Abstract
The retromer is an endosome-localized complex involved in protein trafficking. To better understand its function and regulation in plants, we recently investigated how Arabidopsis retromer subunits assemble and are targeted to endosomal membranes and highlighted original features compared with mammals. We characterized Arabidopsis vps26 null mutant and showed that it displays severe developmental defaults similar to those observed in vps29 mutant. Here, we go further by describing new phenotypic defects associated with loss of VPS26 function, such as inhibition of lateral root initiation. Recently, we showed that VPS35 subunit plays a crucial role in the recruitment of the plant retromer to endosomes, probably through an interaction with the Rab7 homolog RABG3f. In this work, we now show that contrary to mammals, Arabidopsis Rab5 homologs do not seem to be necessary for the recruitment of the core retromer to endosomal membranes, which highlights a new specificity of the plant retromer.
Keywords: retromer, Arabidopsis thaliana, endosomes, Rab GTPases, plant development
The retromer is a multiprotein complex involved in the intracellular trafficking of cargo proteins. In mammals, it is composed of two subcomplexes, the core retromer constituted by the Vacuolar Protein Sorting (VPS) 26, VPS29, VPS35 and a dimer of Sorting Nexin (SNX). These different components can be cytosolic or peripherally associated with endosomal membranes. Although VPS proteins do not exhibit lipid binding domains,1,2 association of the core retromer with endosomal membranes is achieved through a SNX dimer, which interacts with phosphoinositides (PIPs).3 In Arabidopsis thaliana, all members of the retromer are conserved, with two VPS26 isoforms (VPS26a and VPS26b), one VPS29 protein, three VPS35 isoforms (VPS35a, VPS35b and VPS35c) and three SNX proteins named SNX1, SNX2a and SNX2b. However, how the plant retromer assembles and functions remains largely unknown. In a previous work, we investigated the function of the plant retromer by studying the vps29 null mutant that displays a non-functional retromer. We showed that VPS29 is required for endosome homeostasis, organogenesis and proper cycling and localization of the auxin efflux carrier PIN1.4 We further demonstrated that SNXs are dispensable for membrane binding and function of the core retromer in Arabidopsis, revealing some unique features of the plant retromer compared with its mammalian or yeast counterparts.5
In a recent publication, we investigated how plant retromer components are targeted to the endosomal membrane and physically associate to form a functional complex, highlighting again new particularities of the plant retromer.6 To address whether the different Arabidopsis VPS subunits function in the same developmental pathways, we characterized for the first time a vps26a vps26b double mutant and compared its developmental phenotypes to those of vps29. Impaired VPS26 function causes important phenotypic defects similar to those observed in vps29 null mutants such as dwarfism.6 In addition, we report here that vps26a vps26b double null mutant displays severe inhibition of lateral root initiation (Fig. 1A), probably due to alteration of auxin transport as previously observed for vps29.4 We showed that maturation of the major seed storage proteins of Arabidopsis was altered in vps26 double mutant,6 as described in vps29 and vps35a vps35c mutants.7,8 A previous study reported that the Vacuolar Sorting Receptor 1 (VSR1) was involved in the transport of seed storage proteins to the vacuoles9 and that a truncated form of VSR1, whose role remains unknown, was found in wild-type seeds but not in vps29 and vps35 mutants.7 Interestingly, we similarly detected the truncated VSR1 to be missing in vps26a vps26b homozygous seeds after immunoblotting analysis using a specific anti-VSR1 antibody (Fig. 1B). Altogether, our results show that VPS26, VPS29 and VPS35 are involved in the same physiological processes and we assume that the corresponding proteins function exclusively as a multiprotein complex in plants.
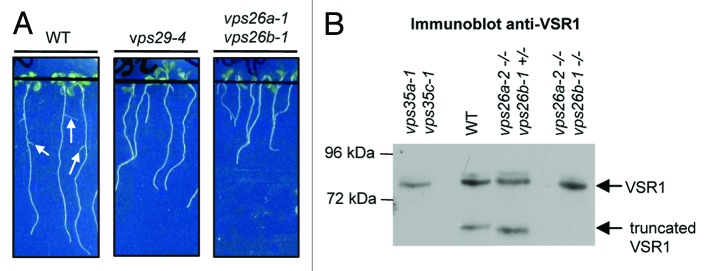
Figure 1.Vps26a vps26b double mutant displays severe inhibition of lateral root initiation and does not accumulate a truncated form of the Vacuolar Sorting Receptor 1 (VSR1). (A) Root phenotype of 10-d-old seedlings from wild-type (WT), vps29-4 single and vps26a-1 vps26b-1 double mutant plants. Arrows indicate lateral roots. (B) Total protein extracts from wild-type (WT), vps35a vps35c, vps26a-2−/− vps26b-1+/− and vps26a-2−/− vps26b-1−/− mutant seeds were analyzed by immunoblotting for the expression of VSR1 and its truncated form. Molecular mass markers are indicated on the left in kDa.
