Abstract
The plant cell wall, a dynamic network of polysaccharides and glycoproteins of significant compositional and structural complexity, functions in plant growth, development and stress responses. In recent years, the existence of plant cell wall integrity (CWI) maintenance mechanisms has been demonstrated, but little is known about the signaling pathways involved, or their components. Examination of key mutants has shed light on the relationships between cell wall remodeling and plant cell responses, indicating a central role for the regulatory network that monitors and controls cell wall performance and integrity. In this review, we present a short overview of cell wall composition and discuss post-synthetic cell wall modification as a valuable approach for studying CWI perception and signaling pathways.
Keywords: cell wall, cell wall integrity, plant growth and development, cell wall signaling plant defense
Plant Cell Wall Composition and Functions
The plant cell wall is a unique, complex molecular network of different polysaccharides with a composition and structure that differs between plant species and can be modified during plant development and in response to stress.1 The flexible and expandable primary cell wall consists of cellulosic microfibrils (1,4-β-D-glucan), hemicellulosic polysaccharides such as β-(1-3,1-4)-glucan, xyloglucan (XG), xylans, mannans and pectic polysaccharides such as homogalacturonan (HG), xylogalacturonan (XGA) and rhamnogalacturonans I and II (RGI and RGII).2,3 In addition, the primary wall contains different types of glycoproteins.4 In contrast to the primary cell wall, the secondary cell wall contains strengthening cross-linked racemic lignin macromolecules and lower amounts of pectins and xyloglucan.5 Most plant cell wall polymers, except cellulose and some glucans, can be substituted with acetyl groups6 and HG and glucuronoxylan can also be methyl-esterified.7,8
The modern view of the plant cell wall is of a dynamic extracellular complex that responds to external or internal cellular signals and forms a continuum with the plasma membrane and cytoskeleton.9 In addition to well established cell wall functions in maintaining and determining cell shape,10,11 resisting internal turgor pressure,12 controlling and directing cell and plant growth,13 contributing to plant morphology14 and regulating diffusion through the apoplast, the cell wall also has recently revealed signaling functions. In particular, mechanisms involved in cell to cell communication,15 cell wall integrity (CWI) maintenance,16-18 as well as perception and signaling in plant development and defense,19-23 have attracted significant interest.
Expression of Cell Wall Enzymes or Their Inhibitors Cause Post-Synthetic Cell Wall Modifications that Affect Plant Growth and Development
Plant cell wall polysaccharide metabolism is regulated by the balance between biosynthesis and degradation; a shift in this balance can lead to dramatic changes in cell wall structure, function and, consequently, in plant growth.16 More than 2,000 genes are predicted to be involved in these processes.24,25 Although significant progress has been made in functional characterization of these genes, using forward and reverse genetics,26-30 these studies are far from complete.
CWI can be affected either by manipulation of biosynthetic pathways or through post-synthetic modification of cell wall structure or composition. When biosynthesis of polysaccharides is disrupted by knocking down or mutating synthetic enzymes, plants react to this modification by activating compensatory mechanisms leading to the alteration of other cell wall components. For example, some mutations in cellulose synthase encoding genes (CesA) lead to increased lignin production31,32 or cause embryo-lethality.33-35 Mutations in pectin-related genes cause a dwarf phenotype and affect cellular adhesion.36 Reduction of fucose in arabinogalactan, a cell wall specific protein, affected root cell elongation.37
Plants themselves express a broad variety of cell wall modifying enzymes (CWMEs) in the apoplast to maintain proper wall plasticity and flexibility and to assist in wall remodeling and degradation during growth and development.38 In addition, CWMEs produced by microorganisms during host invasion cause cell wall modifications that can initiate plant defense responses. Thus, heterologous expression of CWMEs can lead to a broad range of effects that could potentially mimic the plant’s responses to endogenous or external CWMEs.
For example, targeted modification of pectin structure and integrity by the overexpression of pectin modifying enzymes can provide insight into the role of different pectic polysaccharides in plant growth. Expression of rhamnogalacturonan lyase (eRGL) from Aspergillus aculeatus in potato plants resulted in a degradation of the RGI backbone and a decrease in both galactan and arabinan side-chains, leading to wrinkled tubers.39 The specific removal of RGI arabinan side-chains resulting from the expression in the apoplast of an endo-1,5-α-arabinanase of A. aculeatus caused the transgenic potato plants to have severe phenotypic changes including the inability to produce tubers.40 By contrast, the overexpression of a fungal (A. aculeatus) endo-galactanase in potato caused a reduction of galactan side-chains of RGI with a compensatory increase of uronic acids, without affecting tuber development.41 Although RGI galactan and arabinan side-chains have an, as yet unclear, role in planta, the RGI backbone must play an important role in the integrity and function of the cell wall, since its degradation by expression of hydrolases results in morphological changes.
Overexpression of an endopolygalacturonase (AnPGII) of Aspergillus niger in tobacco caused a significant reduction of pectic homogalacturonan partially compensated by an increase in rhamnose, galactose and arabinose in RGI; the resulting transgenic tobacco lines are drastically dwarfed,42 indicating homogalacturonan integrity is a critical factor in plant growth.
Acetyl content depends on the plant organ and developmental stage43 and acetylation of plant cell wall polysaccharides also plays an important role in plant development. De-acetylation of pectins resulting from overexpression of mung bean acetylesterase in potato increased the mechanical strength of tubers due to a decrease in pectin-related cell wall elasticity. In these plants, the altered pectin acetylation can affect the interaction between pectin and cellulose chains.44 The overexpression of pectin acetylesterase 1 (PtPAE1) from cottonwood (Populus trichocarpa) in tobacco resulted in a reduction in acetyl groups of pectin but not in xylan and affected cell expansion and plant organ growth direction as well as altered pollen germination and, consequently, plant reproduction.45 In addition, expression of genes involved in cell wall acetylation was shown to be associated with secondary wall thickening.46
Methylation of cell wall polysaccharides can be involved in growth regulation and polysaccharide accessibility to glycosyl hydrolases.47,48 Arabidopsis plants overexpressing the endogenous PME3 exhibited a dramatic increase in PME activity and significantly longer roots; a pme3 knockout mutant showed the opposite phenotype.49 In aspen trees (Populus tremula ssp. tremuloides), upregulation of PtPME1 inhibited the apical elongation of wood fibers.50 In addition to transcriptional control, PME activity is regulated by endogenous inhibitor proteins (PMEIs), which were discovered in kiwi fruit and subsequently identified in Arabidopsis, pepper, broccoli, wheat and tomato.51-59 PMEIs function in apical meristem development, cell growth acceleration and pollen tube growth.47,53,54,60 Also, overexpression of AtPMEI in Arabidopsis significantly increases the level of pectin methyesterification and improves leaf and root growth.61,62 Methylation of xylan alters lignin structure but does not affects plant growth and wall degradability by cellulases.8,63
Post-Synthetic Modifications of the Cell Wall Affect CWI and Plant Disease Resistance
Pathogenesis involves plant cell wall alteration by CWMEs targeted to wall polysaccharides. It is well established that the plant susceptibility to pathogens depends on the cell wall composition and structure, which determine its recalcitrance to degradation by CWMEs produced by pathogens.64 Moreover, plants have evolved the ability to sense cell wall damage and trigger defense responses leading to an improved resistance to disease.65 Therefore, expression of endogenous or microbial CWMEs and their inhibitors in planta represents a useful tool to improve plant resistance to pathogens and to investigate the role of altered structure and integrity of wall polysaccharides in plant-pathogen interactions.
