Abstract
Plants have the ability to detect invading fungi through the perception of chitin fragments released from the fungal cell walls. Plant chitin receptor consists of two types of plasma membrane proteins, CEBiP and CERK1. However, the contribution of these proteins to chitin signaling is different between Arabidopsis and rice. In Arabidopsis, it seems CERK1 receptor kinase is enough for both ligand perception and signaling, whereas both CEBiP and OsCERK1 are required for chitin signaling in rice. Here we report that Arabidopsis CEBiP homolog, LYM2, is not involved in chitin signaling but contributes to resistance against a fungal pathogen, Alternaria brassicicola, indicating the presence of a novel disease resistance mechanism in Arabidopsis.
Keywords: chitin, receptor, disease resistance, Arabidopsis, Alternaria brassicicola
Detection of invading pathogens through the perception of microbe-associated molecular patterns (MAMPs) by corresponding pattern recognition receptors is an important basis of plant immune system.1,2 Chitin is a representative fungal molecular pattern and its recognition by plant chitin receptor triggers various defense responses in plants.3,4 Both of the infection experiments with the knockout mutants of plant chitin receptors,5,6 as well as the functional studies on fungal effectors that inhibit the perception of chitin oligosaccharides7,8 indicated that the chitin-triggered immunity plays an important role to protect plants from the invasion of fungal pathogens.
So far, two types of lysin motif (LysM)-containing proteins have been identified as the components of cell surface chitin receptor in plants. CEBiP, a receptor-like protein, was identified biochemically as a major chitin oligosaccharide binding protein in the plasma membrane of rice.9 On the other hand, CERK1, a receptor-like kinase, was identified genetically as an essential molecule for chitin signaling in Arabidopsis.5 We recently showed that the rice chitin receptor system requires both CEBiP and OsCERK1 for chitin perception and signaling,10 whereas Arabidopsis does not require CEBiP-like molecule for chitin perception and CERK1 seems sufficient both for chitin perception and membrane signaling.11
There are three closely related CEBiP homologs in Arabidopsis, LYM1-3, LYM1 and LYM3 bind peptidoglycan and constitute peptidoglycan receptor in combination with CERK1.12 Although another CEBiP homolog, LYM2/AtCEBiP, specifically binds chitin oligosaccharides as similar to rice CEBiP, neither the knockout of LYM2 nor all of LYM1-3 affected chitin signaling.11,13 Interestingly, Arabidopsis CERK1 was shown to bind chitin but rice OsCERK1 was not. Thus, it was concluded that CERK1 serves both for chitin perception and membrane signaling in Arabidopsis.11
If so, what can be the function of LYM2, which is biochemically very similar to rice CEBiP but does not contribute to CERK1 mediated chitin signaling? Is it a useless molecule left behind the evolution? Here we show that LYM2 does contribute to disease resistance against fungal pathogens but the mechanism seems independent of chitin signaling mediated by CERK1.
First we compared the disease resistance of a triple mutant for all three LYM proteins, lym1/lym2/lym3, which could respond to chitin oligosaccharide as similar to wild type Col-0 (Fig. 1 and ref. 11), against a fungal pathogen, Alternaria brassicicola (Fig. 2A). Interestingly, the triple mutant showed an increased susceptibility to the pathogen as similar to cerk1 mutant. As the chitin-induced defense responses were completely suppressed in the cerk1 mutant,5 the increased susceptibility of cerk1 mutant against A. brassicicola can be interpreted as the results of the impairment of chitin signaling. On the other hand, as the triple mutant was shown to respond to chitin oligosaccharides normally and CERK1 remained intact in this mutant,11 the results suggested that a resistance mechanism other than CERK1-dependent, chitin-triggered immunity was impaired in this mutant.