Recently, we observed that the strength of the phenotypes of different retromer mutants was correlated with the amount of VPS35 protein in seedlings and showed that VPS35 associates with endosomes independently of VPS26 and VPS29.6 Therefore, contrary to mammals where VPS26 plays a major role in the recruitment of the core retromer to endosomal membranes,10 VPS35 appears as the main component responsible for this mechanism in plants. On the other hand, some actors involved in the association of the retromer with membranes seem to be evolutionarily conserved between plants and mammals. Indeed, we showed that the Arabidopsis Rab7 homolog RABG3f interacted with VPS35 and likely participated in the recruitment of the core retromer to the surface of endosomes,6 as previously reported for Rab7 in mammals.11,12 Interestingly, the mammalian GTPase Rab5 has also been demonstrated to indirectly contribute to the recruitment of retromer to membranes.11 Indeed, overexpression of a dominant negative form of Rab5 results in VPS26 and SNX1 dissociation from endosomes. Because Rab5 does not interact with retromer, the authors hypothesized that this GTPase, by promoting phosphatidylinositol 3-phosphate (PI3P) formation on endosomal membranes, allows the recruitment of SNXs to the endosomal surface, which in turn, participates in the recruitment of the core retromer. Arabidopsis thaliana has three Rab5 homologs: ARA6/RABF1, ARA7/RABF2b and RHA1/RABF2a,13 which are specifically activated by the action of a guanine nucleotide exchange factor (GEF) named VPS9a.14 While vps9a-1 null mutant is embryo lethal, a weaker mutant allele, vps9a-2, displays defects in the elongation of the primary root that result from faulty activation of Rab5 homologs.14 Here, to investigate whether plant Rab5 homologs are necessary for the recruitment of the core retromer to endosomal membranes, we took advantage of vps9a-2 mutant and analyzed by cell fractionation and western blotting the distribution of VPS35a in wild type and vps9a-2 mutant backgrounds. We found that VPS35a was similarly present in the membrane fractions of wild-type and vps9a-2 mutant plants (Fig. 2), suggesting that the activation of Arabidopsis Rab5 homologs is not required for the recruitment of the VPS retromer subcomplex to membranes. This result highlights a new specificity of the plant retromer compared with its mammalian counterpart. Assuming that the role of Rab5 homologs in PI3P production is conserved in plants, this result emphasizes that SNX proteins, which interact with PI3P, are not essential for the recruitment of the core retromer to membranes in plants, as previously proposed in our group.5 Indeed, it appears that the core retromer can work independently of SNXs in protein trafficking and plant development. In mammals, although it has long been thought that the retromer functioned solely as a SNX dimer-core retromer complex, there are now growing evidence showing that cargo proteins and cellular processes can use only one of the retromer subcomplexes without requiring the function of the other.15-17
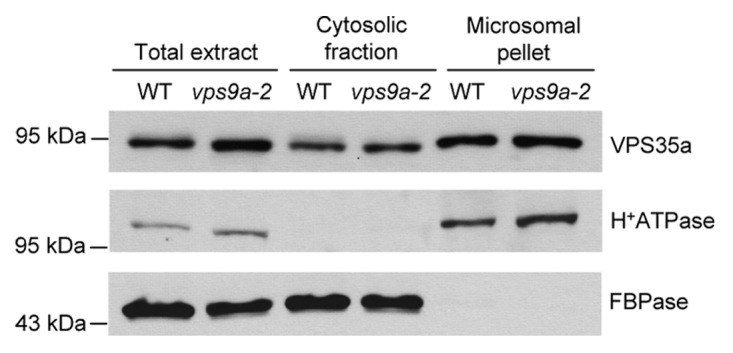
Figure 2. Activation of Rab5 homologs is not required for the recruitment of VPS35 to endosomal membranes in Arabidopsis. Immunoblot analysis on total, cytosolic and microsomal proteins from wild-type (WT) and vps9a-2 seedlings using anti-VPS35a, anti-H+-ATPase and anti-cytosolic fructose-1,6-bisphosphatase (FBPase) antibodies. Cell fractionation was performed as previously described6 and H+-ATPase and FBPase were used as markers of membrane and cytosolic fractions, respectively. Molecular mass markers are indicated on the left in kDa.