The manipulation of cell wall biosynthetic pathways or post-synthetic modifications of cell wall structure or composition can affect the defense against pathogens. For example, some Arabidopsis mutants in cell wall biosynthetic genes are affected in resistance to pathogens.66-68 Cel2, a putative β-1,4-endoglucanase, which targets cellulose or hemi-cellulose, makes plants more susceptible to Botrytis cinerea when overexpressed in ripening inhibited tomato mutants, suggesting a role for cellulose/hemicellulose integrity in plant susceptibility to pathogens.69 Cellodextrins are the end products of cellulose degradation in plant cell walls. Cellodextrins with a degree of polymerization ≥ 7 induce defense responses in grapevine,70 consistent with results indicating that cellulase from Tricoderma viride can release cellodextrins which induce plant defenses.71
Depolymerization of xyloglucan has been proposed to play an important role during both cell wall expansion and pathogen invasion.72-74 Endo-β-1,4-xylanases are key enzymes in the degradation of xylans and a number of endoxylanases are produced by microbial pathogens to break through the plant cell wall. Rice plants expressing a thermostable exogenous xylanase gene (ATX) exhibited upregulation of SOD, CAT and xylanase inhibitor (RIXI) genes, which could play roles in plant defense.75
Acetylation of cell wall polysaccharides affects plant responses to invading pathogens. For example, the Arabidopsis reduced wall acetylation 2 (rwa2) mutant exhibited reduced cell wall acetylation and increased resistance to the necrotrophic fungal pathogen Botrytis cinerea.76 Arabidopsis and Brachypodium plants expressing a fungal Aspergillus nidulans acetylesterase (AnAXE) had reduced cell wall acetylation and increased resistance to B. cinerea and Bipolaris sorokiniana.19 Interestingly, the resistance to pathogens in dicot and monocot AnAXE-expressing plants was mediated by the activation of different defense responses indicating that different plants perceive reduced cell wall acetylation, but exploit different defense signaling pathways to respond to necrotrophic pathogens.19 For protection against invading pathogens, plants have evolved a surveillance system based on proteinaceous inhibitors of microbial CWMEs. To counteract hemicellulose degradation by microbial hemicellulases, plants produce inhibitors such as the Triticum aestivum xylanase inhibitor (TAXI), xylanase inhibitor protein (XIP) and xyloglucan endoglucanase inhibiting protein (XEGIP).58,77 These endogenous inhibitors, induced during pathogen infection, play a role in the inhibition of enzymes produced by microorganisms.58 The transient overexpression of pepper CaXEGIP1 in Arabidopsis, pepper and Nicotiana benthamiana leaves, triggered pathogen-independent, spontaneous cell death.78 Transient overexpression of CaXEGIP1 in Arabidopsis enhanced resistance to biotrophic downy mildew pathogen Hyaloperonospora arabidopsidis. Comparative histochemical and proteomic analyses revealed that CaXEGIP1 overexpression induced defense-related genes as well as cell wall thickening and darkening. Together, these results suggest that pathogen-inducible CaXEGIP1 positively regulates cell death-mediated defense responses and resistance to pathogens in plants.78
Pectin is one of the first structures to be altered during pathogen invasion and accumulating evidence indicates that post-synthetic alteration of pectic polysaccharides affects plant resistance to pathogen.79,80 Pectin degradation requires the combined action of several pectinases, of which the most extensively studied in terms of pathogen attack are the polygalacturonases (PGs). The expression of an attenuated version of endopolygalacturonase II from Aspergillus niger (AnPGII) in tobacco and Arabidopsis caused a reduction of galacturonic acid content due to HG breakdown and improved plant resistance to the fungal and bacterial pathogens B. cinerea and Pseudomonas syringae.42,81 It was proposed that Arabidopsis and tobacco overexpressing microbial AnPGII accumulate oligogalacturonide (OG) fragments, which serve as damage associated molecular patterns (DAMPs) acting in signaling the presence of cell wall damage caused by pathogens and leading to induction of disease resistance. Consistent with this hypothesis, PG-expressing plants showed enhanced constitutive defense responses, such as accumulation of UV-fluorescent metabolites, H2O2, β-1,3-glucanase and peroxidase and expression of defense-related genes.81 The size of OGs (usually between 10 and 15 glycosides) and their degree of methyl- and acetylesterification are important features for their ability to trigger plant defense responses. The de-esterification of OGs by overexpression of a fruit-specific PME in wild strawberry was required to elicit defense-related gene expression and induce plant resistance to B. cinerea.82,83 Consistent with this, chemical methylesterification of OGs greatly diminishes their ability to elicit biological responses.84,85 Treatment with OGs can protect wheat, a grass species with low pectin contents, against Blumeria graminis. Also, partially acetylated OGs are more efficient elicitors and induce additional defense responses, such as the increased accumulation of phenolic autofluorescent compounds in papillae and a decreased formation of haustoria during fungal pathogen infection.86 The degree of methylesterification and acetylation of homogalacturonan is different in various plant species and is regulated during development. Therefore, PGs expressed in different plants could release OGs with different degrees of methylesterification and acetylation capable of eliciting diverse specific biological responses.87
The loss of cell wall integrity can result in the activation of cell wall strengthening mechanisms. Potato tubers expressing an Erwinia carotovora pectate lyase exhibited increased resistance to Erwinia soft rot and increased phenol oxidase activity. These responses may be induced by active unesterified OG fragments, indicating a possible role of phenol biosynthesis and oxidation in the reinforcement of the cell wall against pathogens.88
Esterification of pectic polysaccharides can also affect their susceptibility to degradation by microbial enzymes.89,90 Genetic and molecular evidence indicates a critical role of pectin methylesterification and acetylation in plant defense against pathogens. The expression of inhibitors of pectin methylesterase (PMEI), affecting the activity of endogenous PMEs, has been used as a strategy to increase the level of methylesterification in the cell wall. In Arabidopsis, the overexpression of AtPMEI-1 or AtPMEI-2 causes increased post-synthetic pectin methylesterification and decreased susceptibility to B. cinerea and Pectobacterium carotovorum.62,91 This strategy was effective also in wheat, where the ectopic expression of AcPMEI from kiwi reduced the susceptibility to Fusarium graminearum and B. sorokiniana. Interestingly, PMEI-expressing plants did not show induction of defense responses and their reduced susceptibility was due to the higher resistance of pectins to microbial hydrolases and to the reduced pathogen growth on highly methylated cell walls. The overexpression of different PMEI isoforms can affect plant-pathogen interactions through different mechanisms. For example, overexpression of pepper CaPMEI1 in Arabidopsis improved plant resistance to P. syringae possibly due to the antimicrobial activity of the protein and the activation of the salicylic acid pathway, although a possible contribution of altered cell wall esterification has not been excluded.52 Moreover, CaPMEI overexpressing plants exhibited higher drought and oxidative stress tolerance.52
Pectin acetylation can also affect plant resistance against invading pathogens. Recent work demonstrated that reduction of pectin acetylation increased Arabidopsis resistance to microbial pathogens.19,76 Arabidopsis plants expressing rhamnogalacturonan acetylesterase from A. nidulans (AnRAE) exhibited reduced pectin and xyloglucan acetylation and increased resistance to B. cinerea mediated by the activation of defense responses such as callose deposition, H2O2 accumulation and activation of defense-related genes.19 Since responses observed in these transgenic plants were similar to those usually induced by OGs, it was proposed that increased susceptibility of deacetylated pectin to endogenous and microbial PGs favors the accumulation of active OGs eliciting constitutive and pathogen-induced defense responses. Polygalacturonase-inhibiting proteins (PGIPs) produced by plants are the best-characterized inhibitors of microbial pectic enzymes. PGIPs play a double function in protecting plants: they hinder pectin degradation by inhibiting microbial PG activities and also favor the accumulation of elicitor-active OGs.80,92,93 The expression of PGIPs reduced susceptibility to B. cinerea in Arabidopsis, tomato and grape and induced higher resistance to F. graminearum in wheat.21,94-96
Pectin lyases are produced by pathogenic bacteria and fungi to catalyze the β-elimination cleavage of methylesterified homogalacturonan. A cell wall pectin lyase inhibitor protein that inhibits a pectin lyase from Rhizoctonia solani was identified in sugar beet.97 The level of expression of the inhibitor protein correlated with plant resistance to the pathogen but details on the inhibitor structure, its spatial and temporal regulation and its occurrence in plants were not reported.