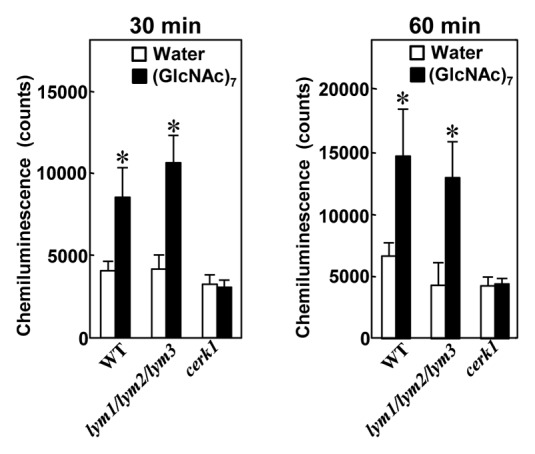
Figure 1. Triple mutant lym1/lym2/lym3 normally responds to chitin oligosaccharide and generates reactive oxygen species. The leaf discs from 7-wk-old Arabidopsis plant were pre-incubated overnight in fresh MGRL medium containing 1% sucrose in a 48-well microtiter plate.11 The medium was replaced with fresh MGRL medium at 2 h before the (GlcNAc)7 or water treatment. After the elicitor treatments, the ROS released by Arabidopsis leaves were quantified by chemiluminescence method with luminol. ROS generation was represented as accumulation for 30 min (left) and 60 min (right) after the treatment of water or 50 μg/ml (GlcNAc)7. Data are shown as means of six leaf discs ± SD. The asterisks indicate statistical significance from the water controls by Student’s t test (p < 0.01). The experiment was repeated twice with the similar results.
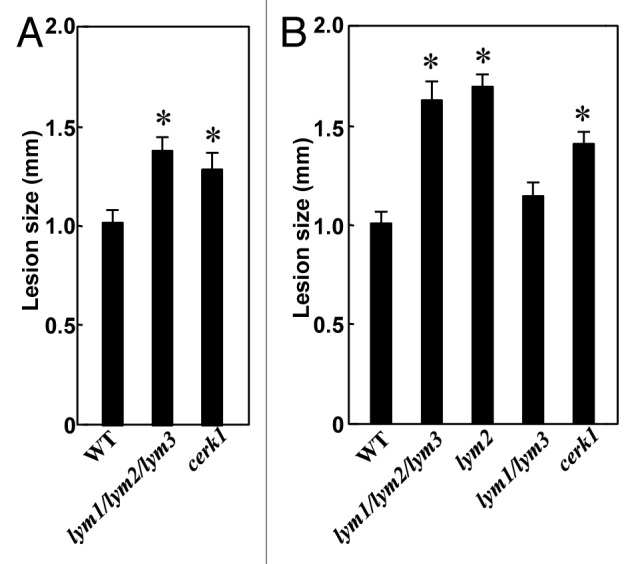
Figure 2. Disease resistance of LYM-protein mutants against Alternaria brassicicola. LYM protein mutants, lym2, lym1/lym3 and lym1/lym2/lym3 (all in Col-0 background), were obtained as described previously.11Arabidopsis plants were grown in soil for 28 d in a growth chamber at 22°C under a 12 h light-dark cycle. Plants were inoculated by spotting 5 μl of a conidial suspension (5 × 105 conidia ml−1 in distilled water) of Alternaria brassicicola (isolate O-264) on each leaf.15 Inoculated plants were then placed in a growth chamber at 22°C with a 12 h light-dark cycle and maintained at 100% relative humidity. Control plants were treated with only distilled water. The size of the lesions was measured at 6 d after inoculation. Lesion development in (A) cerk1, lym1/lym2/lym3 and Col-0 and (B) lym1/lym2/lym3, lym2, lym1/lym3, cerk1 and Col-0 leaves after the inoculation of A. brassicicola was shown. Data shown represent mean ± SE (n > 47). The asterisks indicate statistical significance from the WT controls by Student’s t-test (p < 0.02). This experiment was repeated two times with similar results.
To further analyze the mechanism of such a disease resistance, we compared the resistance of different LYM-protein mutants against A. brassicicola (Fig. 2B). Interestingly again, a single knockout mutant, lym2 showed an increased susceptibility against A. brassicicola, which is comparable to the triple mutant, lym1/lym2/lym3. On the other hand, the double knockout mutant, lym1/lym3, did not show such increase of susceptibility compared with the wild type Col-0.
Considering the differences in the binding specificity of LYM2 and LYM1/3, chitin or peptidoglycan, these results suggest that LYM2 contributes to the disease resistance against A. brassicicola through the perception of chitin. However, at the same time, the fact that the lym2 and the triple mutant were not impaired for the chitin-triggered defense responses such as ROS generation and defense gene expression11,13 indicated that the mechanism of such a disease resistance should be different from the chitin-triggered immunity so far reported.
