B6 and Sle1b mice, minimal IgM or IgG was detected, and levels were similar to that observed in cultures of B cells alone. However, when co-cultured cells were obtained from BW mice, then levels of IgM, and IgG were observed that were significantly higher than in cultures with B cells alone. When the iNKT cell activator, α-GalCer, was added to cultures, then iNKT cells from all 3 strains augmented the B cell secretion of IgM and IgG (Figure 4B). However, the IgG secretion of BW cultures was considerably higher than the others. The pattern of increased total IgG secretion, especially in BW cultures, after addition of α-GalCer was also observed for IgG antibodies to dsDNA (Figure 4D) as well as for a variety of other anti-nuclear antibodies including those directed to U1-70, Histone 2A, Ro60/SSA and U1-A as judged by binding to autoantigens on proteomic microarrays (Supplemental Figure 2). Whereas the iNKT cells from BW mice markedly enhanced IgM, IgG and anti-nuclear antibody secretion, the BW Tcon failed to show helper activity for IgG autoantibodies (Figure 4, B and D; and Supplemental Figure 2).
It was of interest that in the presence of α-GalCer the Sle1b iNKT and B cell cultures produced markedly increased levels of U1-A, Ro60/SSA and anti-Histone 2A autoantibodies as compared to Sle1b B cells alone or as compared to iNKT and B cell cultures from B6 mice (Supplemental Figure 2). It was not clear whether the high levels of IgM and IgG secreted by co-cultures of BW iNKT cells and B cells was due to abnormalities in the iNKT cells, B cells, or both. Figure 4C shows that BW iNKT cells could induce high levels of secretion of IgM and IgG when co-cultured with the B6, Sle1b or BW B cells, but neither the B6 nor the Sle1b iNKT cells could induce high levels of immunoglobulins when co-cultured with B cells from all 3 strains. Thus, the predominant abnormality was in the iNKT cells. Although a clear pattern of helper activity was observed for total IgM and IgG secretion using BW iNKT cells mixed with B cells from 3 strains, a clear pattern of helper activity for autoantibody secretion using the microarray assay was not observed (data not shown).
3.4. Only the Ly108hiCD4+ iNKT cell subset from BW mice secretes IL-21 and induces IgG and IgG autoantibody production by B cells
Further studies were performed to identify the subsets of BW iNKT cells that secrete IL-21, and induce immunoglobulin secretion in BW B cells. Follicular helper CD4+PD-1+CXCR5+ T cells (Tfh) that are not NKT cells have been reported to augment antigen specific IgM and IgG secretion in vivo after cognate antigen dependent interactions with follicular B cells that induce germinal centers in non-autoimmune mice [45]. Similarly, a subset of CD4+PD-1+CXCR5+ follicular helper NKT cells (NKTfh) has been shown to help antigen specific IgM and IgG secretion to hapten conjugated glycolipid by interacting with follicular B cells in vivo [46]. Both types of follicular helper T cells secrete IL-21 that is required for B cell activation and differentiation in normal strains of mice [45, 46]. In contrast to the latter studies, we used in vitro induction of spontaneous immunoglobulin and autoantibody secretion by purified subsets of iNKT cells cultured with B cells, obtained from old BW female mice, without addition of antigen or polyclonal activators as the assay system.
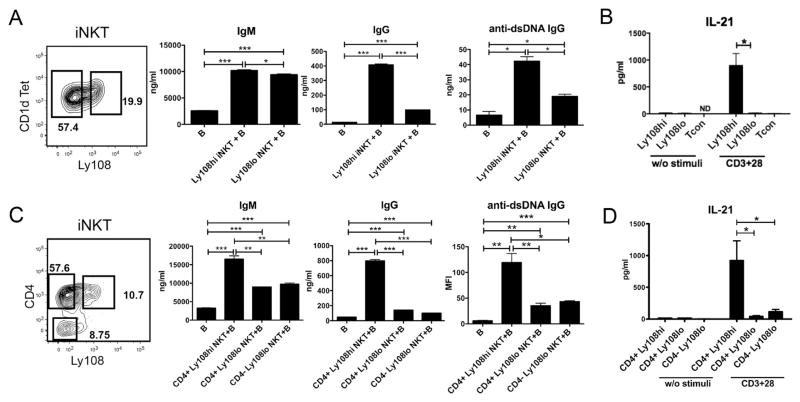
Figure 5A shows the staining pattern separating iNKT cell subsets in the first series of experiments. The iNKT cells were sorted into Ly108hi and Ly108lo cells enclosed in the 2 boxes, and then co-cultured with purified BW B cells. Both subsets of iNKT cells significantly augmented IgM secretion in the cultures, and the Ly108hi cells moderately increased secretion as compared to the Ly108lo cells (Figure 5A). In contrast, the Ly108hi cells augmented IgG and IgG anti-dsDNA secretion about 4 fold as compared to the Ly108lo cells. We then determined whether Ly108hi or Ly108lo iNKT in the BW spleen can secrete IL-21 after addition of anti-CD3 and anti-CD28 mAbs. Whereas the sorted Ly108lo cells and sorted Tcon cells made minimal IL-21 after stimulation, the Ly108hi cells made dramatically higher levels (Figure 5B).