A common plant response to fungal attack is the deposition of callose, a (1,3)-β-glucan polymer, at the sites of pathogen penetration. The role of callose in plant defense is believed to be dependent on the infection strategy of the pathogen. Higher callose accumulation contributes to resistance against nectrophic fungal pathogens, but the role of callose in resistance against biotrophic pathogens is controversial.98,99 However, emerging evidence support the active role of callose synthesis in plant resistance to biotrophic microbial pathogens. Transgenic Arabidopsis plants expressing POWDERY MILDEW RESISTANT 4 (PMR4), which encodes a stress-induced callose synthase, showed a higher callose synthase activity and increased early callose deposition at the sites of pathogen penetration, resulting in prevention of haustoria formation and complete resistance to penetration by powdery mildews Golovinomyces cichoracearum and Blumeria graminis.100 PMR4 overexpressing leaf tissues showed alterations in cell wall composition, decreases in fucose and galactose and an increase in glucose, which are not directly related to callose synthesis. The localized callose synthesis was proposed to be required for an early structural reinforcement of the cell wall supporting complete resistance to the fungal pathogens.
Cell Wall Integrity Perception
The modification of cell wall composition affects plant development and responses to pathogens by two possible mechanisms: through changes in mechanistic properties and through initiation of signaling pathways. For example, changes of sugar content in the plant cell can be sensed through hexokinases, which modify responsive gene expression.101-103 The concept of biologically active oligosaccharides, termed oligosaccharins, was first proposed by Albersheim and colleagues,3 and numerous studies have demonstrated a diversity of oligosaccharin structure and functionality, as described in several comprehensive reviews.104-106
The plant cell wall contains a large number of constituents that could potentially release signals recognizable by specific receptors. During pathogen attack, Damage Associated Molecular Patterns (DAMPs) have been described to function as such signals and the number of possible DAMPs is most likely large.107 Similarly, remodeling of cell walls in response to abiotic stresses such as drought and cold is also believed to be a source of oligosaccharins released during these processes, thus mediating transduction of signals between cell wall and cytoplasm.108,109
Changes in cell wall mechanical properties can also be involved in stress responses; this was strongly evidenced by demonstration of the relationship between cytoskeleton orientation and cellulose deposition.110 During water stress, microtubules are eliminated by proteolytic degradation (Wang et al., 2011) followed by cellulose microfibril re-organization and activation of phospholipase-D dependent signaling during osmotic adaptation.111-113
Release of DAMPs and CWI modifications can be perceived by membrane receptors, communicated by trans-membrane channels and can trigger internal cell signaling cascades to activate appropriate response genes. Thus, the large family of receptor like kinases (RLKs), arabinogalactan proteins, the Mitogen-Activated Protein Kinase (MAPK) pathway and the Target of Rapamycin (TOR) pathway have been suggested to be potential components of CWI perception mechanisms (Fig. 1).9,17,24 The MAPK pathway activates or suppresses gene expression via phosphorylation of appropriate transcription factors or receptors.24,114 Oligogalacturonides (OGs) released from pectins by microbial polygalacturonase elicit signals involved in plant innate immunity.115 OGs serve as DAMPs and are perceived by Wall Associated Kinase-like proteins, which have high affinity to pectin and may act as pectin integrity sensors.18,116-118 RLKs belonging to the Catharanthus roseus (CrRLK)-like protein family were implicated in CWI signaling mechanisms. Among these are THESEUS1 (THE1), HERCULES1 and FERONIA, which have been implicated in brassinosteroid-induced cell elongation.119,120 The THE1 plasma-membrane receptor-like kinase has been proposed to mediate the response of plant cells to perturbations in cellulose content and may act as a cell-wall-integrity sensor.32 THE1 is also required for induction of ROS production and ectopic lignification in Arabidopsis roots in response to inhibition of cellulose biosynthesis.28 Some RLKs have a lectin-like extracellular domain. Lectin receptor kinases may bind cell wall polysaccharides by mediating structural continuity between cell wall and membrane and sensing changes in integrity of the cell wall after pathogen attack.32,121
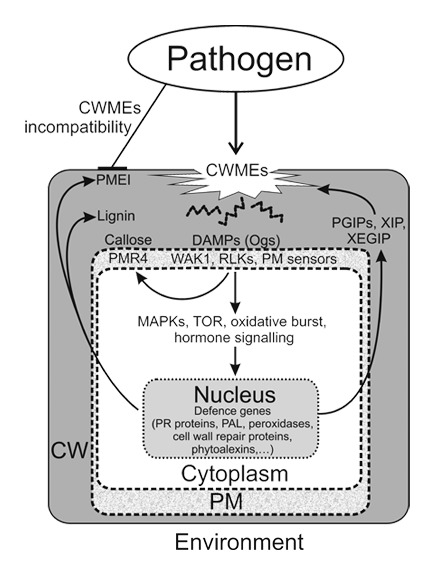
Figure 1. Cell wall integrity signaling components mediate cellular responses upon pathogen attack. Pathogen invasion can be limited by a cell wall structure and composition that is resistant to degradation by cell wall modifying enzymes (CWMEs). Pathogens can be detected by the presence of cell wall fragments (i.e., oligogalacturonides; OGs) generated by CWMEs; these fragments can function as DAMPs and are perceived by Wall Associated Kinases (WAKs), Receptor Like Kinases (RLKs) and Plasma Membrane (PM) sensors. Downstream signaling pathways mediate defense responses (MAPKs, TOR, oxidative burst, hormone mediated pathways of Pathogen Related (PR), CW repair and peroxidases genes, PAL, phytoalexins activation and/or cell wall structural rearrangements such as cell wall repair (PMR4-mediated callose deposition) or cell wall reinforcement (lignin) and de novo synthesis of defensive compounds such as defense proteins including CWME inhibitors (PGIPs, XIP, XEGIP, PMEI).