In conclusion, present study clearly indicated the presence of LYM2 dependent but CERK1 independent disease resistance against fungal pathogens in Arabidopsis. It is difficult to imagine how such a mechanism works at present but the clarification of such a novel disease resistance mechanism will expand our understanding on plant immune system, especially on the significance of chitin perception as a trigger of the battle against invading fungal pathogens.
Acknowledgments
We thank Dr Hanae Kaku for useful discussions. This work was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 22248041 to NS, No. 24780334 to TS and No. 24580071 to YN).
Glossary
Abbreviations:
- ROS
reactive oxygen species
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Note
After we completed the manuscript, Faulkner et al. reported that LYM2 contributes to the regulation of molecular flux via plasmodesmata and contributes to disease resistance against a necrotic fungus, Botrytis cinerea, independently of CERK1.14 This observation completely matches with our finding reported here.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25345
References
- 1.Boller T, He SY. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324:742–4. doi: 10.1126/science.1171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11:539–48. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 3.Shibuya N, Minami E. Oligosaccharide signalling for defense responses in plant. Physiol Mol Plant Pathol. 2001;59:223–33. doi: 10.1006/pmpp.2001.0364. [DOI] [Google Scholar]
- 4.Silipo A, Erbs G, Shinya T, Dow JM, Parrilli M, Lanzetta R, et al. Glyco-conjugates as elicitors or suppressors of plant innate immunity. Glycobiology. 2010;20:406–19. doi: 10.1093/glycob/cwp201. [DOI] [PubMed] [Google Scholar]
- 5.Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:19613–8. doi: 10.1073/pnas.0705147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, et al. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell. 2008;20:471–81. doi: 10.1105/tpc.107.056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jonge R, van Esse HP, Kombrink A, Shinya T, Desaki Y, Bours R, et al. Conserved fungal LysM effector Ecp6 prevents chitin-triggered immunity in plants. Science. 2010;329:953–5. doi: 10.1126/science.1190859. [DOI] [PubMed] [Google Scholar]
- 8.Mentlak TA, Kombrink A, Shinya T, Ryder LS, Otomo I, Saitoh H, et al. Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell. 2012;24:322–35. doi: 10.1105/tpc.111.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, et al. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci USA. 2006;103:11086–91. doi: 10.1073/pnas.0508882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu T, Nakano T, Takamizawa D, Desaki Y, Ishii-Minami N, Nishizawa Y, et al. Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J. 2010;64:204–14. doi: 10.1111/j.1365-313X.2010.04324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinya T, Motoyama N, Ikeda A, Wada M, Kamiya K, Hayafune M, et al. Functional characterization of CEBiP and CERK1 homologs in arabidopsis and rice reveals the presence of different chitin receptor systems in plants. Plant Cell Physiol. 2012;53:1696–706. doi: 10.1093/pcp/pcs113. [DOI] [PubMed] [Google Scholar]
- 12.Willmann R, Lajunen HM, Erbs G, Newman MA, Kolb D, Tsuda K, et al. Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc Natl Acad Sci USA. 2011;108:19824–9. doi: 10.1073/pnas.1112862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan J, Tanaka K, Zhang XC, Son GH, Brechenmacher L, Nguyen TH, et al. LYK4, a lysin motif receptor-like kinase, is important for chitin signaling and plant innate immunity in Arabidopsis. Plant Physiol. 2012;160:396–406. doi: 10.1104/pp.112.201699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faulkner C, Petutschnig E, Benitez-Alfonso Y, Beck M, Robatzek S, Lipka V, et al. LYM2-dependent chitin perception limits molecular flux via plasmodesmata. Proc Natl Acad Sci USA. 2013;110:9166–70. doi: 10.1073/pnas.1203458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narusaka Y, Narusaka M, Seki M, Ishida J, Shinozaki K, Nan Y, et al. Cytological and molecular analyses of non-host resistance of Arabidopsis thaliana to Alternaria alternata. Mol Plant Pathol. 2005;6:615–27. doi: 10.1111/j.1364-3703.2005.00310.x. [DOI] [PubMed] [Google Scholar]