Figure 5.
CD4+Ly108hi iNKT cells provide help for spontaneous antibody secretion of B cells. Sorted B cells with or without sorted iNKT cells, were cultured for 10 days, then supernatant were collected for determination of IgM, IgG and IgG anti-dsDNA antibody concentrations. For IL-21 production assays, cells were sorted and cultured (5X105/ml) for 3 days with anti-CD3 and CD28 mAbs. Different populations of iNKT cells were sorted from three pooled spleens of BW mice. (A) IgM, IgG and IgG anti-dsDNA antibody production from cultures of B cells, with or without sorted Ly108hi or Ly108lo iNKT cells from 6 month old BW mice. (B) IL-21 production by anti-CD3/CD28 stimulated iNKT and Tcon cells of young BW mice. (C) IgM, IgG and IgG anti-dsDNA antibody production from B cells cultures, with or without sorted CD4+Ly108hi, CD4+Ly108lo or CD4−Ly108lo iNKT cells from 6 month old BW mice. (D) IL-21 production by sorted CD4+Ly108hi, CD4+Ly108lo or CD4- Ly108lo iNKT from young BW mice. ND, Not Detected. *, P<0.05; **, P<0.01; ***, P<0.001 (two-tailed Student’s t-test). Data (mean ± s.e.m.) shown are derived from three or four independent experiments (B, D) or from triplicate collected from one representative experiment of two to five with similar results (A, C)
Subsets of BW iNKT cells were also sorted according to their expression of CD4 and Ly108 as shown in Figure 5C. Three subsets were examined according to the boxes shown in the Figure. Whereas the CD4+ cells had 2 distinct populations of Ly108hi and Ly108lo cells, the CD4− cells contained almost all Ly108lo cells. The CD4+Ly108hi subset induced significantly higher levels of IgM and IgG than the CD4+Ly108lo subset. In the case of IgG, the CD4+Ly108hi cells induced about 5 fold more antibody than either the CD4+Ly108lo or CD4−Ly108lo cells. The pattern observed with IgG secretion was observed with IgG anti-dsDNA as well using the microarray assay (Figure 5C). IgG autoantibodies to sm/RNP, Smith, Ro52/SSA, U1-A, Histone 1, U1-70, Ro60/SSA, and LA/SSB nuclear antigens followed this pattern also (Supplemental Figure 3).
These differences in helper activity for B cells were reflected in the considerably higher levels of IL-21 secreted by the CD4+Ly108hi iNKT cells as compared to the CD4+Ly108lo or CD4−Ly108lo iNKT cells (Figure 5D). Although the CD4+Ly108hi cells secreted IL-21 and helped B cell antibody secretion, they did not include a clearly defined population of PD-1+CXCR5+ cells as reported for NKTfh cells obtained after glycolipid challenge in vivo (data not shown).
3.5. High concentrations of IL-21 in the BW serum
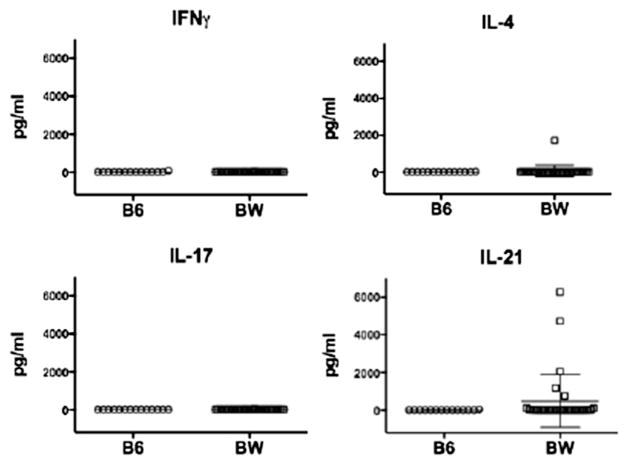
In view of the NKT cell secretion of IL-21 and the associated helper activity for IgG autoantibody production in BW mice, the serum concentrations of IL-21 were compared to that of IFN-gamma, IL-4, and IL-17 in 2–3 month old female BW mice and in control B6 mice. As shown in Figure 6, the serum concentrations of all 4 cytokines was below 50pg/ml in all B6 mice. The concentrations of IFN-gamma and IL-17 were also below 50pg/ml in all BW mice, and in 31 of 32 BW mice for IL-4. Interestingly, the concentrations of IL-21 were between 761 to 6,277 pg/ml in 5 out of 32 BW mice, and the mean was 479 pg/ml. There was no statistically significant correlation between the serum IL-21 and IgG concentrations in these young mice, and the concentration of IL-21 did not increase further in BW female mice that were 6 to 7 months old (data not shown).