Recently, Wolf et al. proposed that brassinosteroid feedback signaling is part of a compensatory response that is activated to protect the plant against the loss of CWI caused by imbalanced pectin modifications.24 Another example is G-proteins that form highly conserved signaling complexes and participate in signal transduction during development and stress responses.122,123 TOR kinase is essential for Arabidopsis development and TOR pathways are involved in regulating cell wall development.124-127 In addition, arabinogalactan proteins, COBRA and extensin-like receptor kinases are examples of GPI-containing proteins that are attached to the plasma membrane and implicated in connecting intracellular and extracellular space, thus affecting cell wall development.17 These transmembrane receptor proteins are good candidates for transducing signals from the extracellular space to the cytoplasm. The high diversity of cell wall structural components and large numbers of potential receptors and transducers suggest the significant complexity of plant CWI maintenance and signaling mechanisms, many of which have yet to be uncovered.
Outlook
The plant cell wall is a dynamic and highly complex structure, consisting of interdependent networks that constantly change during development and in response to environmental cues. Plant cells monitor the status of their cell walls with various types of sensors and receptors at the plasma membrane, some of which may interact with cell wall components, to coordinate mechanical deformations or changes in cell wall structure and cellular responses. Although a number of plant responses to cell wall damage or modification have been described and the existence of CWI maintenance mechanisms in plants is indubitable, detailed transduction pathways involved in CWI signaling via a signal and receptor, leading to a specific output, are yet to be revealed. Most evidence about CWI signaling in plants comes from mutant studies where biosynthesis of various cell wall components was compromised, causing wall structural damage or rearrangement. Post-synthetic modification of cell wall components via their partial remodeling by overexpressed cell wall modifying enzymes/inhibitors is another powerful approach to investigate CWI signaling mechanisms. Fine structural remodeling of particular polysaccharides via side chain cleavage, de-esterification or partial depolymerization leads to the initiation of specific signaling pathways related to these particular modifications. This approach can assist in revealing putative components of signaling pathways initiated in response to such highly specific cell wall modifications and thus allow us to dissect otherwise highly complex cell responses that occur during plant development or defense responses. Because of plant broad adaptability and cell wall complexity, we expect that the near future holds significant discoveries of many new receptors, signal transduction components and transcriptional regulators involved in cell wall-sensing mechanisms and post-synthetic wall modifications.
Post-synthetic cell wall modifications will also find wide application in crop biotechnology and in bioenergy biomass feedstock improvement. Remodeling of particular cell wall components directed toward increasing digestibility can improve biomass saccharification during biofuel production, reducing costs. However, such modifications can also have different effects on plant growth and stress tolerance. Modifications that compromise cell wall mechanical properties can reduce plant resistance to biotic stresses, but other modifications can have the opposite effect by initiating defense mechanisms. Therefore, such modifications require an informed approach to be effective for feedstock improvement. Further detailed studies of post-synthetic modification by overexpression of cell wall modifying enzymes and their effects on plant responses will significantly contribute to improvement of crop productivity and sustainability.
Acknowledgments
The authors acknowledge those authors whose studies were not discussed in this review due to space limitations. The work of Zabotina’s lab was supported by Roy J. Carver Charitable Trust (Grant 09-3384, 2009-2012) and by Plant Science Institute, ISU. The work of Bellincampi’s lab was supported by PRIN 2010-11 (Grant N. 2010T7247Z).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25435
References
- 1.Scheller HV, Ulvskov P. Hemicelluloses. Annu Rev Plant Biol. 2010;61:263–89. doi: 10.1146/annurev-arplant-042809-112315. [DOI] [PubMed] [Google Scholar]
- 2.Caffall KH, Mohnen D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res. 2009;344:1879–900. doi: 10.1016/j.carres.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. Plant cell walls. Garland Science 2010; Taylor&Francis Group, LLC, New York. [Google Scholar]
- 4.Cassab GI. Plant cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:281–309. doi: 10.1146/annurev.arplant.49.1.281. [DOI] [PubMed] [Google Scholar]
- 5.Davin LB, Lewis NG. Lignin primary structures and dirigent sites. Curr Opin Biotechnol. 2005;16:407–15. doi: 10.1016/j.copbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Gille S, Pauly M. O-acetylation of plant cell wall polysaccharides. Front Plant Sci. 2012;3:12. doi: 10.3389/fpls.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohnen D. Pectin structure and biosynthesis. Curr Opin Plant Biol. 2008;11:266–77. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Urbanowicz BR, Peña MJ, Ratnaparkhe S, Avci U, Backe J, Steet HF, et al. 4-O-methylation of glucuronic acid in Arabidopsis glucuronoxylan is catalyzed by a domain of unknown function family 579 protein. Proc Natl Acad Sci USA. 2012;109:14253–8. doi: 10.1073/pnas.1208097109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey TV, Bonetta DT, Goring DR. Sentinels at the wall: cell wall receptors and sensors. New Phytol. 2007;176:7–21. doi: 10.1111/j.1469-8137.2007.02192.x. [DOI] [PubMed] [Google Scholar]
- 10.Singh SP, Montgomery BL. Determining cell shape: adaptive regulation of cyanobacterial cellular differentiation and morphology. Trends Microbiol. 2011;19:278–85. doi: 10.1016/j.tim.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Szymanski DB. Plant cells taking shape: new insights into cytoplasmic control. Curr Opin Plant Biol. 2009;12:735–44. doi: 10.1016/j.pbi.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Ray PM, Green PB, Cleland RE. Role of turgor in plant cell growth. Nature. 1972;239:163–4. doi: 10.1038/239163a0. [DOI] [Google Scholar]
- 13.Mirabet V, Das P, Boudaoud A, Hamant O. The role of mechanical forces in plant morphogenesis. Annu Rev Plant Biol. 2011;62:365–85. doi: 10.1146/annurev-arplant-042110-103852. [DOI] [PubMed] [Google Scholar]
