Abstract
This paper uses data from the U.S. National Health Interview Surveys (N = 1,513,097) to describe and explain temporal patterns in black-white health disparities with models that simultaneously consider the unique effects of age, period, and cohort. First, we employ cross-classified random effects age–period–cohort (APC) models to document black-white disparities in self-rated health across temporal dimensions. Second, we use decomposition techniques to shed light on the extent to which socio-economic shifts in cohort composition explain the age and period adjusted racial health disparities across successive birth cohorts. Third, we examine the extent to which exogenous conditions at the time of birth help explain the racial disparities across successive cohorts. Results show that black-white disparities are wider among the pre-1935 cohorts for women, falling thereafter; disparities for men exhibit a similar pattern but exhibit narrowing among cohorts born earlier in the century. Differences in socioeconomic composition consistently contribute to racial health disparities across cohorts; notably, marital status differences by race emerge as an increasingly important explanatory factor in more recent cohorts for women whereas employment differences by race emerge as increasingly salient in more recent cohorts for men. Finally, our cohort characteristics models suggest that cohort economic conditions at the time of birth (percent large family, farm or Southern birth) help explain racial disparities in health for both men and women.
Keywords: Health inequalities, Racial disparities, Self-rated health, Age–period–cohort, Conditions at birth
Introduction
One of the most disconcerting findings with regards to health disparities is the persistent black–white gap in U.S. morbidity and mortality. Despite declining morbidity and mortality over time, researchers continue to find persistent and significant racial gaps for a host of outcomes, including: self-rated health (SRH), chronic conditions, disability, overall life expectancy, and age- and cause-specific mortality (Geronimus, Bound, Keene, & Hicken, 2007; Hayward, Miles, Crimmins, & Yang, 2000; Hummer & Chinn, 2011; Williams & Sternthal, 2010). Such disparities are well documented on a year-to-year basis (e.g., Harper, Lynch, Burris, & Smith, 2007; Macinko & Elo, 2009) and have also increasingly been tied to life course processes that unfold by age (Geronimus et al., 2010; Jackson et al., 2011; Walsemann, Geronimus, & Gee, 2008). Very little attention in this literature, however, has been given to possible cohort-based differences in health disparities; that is, are black–white gaps in health structured by the cohorts into which individuals are born, in addition to the effects of period factors and aging processes? Further, to what extent can we explain cohort disparities with extant explanations of racial inequality (e.g. socioeconomic conditions)? Addressing these limitations, the intent of this paper is to describe black–white health disparities in the United States using modeling techniques that simultaneously estimate the independent effects of age, period, and cohort. Second, we further attempt to explain cohort-based changes in the race disparity using cohort-based variables.
Age, period and cohort
As an indicator of the internal physiological change due to accumulated exposure to pathogens, genetic manifestation of disease, and the biological breakdown of the human body (Yang & Land, 2006), the effect of aging has been the focus of much research on population health disparities (e.g., Manton & Gu, 2001). However, the persistence of health disparities over time suggests that period effects, temporal social contexts (e.g. historical events) that affect all age groups simultaneously, and cohort effects, unique conditions attributed to individuals within defined birth-year groupings, also play crucial roles. Cohort differences in health also reflect social change and have been attributed to disparate early life experiences and lifetime exposures to pathogens, improvements in nutrition, and advances in health knowledge and medical technologies (Finch & Crimmins, 2004; Fogel, 2005; Manton, Stallard, & Corder, 1997; Masters, 2012; Ryder, 1965; Yang, 2008a). Indeed, rapid and unequal historical changes in educational attainment, family formation, changes in lifestyle (e.g. smoking prevalence) (Preston & Wang, 2006), and labor market participation (Bauman & Graf, 2003, pp. 1–12; Escobedo & Peddicord, 1996; Mosisa & Hipple, 2006; Waite, 1995) have implications for health disparities. However, health disparities within even very new (and young) birth cohorts, such as the racial gaps in low birth weight and infant mortality (Powers, 2013; Powers, Solis, Frisbie, Hummer, & Pullum, 2006; Powers & Song, 2009), imply within-cohort variation in exposures.
Each temporal dimension has a potentially dynamic and unique effect on population health and assessing the impact of one temporal dimension while controlling for other dimensions is essential to understanding temporal changes in health (Masters, 2012; Ryder, 1965; Yang, 2008a). Specifically, analytically omitting cohorts results in model misspecification and potentially biases aging and period temporal trends (Reither, Hauser, & Yang, 2009). There is evidence of cohort influences, modeled within an age–period–cohort (APC) framework, on measures of health such as SRH (Zheng, Yang, & Land, 2011), disability (Lin, Beck, Finch, Hummer, & Masters, 2012), obesity (Reither et al., 2009) and mortality (Masters, 2012; Yang, Fu, & Land, 2004). To date, however, there has been less focus on racial disparities and explaining cohort change, emphasizing the need to fill those gaps using an APC framework. Next, we briefly discuss the importance of SRH as a measure of population health and health disparities.
Trends and disparities in self-rated health
We chose SRH for several reasons: (1) studies show that a poor or fair SRH has strong predictive value for subsequent mortality above and beyond clinical assessments (Benyamini & Idler, 1999; Idler & Benyamini, 1997); (2) although evidence that SRH is associated with specific disease outcomes is limited (Ferraro, Farmer, & Wybraniec, 1997; Menec, Chipperfield, & Perry, 1999), other studies suggest that SRH is associated with current morbidity (Ferraro & Farmer, 1999) and subsequent functional decline and disability (Idler & Angel, 1990); and (3) SRH is often more sensitive to change in response to external factors than are physiologic parameters. Additionally, black and white men and women who report fair/poor SRH have similar mortality risks (McGee, Liao, Cao, & Cooper, 1999), suggesting that our use of this measure for analyses of racial health disparities is valid. We now summarize extant research on the temporal dimensions of SRH, highlighting research which has used an APC approach.
Research suggests that SRH declines with age (Hill & Needham, 2006; McCullough & Laurenceau, 2004) or exhibits a nonlinear decline with improvements at older ages (Zack, Moriarty, Stroup, Ford, & Mokdad, 2004; Zheng et al., 2011). Willson, Shuey, and Elder (2007) found that cohort variation in SRH also declined with age; older cohorts reported better initial health, but more rapid declines than more recent cohorts. Women tend to have worse SRH than men, with greater within-group variation at all ages (Zheng et al., 2011). Although there is some evidence of gender convergence (Ross & Bird, 1994) and racial divergence (Yao & Robert, 2008) in SRH with age, Yang and Lee (2009) found, after accounting for cohort effects, persistent race and gender gaps over the life course.
There are mixed findings for SRH period trends, due, in part, to differences in data source or the measurement of SRH. Hill and Needham (2006) found improvements over time in SRH for women, but a nonlinear pattern for men using the General Social Survey. Salomon, Nordhagen, Oza, and Murray (2009), used the National Health Interview Survey (NHIS) and found that poor/fair SRH declined during the 1980s, increased during the early 1990s, only to flatten out after a sharp decline in 1997, simultaneous with a redesign of the survey. Zack et al. (2004) found a similar increase in fair/poor SRH during the early 1990s using the Behavioral Risk Factor Surveillance System; however, their findings suggested that these increases continued through the remainder of the 1990s. Using the NHIS and APC models, Zheng et al. (2011) found slight cyclical increases in SRH before 1998with significant declines thereafter; this trend was similar among both men and women. Relevant to our focus on racial disparities, one study found that blacks experienced steeper declines in poor/fair SRH than Non-Hispanic whites, resulting in a narrowing period disparity (Salomon et al., 2009). Zheng et al. (2011) found narrowing variation in mean SRH differences, though this is not specific to certain groups (e.g. race/ethnic groups) but rather all variation.
With few exceptions, the cohort temporal dimension of SRH has been neglected. In a community study, Chen, Cohen, and Kasen (2007) compared baby boom women to pre-boomers and found that baby boomers reported overall lower SRH. In contrast, using APC methods, Zheng et al. (2011) found that later baby boom cohorts (1955–1964) had better SRH than other cohorts. Men showed relatively flat cohort trends with the exception of higher SRH among baby boom cohorts; women, in contrast, showed overall worse SRH with declines through the early baby boom cohort (1945–1954) and increases thereafter. Disparities (within cohort variation, not group-specific) decreased through the 1925–1929 cohort, leveled off through WW II cohorts, decreased during baby boom cohorts, and increased among more recent cohorts. These disparities were similar when examined by gender, except that men exhibited substantial declines for the Great Depression through the baby boom cohorts (Zheng et al., 2011). Yang and Lee (2009) find evidence that racial gaps in SRH have diverged for more recent cohorts, but did not examine how this may additionally vary by gender. Within a cohort, however, these gaps do not vary by age and are argued to be attributable to mean cohort differences in characteristics such as socioeconomic status (SES), marital status and other health conditions (Yang & Lee, 2009). Only when the separate effects of age, period, and cohort are estimated simultaneously can we begin to uncover the sources of health disparity trends.
Explanations of racial disparities in health
It has been argued that race in the United States is “an important marker of differential access to societal resources and rewards, and health status is no exception” (Williams, 2005; 53; also see LaVeist, 1994). Although some research suggests that race/ethnic disparities in health and mortality are completely accounted for by individual differences in SES (Rogers, 1992), most studies indicate otherwise—that is, that health disparities are substantially reduced but not completely eliminated (Crimmins, Hayward, & Seeman, 2004; House & Williams, 2000; Hummer & Chinn, 2011; Link & Phelan, 1995). Other contributors to persistent disparities include residential context and job availability and quality (Huffman & Cohen, 2004), differential rates of risky health behaviors (Finch, Frank, & Hummer, 2000; Lantz et al., 2001), discrimination and discrimination-related life course stress (Gee & Ford, 2011; Geronimus et al., 2010; LaVeist, 2000; Sternthal, Slopen, & Williams, 2011; Williams, Neighbors, & Jackson, 2003), differential incarceration (Schnittker, Massoglia, & Uggen, 2011), and racial residential segregation (House, 2002; Williams & Collins, 2001). In fact, one recent study (Do et al., 2008) confirmed the growing supposition that race is a proxy for exposure to differential social conditions; they found that residential context accounted for 15–76% of racial disparities in SRH that were previously unaccounted for by individual-level explanatory variables. More recently, Do, Frank, and Finch (2012) were able to fully account for racial disparities in health using a comprehensive, propensity score adjustment for individual and neighborhood measures of SES.
Cohort characteristics affecting health
Members of larger cohorts are expected to experience persistent disadvantages stemming, in part, from the consequences of increased economic competition once the cohort enters the labor market (e.g. higher stress, unemployment and lower wages) (Easterlin, 1978, 1987). Large cohorts are also expected to put extreme demands on the school and health care systems as well as community resources (O’Brien, Stockard, & Isaacson, 1999). Cohort size is associated with a number of indicators of disadvantage, from crime rates to individual earnings (O’Brien, 1989; O’Brien et al., 1999; Welch, 1979). To date, there has been no application of this characteristic to the study of health trends, despite concerns over future health care burdens of large cohorts (e.g. Baby Boom cohorts). We expect that the scarce resources that arise from relative cohort size will be more detrimental for blacks; this may, however, be ameliorated if racial–ethnic groups are not frequently in competition for similar jobs or if social welfare programs reduce these resource disparities.
Poor economic and health conditions early in life negatively impact health, cognitive functioning, and survival at older ages (Doblhammer, van den Berg, & Fritze, 2011; Van den Berg, Doblhammer, & Christensen, 2009; Van Den Berg, Lindeboom, & Portrait, 2006). One strand of research, aligned with the fetal origins hypothesis, finds that poor conditions (such as under-nutrition) during the critical in utero period of development can have lasting effects on adult health (Barker, 1997; Fogel, 2005). Additionally, early exposure to infectious diseases can lead to chronic inflammation, which in turn influences health and mortality (Crimmins & Finch, 2006; Finch & Crimmins, 2004; McDade, Rutherford, Adair, & Kuzawa, 2010). Other types of harsh environments can also promote a pro-inflammatory response (Miller & Chen, 2010) and, ultimately, contribute to disease later in life (Dong et al., 2004). This early negative exposure may also operate indirectly through stunting educational achievement and labor market outcomes (Case & Paxson, 2010; Kuh & Wadsworth, 1993). We expect that poor cohort economic conditions that impact all groups (e.g. recessions) may, in fact, exacerbate inequalities due to the greater sensitivity of black employment to macroeconomic downturns (Elsby, Hobijn, & SŞahin, 2010, pp. 1–48) and because of inequities in the safety net of wealth accumulation. Further, exposure to economic deprivation, infectious diseases and harsh family environments are conspicuously stratified by race and ethnicity, with blacks far more disadvantaged than whites (Acevedo-Garcia, 2000; Hoynes, Page, & Stevens, 2006; Singh & Yu, 1995); we expect that this differential exposure at the cohort-level will, in part, explain temporal health inequities.