To conclude, although some aspects of plant retromer assembly and functioning appear to be evolutionarily conserved, it is now evident that plants have developed unique mechanisms to regulate this complex, which constitutes an exciting field of investigation for the future.
Acknowledgments
We thank Professor I. Hara-Nishimura for the gift of anti-VPS35a and anti-VSR1 antibodies and for vps35a-1 vps35c-1 seeds and Professor M. Boutry for the gift of anti H+-ATPase antibodies. This work was supported by the Agence Nationale de la Recherche BLANC RETROMER project, grant N°ANR-08-BLAN-0142.
Glossary
Abbreviations:
- VPS
vacuolar protein sorting
- SNX
sorting nexin
- PIP
phosphoinositide
- VSR1
vacuolar sorting receptor 1
- GEF
guanine nucleotide exchange factor
- PI3P
phosphatidylinositol 3-phosphate
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25312
References
- 1.Collins BM, Skinner CF, Watson PJ, Seaman MN, Owen DJ. Vps29 has a phosphoesterase fold that acts as a protein interaction scaffold for retromer assembly. Nat Struct Mol Biol. 2005;12:594–602. doi: 10.1038/nsmb954. [DOI] [PubMed] [Google Scholar]
- 2.Shi H, Rojas R, Bonifacino JS, Hurley JH. The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat Struct Mol Biol. 2006;13:540–8. doi: 10.1038/nsmb1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rojas R, Kametaka S, Haft CR, Bonifacino JS. Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol Cell Biol. 2007;27:1112–24. doi: 10.1128/MCB.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miège C, Gaude T. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell. 2007;130:1057–70. doi: 10.1016/j.cell.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Pourcher M, Santambrogio M, Thazar N, Thierry AM, Fobis-Loisy I, Miège C, et al. Analyses of sorting nexins reveal distinct retromer-subcomplex functions in development and protein sorting in Arabidopsis thaliana. Plant Cell. 2010;22:3980–91. doi: 10.1105/tpc.110.078451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelazny E, Santambrogio M, Pourcher M, Chambrier P, Berne-Dedieu A, Fobis-Loisy I, et al. Mechanisms governing the endosomal membrane recruitment of the core retromer in Arabidopsis. J Biol Chem. 2013;288:8815–25. doi: 10.1074/jbc.M112.440503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki M, Shimada T, Takahashi H, Tamura K, Kondo M, Nishimura M, et al. Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol. 2007;49:142–56. doi: 10.1093/pcp/pcn006. [DOI] [PubMed] [Google Scholar]
- 8.Shimada T, Koumoto Y, Li L, Yamazaki M, Kondo M, Nishimura M, et al. AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol. 2006;47:1187–94. doi: 10.1093/pcp/pcj103. [DOI] [PubMed] [Google Scholar]
- 9.Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I. Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2003;100:16095–100. doi: 10.1073/pnas.2530568100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokool S, Tattersall D, Reddy JV, Seaman MN. Identification of a conserved motif required for Vps35p/Vps26p interaction and assembly of the retromer complex. Biochem J. 2007;408:287–95. doi: 10.1042/BJ20070555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–26. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci. 2009;122:2371–82. doi: 10.1242/jcs.048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueda T, Uemura T, Sato MH, Nakano A. Functional differentiation of endosomes in Arabidopsis cells. Plant J. 2004;40:783–9. doi: 10.1111/j.1365-313X.2004.02249.x. [DOI] [PubMed] [Google Scholar]
- 14.Goh T, Uchida W, Arakawa S, Ito E, Dainobu T, Ebine K, et al. VPS9a, the common activator for two distinct types of Rab5 GTPases, is essential for the development of Arabidopsis thaliana. Plant Cell. 2007;19:3504–15. doi: 10.1105/tpc.107.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nisar S, Kelly E, Cullen PJ, Mundell SJ. Regulation of P2Y1 receptor traffic by sorting Nexin 1 is retromer independent. Traffic. 2010;11:508–19. doi: 10.1111/j.1600-0854.2010.01035.x. [DOI] [PubMed] [Google Scholar]
- 16.Prosser DC, Tran D, Schooley A, Wendland B, Ngsee JK. A novel, retromer-independent role for sorting nexins 1 and 2 in RhoG-dependent membrane remodeling. Traffic. 2010;11:1347–62. doi: 10.1111/j.1600-0854.2010.01100.x. [DOI] [PubMed] [Google Scholar]
- 17.Seaman MN. The retromer complex - endosomal protein recycling and beyond. J Cell Sci. 2012;125:4693–702. doi: 10.1242/jcs.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]