- 14.Hamant O, Traas J, Boudaoud A. Regulation of shape and patterning in plant development. Curr Opin Genet Dev. 2010;20:454–9. doi: 10.1016/j.gde.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Smith C, Corke F, Zheng L, Merali Z, Ryden P, et al. Signaling from an altered cell wall to the nucleus mediates sugar-responsive growth and development in Arabidopsis thaliana. Plant Cell. 2007;19:2500–15. doi: 10.1105/tpc.106.049965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seifert GJ, Blaukopf C. Irritable walls: the plant extracellular matrix and signaling. Plant Physiol. 2010;153:467–78. doi: 10.1104/pp.110.153940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ringli C. Monitoring the outside: cell wall-sensing mechanisms. Plant Physiol. 2010;153:1445–52. doi: 10.1104/pp.110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamann T, Denness L. Cell wall integrity maintenance in plants: lessons to be learned from yeast? Plant Signal Behav. 2011;6:1706–9. doi: 10.4161/psb.6.11.17782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogorelko GV, Lionetti V, Fursova OV, Sundaram RM, Qi M, Whitham SA, et al. Arabidopsis and Brachypodium transgenic plants expressing A. nidulans acetylesterases have decreased degree of polysaccharide acetylation and increased resistance to pathogens. Plant Physiol. 2013;162:9–23. doi: 10.1104/pp.113.214460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lionetti V, Cervone F, Bellincampi D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J Plant Physiol. 2012;169:1623–30. doi: 10.1016/j.jplph.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari S, Sella L, Janni M, Favaron F, D'Ovidio R. Transgenic expression of polygalacturonase-inhibiting proteins in Arabidopsis and wheat increases resistance to the flower pathogen Fusarium graminearum. Plant Biol (Stuttg) 2012;14:31–8. doi: 10.1111/j.1438-8677.2011.00449.x. [DOI] [PubMed] [Google Scholar]
- 22.Moore JP, Farrant JM, Driouich A. A role for pectin-associated arabinans in maintaining the flexibility of the plant cell wall during water deficit stress. Plant Signal Behav. 2008;3:102–4. doi: 10.4161/psb.3.2.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellenc D, Schmitt E, Gallet O. Purification of a plant cell wall fibronectin-like adhesion protein involved in plant response to salt stress. Protein Expr Purif. 2004;34:208–14. doi: 10.1016/j.pep.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Wolf S, Mravec J, Greiner S, Mouille G, Höfte H. Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr Biol. 2012;22:1732–7. doi: 10.1016/j.cub.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 25.Minic Z, Jouanin L. Plant glycoside hydrolases involved in cell wall polysaccharide degradation. Plant Physiol Biochem. 2006;44:435–49. doi: 10.1016/j.plaphy.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Underwood W, Somerville SC. Focal accumulation of defences at sites of fungal pathogen attack. J Exp Bot. 2008;59:3501–8. doi: 10.1093/jxb/ern205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollard M, Beisson F, Li Y, Ohlrogge JB. Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci. 2008;13:236–46. doi: 10.1016/j.tplants.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Maris A, Kaewthai N, Eklof JM, Miller JG, Brumer H, Fry SC, et al. Differences in enzymic properties of five recombinant xyloglucan endotransglucosylase/hydrolase (XTH) proteins of Arabidopsis thaliana. J Exp Bot. 2011;62:261–71. doi: 10.1093/jxb/erq263. [DOI] [PubMed] [Google Scholar]
- 29.Harholt J, Bach IC, Lind-Bouquin S, Nunan KJ, Madrid SM, Brinch-Pedersen H, et al. Generation of transgenic wheat (Triticum aestivum L.) accumulating heterologous endo-xylanase or ferulic acid esterase in the endosperm. Plant Biotechnol J. 2010;8:351–62. doi: 10.1111/j.1467-7652.2009.00490.x. [DOI] [PubMed] [Google Scholar]
- 30.Liepman AH, Wightman R, Geshi N, Turner SR, Scheller HV. Arabidopsis - a powerful model system for plant cell wall research. Plant J. 2010;61:1107–21. doi: 10.1111/j.1365-313X.2010.04161.x. [DOI] [PubMed] [Google Scholar]
- 31.Cano-Delgado A, Penfield S, Smith C, Catley M, Bevan M. Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J. 2003;34:351–62. doi: 10.1046/j.1365-313X.2003.01729.x. [DOI] [PubMed] [Google Scholar]
- 32.Hematy K, Cherk C, Somerville S. Host-pathogen warfare at the plant cell wall. Curr Opin Plant Biol. 2009;12:406–13. doi: 10.1016/j.pbi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Beeckman T, Przemeck GKH, Stamatiou G, Lau R, Terryn N, De Rycke R, et al. Genetic complexity of cellulose synthase A gene function in Arabidopsis embryogenesis. Plant Physiol. 2002;130:1883–93. doi: 10.1104/pp.102.010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillmor CS, Poindexter P, Lorieau J, Palcic MM, Somerville C. Alphaglucosidase I is required for cellulose biosynthesis and morphogenesis in Arabidopsis. J Cell Biol. 2002;156:1003–13. doi: 10.1083/jcb.200111093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persson S, Paredez A, Carroll A, Palsdottir H, Doblin M, Poindexter P, et al. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:15566–71. doi: 10.1073/pnas.0706592104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouton S, Leboeuf E, Mouille G, Leydecker MT, Talbotec J, Granier F, et al. QUASIMODO1 encodes a putativemembrane-bound glycosyltransferase required for normal pectinsynthesis and cell adhesion in Arabidopsis. Plant Cell. 2002;14:2577–90. doi: 10.1105/tpc.004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Hengel AJ, Roberts K. Fucosylated arabinogalactan-proteins are required for full root cell elongation in arabidopsis. Plant J. 2002;32:105–13. doi: 10.1046/j.1365-313X.2002.01406.x. [DOI] [PubMed] [Google Scholar]
- 38.Minic Z. Physiological roles of plant glycoside hydrolases. Planta. 2008;227:723–40. doi: 10.1007/s00425-007-0668-y. [DOI] [PubMed] [Google Scholar]
- 39.Oomen RJFJ, Doeswijk-Voragen CHL, Bush MS, Vincken JP, Borkhardt B, van den Broek LAM, et al. In muro fragmentation of the rhamnogalacturonan I backbone in potato (Solanum tuberosum L.) results in a reduction and altered location of the galactan and arabinan side-chains and abnormal periderm development. Plant J. 2002;30:403–13. doi: 10.1046/j.1365-313X.2002.01296.x. [DOI] [PubMed] [Google Scholar]