We can also think of groups as being born into a race–ethnic-specific opportunity structure that varies in terms of potential social mobility. This opportunity structure is a function of disparities in the pool of race–ethnic-specific resources available to children as they age as well as the level of prejudice and discrimination facing their group. For example, the opportunity to fully participate in the education system or to be exposed to employed co-ethnics have both differentially shifted across time for race and ethnic groups (Chandra, 2000; Walters, 2001; Welch, 1990).
Thus, beyond describing black–white SRH temporal change, this paper also examines some of the major factors thought to account for black–white health disparities, particularly as those disparities are changing across cohorts. While no study can possibly take into account the full range of factors, we focus on some of the most important ones thought to be responsible for continued disparities. As a result, we ask three specific research questions regarding racial disparities in SRH: 1) what are the temporal trends in SRH (age, period, and cohort)?; 2) has the contribution of (now) well-established socio-demographic risk factors changed over time?; and 3) do conditions at the time of birth help to explain racial health disparities in SRH across birth cohorts?
Methods
Data
We utilize the Integrated Health Interview Series (IHIS), the National Health Interview Series (NHIS) harmonized for consistent variable measurement, available through the Minnesota Population Center (Minnesota Population Center and State Health Access Data Assistance Center 2012). We use data from 1972, which is when the question on SRH was first asked, through 2009. The NHIS is a cross-sectional household survey, covering the non-institutionalized civilian population residing in the United States at the time of the interview. Given our primary interest in racial disparities, we restrict our sample to non-Hispanic whites and blacks who are not missing information on the dependent variable of SRH. Prior to 1976, Hispanic ethnicity was not collected; in these cases, individuals were coded according to their stated racial category. (In 1976, Hispanics made up less than 5% of the white population and less than 3% of the black population, respectively.) We also restrict our analysis to those aged 25 and older because most U.S. adults have completed school by that age. Given smaller sample sizes for earlier cohorts, we exclude individuals born before 1893 (what we define as the beginning of the 1895 five-year cohort band). We further excluded observations between 1972 and 1996 that rely on proxy reports when the sample adult was unable to answer questions due to physical or mental health limitations to ensure consistency with survey waves after 1996 in which proxy reporting was eliminated. With these restrictions, our final sample includes 1,304,201 non-Hispanic whites and 208,896 non-Hispanic blacks.
Dependent variable
Our dependent variable is drawn from a measure of SRH available from 1972 continuously through 2009. Respondents who refused to answer the question or responded “unknown” make up less than one percent of the sample in each wave and are dropped from the analysis. Beginning with the 1982 survey, both the question wording and the response categories changed. Before 1982, respondents were asked: “Compared to other persons [person’s] age, would you say that his health is excellent, good, fair or poor?” Thereafter, respondents were asked “Would you say that [person’s] health in general is excellent, very good, good, fair or poor?” To alleviate the comparability issue that arises due to differences in responses categories, we create a dichotomous measure of whether respondents rated their health as fair or poor as compared to good, very good or excellent. There does not appear to be evidence of a discontinuity in the trend line in 1982; this suggests that this measurement issue does not unduly influence our results. Further, given large subsequent mortality risk differences between those who rate themselves in fair/poor health compared to good/very good/excellent health (Benjamins, Hummer, Eberstein, & Nam, 2004), there is empirical precedence for dichotomizing this variable.
Explanatory variables – individual level measures
We use a continuous measure of age as well as an age squared term; in independent tests of various functional forms this provided the best fit and conformed most closely with the use of single-year age dummy variables. Age was top-coded at ninety-nine years old until 1996 and eighty-five years old beginning in 1997; we top-coded age in all waves to eighty-five. We also estimated age-restricted models to age 84 (not shown); models without a standardized top code yielded slightly more modest age disparities among men, but all other results were similar. We centered age on the median age of the cohort band for each individual. This manner of centering protects the age estimates from bias associated with variation in mean age across cohorts (Miyazaki & Raudenbush, 2000). Period is defined as survey year. We group individuals into 5-year cohort bands based on their year of birth to break the linear dependence between age, period and cohort. We define these cohorts by their midpoint; for example, the “1950 cohort” includes individuals born in 1948, 1949, 1950, 1951, and 1952.
We also include a number of indicators of SES and household structure as well as a control for geographic residence. Marital status is measured as married (reference), never-married, and other (widowed, separated, or divorced). A continuous measure of household size was also included. Regional indicators control for residence in the West (reference), South, Northeast, and North Central/Midwest. An individual’s highest level of education is included and has also been cohort median centered to account for improvements in mass education over the century. Respondents are asked whether they were part of the labor force during the preceding one to two weeks (before 1997 the reference period is two weeks, thereafter it is one week). From this question we created indicators of employment: employed (includes “has a job but is not currently working”, reference), unemployed but looking, and unemployed, not looking for work. We also consider anyone sixty-five and older who is not employed or looking for work as retired. Interval level family income was first recoded to its midpoint; we allowed top codes to vary over survey waves (results with a consistent top code yielded similar results). We then adjust income for economies of scale by dividing family income by the square root of household size. We then rescale for inflation dividing income by (CPIyear of survey/100), with 1982–1984 as the reference point (Bureau of Labor Statistics, 2013). We impute the modal marital status and mean income when missing and include indicators of missingness for marital status and income, which are missing for 0.3% and 11–12% of respondents, respectively.
Explanatory variables – cohort characteristics
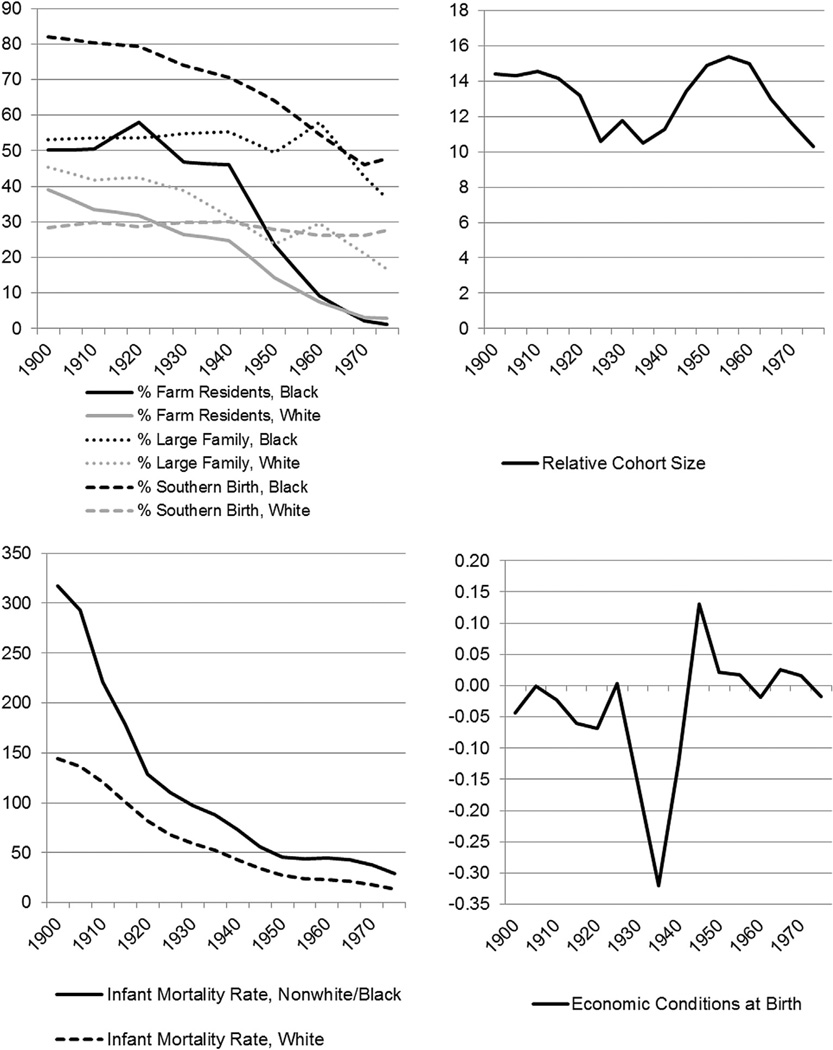
We examine six cohort characteristics (See Appendix Fig. 1): relative cohort size, health and economic conditions at birth. To capture the relative size of the cohort to the overall labor force, we first created year-specific measures of the percent of the working age (15–64) population that was 15–19. These percentages were then averaged across the years that a cohort entered the labor market. For example, the 1940 cohort (born in 1938–1942) entered the labor market as early as 1953 (those aged 15 in the 1938 cohort) to as late as 1961 (those aged 19 for the 1942 cohort); the average of the year-specific percentages of those of working age who were 15–19 was 11.3%. We included only the year each cohort turned age 15 and accounted for crowding or competition for jobs from those slightly older as suggested by O’Brien et al. (1999); these analyses produced similar results (not shown). This measure is limited in that we cannot capture the extent to which blacks and whites may not be competing for the same jobs or the same pool of resources within any given cohort size (i.e. for school funding, hospitals, etc). Following Doblhammer, van den Berg, and Fritze (2011) and a broader literature on measures of aggregate economic conditions, we capture economic conditions at birth using the natural logarithm of the cyclical component of the real GDP per capita. To obtain these estimates we apply the Hodrick–Prescott filter with smoothing value of 500 to time series data derived from historical GDP estimates (Maddison 2010). Using IPUMS decennial census data (Minnesota Population Center) from 1900 through 1980, we create race-specific measures that capture cohort socioeconomic disadvantage, including the percent of children under a year old whose parents report they were born in the South, the percent living in large households (6 or greater members), and the percent living on a farm. We linearly interpolate measures between censuses. We also used historical infant mortality rate (IMR) data from the unpublished tables of the National Vital Statistics System’s historical mortality data to construct our cohort IMR measure. Before 1933, rates include only those states that had complete death registration, thereafter all states are included. Rates specific to the black population are not available before 1960, available inconsistently during the 1960s, and consistently after 1968. Although there is not a sizable discrepancy between rates in 1959 when all non-whites were aggregated and 1960 when blacks were listed separately, the rates that we use to approximate black IMRs before 1960 are biased by the inclusion of other non-white infants. For each cohort characteristic, we standardized the measure to allow comparisons across cohorts.
Appendix Fig. 1.
Descriptive trends in cohort characteristics.
Statistical procedures
Simultaneously modeling all three temporal dimensions is challenging due to the exact linear dependence between age, period and cohort (Period = Age + Cohort). Although many strategies have been developed to address the identification problem (e.g. Fienberg & Mason, 1978, 1985; Heckman & Robb, 1985; Mason, Mason, Winsborough, & Poole, 1973; O’Brien, 2000), we utilize a recently developed methodology for repeated cross-sectional survey data (Yang & Land, 2006, 2008) to estimate a series of cross classified random effects models (CCREM) for binary outcomes. As is conventional to help identify the model (Yang, 2008a), we include a quadratic age term and we group survey respondents into five-year birth cohorts. CCREM, detailed in Yang and Land (2013), accounts for the multi-level structure of the data and, specifically, the possible non-independence of individuals nested within cohorts/periods who may share similar attributes or experiences unique to their cohorts or periods of the surveys. A cross-classified model is appropriate because individuals are not strictly nested within periods and cohorts in a hierarchical fashion.