Figure 6.
IL-21 is increased in the serum of young BW mice. Serum IFNγ, IL-4, IL-17 and IL-21 concentrations in young B6 (n=14) and BW (n=32) mice were determined by Lumenix assays. Bar graphs show mean ± s.e.m.
3.6. T cells infiltrating BW kidneys
After 6 months of age female BW mice develop kidney disease with glomerulonephritis and T cell infiltrates [1, 2]. The T cells are reported to be mainly CD4−CD8− (DN) and to produce IL-17 that contributes to inflammation [3, 37, 39]. We harvested mononuclear cells from female BW kidneys between 6 to 8 months of age, stained for T cell subsets and B cells, and compared the profiles to that in the spleen as shown in Figures 7A and B. T cells accounted for about 30–40% of mononuclear cells from both tissues, and B cells were about 25% in the kidney and 50% in the spleen (Figure 7, A–C).
Figure 7.
Phenotype of infiltrating BW kidney T cells and production of IL-17. (A, B) Representative flow cytometric analyses of 6 month old BW kidney mononuclear cells (KMC) (A) and spleen cells (B). (C) Mean percentages of B, total T, iNKT and Tcon cells among gated lymphocytes. (D) Mean percentages of different subsets of iNKT cells among iNKT cells. (E) Mean percentages of CD4+CD8−, CD4−CD8+ and DN cells among BW kidney Tcon (left) and iNKT cells (middle), and percentages of total T, Tcon and iNKT DN cells among BW live kidney lymphocytes (right). (F) IFNγ and IL-17 production by BW KMC cells (5X105/ml) upon stimulation with anti-CD3 and CD28 mAbs. 5X105/ml KMC cells prepared from one or two kidneys from 6 month old BW mice were cultured in 96 well plates for 3 days. (G) IFNγ and IL-17 production by BW KMC cells with stimulation by α-GalCer. KMC (1X105) cells obtained from two 6 month old BW mice were cultured for 4 days with or without DCs (5X103), in the presence of α-GalCer (100 ng/mL) or not. Culture supernatants were collected for IFNγ and IL-17 measurements by Lumenix. ***, P<0.001 (two-tailed Student’s t-test). Bar graphs show mean ± s.e.m. Data are derived from six mice from three independent experiments (A-E), or from three independent experiments (F), or from two independent experiments (G).
Additional staining showed that about 1–2% of the mononuclear cells were CD1dTet+TCR+ iNKT cells in both tissues. Among the iNKT cells, similar numbers were NK1.1− in the kidney and spleen, but CD4−Ly108lo iNKT cells were significantly higher in the kidney as compared to the spleen (Figure 7D). The latter cells are DN (Figure 7E), and account for about 50% of the kidney iNKT cells. In contrast, only 1% of Tcon cells in the kidney were DN (Figure 7E). Thus, about half of the DN cells in the kidney were iNKT cells and half were Tcon cells (Figure 7E).
The kidney mononuclear cells (KMC) were activated in vitro with anti-CD3/CD28 mAbs, and the secretion of the pro-inflammatory cytokines, IFN-γ and IL-17 was measured. Figure 7F shows that the dominant cytokine was IFN-γ (mean ~1,500 pg/m), with lesser amounts of IL-17 (mean ~300pg/ml). In order to determine whether iNKT cells contributed to the cytokine secretion, the mononuclear cells were co-cultured with purified DCs as a source of antigen presenting cells and stimulated with α-GalCer. As shown in Figure 7G, IFN-γ was detected in supernatants only when mononuclear cells were cultured with DCs in the presence of α-GalCer. IL-17 was also detected under the same conditions, and a significantly reduced amount was observed when only mononuclear cells were stimulated with α-GalCer (Figure 7G). Thus, the iNKT cells in the kidney can contribute to the production of both inflammatory cytokines. There were too few mononuclear cells obtained from the kidney to sort Tcon and iNKT cells to determine their relative contributions to cytokine production.
4. Discussion
The major goals of the study were to determine the predominant T cell sources of the pro-inflammatory and lupus promoting cytokines IL-17 and IL-21 [34–42], as well as the source of help for spontaneous secretion of antibodies in lupus prone BW mice. Ly108 was reported to play an important role in lupus disease development, since introduction of the Ly108-H1 isoform transgene into Sle1b mice with the lupus associated Ly108-1 isoform abolishes SLE related autoimmunity [50]. Several Ly108 isoforms including Ly108-1, -2 and -H1 were found to either promote or suppress lupus[13, 50]. We determined whether surface expression of Ly108 (SLAMF6) in BW mice with the Ly108-1 allele could be used to distinguish subsets of T cells that promote lupus. The Ly108 staining divided Tcon and iNKT cells into Ly108hi and Ly108lo subsets. Almost all Ly108hi cells among iNKT cells were contained within the NK1.1− subset and accounted for about half of the NK1.1− cells. The percentage and absolute number of splenic Ly108hi iNKT cells was significantly increased in both the BW and Sle1b mice as compared to the B6 mice. However, the percentage among Tcon cells in B6 versus Sle1b mice was not significantly different, and indicated that the Sle1b segment had a greater impact on Ly108 expression among iNKT cells than among Tcon cells. SLAM receptors, SLAMF3 and SLAMF6 (Ly108), are increased on blood CD4+ T cells of SLE patients compared to healthy controls [51], but our results showed that Ly108 expression (MFI) on either young or old BW splenic CD4+ T cells was decreased as compared to B6 mice (data not shown). The explanation of the human and mouse difference is not clear, and may reflect differences in the lymphoid tissues analyzed in addition to other factors.