- 40.Skjùt M, Pauly M, Bush MS, Borkhardt B, McCann MC, Ulvskov P. Direct interference with rhamnogalacturonan I biosynthesis in Golgi vesicles. Plant Physiol. 2002;129:95–102. doi: 10.1104/pp.010948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oxenboll Sorensen S, Pauly M, Bush M, Skjot M, McCann MC, Borkhardt B, et al. Pectin engineering: modification of potato pectin by in vivo expression of an endo-1,4-beta-D-galactanase. Proc Natl Acad Sci USA. 2000;97:7639–44. doi: 10.1073/pnas.130568297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capodicasa C, Vairo D, Zabotina O, McCartney L, Caprari C, Mattei B, et al. Targeted modification of homogalacturonan by transgenic expression of a fungal polygalacturonase alters plant growth. Plant Physiol. 2004;135:1294–304. doi: 10.1104/pp.104.042788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obel N, Erben V, Schwarz T, Kühnel S, Fodor A, Pauly M. Microanalysis of plant cell wall polysaccharides. Mol Plant. 2009;2:922–32. doi: 10.1093/mp/ssp046. [DOI] [PubMed] [Google Scholar]
- 44.Orfila C, Dal Degan F, Jørgensen B, Scheller HV, Ray PM, Ulvskov P. Expression of mung bean pectin acetyl esterase in potato tubers: effect on acetylation of cell wall polymers and tuber mechanical properties. Planta. 2012;236:185–96. doi: 10.1007/s00425-012-1596-z. [DOI] [PubMed] [Google Scholar]
- 45.Gou JY, Miller LM, Hou G, Yu XH, Chen XY, Liu CJ. Acetylesterase-mediated deacetylation of pectin impairs cell elongation, pollen germination, and plant reproduction. Plant Cell. 2012;24:50–65. doi: 10.1105/tpc.111.092411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee C, Teng Q, Zhong R, Ye ZH. The four Arabidopsis reduced wall acetylation genes are expressed in secondary wall-containing cells and required for the acetylation of xylan. Plant Cell Physiol. 2011;52:1289–301. doi: 10.1093/pcp/pcr075. [DOI] [PubMed] [Google Scholar]
- 47.Pelletier S, Van Orden J, Wolf S, Vissenberg K, Delacourt J, Ndong YA, et al. A role for pectin de-methylesterification in a developmentally regulated growth acceleration in dark-grown Arabidopsis hypocotyls. New Phytol. 2010;188:726–39. doi: 10.1111/j.1469-8137.2010.03409.x. [DOI] [PubMed] [Google Scholar]
- 48.Protsenko MA, Buza NL, Krinitsyna AA, Bulantseva EA, Korableva NP. Polygalacturonase-inhibiting protein is a structural component of plant cell wall. Biochemistry (Mosc) 2008;73:1053–62. doi: 10.1134/S0006297908100015. [DOI] [PubMed] [Google Scholar]
- 49.Hewezi T, Howe P, Maier TR, Hussey RS, Mitchum MG, Davis EL, et al. Cellulose binding protein from the parasitic nematode Heterodera schachtii interacts with Arabidopsis pectin methylesterase: cooperative cell wall modification during parasitism. Plant Cell. 2008;20:3080–93. doi: 10.1105/tpc.108.063065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siedlecka A, Wiklund S, Péronne MA, Micheli F, Lesniewska J, Sethson I, et al. Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol. 2007;146:554–65. doi: 10.1104/pp.107.111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balestrieri C, Castaldo D, Giovane A, Quagliuolo L, Servillo L. A glycoprotein inhibitor of pectin methylesterase in kiwi fruit (Actinidia chinensis) Eur J Biochem. 1990;193:183–7. doi: 10.1111/j.1432-1033.1990.tb19321.x. [DOI] [PubMed] [Google Scholar]
- 52.An SH, Sohn KH, Choi HW, Hwang IS, Lee SC, Hwang BK. Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta. 2008;228:61–78. doi: 10.1007/s00425-008-0719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peaucelle A, Louvet R, Johansen JN, Höfte H, Laufs P, Pelloux J, et al. Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr Biol. 2008;18:1943–8. doi: 10.1016/j.cub.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 54.Zhang GY, Feng J, Wu J, Wang XW. BoPMEI1, a pollen-specific pectin methylesterase inhibitor, has an essential role in pollen tube growth. Planta. 2010;231:1323–34. doi: 10.1007/s00425-010-1136-7. [DOI] [PubMed] [Google Scholar]
- 55.Hong MJ, Kim DY, Lee TG, Jeon WB, Seo YW. Functional characterization of pectin methylesterase inhibitor (PMEI) in wheat. Genes Genet Syst. 2010;85:97–106. doi: 10.1266/ggs.85.97. [DOI] [PubMed] [Google Scholar]
- 56.Reca IB, Lionetti V, Camardella L, D’Avino R, Giardina T, Cervone F, et al. A functional pectin methylesterase inhibitor protein (SolyPMEI) is expressed during tomato fruit ripening and interacts with PME-1. Plant Mol Biol. 2012;79:429–42. doi: 10.1007/s11103-012-9921-2. [DOI] [PubMed] [Google Scholar]
- 57.Raiola A, Camardella L, Giovane A, Mattei B, De Lorenzo G, Cervone F, et al. Two Arabidopsis thaliana genes encode functional pectin methylesterase inhibitors. FEBS Lett. 2004;557:199–203. doi: 10.1016/S0014-5793(03)01491-1. [DOI] [PubMed] [Google Scholar]
- 58.Juge N. Plant protein inhibitors of cell wall degrading enzymes. Trends Plant Sci. 2006;11:359–67. doi: 10.1016/j.tplants.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Jolie RP, Duvetter T, Vandevenne E, Van Buggenhout S, Van Loey AM, Hendrickx ME. A pectin-methylesterase-inhibitor-based molecular probe for in situ detection of plant pectin methylesterase activity. J Agric Food Chem. 2010;58:5449–56. doi: 10.1021/jf100248u. [DOI] [PubMed] [Google Scholar]
- 60.Röckel N, Wolf S, Kost B, Rausch T, Greiner S. Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J. 2008;53:133–43. doi: 10.1111/j.1365-313X.2007.03325.x. [DOI] [PubMed] [Google Scholar]
- 61.Lionetti V, Francocci F, Ferrari S, Volpi C, Bellincampi D, Galletti R, et al. Engineering the cell wall by reducing demethylesterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc Natl Acad Sci USA. 2010;107:616–21. doi: 10.1073/pnas.0907549107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M, et al. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol. 2007;143:1871–80. doi: 10.1104/pp.106.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee C, Teng Q, Zhong R, Yuan Y, Haghighat M, Ye ZH. Three Arabidopsis DUF579 domain-containing GXM proteins are methyltransferases catalyzing 4-o-methylation of glucuronic acid on xylan. Plant Cell Physiol. 2012;53:1934–49. doi: 10.1093/pcp/pcs138. [DOI] [PubMed] [Google Scholar]
- 64.Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell AL. Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008;13:610–7. doi: 10.1016/j.tplants.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Hematy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, et al. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol. 2007;17:922–31. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 66.Ellis C, Karafyllidis I, Turner JG. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol Plant Microbe Interact. 200:1025–30. doi: 10.1094/MPMI.2002.15.10.1025. [DOI] [PubMed] [Google Scholar]
- 67.Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell. 2002;14:1557–66. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernandez-Blanco C, Feng DX, Hu J, Sanchez-Vallet A, Deslandes L, Llorente F, et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell. 2007;19:890–903. doi: 10.1105/tpc.106.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flors V, Leyva MD, Vicedo B, Finiti I, Real MD, Garcia-Agustin P, et al. Absence of the endo-beta-1,4-glucanases Cel1 and Cel2 reduces susceptibility to Botrytis cinerea in tomato. Plant J. 2007;52:1027–40. doi: 10.1111/j.1365-313X.2007.03299.x. [DOI] [PubMed] [Google Scholar]