Our race-specific baseline models of SRH are specified as follows:
| (1) |
where Yijk is the presence or absence of fair/poor SRH for i = 1, 2, … njk individuals within cohort j and period k. Age and age-squared are represented by Aijk and , we cohort-median centered these terms to reduce bias.
| (2) |
At level 2, β0jk is the outcome, which in this model, represents the cell mean of individuals who belong to birth cohort j and surveyed in year k. γ0 is the model intercept, or expected mean at zero values for all level 1 covariates averaged across all periods and cohorts; u0j is the residual random effect of cohort j (i.e., the contribution of cohort j averaged over all periods on β0jk) and is assumed to be normally distributed with mean 0 and a within-cell variance τu; and ν0k is the residual random effect of period k (i.e., the contribution of period k averaged over all cohorts) and is assumed to be normally distributed with mean 0 and a within-cell variance τv. We use Stata version 12 xtmelogit for model estimation.
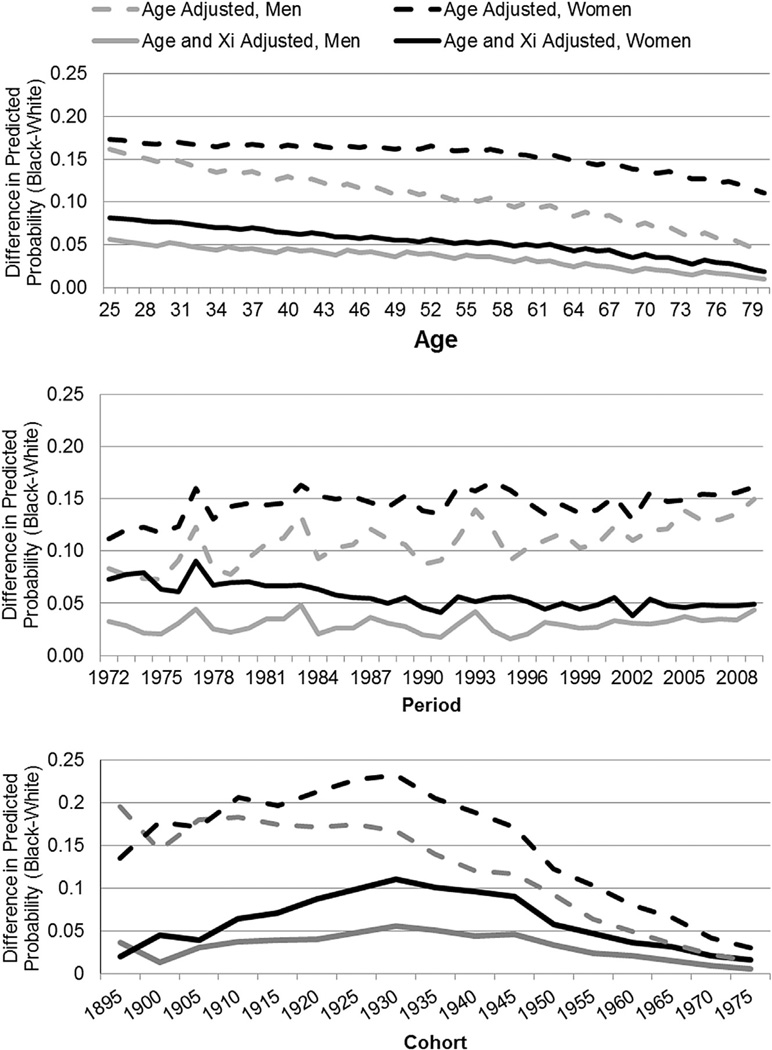
To aid interpretation of the CCREM models, we generate the predicted probabilities of fair/poor health from recovered random cohort and period effects which have been adjusted by the covariates held at their mean. Given that we used median centered age, age probabilities are first calculated for every age within a cohort and then averaged across cohorts to arrive at a probability for a given age. The disparities presented in Fig. 2 are measured by an absolute measure of the black probability of poor/fair SRH minus the white probably of poor/fair SRH, but we also highlight results from a relative measure (black divided by white) in our conclusion.
Fig. 2.
Racial difference in the predicted probability of fair/poor health across age, period and cohort for men and women: Adjusted for age and socio-demographic covariates.
Decomposition methods are a standard demographic technique to understand the role that compositional differences play in between group inequality. We utilize a variant for nonlinear outcomes (Fairlie, 1999, 2005) which decomposes the predicted probability gap in fair/poor health from cohort-specific models. Using 1950 as an illustrative cohort:
| (3) |
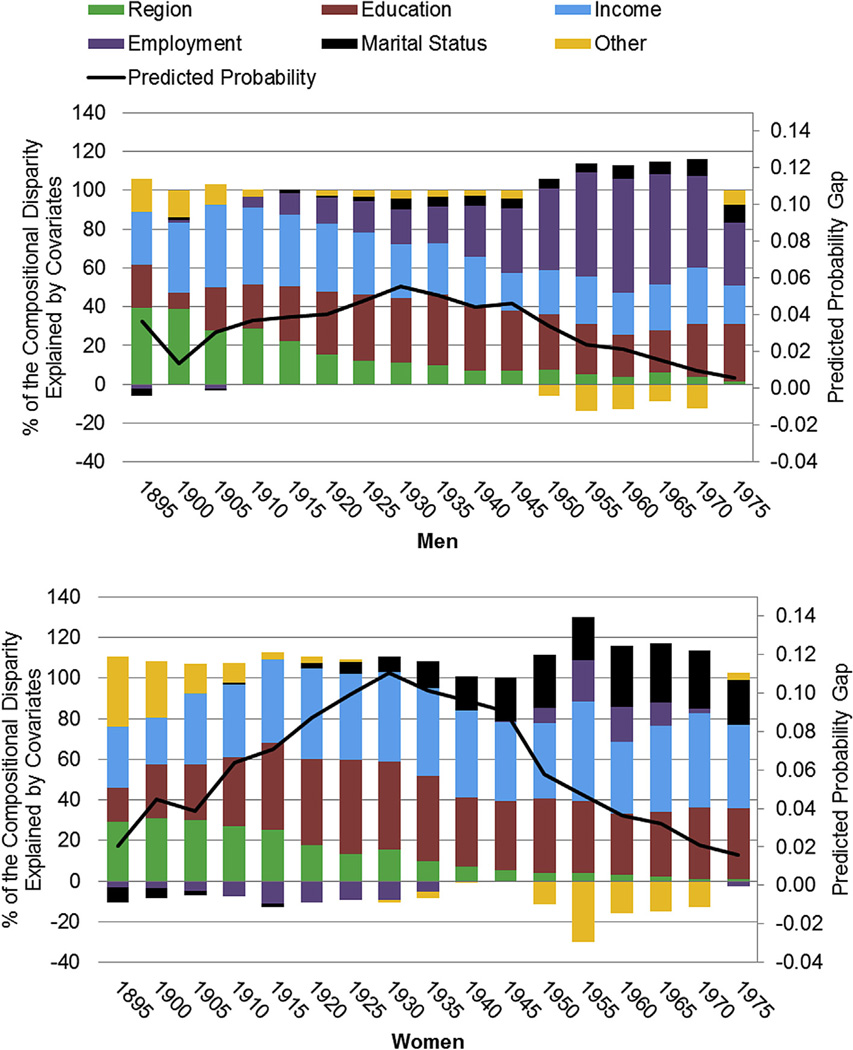
where is the mean probability of fair/poor SRH of whites in 1950 and is the mean probability of fair/poor SRH for blacks in the 1950 cohort. The first term represents the contribution of compositional changes to the overall change in the probability of reporting poor health between whites and blacks. That is, it is the difference in rates of poor/fair health had blacks faced the same returns to risk—measured by individual-level covariates such as marital status, education, and income—as whites in the 1950 cohort. This compositional part of the disparity elucidates, for example, how much of the inequality in fair/poor health is due to lower average educational attainment of blacks relative to whites. The second term is the portion of the difference due to differences in the effects of the coefficients for the measured covariates. Specifically, it assesses the contribution to the gap that would have occurred if black returns to risk equaled those of whites in the 1950 cohort and if group characteristics were held fixed at the white levels. Given that the second term often includes the influence of any unmeasured covariates, we focus only on the influence of compositional factors in explaining the racial health gap; we refer to this as the compositional disparity in Fig. 3. This decomposition strategy is repeated and summarized across the range of our birth cohorts. To more closely approximate the CCREM models, we have included controls for period and age. Given our focus on social explanations for cohort disparities, we present the contributions of the remaining demographic and SES factors net of period and age. This approach allows us to determine the primary SES determinants of racial health disparities and, perhaps more importantly, to see if these determinants have changed across cohorts. We can explore both whether their contribution to disparities has increased or decreased and we can explore whether other factors have become more or less salient over time.
Fig. 3.
Contributions of covariates (%) to the compositional disparity by cohort (primary y-axis) and racial difference in the predicted probability of fair/poor health adjusted for socio-demographic controls by cohort (secondary y-axis).
Analytic plan
Using CCREM, we examine gender-specific health patterns across time, adjusted for age (Model 1), and individual-level characteristics (Model 2), to illustrate the underlying temporal trends. Using race-specific models, we then examine the racial disparities for each of these temporal dimensions. Given the length and level of detail in each regression model, we do not report the results of all regressions but rather summarize the results in a series of graphical displays (the race-specific models are shown in Appendix Table 1 for illustrative purposes; all other results are available upon request). Using decomposition methods, we then examine the role of changing endowments of individual-level characteristics across cohorts. We finally return to our CCREM framework and introduce a series of cohort characteristics one by one, and then in combination, to determine the extent to which we can explain cohort variation in health disparities.
Appendix Table 1.
CCREM estimates of self-rated health, by race.