Purified iNKT and Tcon cells from the fresh spleens of BW mice were obtained by flow cytometry, and activated in vitro with plate bound anti-CD3 and anti-CD28 mAbs. Whereas the Tcon cells secreted similar levels of IFN-γ compared to the iNKT cells, significantly higher levels of IL-4 and IL-17 were produced by the iNKT cells. Subsets of the BW iNKT cells were purified, and the IL-17 secretion was confined to a small subset of Ly108loCD4−Nrp-1+NK1.1− cells that accounted for about 5% of BW iNKT cells. The Ly108 marker was useful in defining a small subset among Nrp-1+NK1.1− iNKT cells. This subset was increased about 20 fold among all splenic lymphocytes in the BW mice as compared to the B6 or Sle1b mice, and was able to secrete IL-4 and IFN-γ in addition to IL-17. This CD4− subset also failed to express CD8, and was DN (data not shown).
The results of the current studies of IL-17 secreting cells among NKT cells are in agreement with those reported previously, since IL-17 secretion was confined to a subset of iNKT cells that does not express CD4, CD8, or NK1.1 but does express Nrp-1 [44]. The Nrp-1+ NKT cells in B6 mice were identified as recent thymic emigrants [44]. However, it is unclear if that is the case in the present study, since the Ly108lo cells represent about 15–20% of the Nrp-1+ NKT cells in the BW mice, and we did not determine the impact of adult thymectomy on these cells. Since Tcon cells from BW mice secreted significantly less IL-17 than iNKT cells after activation, the results suggest that iNKT cells may be an important source of this pro-inflammatory cytokine in the pathogenesis of lupus [37–41].
We also studied the secretion of IL-21 by iNKT cells and Tcon cells in the BW mice. IL-21 promotes follicular and extrafollicular antibody formation, as well as the formation and maintenance of germinal centers in non-autoimmune mice [45]. The predominant sources of IL-21 in non-autoimmune mice are PD-1+CXCR5+ follicular helper cells including conventional Tfh cells or NKTfh cells that are activated and induced to differentiate after the injection of protein or glycolipid antigens respectively [45, 46]. However, ICOS+PSGL-1loCXCR5− extrafollicular helper T cells that secrete IL-21 have been identified in lupus prone mice [52]. When the purified BW Tcon cells used in the current study were activated in vitro, there was minimal IL-21 secretion. Interestingly, the iNKT cells of the BW mice secreted large amounts of IL-21, as compared to those from B6 and Sle1b mice. The results are consistent with previous studies that showed iNKT cells are the predominant source of IL-21 even in non-autoimmune mice [32].
In contrast to the studies of IL-17, the secretion of IL-21 was confined to the CD4+Ly108hiNK1.1− NKT cells instead of the CD4−Ly108lo NK1.1− cells. In addition, the expression of Ly108 clearly distinguished two subsets of CD4+ NKT cells that did or did not secrete IL-21. Since the IL-21 secreting subset did not express the PD-1 or CXCR5 markers reported on NKTfh cells [46], it is possible that they are more closely related to the ICOS+PSGL-1loCXCR5− extrafollicular helper T cells [52]. Since IL-21 is a potent B cells helper factor, we also studied the ability of iNKT cells and their subsets to enhance B cell secretion of IgM, IgG, and anti-nuclear autoantibodies. Whereas whole spleen cells of more than 6 month old female BW mice spontaneously secreted all these antibodies, the spleen cells from the old female B6 and Sle1b mice secreted minimal amounts. Nevertheless, the Sle1b cells secreted significantly more than the B6 cells.