- 70.Aziz A, Gauthier A, Bezier A, Ponssot B, Joubert J-M, Pugin A, et al. Elicitor and resistance-inducing activities of β-1,4 cellodextrins in grapevine, comparison with β-1,3 glucans and α-1,4 oligogalacturonides. J Exp Bot. 2007;58:1463–1472. doi: 10.1093/jxb/erm008. [DOI] [PubMed] [Google Scholar]
- 71.Calderon AA, Zapata JM, Munoz R, Pedreno MA, Barcelo AR. Resveratrol production as a part of the hypersensitive-like response of grapevine cells to an elicitor from Trichoderma viride. New Phytol. 1993;124:455–63. doi: 10.1111/j.1469-8137.1993.tb03836.x. [DOI] [Google Scholar]
- 72.Bourquin V, Nishikubo N, Abe H, Brumer H, Denman S, Eklund M, et al. Xyloglucan endotransglycosylases have a function during the formation of secondary cell walls of vascular tissues. Plant Cell. 2002;14:3073–88. doi: 10.1105/tpc.007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qin Q, Bergmann CW, Rose JK, Saladie M, Kolli VS, Albersheim P, et al. Characterization of a tomato protein that inhibits a xyloglucan-specific endoglucanase. Plant J. 2003;34:327–38. doi: 10.1046/j.1365-313X.2003.01726.x. [DOI] [PubMed] [Google Scholar]
- 74.Baumann MJ, Eklöf JM, Michel G, Kallas AM, Teeri TT, Czjzek M, et al. Structural evidence for the evolution of xyloglucanase activity from xyloglucan endo-transglycosylases: biological implications for cell wall metabolism. Plant Cell. 2007;19:1947–63. doi: 10.1105/tpc.107.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weng XY, Huang YY, Hou CX, Jiang DA. Effects of an exogenous xylanase gene expression on the growth of transgenic rice and the expression level of endogenous xylanase inhibitor gene RIXI. J Sci Food Agric. 2013;93:173–9. doi: 10.1002/jsfa.5746. [DOI] [PubMed] [Google Scholar]
- 76.Manabe Y, Nafisi M, Verhertbruggen Y, Orfila C, Gille S, Rautengarten C, et al. Loss-of-function mutation of REDUCED WALL ACETYLATION2 in Arabidopsis leads to reduced cell wall acetylation and increased resistance to Botrytis cinerea. Plant Physiol. 2011;155:1068–78. doi: 10.1104/pp.110.168989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brutus A, Reca IB, Herga S, Mattei B, Puigserver A, Chaix JC, et al. A family 11 xylanase from the pathogen Botrytis cinerea is inhibited by plant endoxylanase inhibitors XIP-I and TAXI-I. Biochem Biophys Res Commun. 2005;337:160–6. doi: 10.1016/j.bbrc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 78.Choi HW, Kim NH, Lee YK, Hwang BK. The pepper extracellular xyloglucan-specific endo-beta-1,4-glucanase inhibitor protein gene, CaXEGIP1, is required for plant cell death and defense responses. Plant Physiol. 2013;161:384–96. doi: 10.1104/pp.112.203828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Lorenzo G, Castoria R, Bellincampi D, Cervone F. Fungal invasion enzymes and their inhibition. In The Mycota. V. Plant Relationships, Part B, (Berlin: Springer-Verlag) 1997; 61-83 [Google Scholar]
- 80.De Lorenzo G, Ferrari S. Polygalacturonase-inhibiting proteins in defense against phytopathogenic fungi. Curr Opin Plant Biol. 2002;5:295–9. doi: 10.1016/S1369-5266(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 81.Ferrari S, Galletti R, Pontiggia D, Manfredini C, Lionetti V, Bellincampi D, et al. Transgenic expression of a fungal endo-polygalacturonase increases plant resistance to pathogens and reduces auxin sensitivity. Plant Physiol. 2007;146:669–81. doi: 10.1104/pp.107.109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osorio S, Castillejo C, Quesada MA, Medina-Escobar N, Brownsey GJ, Suau R, et al. Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca) Plant J. 2008;54:43–55. doi: 10.1111/j.1365-313X.2007.03398.x. [DOI] [PubMed] [Google Scholar]
- 83.Osorio S, Bombarely A, Giavalisco P, Usadel B, Stephens C, Araguez I, et al. Demethylation of oligogalacturonides by FaPE1 in the fruits of the wild strawberry Fragaria vesca triggers metabolic and transcriptional changes associated with defense and development of the fruit. J Exp Bot. 2011;62:2855–73. doi: 10.1093/jxb/erq465. [DOI] [PubMed] [Google Scholar]
- 84.Jin DF, West CA. Characteristics of galacturonic Acid oligomers as elicitors of casbene synthetase activity in castor bean seedlings. Plant Physiol. 1984;74:989–92. doi: 10.1104/pp.74.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navazio L, Moscatiello R, Bellincampi D, Baldan B, Meggio F, Brini M, et al. The role of calcium in oligogalacturonide-activated signalling in soybean cells. Planta. 2002;215:596–605. doi: 10.1007/s00425-002-0776-7. [DOI] [PubMed] [Google Scholar]
- 86.Randoux B, Renard-Merlier D, Mulard G, Rossard S, Duyme F, Sanssene J, et al. Distinct defenses induced in wheat against powdery mildew by acetylated and nonacetylated oligogalacturonides. Phytopathology. 2010;100:1352–63. doi: 10.1094/PHYTO-03-10-0086. [DOI] [PubMed] [Google Scholar]
- 87.Wolf S, Rausch T, Greiner S. The N-terminal pro region mediates retention of unprocessed type-I PME in the Golgi apparatus. Plant J. 2009;58:361–75. doi: 10.1111/j.1365-313X.2009.03784.x. [DOI] [PubMed] [Google Scholar]
- 88.Wegener GB. Induction of defense responses against Erwinia soft rot by an endogenous pectate lyase in potatoes. Physiol Mol Plant Pathol. 2002;60:91–100. doi: 10.1006/pmpp.2002.0377. [DOI] [Google Scholar]
- 89.Limberg G, Korner R, Buchholt HC, Christensen TM, Roepstorff P, Mikkelsen JD. Analysis of different de-esterification mechanisms for pectin by enzymatic fingerprinting using endopectin lyase and endopolygalacturonase II from A. niger. Carbohydr Res. 2000;327:293–307. doi: 10.1016/S0008-6215(00)00067-7. [DOI] [PubMed] [Google Scholar]
- 90.Selig MJ, Adney WS, Himmel ME, Decker SR. The impact of cell wall acetylation on corn stover hydrolysis by cellulolytic and xylanolytic enzymes. Cellulose. 2009;16:711–22. doi: 10.1007/s10570-009-9322-0. [DOI] [Google Scholar]
- 91.Raiola A, Lionetti V, Elmaghraby I, Immerzeel P, Mellerowicz EJ, Salvi G, et al. Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol Plant Microbe Interact. 2011;24:432–40. doi: 10.1094/MPMI-07-10-0157. [DOI] [PubMed] [Google Scholar]
- 92.De Lorenzo G, Cervone F, Bellincampi D, Caprari C, Clark AJ, Desiderio A, et al. Polygalacturonase, PGIP and oligogalacturonides in cell-cell communication. Biochem Soc Trans. 1994;22:394–7. doi: 10.1042/bst0220394. [DOI] [PubMed] [Google Scholar]
- 93.De Lorenzo G, D'Ovidio R, Cervone F. The role of polygalacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu Rev Phytopathol. 2001;39:313–35. doi: 10.1146/annurev.phyto.39.1.313. [DOI] [PubMed] [Google Scholar]