| White women | Black women | White men | Black men | |||||
|---|---|---|---|---|---|---|---|---|
| Intercept | −1.854** | −2.559** | −.938** | −1.908** | −1.849** | −2.557** | −1.122** | −2.177** |
| Age-CMC | .041** | .035** | .022** | .033** | .052** | .030** | .039** | .027** |
| Age-Squared-CMC | −.001** | −.002** | −.001** | −.002** | −.001** | −.001** | −.001** | −.001** |
| Education-CMC | −.147** | −.092** | −.127** | −.086** | ||||
| Income (in 10,000s) | −.493** | −.488** | −.465** | −.476** | ||||
| Unemployed (Employed) | .584** | .496** | .591** | .459** | ||||
| Not in labor force | 1.014** | 1.234** | 1.787** | 1.832** | ||||
| Retired | .618** | .674** | .811** | .865** | ||||
| Family size | −.051** | −.037** | .002 | −.025** | ||||
| Never married (married) | .236** | .109** | .102** | .011 | ||||
| Divorced, separated, widowed | .177** | .121** | .154** | .050 | ||||
| Northeast residence (West) | −.206** | −.248** | −.108** | −.257** | ||||
| North Central/Midwest | −.081** | −.072* | .016 | −.024 | ||||
| South | .191** | −.066* | .285** | .077** | ||||
| Random effects | ||||||||
| Cohort | ||||||||
| 1895 | .685** | .557** | .415** | .087 | .662** | .382** | .839** | .350* |
| 1900 | .735** | .445** | .632** | .194* | .663** | .271** | .627** | .044 |
| 1905 | .801** | .649** | .659** | .307** | .788** | .535** | .872** | .425** |
| 1910 | .804** | .740** | .800** | .543** | .919** | .758** | .986** | .653** |
| 1915 | .796** | .561** | .755** | .459** | .951** | .667** | .978** | .593** |
| 1920 | .658** | .553** | .716** | .557** | .803** | .665** | .848** | .601** |
| 1925 | .462** | .454** | .642** | .570** | .632** | .573** | .729** | .581** |
| 1930 | .262** | .328** | .539** | .567** | .405** | .417** | .535** | .524** |
| 1935 | .066** | .216** | .312** | .450** | .189** | .291** | .723** | .401** |
| 1940 | −.138** | .112** | .135** | .363** | −.060** | .166** | .024 | .258** |
| 1945 | −.310** | .048** | −.035 | .291** | −.278** | .086** | −.124** | .225** |
| 1950 | −.485** | −.320** | −.383** | −.173** | −.483** | −.257** | −.386** | −.131** |
| 1955 | −.588** | −.453** | −.555** | −.356** | −.668** | −.454** | −.707** | −.397** |
| 1960 | −.765** | −.705** | −.814** | −.629** | −.854** | −.700** | −.944** | −.605** |
| 1965 | −.860** | −.863** | −.985** | −.783** | −1.028** | −.903** | −1.212** | −.859** |
| 1970 | −.987** | −1.038** | −1.298** | −1.087** | −1.229** | −1.118** | −1.528** | −1.160** |
| 1975 | −1.132** | −1.281** | −1.525** | −1.352** | −1.406** | −1.372** | −1.792** | −1.487** |
| Period | ||||||||
| 1972 | .354** | .229** | .063 | .263** | .516** | .383** | .235** | .316** |
| 1973 | .370** | .262** | .116* | .316** | .469** | .359** | .166^ | .263** |
| 1974 | .442** | .300** | .180** | .349** | .571** | .487** | .236** | .309** |
| 1975 | .307** | .113* | .056 | .118^ | .404** | .248** | .094 | .101 |
| 1976 | .216** | .076 | .025 | .075 | .295** | .189** | .107 | .156^ |
| 1977 | .111* | −.012 | .132* | .263** | .222** | .141** | .211* | .242* |
| 1978 | .146** | −.021 | .015 | .073 | .266** | .158** | .043 | .075 |
| 1979 | .182** | .011 | .094^ | .114^ | .288** | .186** | .032 | .062 |
| 1980 | .134** | −.096^ | .080 | .063 | .207** | .060 | .053 | .005 |
| 1981 | .103* | −.149** | .052 | −.0004 | .151* | −.034 | .087 | .035 |
| 1982 | .097* | −.060 | .057 | .047 | .157* | .040 | .117 | .083 |
| 1983 | −.011 | −.165** | .077 | −.003 | .075 | −.050 | .173* | .160^ |
| 1984 | −.034 | −.179** | .016 | −.047 | −.001 | −.073 | −.093 | −.165^ |
| 1985 | −.069^ | −.188** | −.020 | −.103^ | −.010 | −.071 | −.041 | −.097 |
| 1986 | −.121* | −.219** | −.037 | −.141^ | −.122^ | −.148* | −.096 | −.154 |
| 1987 | −.126** | −.212** | −.065 | −.142* | −.152* | −.154** | −.030 | −.035 |
| 1988 | −.175** | −.245** | −.119** | −.214** | −.223** | −.210** | −.124^ | −.136^ |
| 1989 | −.189** | −.251** | −.067 | −.159** | −.244** | −.230** | −.168* | −.188* |
| 1990 | −.195** | −.248** | −.144** | −.253** | −.282** | −.278** | −.307** | −.330** |
| 1991 | −.172** | −.226** | −.151** | −.293** | −.214** | −.216** | −.242** | −.314** |
| 1992 | −.146** | −.196** | −.002 | −.121* | −.174** | −.194** | −.092 | −.136^ |
| 1993 | −.110* | −.149** | −.002 | −.141* | −.202** | −.199** | .040 | −.004 |
| 1994 | −.144** | −.171** | .022 | −.119^ | −.188** | −.172** | −.055 | −.197* |
| 1995 | −.101* | −.118* | .005 | −.078 | −.104^ | −.094^ | −.172* | −.235* |
| 1996 | −.082^ | −.077 | −.041 | −.099 | −.146* | −.124^ | −.129 | −.199^ |
| 1997 | −.188** | −.132* | −.159** | −.199** | −.341** | −.275** | −.196** | −.173* |
| 1998 | −.198** | −.111* | −.116* | −.138* | −.301** | −.220** | −.134^ | −.156* |
| 1999 | −.196** | −.082^ | −.157** | −.169** | −.291** | −.213** | −.215^ | −.196* |
| 2000 | −.157** | −.054 | −.121* | −.117^ | −.247** | −.181** | −.168* | −.164* |
| 2001 | −.118** | .018 | −.033 | −.007 | −.206** | −.095^ | −.047 | −.028 |
| 2002 | −.063 | .095^ | −.117 | −.119^ | −.137* | −.044 | −.083 | −.022 |
| 2003 | −.088* | .080 | .002 | .022 | −.137* | −.051 | −.029 | −.037 |
| 2004 | −.023 | .181** | −.005 | .027 | −.082 | .014 | .012 | .039 |
| 2005 | −.008 | .217** | .010 | .039 | −.078 | .030 | .107^ | .097 |
| 2006 | −.001 | .215** | .042 | .061 | −.016 | .062 | .094 | .085 |
| 2007 | .066 | .482** | .077 | .244** | .037 | .286** | .130^ | .262** |
| 2008 | .095* | .529** | .104^ | .279** | .106^ | .342** | .200** | .302** |
| 2009 | .095* | .554** | .132** | .308** | .135* | .338** | .286** | .374** |
| Variance components | ||||||||
| Cohort | .176 | .221 | .097 | .177 | .248 | .212 | .160 | .198 |
| Period | .685 | .631 | .755 | .606 | .789 | .656 | .909 | .654 |
Results
Descriptive results
There are considerable disparities in SRH by race (Table 1). For men, only 14.6% of whites, but 22.3% of blacks indicated they were in fair or poor health. Likewise, 14.5% of white women compared to 26.5% of black women responded that they were infair or poor health. Blacks are, on average, approximately one to two years younger than whites in the sample. Marital status differs markedly by race; blacks are less apt to be married (only 54.5% of men and 39.9% of women), and more likely to be never-married, separated, divorced or widowed. For example, 7.0% of white women are never-married compared to 20.2% of black women and 26.0% of white women are divorced, separated or widowed relative to 39.9% of black women. Blacks are more likely to reside in the South and less likely to reside in the West relative to Whites. Although the proportion with only some college experience is similar by race, there is greater inequity at the tails of the educational distribution. Blacks are less likely to have a college degree and more likely to have less than a high school degree. White and black women have similar employment profiles; in contrast, black men are less likely to be employed (64.6%) relative to white men (68.9%), and more likely to be unemployed and not looking for work. Racial disparities in income are also apparent despite our truncated income distribution; black men have family incomes that are, on average, approximately $3000 less than white men whereas black women have family incomes that are on average $4000 less than white women.
Table 1.
Descriptive statistics of self-rated health and covariates, by gender and race.
| Men | Women | White | Black | |||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||
| Outcome | ||||||
| Fair/Poor Health (%) | 15.5 | 16.2 | 14.6 | 14.5 | 22.3 | 26.5 |
| Covariates | ||||||
| Age | 49.9 | 49.9 | 50.1 | 50.2 | 48.0 | 47.7 |
| Period | 1992.1 | 1989.6 | 1991.8 | 1989.2 | 1994.2 | 1992.2 |
| Cohort | 1942.2 | 1939.7 | 1941.7 | 1938.9 | 1946.2 | 1944.5 |
| Region of residence | ||||||
| Northeast (%) | 21.2 | 21.7 | 21.7 | 22.3 | 17.6 | 18.6 |
| North Central/Mid-west (%) | 26.3 | 26.8 | 27.3 | 27.9 | 19.4 | 19.7 |
| South (%) | 33.9 | 34.4 | 31.2 | 31.3 | 52.6 | 52.9 |
| West (%) | 18.6 | 17.1 | 19.8 | 18.5 | 10.4 | 8.8 |
| Marital status | ||||||
| Married (%) | 69.6 | 63.1 | 72.0 | 67.0 | 54.5 | 39.9 |
| Never married (%) | 13.5 | 8.9 | 12.4 | 7.0 | 20.3 | 20.2 |
| Divorced/Widowed/Separated (%) | 16.9 | 28.0 | 15.6 | 26.0 | 25.2 | 39.9 |
| Missing marital status (%) | 0.4 | 0.4 | 0.4 | 0.3 | 0.9 | 1.1 |
| Family size | 2.5 | 2.7 | 2.5 | 2.7 | 2.6 | 2.9 |
| Education level | ||||||
| High school degree or less (%) | 57.5 | 62.9 | 53.0 | 61.2 | 65.9 | 67.9 |
| Some college (%) | 20.9 | 20.2 | 20.9 | 20.2 | 21.0 | 20.3 |
| College degree or more (%) | 24.5 | 17.6 | 26.1 | 18.6 | 13.1 | 11.8 |
| Employment status | ||||||
| Employed (%) | 68.4 | 49.9 | 68.9 | 49.1 | 64.6 | 54.2 |
| Unemployed, Looking (%) | 3.2 | 2.6 | 2.9 | 2.3 | 5.1 | 4.5 |
| Unemployed, Not Looking (%) | 10.8 | 27.6 | 10.0 | 27.9 | 16.2 | 25.9 |
| Retired (%) | 17.6 | 19.9 | 18.2 | 20.7 | 14.1 | 15.4 |
| Missing employment status (%) | 0.4 | 0.3 | 0.4 | 0.3 | 0.7 | 0.6 |
| Family income | 15,634 | 14,045 | 16,055 | 14,636 | 12,782 | 10,531 |
| Missing family income | 9.8 | 11.5 | 9.4 | 11.1 | 12.5 | 14.0 |
| Cohort characteristics | ||||||
| Relative cohort size at labor market entry | 13.2 | 13.2 | 13.2 | 13.2 | 13.2 | 13.2 |
| Macro-economic conditions at birth | −0.03 | −0.04 | −0.03 | −0.04 | −0.03 | −0.03 |
| % of cohort with Southern residencea | 28.32 | 28.49 | 52.03 | 52.35 | ||
| % of cohort with farm residencea | 19.46 | 21.02 | 27.98 | 29.75 | ||
| % of cohort with large familya | 31.80 | 32.80 | 52.03 | 52.35 | ||
| Infant mortality ratea | 48.73 | 52.92 | 74.14 | 78.14 | ||
| N | 592,141 | 920,956 | 515,975 | 788,226 | 76,166 | 155,714 |
Race-specific measures.
Overall trends in self-rated health
Given the structure of the data (i.e. the oldest cohorts are not observed at young ages and, likewise, the youngest cohorts are not observed at the oldest ages), as well as concerns with differential mortality, we use caution in interpreting patterns in the cohort tails as well as patterns for older individuals. Additionally, in results not shown, we estimated models without cohort median centering age to reduce bias and our trend results were similar to extant results on SRH trends (i.e., a curvilinear aging trend, and more flat cohort trend) (Zheng et al., 2011); small differences between their results and ours suggest that there may be movement within the health categories that affect the conditional expected value of SRH but not the probability of poor/fair health. Thus, our results should be interpreted as temporal trends after reducing the age-related bias; they vary from prior research due to the extent to which age composition effects are present.