The addition of purified BW Tcon cells to the purified BW CD19+ B cells did not significantly increase the secretion of IgM, IgG or anti-dsDNA antibodies. On the other hand, addition of purified BW iNKT cells provided considerable augmentation for antibody and autoantibody secretion as reported previously [19]. Addition of the iNKT cell activator α-GalCer increased the ability of the purified iNKT cells to augment IgG and IgG autoantibody secretion. Although purified iNKT cells from old B6 and Sle1b mice did not significantly increase IgG secretion of purified syngeneic B cells, the addition of α-GalCer to the cultures resulted in significant increases. Although the B6 cells produced little or no autoantibodies, the Sle1b cells secreted high levels of anti-histone and anti-RNA associated protein antibodies after α-GalCer addition. This may be due to impaired tolerance in Sle1b B cells [13], and/or to abnormalities in Sle1b iNKT cells. The results suggest that activation of iNKT cells with the glycolipid enhances their helper activity. Previous lupus studies that added α-GalCer to cultures of whole spleen cells [25], rather than to cultures of purified NKT cells and B cells, reported an increase in immunoglobulin secretion but a decrease in autoantibody secretion, whereas in the present study both increased. The reasons for the different results are not clear. Importantly, in vivo activation of iNKT cells with α-GalCer administered to BW mice, resulted in increased levels of both IgG and IgG anti-dsDNA antibodies in the serum, and an increase in lupus disease activity and death [18, 21].
In a series of “mix and match experiments” in the current study, the BW iNKT cells were shown to induce antibody secretion of the B6 and Sle1b B cells. In contrast, the B6 and Sle1b iNKT cells did not induce antibody secretion even of BW B cells. Thus, the increased BW iNKT cell helper function was consistent with their increased secretion of the helper factor, IL-21. Our previous studies showed that old, but not young, iNKT cells mediated helper function for IgG [19].
In order to determine which subsets of invariant NKT cells mediated helper activity for BW B cells, subsets were purified as described in the IL-17 and IL-21 cytokine studies, and compared for their ability to help the spontaneous antibody secretion. As expected, the CD4+Ly108hi subset of iNKT cells that was the predominant source of IL-21 was also the predominant source of help for spontaneous antibody and autoantibody secretion. Importantly, the BW CD4−Ly108lo subset that was the predominant source of IL-17 did not provide helper activity. We were unable to block the helper activity of the BW iNKT cells by adding anti-IL-21 mAb to the cultures (data not shown), and this may be due in part to the presence of NKT cell derived IL-4 and IFNγ in the cultures. The latter 2 cytokines also contribute to augmentation of antibody secretion by B cells, and switching to the pathogenic IgG2a isotype.
In view of the requirement for IL-21 to promote lupus in the MRL/lpr, BXSB-yaa, and sanroque mice [34–36], the elevated serum levels of IL-21 in the BXSB-yaa mice [53], and the elevated levels of the mRNA encoding IL-21 in the PBMC of humans with lupus [54], we compared the concentrations of IL-21 in female BW mice and B6 mice to that of IFNγ, IL-17 and IL-4. Whereas the concentrations of all the cytokines were below 50 pg/ml in all B6 mice, the concentration if IL-21 was markedly elevated in a fraction of BW mice that had low serum levels of IFNγ, IL-17 and IL-4. Since NKT cells were the predominant source of IL-21 after stimulation in vitro, the elevated serum levels may reflect spontaneous NKT activation and secretion of IL-21 in vivo.
Since T cells expressing IL-17 have been reported to infiltrate the diseased kidneys of mice and humans with lupus [3, 37, 38], we examined the phenotype and cytokine secretion of the kidney infiltrating T cells of old BW mice. T cells made up about a 35% of the mononuclear cell infiltrate, and B cells made up about 25%. Among the lymphocytes, only about 1% were iNKT cells as judged by CD1d tetramer staining, and about 50% were DN. In contrast, only about 1% of Tcon cells were DN. These results are consistent with those of previous studies that showed NKT cells and DN T cells infiltrated the kidneys of old female BW mice [22, 31, 37, 39]. Although iNKT cells were reported not to be present in the blood DN T cells from SLE patients [55], their presence in the DN T cells of human lupus kidneys was not studied. Activation of the mononuclear cells from BW kidneys with ant-CD3/CD28 mAbs resulted in secretion of the pro-inflammatory cytokines, IFN-γ and IL-17. α-GalCer was used to selectively activate kidney iNKT cells and both IFN-γ and IL-17 were detected. The results suggested that iNKT cells can contribute to the secretion of the pro-inflammatory cytokines in the kidney, and thereby contribute to kidney inflammation. Our preliminary studies indicated that kidney disease in T cell depleted BW female mice is markedly attenuated, and transfer of purified BW iNKT cells induces kidney disease (Tang, X., unpublished observations).
In summary, two different subsets of BW iNKT cells that produce large amounts of IL-17 or IL-21 were identified with characteristics that were consistent with some but not all of the markers on NKT17 and NKTfh cells reported previously [44, 46]. The subset that secreted large amounts of IL-21 could be clearly identified among the CD4+ cells by the Ly108hi phenotype, and provided help for the spontaneous secretion of antibodies and autoantibodies by purified B cells. The IL-17 secreting subset expressed the Ly108loCD4−NK1.1−Nrp-1+ phenotype. The murine studies indicate that iNKT cells play a role in the pathogenesis of lupus. Since autoantibody formation, IL-17 and IL-21 contribute to disease development [34–38], NKT cell may be a valuable therapeutic target.