- 94.Ferrari S, Vairo D, Ausubel FM, Cervone F, De Lorenzo G. Tandemly duplicated Arabidopsis genes that encode polygalacturonase-inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell. 2003;15:93–106. doi: 10.1105/tpc.005165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Powell ALT, van Kan J, ten Have A, Visser J, Greve LC, Bennett AB, et al. Transgenic expression of pear PGIP in tomato limits fungal colonization. Mol Plant Microbe Interact. 2000;13:942–50. doi: 10.1094/MPMI.2000.13.9.942. [DOI] [PubMed] [Google Scholar]
- 96.Aguero CB, Uratsu SL, Greve C, Powell ALT, Labavitch JM, Meredith CP, et al. Evaluation of tolerance to Pierce's disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Mol Plant Pathol. 2005;6:43–51. doi: 10.1111/j.1364-3703.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- 97.Bugbee WM. A pectin lyase inhibitor protein from cell walls of sugar beet. Phytopathology. 1993;83:63–8. doi: 10.1094/Phyto-83-63. [DOI] [Google Scholar]
- 98.Underwood W. The plant cell wall: a dynamic barrier against pathogen invasion. Fronties in Plant Science. 2012;3:85. doi: 10.3389/fpls.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ton J, Mauch-Mani B. Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J. 2004;38:119–30. doi: 10.1111/j.1365-313X.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 100.Ellinger D, Naumann M, Falter C, Zwikowics C, Jamrow T, Manisseri C, et al. Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiol. 2013;161:1433–44. doi: 10.1104/pp.112.211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fujiki Y, Ito M, Nishida I, Watanabe A. Multiplesignaling pathways in gene expression during sugar starvation. Plant Physiol. 2000;124:1139–48. doi: 10.1104/pp.124.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jang J-C, Sheen J. Sugar sensing in higher plants. Plant Cell. 1994;6:1665–79. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Graham IA, Denby KJ, Leaver CJ. Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell. 1994;6:761–72. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fry SC, Aldington S, Hetherington PR, Aitken J. Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiol. 1993;103:1–5. doi: 10.1104/pp.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cote F, Hahn MG. Oligosaccharins: structures and signal transduction. Plant Mol Biol. 1994;26:1379–411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- 106.Zabotina OA, Zabotin AI. Biologically active oligosaccharide functions in plant cell: updates and prospects. Oligosaccharides: Sources, properties and applications (ed. Nicole S. Gordon) Nova Science Publishers NY 2010; 209-244 [Google Scholar]
- 107.Zipfel C. Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol. 2009;12:414–20. doi: 10.1016/j.pbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 108.Boyer JS. Evans Review: Cell wall biosynthesis and the molecular mechanism of plant enlargement. Funct Plant Biol. 2009;36:383–94. doi: 10.1071/FP09048. [DOI] [PubMed] [Google Scholar]
- 109.Zabotin AI, Barisheva TS, Trofimova OI, Toroschina TE, Larskaya IA, Zabotina OA. Oligosaccharin and ABA synergistically affect the acquisition of freezing tolerance in winter wheat. Plant Physiol Biochem. 2009;47:854–8. doi: 10.1016/j.plaphy.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Geitmann A, Ortega JK. Mechanics and modeling of plant cell growth. Trends Plant Sci. 2009;14:467–78. doi: 10.1016/j.tplants.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 111.Wang S, Kurepa J, Hashimoto T, Smalle JA. Salt stress-induced disassembly of Arabidopsis cortical microtubule arrays involves 26S proteasome-dependent degradation of SPIRAL1. Plant Cell. 2011;23:3412–27. doi: 10.1105/tpc.111.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fernandes JC, García-Angulo P, Goulao LF, Acebes JL, Amâncio S. Mineral stress affects the cell wall composition of grapevine (Vitis vinifera L.) callus. Plant Sci. 2013;205-206:111–20. doi: 10.1016/j.plantsci.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 113.Nick P. Microtubules, signalling and abiotic stress. Plant J. 2013 doi: 10.1111/tpj.12102. [DOI] [PubMed] [Google Scholar]
- 114.Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signaling. Trends Plant Sci. 2005;10:339–46. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 115.Galletti R, De Lorenzo G, Ferrari S. Host-derived signals activate plant innate immunity. Plant Signal Behav. 2009;4:33–4. doi: 10.4161/psb.4.1.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci USA. 2010;107:9452–7. doi: 10.1073/pnas.1000675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.He ZH, Fujiki M, Kohorn BD. A cell wall-associated, receptor-like protein kinase. J Biol Chem. 1996;271:19789–93. doi: 10.1074/jbc.271.33.19789. [DOI] [PubMed] [Google Scholar]
- 118.Kohorn BD, Lane S, Smith TA. An Arabidopsis serine/threonine kinase homologue with an epidermal growth factor repeat selected in yeast for its specificity for a thylakoid membrane protein. Proc Natl Acad Sci USA. 1992;89:10989–92. doi: 10.1073/pnas.89.22.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guo H, Li L, Ye H, Yu X, Algreen A, Yin Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2009;106:7648–53. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol Plant. 2010;3:626–40. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- 121.Gouget A, Senchou V, Govers F, Sanson A, Barre A, Rouge P, et al. Lectin receptor kinases participate in protein-protein interactions to mediate plasma membrane-cell wall adhesions in Arabidopsis. Plant Physiol. 2005;140:81–90. doi: 10.1104/pp.105.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Temple BR, Jones AM. The plant heterotrimeric G-protein complex. Annu Rev Plant Biol. 2007;58:249–66. doi: 10.1146/annurev.arplant.58.032806.103827. [DOI] [PubMed] [Google Scholar]
- 123.Delgado-Cerezo M, Sánchez-Rodríguez C, Escudero V, Miedes E, Fernández PV, Jordá L, et al. Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi. Mol Plant. 2012;5:98–114. doi: 10.1093/mp/ssr082. [DOI] [PubMed] [Google Scholar]
- 124.Deprost D, Yao L, Sormani R, Moreau M, Leterreux G, Nicolaï M, et al. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep. 2007;8:864–70. doi: 10.1038/sj.embor.7401043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baumberger N, Ringli C, Keller B. The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes Dev. 2001;15:1128–39. doi: 10.1101/gad.200201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baumberger N, Steiner M, Ryser U, Keller B, Ringli C. Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J. 2003;35:71–81. doi: 10.1046/j.1365-313X.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 127.Leiber RM, John F, Verhertbruggen Y, Diet A, Knox JP, Ringli C. The TOR pathway modulates the structure of cell walls in Arabidopsis. Plant Cell. 2010;22:1898–908. doi: 10.1105/tpc.109.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]