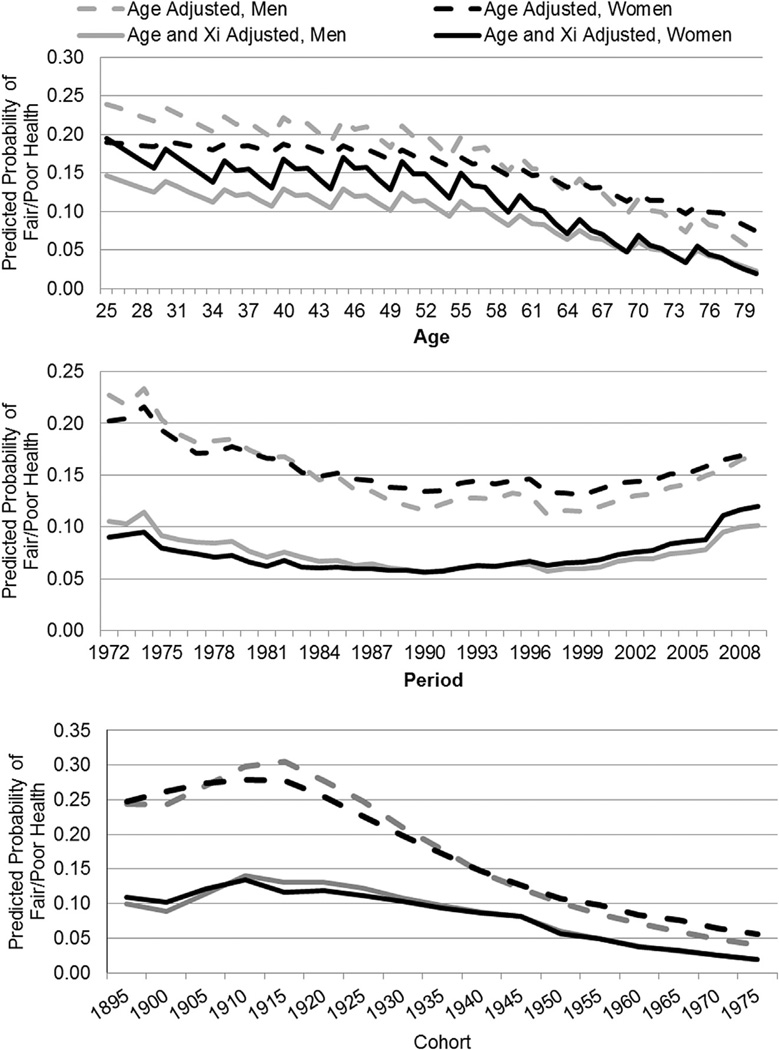
Fig. 1 presents predicted probabilities of fair/poor SRH by gender; these overall trends, combining blacks and whites, are plotted for age, period and cohort. Each panel of the figure shows predicted probabilities of poor/fair health after adjustment for age only and then after adjustment for age and other covariates. Age-adjusted fair/poor SRH decreases for both men and women in a curvilinear fashion with age; gender differences that initially favor women converge around retirement age and then begin to crossover thereafter. After controlling for covariates (solid black and gray lines), the probabilities of poor/fair health are higher for women than men at younger ages. In race-specific trends (not shown), fair/poor probabilities are higher among blacks than whites; the overall shape of aging patterns are similar; however in the age only model the gender cross-over happens earlier among blacks—around age 45, than whites at approximately age 65. Our centered aging estimates vary from conventionally estimated ones in that they have been cohort-centered to purge bias from cohort variation in age; non-centered estimates (not shown) support a curvilinear increase in fair/poor health with increasing age.
Fig. 1.
Predicted probability of fair/poor health across age, period and cohort for men and women: Adjusted for age and socio-demographic covariates.
After controlling for age, predicted rates of poor/fair health are at their highest in the 1970’s and there is a decline across periods through the late 1990’s followed by a slight increase for both women and men. With demographic and SES indicators in the model, period trends flatten and the predicted probability of fair/poor health is similarly high in the contemporary period as in the early 1970’s. The trends for men and women are extremely similar, though there is evidence of a crossover such that women have slightly worse health in recent years. Race-specific trends suggest similar period trends across all groups, but higher predicted probabilities, wider gender gaps and more year-to-year fluctuation among blacks than whites. After accounting for age, cohort trends are consistent with extant literature on pre-Depression cohorts (Idler, 1993); the predicted probability of fair/poor health initially increases across cohorts for women and men, peaking around the 1915/1920 cohorts before beginning a decline. Accounting for demographic and SES covariates illustrate the importance of such factors in driving cohort trends in fair/poor health: the probabilities for men and women become strikingly lower across cohorts. Similar to the age and period results, women and men have a similar predicted probability of fair/poor health across all cohorts; there is evidence of a subtle cross over with the 1915 cohort, followed by convergence around the Great Depression cohorts. Race-specific trends again show higher predicted probabilities among blacks; there is a similar shape in the age-adjusted trend for blacks and whites, however, in the covariate adjusted model there is a greater gender difference among blacks and the trends decline more slowly for mid-century cohorts of black women than white women.
Racial disparities in self-rated health
Fig. 2 shows the black–white disparity in SRH for men and women, measured as the black predicted probability of poor/fair SRH minus the white predicted probability of poor/fair SRH. The first graph (Fig. 2) illustrates relatively flat racial disparities for women through roughly age 60, declining thereafter; racial disparities for men decline much more rapidly across all ages. Racial disparities are greater at all ages for women. These disparities are smaller and, for men, decline less precipitously, after accounting for socio-demographic factors. The second graph (Fig. 2) illustrates racial disparities by period. Again, disparities are larger for women than men; the inequity in SRH follows an uneven, but generally flat pattern across periods for both sexes.
Finally, the last graph in Fig. 2 illustrates disparities by cohort. Turning first to the age-only adjusted disparities, our results show that the disparity is higher for women than men for most of the cohorts. For women, the disparity is largest between the 1925 and 1945 cohorts and declines thereafter; for men, the disparity declines across cohorts beginning after the earliest surviving cohorts (e.g. 1895–1910). The sharp decline in the racial disparity for women, and more modest decline for men coincide with cohorts that entered the labor market after the civil and women’s rights movements. The gaps become substantially more modest after accounting for social, demographic and economic differences between blacks and whites, particularly for the earliest cohorts, but are otherwise similar in pattern.
To summarize, the age-adjusted racial disparities in SRH for women increased through the mid-20th century cohorts; declines after the 1945 cohort brought disparities to their lowest historic levels. On the other hand, the racial disparities for men, despite a relatively flat pattern across the earliest available 20th century cohorts, show declines from the 1915 cohort, which bodes well for future health and mortality disparities. Our covariate adjusted models suggest that at least part of these dramatic disparities observed over successive cohorts born in the first half of the 20th century can be accounted for by differences in social and economic characteristics. However, the question remains as to which characteristics are the most important for particular cohorts; we utilize decomposition methods to explore these contributions.
Explaining racial cohort disparities in health: individual-level differences
Using decomposition techniques, we examine how the socio-demographic controls in our models contribute to explaining the age–period adjusted racial health gap in successive birth cohorts (the solid lines in Fig.2), with a particular focus on the part of the gap due to differences in composition. The importance of composition relative to the “effects” (a component which also includes the influence of unmeasured covariates) varies across cohorts and by gender. In results not shown, compositional differences explain approximately seventy to eighty percent of the total gap for men quite consistently across cohorts. In contrast, compositional differences only account for forty to fifty percent of the total female gap in fair/poor health. This suggests that unmeasured factors (e.g. residential segregation or risky health behaviors) are particularly important in understanding inequality for the female cohorts for which the disparity is greatest.
Fig. 3 illustrates the percent of the compositional gap that is due to each of the factors considered: education, employment, income, marriage, geographic region and other factors. For men and women, differences between blacks and whites in mean education levels contribute consistently to explaining compositional differences in fair/poor health. For women, for example, education differences explain approximately fifty percent of the compositional gap for cohorts entering the labor market around WWII. Regional distributions, particularly racial differences in Southern residence, decline in importance across cohorts for both men and women; this is consistent with migration patterns of blacks out of the South from post-WWI through the 1970s (Fuguitt, Fulton, & Beale, 2001).
For men, differences in income decline in importance, whereas differences in employment, driven mostly by differences in those not in the labor force, are increasingly important in understanding recent cohort disparities. This is consistent with dramatic increases in joblessness among young black men beginning in the 1970s related to trends of increasing incarceration and stricter child support enforcement (Holzer, Offner, & Sorensen, 2005; Small & Newman, 2001). For women, mean differences in income consistently contribute to the gap across the range of cohorts. Also in contrast to men, differences in employment initially favor black women for cohorts before 1950. For example, for the 1920 cohort, the race gap in SRH would actually be larger if black women were distributed in the labor market in a similar way to white women, specifically, retired at similarly high rates. This suggests that black women’s longer tenure in the labor force later in life serves to reduce disparities. However, consistent with period-based research (i.e., studies that ignore cohort effects), finding a racial crossover in labor force participation (i.e. that historically higher black female employment rates relative to whites have reversed themselves) (Browne, 1997) appears to change in more recent cohorts of women, such that employment differences now explain a small portion of the fair/poor gap. Finally, for both men and women, racial differences in marriage patterns contribute to the fair/poor gap in health, starting, most noticeably, with the 1925 cohort. For men, differences in marriage is a minor factor in understanding health disparities whereas these differences are much more important in understanding gaps in health for more recent cohorts of women. These patterns are consistent with changes in marriage over time, including more rapid increases in age at first marriage for blacks relative to whites, greater increases in being never-married for blacks (and particularly for women) and higher rates of remarriage for whites (Bennett, Bloom, & Craig, 1989;Waite, 1995).
Explaining racial cohort disparities in health: cohort-level differences
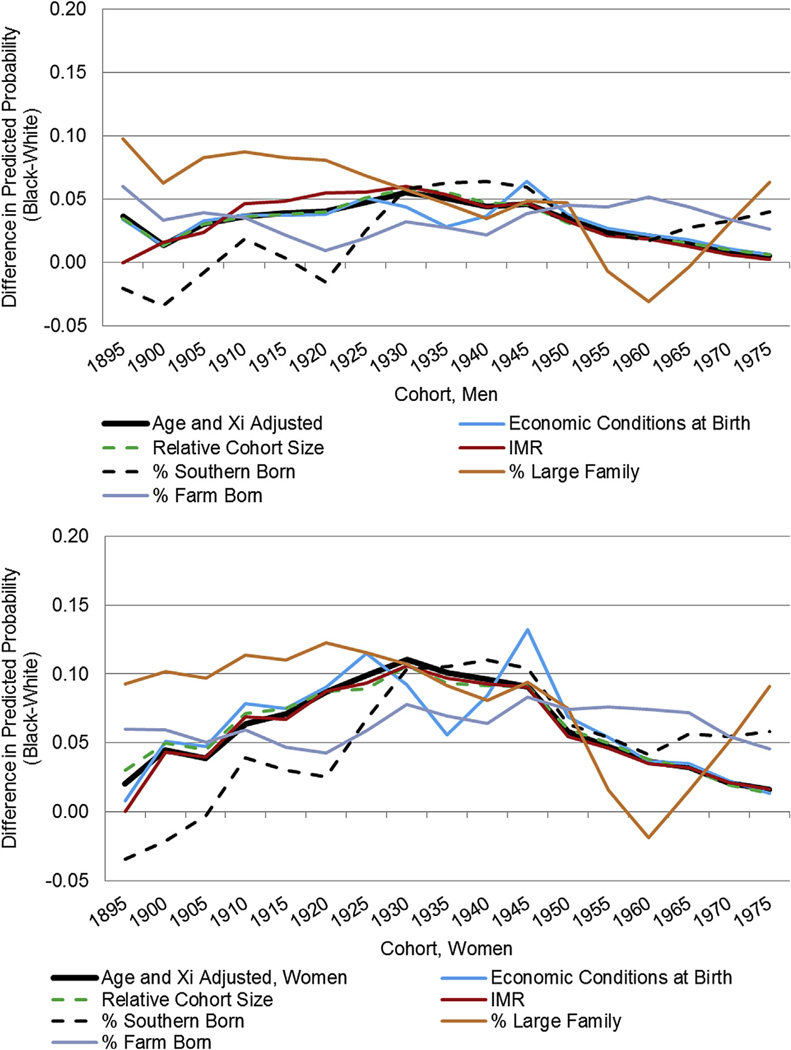
Finally, we introduced cohort characteristics to our CCREM models to understand how disparities would change after accounting for relative cohort size, health and economic conditions at birth; these disparities are graphed in Fig. 4. Table 2 displays the cohort characteristic coefficients from gender and race-specific models as well as the cohort variance parameters. From the latter we can discern how much of the cohort variation from the covariate adjusted model is explained by the addition of the cohort characteristics. This represents a conservative approach because many individual characteristics are expected to be endogenous to the cohort characteristics; consequently, we are understating the extent to which given cohort characteristics explain cohort variation.
Fig. 4.
Racial difference in the predicted probability of fair/poor health across cohort for men and women: adjusted for socio-demographic covariates and cohort characteristics.
Table 2.