5. Conclusion
We found that iNKT cells from lupus prone NZB/W female mice were markedly more potent than conventional T cells in providing help for autoantibody secretions and secreting IL-21 and IL-17 after stimulation through the TCR. Analysis of iNKT cell subsets showed that helper activity and IL-21 secretion was confined to CD4+NK1.1− iNKT cells that expressed high levels of the SLAM family member, Ly108, that is required for NKT development. In contrast, the iNKT cell subset that secreted abundant IL-17 expressed the CD4− Ly108loNK1.1−Nrp-1+ phenotype. Both subsets are likely to contribute to the development of lupus, since previous studies have shown that autoantibody formation, IL-21 and IL-17 secretion contribute to disease activity especially in the kidney.
Supplementary Material
Supplemental Figure 1. (A) Absolute splenic cell number of iNKT (TCR+CD1dtet+), Tcon (TCR+CD1dtet−), Ly108hi iNKT and Ly108hi Tcon cells from B6, Sle1b and BW mice. Each symbol in graphs represents an individual mouse. (B) Ly108 expression on iNKT (upper) and Tcon (lower) cells of B6, Sle1b and BW mice. Isolated splenic single cell suspensions were analyzed by flow cytometry with Pacific Blue conjugated anti-Ly108 mAb (open histograms) or mouse isotype control IgG2a (closed histograms), green line indicates B6, blue line indicates Sle1b, and red line indicates BW in the Ly108 FACS overlay. Data are presented as the mean ± s.e.m. Group differences with P>0.05 were not considered statistically significant (ns). *, P<0.05; **, P<0.01; ***, P<0.001 (two-tailed Student’s t-test).
Supplemental Figure 2. Sle1b and BW iNKT cells provide help for IgG autoantibody production of Sle1b and BW B cells, respectively, and α-GalCer augments the production. Sorted CD19+ B cells with or without sorted iNKT cells from 6 month old B6, Sle1b and NZB/W mice were cultured for 10 days, and supernatants were collected for determination IgG autoantibody concentrations.
Supplemental Figure 3. CD4+Ly108hi iNKT cells provide help for IgG autoantibody secretion of B cells. Sorted B cells with or without sorted CD4+Ly108hi, CD4+Ly108lo or CD4−Ly108lo iNKT cells from 6 month old BW mice, were cultured for 10 days, then supernatant were collected for determination of IgG autoantibody production. Data shown are one of two independent experiments with similar results.
Highlights.
iNKT cell helper activity in NZB/W mice is mediated by the Ly108hiCD4+NK1.1− subset
iNKT IL-21 secretion in NZB/W mice is mediated by the Ly108hiCD4+NK1.1− subset
Ly108loCD4+NK1.1− NKT cells mediated minimal helper activity or IL-21 secretion
iNKT IL-17 secretion is mediated by Ly108loCD4−Nrp-1+ but not Ly108hiCD4−Nrp-1+ subset
Acknowledgments
We would like to thank Dr. Edward K. Wakeland (University of Texas Southwestern Medical Center) for generous gift of Sle1b mice, Dr P. Savage (Brigham Young University) for providing the α-GalCer, the NIH Tetramer Facility (Atlanta, GA) for providing the CD1d-tetramer, and Kent Jensen for his technical assistant on Luminex assays. This work was supported by NIH research grant RO1 DK082537.
Abbreviations
- SLE
Systemic lupus erythematosus
- BW
NZB/W F1
- B6
C57BL/6
- Sle1b
B6.Sle1b
- iNKT
invariant NKT
- Tcon
conventional T cells
- Nrp-1
neuropillin-1
- α-GalCer
α-galactocylceramide
- Tfh
Follicular helper T cells
- NKTfh
follicular helper NKT cells
- DN
CD4−CD8−
- KMC
kidney mononuclear cells
Footnotes
Contribution: X.T. designed and performed research, contributed vital analytical methods, collected, analyzed and interpreted data, and wrote the paper; B.Z., J.J., J.P., H.D. and P.U. performed and supervised the microarray assay for autoantibodies in cell culture supernatants; and S.S. provided overall research supervision and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–6. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Theofilopoulos AN, Kofler R, Singer PA, Dixon FJ. Molecular genetics of murine lupus models. Adv Immunol. 1989;46:61–109. doi: 10.1016/s0065-2776(08)60651-3. [DOI] [PubMed] [Google Scholar]
- 3.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 4.Ando DG, Sercarz EE, Hahn BH. Mechanisms of T and B cell collaboration in the in vitro production of anti-DNA antibodies in the NZB/NZW F1 murine SLE model. J Immunol. 1987;138:3185–90. [PubMed] [Google Scholar]
- 5.Borchers A, Ansari AA, Hsu T, Kono DH, Gershwin ME. The pathogenesis of autoimmunity in New Zealand mice. Semin Arthritis Rheum. 2000;29:385–99. doi: 10.1053/sarh.2000.7173. [DOI] [PubMed] [Google Scholar]
- 6.Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–68. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 7.La Cava A, Fang CJ, Singh RP, Ebling F, Hahn BH. Manipulation of immune regulation in systemic lupus erythematosus. Autoimmun Rev. 2005;4:515–9. doi: 10.1016/j.autrev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi T. Spontaneous production of antibodies to deoxyribonucleic acids in patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1985;35:47–56. doi: 10.1016/0090-1229(85)90077-7. [DOI] [PubMed] [Google Scholar]