Cohort characteristic estimates and variance parameters from race and gender stratified models.
| Model | White women | Black women | White men | Black men | ||||
|---|---|---|---|---|---|---|---|---|
| β | Var | β | Var | β | Var | β | Var | |
| Covariate adjusted | – | 0.398 | – | 0.367 | – | 0.432 | – | 0.428 |
| % Farm residents | 0.049** | 0.029 | 0.039** | 0.029 | 0.049** | 0.064 | 0.032** | 0.032 |
| % Southern residents | 0.398** | 0.144 | 0.046** | 0.058 | 0.428** | 0.137 | 0.051** | 0.045 |
| % Large family | 0.065** | 0.054 | 0.090** | 0.173 | 0.066** | 0.086 | 0.097** | 0.200 |
| IMR | 0.011** | 0.141 | 0.003^ | 0.287 | 0.011** | 0.210 | 0.004^ | 0.323 |
| Relative cohort size | 0.084 | 0.378 | 0.029 | 0.365 | 0.069 | 0.419 | 0.062 | 0.417 |
| Economic conditions at birth | −1.899 | 0.309 | −2.307 | 0.320 | −2.179 | 0.389 | −2.303 | 0.381 |
p < 0.001,
p < 0.01,
p < 0.05.
Note: All coefficients are from separate models. Cohort variance parameters from the model are listed to the right of the coefficient; the covariate adjusted variance parameter is listed at top for reference.
Looking first at how the cohort characteristics impact the disparities for men, Fig. 4 indicates that only some of the characteristics we introduce reduce the disparity. For example, the measure of relative cohort size does not reduce cohort disparities. The falling IMR only modestly suppressed the disparities for a few cohorts before the Great Depression, but otherwise did not reduce cohort disparities. Macroeconomic conditions at birth also has weak explanatory power with two important exceptions: the disparities are reduced for cohorts born around 1935, suggesting that part of their elevated probability is a function of the extremely negative economic conditions at birth that impacted blacks more so than whites. Likewise, the post-WW II boom served to reduce the disparity below what it would have been otherwise because once we control for these conditions, the disparity is elevated for these cohorts. These results are reflected in the regression estimates in Table 2; relative cohort size and macro-economic conditions at birth do not attain statistical significance for men and do very little in terms of explaining cohort variance in SRH. The race-specific cohort IMR does, however, explains approximately 51 [((0.432 − 0.210)/0.432)*100] and 25 [((0.428 − 0.323)/0.428)*100] percent of the residual cohort variance among white and black men, respectively.
The percent of the cohort that is Southern born results in similar reductions in the disparities to the other characteristics mentioned after the 1930 cohort, but results in the largest declines in the disparity for the earlier cohorts. The cohort’s percent farm born dramatically reduces the disparity from the 1910 through the 1960 cohort, consistent with the converging percentages of blacks relative to whites born on a farm across cohorts (See Appendix Fig. 1). Table 2 likewise indicates that alongside percent farm residents, the percent Southern born emerges as one of the most important explanatory factors with respect to the cohort variance in health. For example, for white women, the percent farm residents explains as much as 93% [((0.398 − 0.029)/0.398)*100] and the percent Southern born as much as 64% [((0.398 − 0.144)/0.398)*100] of the residual cohort variance. The percent of the cohort born to large families suggests that disparities would have been even larger before 1930 if not for the relatively parallel trends in cohort family formation among blacks and whites. Family patterns then explain some of the gap until the 1950 cohort, consistent with a more rapid reduction in white family size relative to black families following the Great Depression. After the 1950 cohort, family size results in a dramatic decline in disparities; this coincides historically with an increase in the percent with large families among blacks and continued decline among whites. The characteristics impact cohort disparities in a similar manner for women.
Discussion
In order to better understand temporal changes in U.S. health, and the factors that contribute to these changes, researchers should consider incorporating the full demographic toolkit of age, period, and cohort effects into their studies of temporal population health change (e.g., Yang, 2008a, 2008b). Each of these temporal components have important theoretical meanings, which can inform studies of population health disparities. Using a repeated cross-sections of NHIS data, normalized over survey years, we were able to simultaneously explore the temporal patterns in SRH in addition to exploring the temporal patterns in SRH disparity by further decomposing the composition of cohorts to determine the individual-level factors that contributed to the observed dramatic changes in disparities over time.
Our results for aging exhibit a pattern of declining fair/poor SRH with increasing age and also show a persistent, declining racial disparity that is inconsistent with a weathering process at later ages (e.g., Geronimus, Hicken, Keene, & Bound, 2006; Walsemann et al., 2008). However, these aging results should be interpreted within the context of the cohort-centering approach. Additionally, as we later discuss, relative disparities (ratio of black to white) show a flat (men) or slight increase (women) in the age disparity, cautioning against making strong inferences based on small predicted probabilities at later ages. Our results for period indicate broad downward trends in the probability of reporting fair/poor SRH through the 1990’s followed by an increase thereafter. These trends are consistent with improvements in health through the 1980s shown in other research (Hill & Needham, 2006; Salomon et al., 2009), and consistent with research showing increases in fair/poor health during the early 1990s (Salomon et al., 2009). Although Salomon et al. (2009) find evidence that the period disparity has narrowed considerably, our relatively flat racial disparities are more consistent with Zheng et al.’s (2011) conclusion that shows no significant period variation in SRH dispersion, using APC methods. Third, our results indicate a decrease in the probability of rep orting fair/poor health after the 1915/1920 birth cohorts. In terms of disparities, among men, there is a very slight increasing cohort-based racial disparity that flattens out for most of the cohorts born in the mid-1900’s, before declining for more recent cohorts. On the other hand, women experienced a long and significant trend of increasing disparities that has only recently found its way back to pre-1910 cohort levels.
Our decomposition results also demonstrate some very interesting trends in terms of what factors are contributing to cohort disparities as well as which factors are gaining or losing importance over time. While the usual suspects of SES factors (education, income, employment) continue to demonstrate their relevance for racial disparities in health, factors such as residence in the South begin to decline in importance in more recent birth cohorts for both men and women. Of particular and emerging importance however, marital status becomes a particularly salient contributor to observed racial disparities in health among women in cohorts from 1925 onwards while employment status emerges in the 1910 cohort for men and becomes a major contributor to observed racial health disparities from 1950 onward. Although deleterious trends in marriage and employment may re-exacerbate inequalities as they continue to play out over the life course for younger cohorts, our extant results are heartening for the prospect of future cohort inequities. For both men and women, not only are recent racial disparities in health declining, but these disparities, particularly for men, are disproportionately explained by differences in average socio-demographic characteristics (modifiable factors such as educational attainment or employment).
Our analysis of cohort characteristics also highlights the critical role of early life conditions on both late-life health as well as on racial inequalities in health. We find that race-specific shifts across cohorts in terms of economic deprivation early in life explain as much as 93% of the residual cohort variance in health, and that after accounting for these early life conditions disparities are dramatically reduced. Most crucial to understanding disparities is the composition of the cohort in terms of SES/deprivation (i.e. measured as living on a farm, in the South or in a large family). Less crucial are the macro-economic conditions at birth with the exception of the two dramatic historical periods—the Great Depression and the post-WW II boom. Although we cannot sp-tnqh_0009;eak to the possible biological pathways through which these economic conditions may exert their influence, our results support previous literature with respect to the salience of economic conditions at birth.
As with all health analyses of the adult population, our results must be interpreted with caution due to potential sources of selection effects that might exclude the most frail or socioeconomically disadvantaged members of the U.S. population. Although our data are nationally representative, selective mortality excludes the least healthy from our estimates of racial health disparities, and mortality selection may disproportionately affect older cohorts. We have no way of knowing what these disparities would have looked like if mortality had not previously occurred to create a more select sub-sample (Hayward et al., 2000). Clearly, with increasing age and for the earliest cohorts, mortality selection plays a greater role. We know, based on differential mortality rates, that black and white adults do not die at the same rates and as such, our disparity estimates are likely biased by this non-random mortality. Further, the NHIS surveys the non-institutionalized population so selective migration into nursing homes and long-term care facilities further increases the potential for bias. Again, these biases will be magnified by age and within the older birth cohorts. Finally, omitting the incarcerated population, which grossly disadvantages the black population relative to the white population, may serve to underestimate racial health disparities, particularly for men. These potential sources of bias are not unique to our chosen APC approach—rather, they are endemic to all studies of population health that measure non-institutionalized populations with selective mortality. However, they are further magnified in our study when looking at especially old cohorts, and as such, patterns in the tails of our distributions should be interpreted cautiously.
Racial health disparities have been presented in the literature in two ways: predominately in terms of the relative difference (either odds ratios or a ratio of the race-specific trends) but also in terms of the absolute difference (the difference in the health of one group minus the other). Although relative measures, as dimensionless, may be preferable for understanding change over time, they may overestimate inequalities in some cases (i.e. when the level of a negative outcome is falling) and lack information on overall population health (Houweling, Kunst, Huisman, & Mackenbach, 2007; King, Harper, & Young, 2012)–necessitating absolute measures. Theoretically, absolute measures are consistent with our decomposition framework which examines the absolute difference in probabilities; however, absolute differences can be especially small and seemingly unimportant when the prevalence of the outcome is low (Houweling et al., 2007).When such measures diverge, the overreliance on one can lead to fundamentally different conclusions; thus, we also examined differences based on relative racial differences (results not shown). Our period results are similar with respect to an overall flat and uneven trend regardless of the choice of disparity measure; there is however, less difference between the age and covariate adjusted estimates when disparities are measured as relative differences compared to absolute differences. Our relative cohort disparities also show the same upward peak for cohorts born in the middle of the century as the absolute disparities; however the relative disparities are lower for the earliest cohorts not only in the covariate adjusted model, but the age-adjusted one as well. Our age results at older ages are most sensitive to the choice of disparity: the relative disparity measures show a slight increase in the disparity after age 60 for women and are relatively flat for men. This suggests that for men, the absolute difference at older ages is declining, albeit in a very small manner, but the underlying magnitude of the probabilities is relatively stagnant with black probabilities approximately 1.5 times that of whites. Synthesizing these patterns suggests that age disparities may be relatively constant at older ages, or only very modestly declining, with age for men. For women, a divergence occurs at older ages as well; absolute differences suggest a slight decline, whereas relative differences suggest a slight increase. We suggest that regardless of the preferred measure, interpreting the differences at older ages should be done with caution given the small predicted probabilities and that overall our results are robust to the choice of measure.
It is also worth noting that our cohort measures are national measures that are assumed to be consistent across context. Although national-level measures may have broad influences upon a cohort and may show significant effects, regional measures of context would be much preferred to uncover some of the national heterogeneity. Currently, there are no data sources that would pinpoint the place of birth for respondents to surveys that have similar characteristics to the IHIS that allow for the type of analysis undertaken here. Further, it is possible that macro-economic period effects may be very important sources of population health and health disparities, and these should be explored in future studies. Indeed, current place of residence is available in repeated cross-sections such as these and geo-coding techniques are now time- and cost-efficient. Point-in-time estimates may also underestimate the effects of context and repeated panel data sets may allow for a more nuanced APC approach, although data systems in the United States currently do not support such analyses.
In summary, it is clear that the concept of the cohort has important theoretical implications for population health research and has particular import for the study of racial health disparities. While the dimensions of age and period have retained seemingly exclusive rights over population health approaches, the time for the theoretical and analytical cohort approach has arrived. In order to thoroughly and accurately study temporal changes in population health and health disparities, we need to carefully begin to incorporate the concept of the cohort into our studies. Future studies should pay careful attention to the potential biases of excluding any of these temporal factors and should move beyond the descriptive aim of most such studies. We briefly explored how changing cohort composition can lead to not only differential health disparities over time, but also can have differential explanatory power over time. However, this focus on cohort composition can and should be extended to other macro-characteristics of cohorts in order to explain how temporal characteristics and social conditions and change can affect the health of the population as well as population health disparities. To summarize, future studies should explore cohort and period characteristics that may be responsible for observed temporal health disparities. An approach to population he alth change that takes the dimensions of age, period, and cohort into account should lead to a better understanding of racial health disparities.
Acknowledgment
This manuscript was supported by Award Number R01MD004025 from the National Institute on Minority Health and Health Disparities (Brian K. Finch PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Minority Health and Health Disparities or the National Institutes of Health. The authors gratefully acknowledge the programming assistance of Ms. Aimee Bower.
References
- Acevedo-Garcia D. Residential segregation and the epidemiology of infectious diseases. Social Science & Medicine. 2000;51(8):1143–1161. doi: 10.1016/s0277-9536(00)00016-2. http://dx.doi.org/10.1016/S0277-9536(00)00016-2. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Fetal nutrition and cardiovascular disease in later life. British Medical Bulletin. 1997;53:96–108. doi: 10.1093/oxfordjournals.bmb.a011609. [DOI] [PubMed] [Google Scholar]
- Bauman KJ, Graf NL. Educational Attainment: 2000. US Census Bureau; 2003. (No. C2KBR-24). Retrieved from http://www.census.gov/prod/2003pubs/c2kbr-24.pdf. [Google Scholar]
- Benjamins MR, Hummer RA, Eberstein IW, Nam CB. Self-reported health and adult mortality risk: an analysis of cause-specific mortality. Social Science & Medicine. 2004;59(6):1297–1306. doi: 10.1016/j.socscimed.2003.01.001. http://dx.doi.org/10.1016/j.socscimed.2003.01.001. [DOI] [PubMed] [Google Scholar]
- Bennett NG, Bloom DE, Craig PH. The divergence of black and white marriage patterns. American Journal of Sociology. 1989;95(3):692–722. http://dx.doi.org/10.2307/2780552. [Google Scholar]
- Benyamini Y, Idler EL. Community studies reporting association between self-rated health and mortality additional studies, 1995 to1998. Research on Aging. 1999;21(3):392–401. http://dx.doi.org/10.1177/0164027599213002. [Google Scholar]
- Browne I. Explaining the black–white gap in labor force participation among women heading households. American Sociological Review. 1997;62(2):236–252. http://dx.doi.org/10.2307/2657302. [Google Scholar]
- Bureau of Labor Statistics. Consumer price index history table. 2013 Available at ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt.
- Case A, Paxson C. Causes and consequences of early-life health. Demography. 2010;47(1):S65–S85. doi: 10.1353/dem.2010.0007. http://dx.doi.org/10.1353/dem.2010.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A. Labor-market dropouts and the racial wage gap: 1940–1990. The American Economic Review. 2000;90(2):333–338. http://dx.doi.org/10.2307/117246. [Google Scholar]
- Chen H, Cohen P, Kasen S. Cohort differences in self-rated health: evidence from a three-decade, community-based, longitudinal study of women. American Journal of Epidemiology. 2007;166(4):439–446. doi: 10.1093/aje/kwm100. http://dx.doi.org/10.1093/aje/kwm100. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proceedings of the National Academy of Science. 2006;103(2):498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Hayward MD, Seeman TE. Race/ethnicity, socioeconomic status, and health. In: Anderson NB, Bulatao RA, Cohen B, editors. Critical perspectives on racial and ethnic differences in health in late life. Washington, D.C.: National Academies Press; 2004. pp. 310–352. [PubMed] [Google Scholar]
- Do DP, Finch BK, Basurto-Davila R, Bird C, Escarce J, Lurie N. Does place explain racial health disparities? Quantifying the contribution of residential context to the black/white health gap in the United States. Social Science & Medicine (1982) 2008;67(8):1258–1268. doi: 10.1016/j.socscimed.2008.06.018. http://dx.doi.org/10.1016/j.socscimed.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do DP, France R, Finch BK. Does SES explain more of the black/white health gap than we thought? Revisiting our approach toward understanding racial disparities in health. Social Science & Medicine. 2012;74:1385–1393. doi: 10.1016/j.socscimed.2011.12.048. http://dx.doi.org/10.1016/j.socscimed.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Doblhammer G, van den Berg GJ, Fritze T. Economic conditions at the time of birth and cognitive abilities late in life: Evidence from eleven European countries; Institute for the Study of Labor (IZA) Working Paper; Germany. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110(13):1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. http://dx.doi.org/10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Easterlin RA. What will 1984 be like? Socioeconomic implications of recent twists in age structure. Demography. 1978;15(4):397–432. [PubMed] [Google Scholar]
- Easterlin RA. Birth and fortune: The impact of numbers on personal welfare. Chicago: University of Chicago Press; 1987. [Google Scholar]
- Elsby MWL, Hobijn B, Şhin A. The labor market in the great recession; Brookings Papers on Economic Activity, (Spring).2010. [Google Scholar]
- Escobedo LG, Peddicord JP. Smoking prevalence in US birth cohorts: the influence of gender and education. American Journal of Public Health. 1996;86(2):231–236. doi: 10.2105/ajph.86.2.231. http://dx.doi.org/10.2105/AJPH.86.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairlie RW. The absence of the African-American owned business: an analysis of the dynamics of self-employment. Journal of Labor Economics. 1999;17(1):80–108. http://dx.doi.org/10.1086/209914. [Google Scholar]
- Fairlie RW. An extension of the Blinder-Oaxaca decomposition technique to logit and probit models. Journal of Economic and Social Measurement. 2005;30(4):305–316. [Google Scholar]
- Ferraro KF, Farmer MM. Utility of health data from social surveys: is there a gold standard for measuring morbidity? American Sociological Review. 1999;64(2):303–315. http://dx.doi.org/10.2307/2657534. [Google Scholar]
- Ferraro KF, Farmer MM, Wybraniec JA. Health trajectories: long-term dynamics among black and white adults. Journal of Health and Social Behavior. 1997;38(1):38–54. http://dx.doi.org/10.2307/2955360. [PubMed] [Google Scholar]
- Fienberg SE, Mason WM. Identification and estimation of age-period-cohort models in the analysis of discrete archival data. In: Schuessler KF, editor. Sociological methodology. San Francisco: Jossey-Bass; 1978. pp. 1–67. [Google Scholar]
- Fienberg SE, Mason WM. Specification and implementation of age period and cohort models. In: Mason WM, Fienberg SE, editors. Cohort analysis in social research. New York: Springer; 1985. pp. 45–88. Retrieved from http://link.springer.com/chapter/10.1007/978-1-4613-8536-3_3. [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Finch BK, Frank R, Hummer RA. Racial/ethnic disparities in infant mortality: the role of behavioral factors. Biodemography and Social Biology. 2000;47(3–4):244–263. doi: 10.1080/19485565.2000.9989021. http://dx.doi.org/10.1080/19485565.2000.9989021. [DOI] [PubMed] [Google Scholar]
- Fogel RW. Changes in the disparities in chronic diseases during the course of the 20th century. Perspectives in Biology and Medicine. 2005;48(1 Suppl):S150–S165. [PubMed] [Google Scholar]
- Fuguitt GV, Fulton JA, Beale CL. The shifting patterns of black migration from and into the nonmetropolitan South. US Department of Agriculture; 2001. pp. 1965–1995. [Google Scholar]
- Gee GC, Ford CL. Structural racism and health inequalities. Du Bois Review: Social Science Research on Race. 2011;8(01):115–132. doi: 10.1017/S1742058X11000130. http://dx.doi.org/10.1017/S1742058X11000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Bound J, Keene D, Hicken M. Black-white differences in age trajectories of hypertension prevalence among adult women and men 1999–2002. Ethnicity & Disease. 2007;17(1):40–48. [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749. http://dx.doi.org/10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US Black women experience stress-related accelerated biological aging? In: Hawthorne NY, editor. Human Nature. 1. Vol. 21. 2010. pp. 19–38. http://dx.doi.org/10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper S, Lynch J, Burris S, Smith GD. Trends in the black–white life expectancy gap in the united states 1983–2003. JAMA. 2007;297(11):1224–1232. doi: 10.1001/jama.297.11.1224. http://dx.doi.org/10.1001/jama.297.11.1224. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Miles TP, Crimmins EM, Yang Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. American Sociological Review. 2000;65(6):910–930. http://dx.doi.org/10.2307/2657519. [Google Scholar]
- Heckman J, Robb R. Using longitudinal data to estimate age period and cohort effects in earnings equations. In: Mason WM, Fienberg SE, editors. Cohort analysis in social research. New York: Springer; 1985. pp. 137–150. Retrieved from http://link.springer.com/chapter/10.1007/978-1-4613-8536-3_5. [Google Scholar]
- Hill TD, Needham BL. Gender-specific trends in educational attainment and self-rated health 1972–2002. American Journal of Public Health. 2006;96(7):1288–1292. doi: 10.2105/AJPH.2004.061119. http://dx.doi.org/10.2105/AJPH.2004.061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer HJ, Offner P, Sorensen E. Declining employment among young black less-educated men: the role of incarceration and child support. Journal of Policy Analysis and Management. 2005;24(2):329–350. http://dx.doi.org/10.1002/pam.20092. [Google Scholar]
- House JS. Understanding social factors and inequalities in health 20th century progress and 21st century prospects. Journal of Health and Social Behavior. 2002;43(2):125–142. http://dx.doi.org/10.2307/3090192. [PubMed] [Google Scholar]
- House J, Williams DR. Understanding and reducing socioeconomic and racial/ethnic disparities. In: Syme SA, editor. Promoting health: Intervention strategies from social and behavioral research. Washington, DC: National Academy Press; 2000. pp. 81–124. [Google Scholar]
- Houweling TA, Kunst AE, Huisman M, Mackenbach JP. Using relative and absolute measures for monitoring health inequalities: experiences from cross-national analyses on maternal and child health. International Journal for Equity in Health. 2007;6(1):15. doi: 10.1186/1475-9276-6-15. http://dx.doi.org/10.1186/1475-9276-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoynes HW, Page ME, Stevens AH. Poverty in America: trends and explanations. The Journal of Economic Perspectives. 2006;20(1):47–68. http://dx.doi.org/10.2307/30033633. [Google Scholar]
- Huffman ML, Cohen PN. Racial wage inequality: job segregation and devaluation across U.S. labor markets. American Journal of Sociology. 2004;109(4):902–936. http://dx.doi.org/10.1086/378928. [Google Scholar]
- Hummer RA, Chinn JJ. Race/ethnicity and U.S. adult mortality. Du Bois Review: Social Science Research on Race. 2011;8(01):5–24. doi: 10.1017/S1742058X11000051. http://dx.doi.org/10.1017/S1742058X11000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler EL. Age differences in self-assessments of health: age changes, cohort differences, or survivorship? Journal of Gerontology. 1993;48(6):S289–S300. doi: 10.1093/geronj/48.6.s289. http://dx.doi.org/10.1093/geronj/48.6.S289. [DOI] [PubMed] [Google Scholar]
- Idler EL, Angel RJ. Self-rated health and mortality in the NHANES-I Epidemiologic Follow-up Study. American Journal of Public Health. 1990;80(4):446–452. doi: 10.2105/ajph.80.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38(1):21–37. http://dx.doi.org/10.2307/2955359. [PubMed] [Google Scholar]
- Jackson JS, Hudson D, Kershaw K, Mezuk B, Rafferty J, Tuttle KK. Discrimination, chronic stress, and mortality among black Americans: a life course framework. In: Rogers RG, Crimmins EM, editors. International handbook of adult mortality. Netherlands: Springer; 2011. pp. 311–328. Retrieved from http://link.springer.com/chapter/10.1007/978-90-481-9996-9_15. [Google Scholar]
- King NB, Harper S, Young ME. Use of relative and absolute effect measures in reporting health inequalities: structured review. BMJ. 2012;345:e5774. doi: 10.1136/bmj.e5774. http://dx.doi.org/10.1136/bmj.e5774 (sep 03). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh DJ, Wadsworth ME. Physical health status at 36 years in a British national birth cohort. Social Science & Medicine (1982) 1993;37(7):905–916. doi: 10.1016/0277-9536(93)90145-t. [DOI] [PubMed] [Google Scholar]
- Lantz PM, Lynch JW, House JS, Lepkowski JM, Mero RP, Musick MA, et al. Socioeconomic disparities in health change in a longitudinal study of US adults: the role of health-risk behaviors. Social Science & Medicine (1982) 2001;53(1):29–40. doi: 10.1016/s0277-9536(00)00319-1. [DOI] [PubMed] [Google Scholar]