- 9.Tsao BP, Ebling FM, Roman C, Panosian-Sahakian N, Calame K, Hahn BH. Structural characteristics of the variable regions of immunoglobulin genes encoding a pathogenic autoantibody in murine lupus. J Clin Invest. 1990;85:530–40. doi: 10.1172/JCI114469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–81. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang A, Batteux F, Wakeland EK. The role of SLAM/CD2 polymorphisms in systemic autoimmunity. Curr Opin Immunol. 2010;22:706–14. doi: 10.1016/j.coi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, Yim YS, Pertsemlidis A, Garner HR, Jr, Morel L, et al. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–80. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, Mooney JM, Schatzle JD, Wakeland EK, Mohan C. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312:1665–9. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 14.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–62. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu T, Simmons A, Yuan J, Bender TP, Alberola-Ila J. The transcription factor c-Myb primes CD4+CD8+ immature thymocytes for selection into the iNKT lineage. Nat Immunol. 2010;11:435–41. doi: 10.1038/ni.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–65. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–9. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng D, Liu Y, Sidobre S, Kronenberg M, Strober S. Activation of natural killer T cells in NZB/W mice induces Th1-type immune responses exacerbating lupus. J Clin Invest. 2003;112:1211–22. doi: 10.1172/JCI17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, Strober S. Natural killer T cells and innate immune B cells from lupus-prone NZB/W mice interact to generate IgM and IgG autoantibodies. Eur J Immunol. 2008;38:156–65. doi: 10.1002/eji.200737656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morshed SR, Takahashi T, Savage PB, Kambham N, Strober S. Beta-galactosylceramide alters invariant natural killer T cell function and is effective treatment for lupus. Clin Immunol. 2009;132:321–33. doi: 10.1016/j.clim.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng D, Lee MK, Tung J, Brendolan A, Strober S. Cutting edge: a role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J Immunol. 2000;164:5000–4. doi: 10.4049/jimmunol.164.10.5000. [DOI] [PubMed] [Google Scholar]
- 22.Forestier C, Molano A, Im JS, Dutronc Y, Diamond B, Davidson A, Illarionov PA, Besra GS, Porcelli SA. Expansion and hyperactivity of CD1d-restricted NKT cells during the progression of systemic lupus erythematosus in (New Zealand Black x New Zealand White)F1 mice. J Immunol. 2005;175:763–70. doi: 10.4049/jimmunol.175.2.763. [DOI] [PubMed] [Google Scholar]
- 23.Chan OT, Paliwal V, McNiff JM, Park SH, Bendelac A, Shlomchik MJ. Deficiency in beta(2)-microglobulin, but not CD1, accelerates spontaneous lupus skin disease while inhibiting nephritis in MRL-Fas(lpr) nice: an example of disease regulation at the organ level. J Immunol. 2001;167:2985–90. doi: 10.4049/jimmunol.167.5.2985. [DOI] [PubMed] [Google Scholar]
- 24.Yang JQ, Wen X, Liu H, Folayan G, Dong X, Zhou M, Van Kaer L, Singh RR. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 2007;56:1219–33. doi: 10.1002/art.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang JQ, Wen X, Kim PJ, Singh RR. Invariant NKT cells inhibit autoreactive B cells in a contact- and CD1d-dependent manner. J Immunol. 2011;186:1512–20. doi: 10.4049/jimmunol.1002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wermeling F, Lind SM, Jordo ED, Cardell SL, Karlsson MC. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J Exp Med. 2010;207:943–52. doi: 10.1084/jem.20091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wither J, Cai YC, Lim S, McKenzie T, Roslin N, Claudio JO, Cooper GS, Hudson TJ, Paterson AD, Greenwood CM, et al. Reduced proportions of natural killer T cells are present in the relatives of lupus patients and are associated with autoimmunity. Arthritis Res Ther. 2008;10:R108. doi: 10.1186/ar2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higuchi M, Zeng D, Shizuru J, Gworek J, Dejbakhsh-Jones S, Taniguchi M, Strober S. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169:5564–70. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- 29.Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–99. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, Garcia-Ojeda M, Sibley R, Strober S. Bone marrow NK1.1(−) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189:1073–81. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morshed SR, Mannoor K, Halder RC, Kawamura H, Bannai M, Sekikawa H, Watanabe H, Abo T. Tissue-specific expansion of NKT and CD5+B cells at the onset of autoimmune disease in (NZBxNZW)F1 mice. Eur J Immunol. 2002;32:2551–61. doi: 10.1002/1521-4141(200209)32:9<2551::AID-IMMU2551>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 32.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–34. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 33.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 34.Rankin AL, Guay H, Herber D, Bertino SA, Duzanski TA, Carrier Y, Keegan S, Senices M, Stedman N, Ryan M, et al. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Fas(lpr/lpr)/J mice. J Immunol. 