- LaVeist TA. Beyond dummy variables and sample selection: what health services researchers ought to know about race as a variable. Health Services Research. 1994;29(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- LaVeist TA. On the study of race, racism, and health: a shift from description to explanation. International Journal of Health Services. 2000;30(1):217–219. doi: 10.2190/LKDF-UJQ5-W1KU-GLR1. http://dx.doi.org/10.2190/LKDF-UJQ5-W1KU-GLR1. [DOI] [PubMed] [Google Scholar]
- Lin S, Beck AN, Finch BK, Hummer RA, Masters RK. Trends in US older adult disability: exploring age, period, and cohort effects. American Journal of Public Health. 2012;102(11):2157–2163. doi: 10.2105/AJPH.2011.300602. http://dx.doi.org/10.2105/AJPH.2011.300602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. Journal of Health and Social Behavior. 1995;35:80–94. http://dx.doi.org/10.2307/2626958. [PubMed] [Google Scholar]
- Macinko J, Elo IT. Black–white differences in avoidable mortality in the USA, 1980–2005. Journal of Epidemiology and Community Health (1979) 2009;63(9):715–721. doi: 10.1136/jech.2008.081141. http://dx.doi.org/10.2307/20721043. [DOI] [PubMed] [Google Scholar]
- Maddison A. Statistics on World Population, GDP and Per Capita GDP, 1-2008 AD. University of Groningen; 2010. Available at www.ggdc.net/maddison/Historical_Statistics/horizontal-file_02-2010.xls. [Google Scholar]
- Manton KG, Gu X. Changes in the prevalence of chronic disability in the United States black and nonblack population above age 65 from 1982 to 1999. Proceedings of the National Academy of Science. 2001;98:6354–6359. doi: 10.1073/pnas.111152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton KG, Stallard E, Corder L. Changes in the age dependence of mortality and disability: cohort and other determinants. Demography. 1997;34(1):135–157. http://dx.doi.org/10.2307/2061664. [PubMed] [Google Scholar]
- Mason KO, Mason WM, Winsborough HH, Poole WK. Some methodological issues in cohort analysis of archival data. American Sociological Review. 1973;38(2):242–258. http://dx.doi.org/10.2307/2094398. [Google Scholar]
- Masters RK. Uncrossing the U.S. black-white mortality crossover: the role of cohort forces in life course mortality risk. Demography. 2012;49(3):773–796. doi: 10.1007/s13524-012-0107-y. http://dx.doi.org/10.1007/s13524-012-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ME, Laurenceau J-P. Gender and the natural history of self-rated health: a 59-year longitudinal study. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 2004;23(6):651–655. doi: 10.1037/0278-6133.23.6.651. http://dx.doi.org/10.1037/0278-6133.23.6.651. [DOI] [PubMed] [Google Scholar]
- McDade TW, Rutherford J, Adair L, Kuzawa CW. Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proceedings of Biological Sciences. 2010;277(1684):1129–1137. doi: 10.1098/rspb.2009.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee DL, Liao Y, Cao G, Cooper RS. Self-reported health status and mortality in a multiethnic US cohort. American Journal of Epidemiology. 1999;149(1):41–46. doi: 10.1093/oxfordjournals.aje.a009725. [DOI] [PubMed] [Google Scholar]
- Menec VH, Chipperfield JG, Perry RP. Self-perceptions of health: a prospective analysis of mortality, control, and health. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1999;54B(2):P85–P93. doi: 10.1093/geronb/54b.2.p85. http://dx.doi.org/10.1093/geronb/54B.2.P85. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21(6):848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnesota Population Center and State Health Access Data Assistance Center. Integrated Health Interview Series: Version 5.0. Minneapolis: University of Minnesota; 2012. [Google Scholar]
- Miyazaki Y, Raudenbush SW. Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychological Methods. 2000;5(1):44–63. doi: 10.1037/1082-989x.5.1.44. [DOI] [PubMed] [Google Scholar]
- Mosisa A, Hipple S. Trends in labor force participation in the United States. Monthly Labor Review. 2006 Oct;35:37–57. [Google Scholar]
- O’Brien RM. Relative cohort size and age-specific crime rates: an age-period-relative-cohort-size model. Criminology. 1989;27:57–78. [Google Scholar]
- O’Brien RM. Age period cohort characteristic models. Social Science Research. 2000;29(1):123–139. http://dx.doi.org/10.1006/ssre.1999.0656. [Google Scholar]
- O’Brien RM, Stockard J, Isaacson L. The enduring effects of cohort characteristics on age-specific homicide rates, 1960–1995. American Journal of Sociology. 1999;104(4):1061–1095. doi: 10.1086/210136. [DOI] [PubMed] [Google Scholar]
- Powers DA. Black–white differences in maternal age, maternal birth cohort, and period effects on infant mortality in the US (1983–2002) Social Science Research. 2013;42(4):1033–1045. doi: 10.1016/j.ssresearch.2013.03.005. http://dx.doi.org/10.1016/j.ssresearch.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers DA, Solis P, Frisbie WP, Hummer RA, Pullum SG. Race/ethnic differences and age-variation in the effects of birth outcomes on infant mortality in the U.S. Demographic Research. 2006;14:179–216. http://dx.doi.org/10.4054/DemRes.2006.14.10. [Google Scholar]
- Powers DA, Song SE. Absolute change in cause-specific infant mortality for blacks and whites in the US: 1983–2002. Population Research and Policy Review. 2009;28(6):817–851. http://dx.doi.org/10.1007/s11113-009-9130-0. [Google Scholar]
- Preston SH, Wang H. Sex mortality differences in the United States: the role of cohort smoking patterns. Demography. 2006;43(4):631–646. doi: 10.1353/dem.2006.0037. http://dx.doi.org/10.1353/dem.2006.0037. [DOI] [PubMed] [Google Scholar]
- Reither EN, Hauser RM, Yang Y. Do birth cohorts matter? Age–period–cohort analyses of the obesity epidemic in the United States. Social Science & Medicine. 2009;69(10):1439–1448. doi: 10.1016/j.socscimed.2009.08.040. http://dx.doi.org/10.1016/j.socscimed.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RG. Living and dying in the U.S.A.: sociodemographic determinants of death among blacks and whites. Demography. 1992;29(2):287–303. http://dx.doi.org/10.2307/2061732. [PubMed] [Google Scholar]
- Ross CE, Bird CE. Sex stratification and health lifestyle: consequences for men’s and women’s perceived health. Journal of Health and Social Behavior. 1994;35(2):161–178. http://dx.doi.org/10.2307/2137363. [PubMed] [Google Scholar]
- Ryder NB. The cohort as a concept in the study of social change. American Sociological Review. 1965;30(6):843–861. http://dx.doi.org/10.2307/2090964. [PubMed] [Google Scholar]
- Salomon JA, Nordhagen S, Oza S, Murray CJL. Are Americans feeling less healthy? The puzzle of trends in self-rated health. American Journal of Epidemiology. 2009;170(3):343–351. doi: 10.1093/aje/kwp144. http://dx.doi.org/10.1093/aje/kwp144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittker J, Massoglia M, Uggen C. Incarceration and the health of the African American community. Du Bois Review: Social Science Research on Race. 2011;8(01):133–141. http://dx.doi.org/10.1017/S1742058X11000026. [Google Scholar]
- Singh GK, Yu SM. Infant mortality in the United States: trends, differentials, and projections, 1950 through 2010. American Journal of Public Health. 1995;85(7):957–964. doi: 10.2105/ajph.85.7.957. http://dx.doi.org/10.2105/AJPH.85.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small ML, Newman K. Urban poverty after the truly disadvantaged: the rediscovery of the family, the neighborhood, and culture. Annual Review of Sociology. 2001;27:23–45. http://dx.doi.org/10.2307/2678613. [Google Scholar]
- Sternthal MJ, Slopen N, Williams DR. Racial disparities in health. Du Bois Review: Social Science Research on Race. 2011;8(01):95–113. doi: 10.1017/S1742058X11000087. http://dx.doi.org/10.1017/S1742058X11000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg GJ, Doblhammer G, Christensen K. Exogenous determinants of early-life conditions, and mortality later in life. Social Science & Medicine. 2009;68(9):1591–1598. doi: 10.1016/j.socscimed.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Van Den Berg GJ, Lindeboom M, Portrait F. Economic conditions early in life and individual mortality. American Economic Review. 2006;96(1):290–302. doi: 10.1257/000282806776157740. [DOI] [PubMed] [Google Scholar]
- Waite LJ. Does marriage matter? Demography. 1995;32(4):483–507. http://dx.doi.org/10.2307/2061670. [PubMed] [Google Scholar]
- Walsemann KM, Geronimus AT, Gee GC. Accumulating disadvantage over the life course evidence from a longitudinal study investigating the relationship between educational advantage in youth and health in middle age. Research on Aging. 2008;30(2):169–199. http://dx.doi.org/10.1177/0164027507311149. [Google Scholar]
- Walters PB. Educational access and the state: historical continuities and discontinuities in racial inequality in American education. Sociology of Education. 2001;74:35–49. http://dx.doi.org/10.2307/2673252. [Google Scholar]
- Welch F. Effects of cohort size on earnings: baby boom babies’ financial bust. Journal of Political Economy. 1979;87(5):S65–S97. [Google Scholar]
- Welch F. The employment of black men. Journal of Labor Economics. 1990;8(1):S26–S74. http://dx.doi.org/10.2307/2535207. [Google Scholar]
- Williams DR. The health of U.S. racial and ethnic populations. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005:53–62. doi: 10.1093/geronb/60.special_issue_2.s53. 60 Spec No 2. [DOI] [PubMed] [Google Scholar]
- Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Reports. 2001;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. American Journal of Public Health. 2003;93(2):200–208. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Sternthal M. Understanding Racial/ethnic disparities in health: sociological contributions. Journal of Health and Social Behavior. 2010;51(Suppl):S15–S27. doi: 10.1177/0022146510383838. http://dx.doi.org/10.1177/0022146510383838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson AE, Shuey KM, Elder GH. Cumulative advantage processes as mechanisms of inequality in life course health. American Journal of Sociology. 2007;112(6):1886–1924. http://dx.doi.org/10.1086/512712. [Google Scholar]
- 100.Yang Y. Trends in U.S. adult chronic disease mortality, 1960–1999: age, period, and cohort variations. Demography. 2008a;45(2):387–416. doi: 10.1353/dem.0.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Y. Encyclopedia of the life course and human development. New York: Gale Publishing; 2008b. Age, period, cohort effects. [Google Scholar]
- 102.Yang Y, Fu WJ, Land KC. A methodological comparison of age-period-cohort models: the intrinsic estimator and conventional generalized linear models. Sociological Methodology. 2004;34(1):75–110. http://dx.doi.org/10.1111/j.0081-1750.2004.00148.x. [Google Scholar]
- 103.Yang Y, Land KC. A mixed models approach to the age-period-cohort analysis of repeated cross-section surveys, with an application to data on trends in verbal test scores. Sociological Methodology. 2006;36(36):75–97. [Google Scholar]
- 104.Yang Y, Land KC. Age-period-cohort analysis of repeated cross-section surveys: fixed or random effects? Sociological Methods & Research. 2008;36(3):297–326. [Google Scholar]
- 105.Yang Y, Land KC. Age-period-cohort analysis: New models, methods, and empirical applications. Boca Raton, FL: CRC Press; 2013. [Google Scholar]
- 106.Yang Y, Lee LC. Sex and race disparities in health: cohort variations in life course patterns. Social Forces. 2009;87(4):2093–2124. http://dx.doi.org/10.1353/sof.0.0183. [Google Scholar]
- 107.Yao L, Robert SA. The contributions of race, individual socioeconomic status, and neighborhood socioeconomic context on the self-rated health trajectories and mortality or older adults. Research on Aging. 2008;30(2):251–273. http://dx.doi.org/10.1177/0164027507311155. [Google Scholar]
- 108.Zack MM, Moriarty DG, Stroup DF, Ford ES, Mokdad AH. Worsening trends in adult health-related quality of life and self-rated health: United States, 1993–2001. Public Health Reports (1974) 2004;119(5):493–505. doi: 10.1016/j.phr.2004.07.007. http://dx.doi.org/10.2307/20056716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng H, Yang Y, Land KC. Variance function regression in hierarchical age–period–cohort models: applications to the study of self-reported health. American Sociological Review. 2011;76(6):955–983. doi: 10.1177/0003122411430940. http://dx.doi.org/10.1177/0003122411430940. [DOI] [PMC free article] [PubMed] [Google Scholar]