2012;188:1656–67. doi: 10.4049/jimmunol.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, 3rd, Leonard WJ, Roopenian DC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009;106:1518–23. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–8. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 37.Crispin JC, Kyttaris VC, Terhorst C, Tsokos GC. T cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:317–25. doi: 10.1038/nrrheum.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Chu Y, Yang X, Gao D, Zhu L, Wan L, Li M. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–83. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–9. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang HK, Liu M, Datta SK. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J Immunol. 2007;178:7849–58. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- 41.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–85. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 42.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–75. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 43.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 44.Milpied P, Massot B, Renand A, Diem S, Herbelin A, Leite-de-Moraes M, Rubio MT, Hermine O. IL-17-producing invariant NKT cells in lymphoid organs are recent thymic emigrants identified by neuropilin-1 expression. Blood. 2011;118:2993–3002. doi: 10.1182/blood-2011-01-329268. [DOI] [PubMed] [Google Scholar]
- 45.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 46.Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, Hogan JJ, Cerundolo V, Tangye SG, Bittman R, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2012;13:35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 47.Hongo D, Tang X, Dutt S, Nador RG, Strober S. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood. 2012;119:1581–9. doi: 10.1182/blood-2011-08-371948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabakman SM, Lau L, Robinson JT, Price J, Sherlock SP, Wang H, Zhang B, Chen Z, Tangsombatvisit S, Jarrell JA, et al. Plasmonic substrates for multiplexed protein microarrays with femtomolar sensitivity and broad dynamic range. Nat Commun. 2011;2:466. doi: 10.1038/ncomms1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang B, Price J, Hong GS, Tabakman SM, Wang HL, Jarrell JA, Feng J, Utz PJ, Dai HJ. Multiplexed cytokine detection on plasmonic gold substrates with enhanced near-infrared fluorescence. Nano Research. 2013;6:113–120. [Google Scholar]
- 50.Keszei M, Detre C, Rietdijk ST, Munoz P, Romero X, Berger SB, Calpe S, Liao G, Castro W, Julien A, et al. A novel isoform of the Ly108 gene ameliorates murine lupus. J Exp Med. 2011;208:811–22. doi: 10.1084/jem.20101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatterjee M, Rauen T, Kis-Toth K, Kyttaris VC, Hedrich CM, Terhorst C, Tsokos GC. Increased expression of SLAM receptors SLAMF3 and SLAMF6 in systemic lupus erythematosus T lymphocytes promotes Th17 differentiation. J Immunol. 2012;188:1206–12. doi: 10.4049/jimmunol.1102773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–86. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–71. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 54.Nakou M, Papadimitraki ED, Fanouriakis A, Bertsias GK, Choulaki C, Goulidaki N, Sidiropoulos P, Boumpas DT. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to the generation of plasma B cells. Clin Exp Rheumatol. 2013;31:172–9. [PubMed] [Google Scholar]
- 55.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–6. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. (A) Absolute splenic cell number of iNKT (TCR+CD1dtet+), Tcon (TCR+CD1dtet−), Ly108hi iNKT and Ly108hi Tcon cells from B6, Sle1b and BW mice. Each symbol in graphs represents an individual mouse. (B) Ly108 expression on iNKT (upper) and Tcon (lower) cells of B6, Sle1b and BW mice. Isolated splenic single cell suspensions were analyzed by flow cytometry with Pacific Blue conjugated anti-Ly108 mAb (open histograms) or mouse isotype control IgG2a (closed histograms), green line indicates B6, blue line indicates Sle1b, and red line indicates BW in the Ly108 FACS overlay. Data are presented as the mean ± s.e.m. Group differences with P>0.05 were not considered statistically significant (ns). *, P<0.05; **, P<0.01; ***, P<0.001 (two-tailed Student’s t-test).
Supplemental Figure 2. Sle1b and BW iNKT cells provide help for IgG autoantibody production of Sle1b and BW B cells, respectively, and α-GalCer augments the production. Sorted CD19+ B cells with or without sorted iNKT cells from 6 month old B6, Sle1b and NZB/W mice were cultured for 10 days, and supernatants were collected for determination IgG autoantibody concentrations.
Supplemental Figure 3. CD4+Ly108hi iNKT cells provide help for IgG autoantibody secretion of B cells. Sorted B cells with or without sorted CD4+Ly108hi, CD4+Ly108lo or CD4−Ly108lo iNKT cells from 6 month old BW mice, were cultured for 10 days, then supernatant were collected for determination of IgG autoantibody production. Data shown are one of two independent experiments with similar results.